Abstract
Background:
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, in part because one-third of Americans fail to get screened. In a prior randomized controlled trial, we found that an iPad patient decision aid called Mobile Patient Technology for Health-CRC (mPATH-CRC) doubled the proportion of patients who completed CRC screening.
Methods:
All data for the present analysis was collected as part of a randomized-controlled trial to determine the impact of mPATH-CRC on receipt of CRC screening within 24 weeks. Participants were enrolled from 6 community-based primary care practices between June 2014 and May 2016 and randomized to either usual care or mPATH-CRC. Six potential mediators of the intervention effect on screening were considered. The Iacobucci method was used to assess the significance of the mediation.
Results:
A total of 408 patients had complete data for all potential mediators. Overall, the potential mediators accounted for approximately three-fourths (76.3%) of the effect of the program on screening completion. Perceived benefits, self-efficacy, ability to state a screening decision, and patient/provider discussion were statistically significant mediators. Patient/provider discussion accounted for the largest proportion of the effect of mPATH-CRC (70.7%).
Conclusions:
mPATH-CRC increased completion of CRC screening by affecting patient-level and system-level mediators. However, the most powerful mediator was the occurrence of a patient-provider discussion about screening. Digital interventions like mPATH-CRC are an important adjunct to the patient/provider encounter.
Impact:
Understanding the factors that mediated mPATH-CRC’s success is paramount to developing other effective interventions.
Keywords: Colorectal Cancer, Colorectal Cancer Screening, Digital Health, Mediators of Screening, Cancer Prevention
Introduction
Colorectal cancer (CRC) is a major health problem throughout the world, [1] and it is the second leading cause of cancer death in the United States [2]. While screening reduces CRC incidence and mortality, only two-thirds of age-eligible Americans are screened for CRC [3]. The burden of CRC is even greater in groups prone to health disparities. Past studies have shown that participation in CRC screening is lower among racial/ethnic minorities, individuals with low health literacy, and individuals with lower socioeconomic status [4] [5].
Understanding the factors that influence a patient’s screening decision and subsequent completion of screening is paramount to improving CRC screening rates. Several qualitative studies have investigated perceptions and experiences with CRC screening. One meta-analysis examined 94 studies to generate an expanded theory of CRC screening participation [6]. The barriers to CRC screening included language barriers, logistical challenges, and cultural beliefs. Facilitators of CRC screening included awareness of CRC screening, attitudes towards CRC screening, and motivation for screening [6].
Past studies have looked at different interventions to improve CRC screening such as decision aids, direct-mailed fecal immunochemical test (FIT), colonoscopy outreach, reminder systems and patient navigation. In our prior randomized controlled trial of a Web-based decision aid, although the intervention increased desire to receive CRC screening, it did not significantly impact screening test ordering or completion [7]. Several small comparative effectiveness trials and pilot studies have shown improved colon cancer screening rates in safety net clinics with direct mailed FIT cards [8]. The Strategies and Opportunities to STOP Colon Cancer in Priority Populations (STOP CRC) study was a large scale pragmatic study that evaluated effectiveness of a mailed FIT intervention using electronic health record (EHR) tools delivered by clinic staff at federally qualified health centers (FQHC) patient populations [9]. The trial showed a higher adjusted clinic-level proportion of participants who completed a FIT (13.9% in intervention arm versus 10.4% in the usual care arm) [9]. Mailed outreach with colonoscopy invitation has also been tested among vulnerable populations and showed some improvement in screening rates [10]. The success of many direct mailed interventions seems to be linked to reminder systems and patient navigation. Many patients fail to return cards based on fear of results, concern for cost of follow up test, unease of mailing fecal matter or forgetfulness [11]. Live phone call reminder systems seem to be more effective than written or automated communication [12]. Patient navigation has also been found to be an effective adjunct to screening interventions with one study showing intervention patients were more likely to undergo CRC screening than control patients (33.6% vs 20.0%; P < .001) [13] [14].
In our randomized controlled trial looking at the effect of digital health intervention on CRC screening in vulnerable patients, we found that patients who used the Mobile Patient Technology for Health-CRC (mPATH-CRC) iPad program were twice as likely to complete screening [15]. mPATH-CRC is an iPad application that informs patients of the need for screening, helps them make a decision, lets them “self-order” a test, and sends automated electronic messages to help them complete the test. Patients use mPATH-CRC at their primary care provider’s office on a device owned by the practice and can receive follow-up support via text messages or emails on their own devices [15]. The mPATH-CRC study targeted a population prone to health disparities, with greater than 50% of the population having incomes less than $20,000 and 37% of the population reporting poor to fair health status. Therefore we wanted to evaluate what factors led to the success of the intervention in this vulnerable patient population and mediated the common known barriers and facilitators of CRC screening. Mediation analysis allows us to determine the most effective components of the intervention to inform future implementation and scaling.
The key mediators of the intervention can be thought of in three categories: traditional targets of decision aids (knowledge, attitudes, and beliefs), patient provider discussions, and intent to screen in the next 30 days. We examined these mediators as they relate to CRC screening completion.
MATERIALS AND METHODS
Design Overview
All data for the present analysis was collected as part of a randomized-controlled trial to determine the impact of mPATH-CRC on receipt of colorectal cancer screening within 24 weeks. Primary results and details regarding methods have been described previously [15]. Briefly, participants were enrolled from 6 community-based primary care practices between June 2014 and May 2016 and randomized to either usual care or mPATH-CRC. Eligible patients included English-speaking individuals aged 50 to 74 years who were scheduled to see a primary care provider and were due for CRC screening. The intervention was administered to patients prior to the visit to provide “just in time” information that patients could discuss with their provider if they had questions. After patients used mPATH the research assistant attached a brief flyer to their clinic paperwork to let the provider know what the patient chose in terms of screening. If the patient was unsure or did not want screening the flyer encouraged the provider to discuss with the patient. In addition the mPATH program prompted patients who chose not be to be screened with a message that stated “if you have any questions or concerns about getting tested, please talk to your doctor”. Twenty-four weeks after the visit, study staff interviewed patients and reviewed charts to determine if CRC screening had been ordered and completed. Study interviewers and outcome assessors were blinded to participant allocation. The Wake Forest University Health Sciences Institutional Review Board approved the study, all participants provided written informed consent and the study was conducted in accordance with recognized ethical guidelines.
Interventions
Content and usability of the two programs (mPATH-CRC and the Control Program) have been previously published [15] [16]. Briefly, the mPATH-CRC intervention had three primary components: 1) a previously validated brief decision aid about CRC screening reviewing the two most commonly recommended CRC screening tests, fecal testing for blood and colonoscopy, 2) patient self-ordering of screening tests which triggered the research assistant to enter a co-signature required order under the primary care provider’s name in the EHR, and 3) follow-up electronic messages delivered by text or e-mail designed to promote screening test completion. The control program consisted of a brief video about diet and exercise that was produced by the Centers for Disease Control.
Measures
Participants viewed mPATH-CRC or the Control Program and completed a post-program survey that captured attitudes and beliefs about screening immediately prior to their regularly scheduled primary care appointment. (Figure 1) Telephone surveys were administered the next business day to determine if the patients discussed screening with their PCPs; and 24 weeks after the screening visit to determine if screening was ordered and completed. Sociodemographic variables assessed at baseline included age, sex, race and ethnicity, educational attainment, marital status, health insurance coverage, employment, and income. Health literacy was estimated using the single-item health literacy screening question (“How confident are you filling out medical forms by yourself?” [17]).
Figure 1: Overview of timing of study measures.
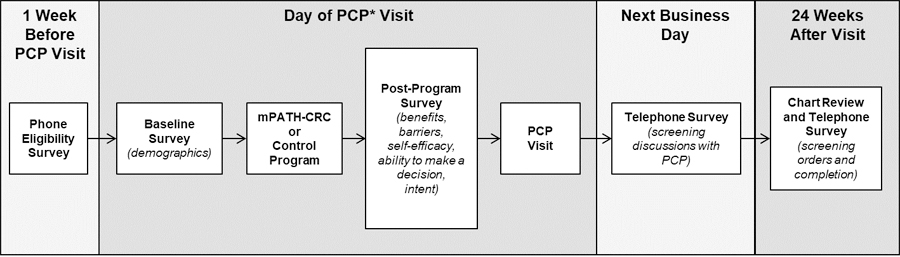
The figure illustrates how the study design was administered. One week prior to the PCP visit the patient underwent a phone eligibility survery. At the day of the visit participants viewed mPATH-CRC or the Control Program and completed a post-program survey that captured attitudes and beliefs about screening immediately prior to their regularly scheduled primary care appointment. Telephone surveys were administered the next business day to determine if the patients discussed screening with their PCPs; and 24 weeks after the screening visit to determine if screening was ordered and completed.
*PCP = Primary care provider
Outcome.
The primary trial outcome was chart-verified completion of a CRC screening test within 24 weeks of study enrollment.
Mediators.
We assessed six potential mediators across three categories: traditional targets of decision aids (benefits, barriers, self-efficacy, and ability to make a decision), patient provider discussions, and intent to screen in the next 30 days. Targets of decision aids were measured in the post-program survey. CRC screening attitudes and beliefs were assessed with 5 point Likert-type items modified from a validated instrument [18]. Five items addressed the perceived benefits of screening (i.e., ability to detect cancer early, ability to save lives) and five item assessed patients’ fears and concerns (i.e., fear of receiving bad news, fear that tests are painful, concerns that tests are embarrassing). We measured self-efficacy to complete CRC screening with a 1-item validated instrument from Vernon et al. [19] Higher scores on these three measures indicate a more positive attitude toward colorectal screening. Ability to make a CRC screening decision was measured with the ability to state a screening preference item. The intention to receive screening was also measured on the post-program survey. We assessed ability to state a preference with the single item, “If you were going to be tested for colon cancer, which test would you want to have?”; possible answers included “stool test for blood”, “colonoscopy”, “I never want to be tested”, and “I don’t know enough to decide.” Intention to receive screening was measured with the item “Are you seriously thinking about getting tested for colon cancer?” with possible answers ranging from “Yes, within the next 30 days” to “No, I am not thinking of getting tested.” Patient-provider discussion about colorectal screening (yes or no) was assessed during the next day telephone survey using the item “At this visit, did you and your doctor discuss getting tested (or screened) for colon cancer?
Statistical analysis
Logistic or linear regression was used to assess the effect of the intervention on the potential mediators and to evaluate the association between mediators and CRC screening completion. The method proposed by Iacobucci [20] was used to assess the significance of each mediator separately. The product of coefficients approach [21] was used to extend Icaobucci’s method to allow for multiple mediators. For the multiple mediator model, the standard error was calculated using bootstrap. Percent mediation was calculated using the crude estimate of ab/ (ab + c’) [22].
Results:
450 participants who were between the ages of 50 and 74 and due for colorectal screening were accrued from six community-based primary care clinics in North Carolina between June 2014 and May 2016; 223 were randomized to the mPATH-CRC arm and 227 to the Control arm. Participant characteristics are shown in Table 1. Briefly, 54% were female, 38% self-identified as African-American, 63% were unemployed, 53% had annual incomes less than $20,000, and 37% had limited health literacy. As previously published, participants in the mPATH-CRC arm were twice as likely to have colorectal cancer screening compared to participants in the Control arm (30% vs 15%), with an accompanying difference in test ordering in the arms (69% vs 32%) [15]. The increase in ordering was apparent for both fecal blood tests (36% vs 11%) and colonoscopies (32% vs 21%). Overall, patients in both the mPATH and Control arms were equally likely to complete CRC tests once they were ordered (43% and 46% respectively, p=0.70); however, participants were more likely to complete an ordered colonoscopy (61%) compared to an ordered fecal test (26%).
Table 1.
Baseline Characteristics of mPATH-CRC Colorectal Cancer Screening Trial Participants
| Characteristic* | mPATH (n=223) |
Control (n=227) |
Total (N=450) |
|---|---|---|---|
| # (%) | # (%) | # (%) | |
| Age, Median (Range) | 58 (50–74) | 57 (50–74) | 57 (50–74) |
| Female | 124 (56) | 118 (52) | 242 (54) |
| Race/Ethnicity | |||
| White, non-Hispanic | 125 (56) | 133 (59) | 258 (57) |
| African-American | 87 (39) | 82 (36) | 169 (38) |
| Hispanic/Latino | 5 (2) | 5 (2) | 10 (2) |
| Other | 6 (3) | 7 (3) | 13 (3) |
| Clinic Site | |||
| 1 | 89 (40) | 92 (41) | 181 (40) |
| 2 | 56 (25) | 57 (25) | 113 (25) |
| 3 | 18 (8) | 18 (8) | 36 (8) |
| 4 | 13 (6) | 14 (6) | 27 (6) |
| 5 | 33 (15) | 32 (14) | 65 (14) |
| 6 | 14 (6) | 14 (6) | 28 (6) |
| Education | |||
| < High school | 46 (21) | 49 (22) | 95 (21) |
| High school | 70 (31) | 74 (33) | 144 (32) |
| Some college or more | 107 (48) | 104 (46) | 211 (47) |
| Married/Living as Married | 104 (47) | 105 (46) | 209 (46) |
| Health insurance | |||
| Uninsured | 28 (13) | 33 (15) | 61 (14) |
| Medicaid | 46 (21) | 34 (15) | 80 (18) |
| Medicare | 58 (26) | 50 (22) | 108 (24) |
| Commercial | 91 (41) | 110 (48) | 201 (45) |
| Employed | 85 (38) | 83 (37) | 168 (37) |
| Income < $20,0001 | 118 (55) | 113 (51) | 231 (53) |
| Limited health literacy | 90 (40) | 76 (33) | 166 (37) |
| Last visit for routine checkup4 | |||
| Within last year | 139 (64) | 135 (62) | 274 (63) |
| General Health5 | |||
| Very good to excellent | 51 (23) | 55 (25) | 106 (24) |
| Good | 87 (40) | 84 (38) | 171 (39) |
| Poor to fair | 81 (37) | 83 (37) | 164 (37) |
| Lives in rural area^ | 51 (23) | 51 (22) | 102 (23) |
A few participants skipped some questions on the self-surveys
n=214 in the mPATH arm and 223 in the Control arm
n=217 and 218
n=219 and 222
rural dwelling defined by Rural-Urban Continuum Areas codes < 4(43)
Mediators of Completion of Screening
As seen in Table 2, the mPATH-CRC program had a significant effect on all the potential mediators of test completion. Participants randomized to the mPATH-CRC arm were more likely: to state a screening decision, to report intent to have a screening test in the next month, and to report a patient/provider discussion about colorectal screening. In addition, perceived benefits, lack of barrier concern, and self-efficacy scores were higher for the mPATH-CRC participants.
Table 2.
Effect of the mPATH-CRC Intervention on Hypothesized Mediators
| Mediator | mPATH (n=223) |
Control (n=227) |
p-value |
|---|---|---|---|
| #/N (%) | #/N (%) | ||
| Ability to form a screening decision | 155/196 (79) | 111/213 (52) | <.001 |
| Intent to Screen within 30 days | 51/223 (23) | 31/227 (14) | .011 |
| Patient/Provider Discussion about screening | 150/197 (76) | 103/213 (48) | <.001 |
| Mean (SD) |
Mean (SD) |
||
| Benefits of Screening* | 4.54 (0.51) | 4.34 (0.59) | <.001 |
| Barriers to Screening* | 3.61 (0.67) | 3.49 (0.66) | <.001 |
| Self-Efficacy for Screening* | 3.89 (0.84) | 3.64 (1.00) | .004 |
Higher scores indicate more positive attitudes towards screening
As shown in Table 3 all proposed mediators were significantly associated CRC test completion in univariate analyses.
Table 3.
Association between Mediators and Colorectal Cancer Screening Completion within the mPATH-CRC Trial (N=450).
| Mediator | Screened (n=101) |
Not Screened (n=349) |
p-value |
|---|---|---|---|
| #/N (%) | #/N (%) | ||
| Ability to form a screening decision | 75/94 (80) | 191/315 (61) | .001 |
| Intent to Screen within 30 days | 30/101 (30) | 52/349 (15) | .001 |
| Patient/Provider Discussion about screening | 85/94 (90) | 168/316 (53) | <.001 |
| Mean (SD) |
Mean (SD) |
||
| Benefits of Screening* | 4.60 (0.51) | 4.39 (0.56) | <.001 |
| Barriers to Screening* | 3.79 (0.63) | 3.48 (0.66) | <.001 |
| Self-Efficacy for Screening * | 4.00 (0.77) | 3.69 (0.96) | .004 |
Higher scores indicate more positive attitudes towards screening
After including all potential mediators in a multivariable model (Table 4), the odds ratio for the effect of the mPATH-CRC program on colorectal cancer screening completion dropped from 2.70 (95% CI: 1.66, 4.39) to 1.68 (95% CI: 0.98, 2.88). Overall, the potential mediators accounted for approximately three fourths (76.3%) of the effect of the program on screening completion. Perceived benefits, self-efficacy, ability to state a screening decision, and patient/provider discussion were statistically significant mediators. Patient/provider discussion accounted for the largest proportion of the effect of mPATH-CRC (70.7%).
Table 4.
Association between mPATH Intervention Arm and Colorectal Cancer Screening Test Completion – With and Without Adjustment for Mediators
| Adjusted for Mediator | Mediation | |||||
|---|---|---|---|---|---|---|
| Mediator | beta | SE | OR | 95% CI | % | p-value |
| Unadjusted Intervention Effect | .9938 | .2477 | 2.70 | 1.66, 4.39 | ||
| Adjusted for… | ||||||
| Benefit Score | .8851 | .2518 | 2.42 | 1.48, 3.97 | 12.4 | .029 |
| Barriers Score | .9476 | .2518 | 2.58 | 1.58, 4.23 | 7.1 | .144 |
| Self-Efficacy | .9149 | .2508 | 2.50 | 1.53, 4.08 | 10.1 | .047 |
| Decision | .8309 | .2562 | 2.30 | 1.39, 3.79 | 46.8 | .031 |
| Intend to Screen (30d) | .9344 | .2510 | 2.55 | 1.56, 4.16 | 33.7 | .086 |
| Pt/Doc Discussion | .6289 | .2620 | 1.88 | 1.12, 3.13 | 70.7 | <.001 |
| All Mediators | .5193 | .2754 | 1.68 | 0.98, 2.88 | 76.3 | 0.006 |
This analysis was conducted using only complete cases (N=408).
Discussion:
Previous studies have shown that patients’ decision to participate in colorectal cancer screening depends on their views of cancer, attitudes towards colorectal cancer screening modalities, and motivation for screening [6]. The mPATH-CRC digital health intervention includes a decision aid that targets these patient-level mediators as well as system changes that target structural barriers. Decision aids, like the one incorporated in mPATH-CRC, have been shown to positively impact patients’ decision-making abilities and screening intentions [23] [24]. We found a similar effect of mPATH-CRC on these patient-level mediators in our analyses. However, the most powerful mediator of screening completion we observed was the occurrence of a patient-provider discussion about screening.
Other studies have found that a physician’s recommendation is one of the most powerful motivators for patients to accept screening [25] [26] [27] Patients’ trust and value physician guidance on important decisions, especially in vulnerable populations [28] [29]. In our randomized controlled trial, half of patients who used mPATH-CRC ordered their own screening test via the program, but 76% reported discussing screening with their provider. This finding illustrates that even patients who order their own screening may value discussing their decision with their provider. By administering mPATH-CRC immediately before a medical visit, the program provides “just in time” information delivery to patients and may prompt patients to engage in screening discussions with their providers. The program also ends with a prompt to ask your provider questions or clarify issues of concern which also helps to foster patient-provider discussion. Whether mPATH-CRC would yield similar effects on screening rates if it were administered separate from a medical visit is unknown.
Our analysis also found that all mediators accounted for approximately three-fourths of the effect of mPATH-CRC, which means that a quarter of mPATH-CRC’s effect on screening is due to other aspects of the intervention. One key feature of mPATH-CRC is the ability for patients to self-order a screening test. In an earlier trial of a CRC screening decision aid that did not include the option for patients to order their own screening, we found that only one-third of patients who indicated a desire for immediate screening had a screening test ordered by their provider, and there was no significant difference in completion of screening compared to the control group [7]. Healthcare providers identify lack of time as a major barrier to delivering preventive care services, which may explain this significant gap between patients’ desire for screening and receipt of screening [30]. By allowing patients to self-order tests, mPATH-CRC reduces time barriers and empowers patients to manage their own care.
Other effective strategies for off-loading work from busy clinicians and facilitating care delivery include patient navigation. Reuland et al. combined a CRC screening decision aid with patient navigation and found CRC screening rates more than doubled [13]. While effective, in-person patient navigation is resource-intensive. In contrast, mPATH-CRC functions as a digital health navigator by facilitating test ordering and encouraging test completion via automated patient reminders. Although we found mPATH-CRC doubled screening rates, we also observed that patients completed only half of ordered screening tests suggesting that some patients will need more than digital navigation. Starting with mPATH-CRC and then stepping-up to in-person navigation as needed may yield higher screening rates with less resources than a pure in-person navigation approach.
Our study has limitations. While our study sample represented socioeconomic diversity, it was limited to English-speakers and a single health system. We have since developed a Spanish version of the application that is now being implemented in clinics. The barriers and facilitators of screening and how they mediate completion of screening could differ in other subgroups. Additionally, the occurrence of patient-provider discussions about CRC screening was based on patient report, and we did not audiotape encounters to explore the content of those discussions.
In summary, mPATH-CRC increased completion of CRC screening by affecting traditional targets of decision aids including perceived benefits, self-efficacy, and ability to form a screening decision, however, the most powerful mediator was the occurrence of a patient-provider discussion about screening. The ability of patients to self-order a screening test may also account for a significant proportion of mPATH-CRC’s effect. Given the importance of patient-provider discussions, digital interventions like mPATH-CRC are an important adjunct to the patient/doctor encounter to allow for better shared-decision making discussions but are not a replacement. Future research should investigate factors that lead to test completion and strategies for helping patients complete ordered tests. This could consist of a more robust reminder system, live phone calls from clinic staff, and phased in patient navigation.
Financial support:
The study received funding from the National Cancer Institute (R01CA178941). The study was supported by the Wake Forest Clinical and Translational Science Institute study coordinator pool (UL1TR001420) and by the Wake Forest Comprehensive Cancer Center shared resources (CCSG P30CA012197).
Footnotes
Conflict of Interest Policy:
“Dr. David Miller is the developer of the mPATH application. Dr. Miller and Wake Forest University Health Sciences have an ownership interest in the application.”
References:
- [1].Ferlay J, Hai-Rim S, Bray F, Forman D, Mathers C and Parkin D, “Estimates of worldwide burden of cancer in 2008:GLOBOCAN 2008,” Int J Cancer, pp. 127:2893–2917, 2010. [DOI] [PubMed] [Google Scholar]
- [2].A. C. Society, “Cancer facts and figures, 2017,” American Cancer Society, 2017.
- [3].K. J. R. T. T. C. R. L. Joseph DA, “Use of Colorectal Cancer Screening Tests by State,” Preventing Chronic Disease, vol. 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martin D. e. a., “Recommendations for Cancer Epidemiologic Research in Understudied Populations and Implications for Future Needs,” Cancer Epidemiol Biomarkers Prev, vol. 25, no. 4, pp. 573–80, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].S. C. G. A. W. J. von Wagner C, “Health literacy and self-efficacy for participating in colorectal cancer screening: the role of information processing,” Patient Educ Couns, vol. 75, pp. 352–357, 2009. [DOI] [PubMed] [Google Scholar]
- [6].Honein-AbouHaidar GN, Kastner M, Vuong V, Perrier L, Daly C, Rabeneck L, Straus S and Baxter N, “Systematic Review and Meta-Study Synthesis of Qualitative Studies Evaluating Facilitators and Barriers to Particpation in Colorectal Cancer Screening,” Cancer Eidemiol Biomarkers Prev, vol. 25, no. 6, pp. 907–917, 2016. [DOI] [PubMed] [Google Scholar]
- [7].M. D. Jr, JG S, LD C, DC G, S S and MP P, “Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population,” Am J Prev Med, vol. 40, pp. 608–615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].V. W. P. A. e. a. Coronado GD, “Strategies and opportunities to STOP colon cancer in priority populations: pragmatic pilot study design and outcomes,” BMC Cancer, vol. 14, p. 55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coronado G, Petrik A, Vollmer W, Taplin S, Keast E and Field SGB, “Effectivenss of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial,” JAMA Internal Medicine, vol. 178, no. 9, pp. 1174–1181, August 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gupta S, Halm E, Rockey D, Hammons Marcia, Koch M, Carter E and et al. , “Comparative Effectiveness of Fecal Immunochemical Test Outreach, Colonoscopy Outreach, and Usual Care for Boosting Colorectal Cancer Screening Among the Underserved A Randomized Control Trial,” JAMA Internal Medicine, vol. 173, no. 18, pp. 1725–1732, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].J. L. S. J. J. S. A. F. P. B. G. Coronado Gloria D, “Reasons for non-response to a direct-mailed FIT kit program: lessons learned from a pragmatic colorectal-cancer screening study in a federally sponsored health center,” Translational Behavioral Medicine, vol. 5, no. 1, pp. 60–67, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].R. J. F. M. e. a. Coronado GD, “Effect of Reminding Patients to Complete Fecal Immunochemical Testing: A Comparative Effectiveness Study of Automated and Live Approaches,” Journal of General Internal Medicine, vol. 33, no. 1, pp. 72–78, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reuland D. e. a., “Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population,” JAMA Internal Medicine, vol. 177, no. 7, pp. 967–974, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].M. J. L. S. e. a. Lasser KE, “Colorectal Cancer Screening Among Ethnically Diverse, Low-Income Patients: A Randomized Controlled Trial.,” Archives of Internal Medicine, vol. 171, no. 10, pp. 906–912, 2011. [DOI] [PubMed] [Google Scholar]
- [15].M. e. al., “Effect of a Digital Health Intervention on Reciept of Colorectal Cancer Screening in Vulnerable,” Annals of Internal Medicine, vol. 168, pp. 550–557, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].D. W. K. C. L. B. D. L. D. D.-T. N. e. a. Miller, “Usability of a Novel Mobile Health iPad App by Vulnerable Populations,” JMIR MHealth UHealth, vol. 5, no. 4, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].R. E. R. S. H. D. W. B. Wallace LS, “Brief report: screening items to identify patients with limited health literacy skills,” Journal of General Internal Medicine, vol. 21, pp. 874–877, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].C. V. M. U. L. P. V. G. S. C. Rawl S, “Validation of scales to measure benefits of and barriers to colorectal cancer screening,” Journal of Psychosocial Oncology, vol. 19, pp. 47–63, 2011. [Google Scholar]
- [19].M. R. T. B. Vernon SW, “Development and validation of an instrument to measure factors related to colorectal cancer screening adherence,” Cancer Epidemiologoy Biomarkers & Prevention, vol. 6, pp. 825–832, 1997. [PubMed] [Google Scholar]
- [20].I. D. e. al, “Medication analysis and categorical variables: The final frontier,” Journal of Consumer Psychology, vol. 22, pp. 582–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Preacher IA, “Asymptotic and reseamline strategies for assessing and comparing indirect effects in multiple mediator models,” Behavior Research Methods, vol. 40, pp. 879–891, 2008. [DOI] [PubMed] [Google Scholar]
- [22].Rijnhart J. T. J. E. I. e. a., “Comparison of logistic-regression based methods for simple mediation analysis with dichotomous outcome variable,” BMC Med Res Methodol, vol. 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].RJ V, SK L, MA L-O, GR K, DS R and e. a. Saraykar SS, “Patient decison aids for colorectal cancer screening: a systematic reviw and meta-analysis,” Am J Prev Med, vol. 51, no. 5, pp. 779–791, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].S. D. e. al., “Patient Decision Aids to Engage Adults in Treatment or Screening Decisions,” JAMA, vol. 318, no. 7, pp. 657–658, 2017. [DOI] [PubMed] [Google Scholar]
- [25].McLachian S. e. a., “Patients’ experiences and reported bariers to colonoscopy in the screening context- a systematic review of the literature.,” Patient education and counseling, vol. 86, no. 2, pp. 137–146, 2012. [DOI] [PubMed] [Google Scholar]
- [26].K. N. Gilbert A., “Colorectal caner screening: physician recommendations is influential advice to Marylanders,” Preventive Medicine, vol. 41, no. 2, pp. 367–379, 2005. [DOI] [PubMed] [Google Scholar]
- [27].T. V. e. al, “Colorectal cancer screening among African Americans: the importance of physician recommendations,” Journal of National Medical Association, vol. 95, no. 9, p. 806, 2003. [PMC free article] [PubMed] [Google Scholar]
- [28].Becker ER and Roblin DW, “Translating primary care practice climate into patient activation: the role of patient trust in physician,” Med Care, vol. 46, no. 3, pp. 795–805, 2008. [DOI] [PubMed] [Google Scholar]
- [29].W. RO, C. RJ, P. CA, B. A, S. JS, W. K. B. S. K. S and R. R, “Perceptions of Provider Communication Among Vulnerable Patients With Diabetes: Influences of Medical Mistrust and Health Literacy,” J Health Communication, vol. 21, pp. 127–134, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Y. KS, P. Kl, O. T, K. KM and M. JL., “Primary Care: is there enough time for prevention?,” AM J Public Health, vol. 93, pp. 635–641, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


