Abstract
Two neurons can only form a synapse if their axonal and dendritic projections meet at the same time and place. While spatiotemporal proximity is necessary for synapse formation, it remains unclear to what extent the underlying positional strategies are sufficient to ensure synapse formation between the right partners. Many neurons readily form synapses with wrong partners if they find themselves at the wrong place or time. Minimally, restricting spatiotemporal proximity can prevent incorrect synapses. Maximally, restricting encounters in time and space could be sufficient to ensure correct partnerships between neurons that can form synapses promiscuously. In this review we explore recent findings on positional strategies during developmental growth that contribute to precise outcomes in brain wiring.
Introduction
Brain wiring is a developmental growth process. As in any other growth process, cellular interactions are restricted in space and time. Correspondingly, surface molecular interactions are restricted to those that ‘get to see’ each other during development. Many of these interactions contribute to such positional effects during brain wiring, others directly ensure synaptic partnerships [1–5]. Disruption of either of these two types of molecular functions is likely to lead to brain wiring defects.
Since the late 1980s, the quest for molecular mechanisms that contribute to brain wiring focused on attractive and repulsive molecular signals that specify where to grow an axon or to make a synapse. This approach has blossomed over the years and is considered in excellent recent reviews [1,5]. In addition, recent years have seen an increasing number of remarkable molecular and cellular mechanisms that contribute in less expected ways to developmental growth, and ultimately specificity in brain wiring. Processes like axon pre-sorting, branch self-avoidance and tiling, or approach angles and speed are all essential for the right neuronal partners to meet each other prior to synapse formation [6–8]. As the brain grows, every neuron runs a cell-intrinsic growth program and responds to environmental cues. The sum of all the mechanisms that restrict encounters between potential partners during the time when both are able to form synapses is a significant contributor to synaptic specificity.
The study of dynamically changing processes during development, as opposed to a focus on final outcomes, is key to the recognition of the developmental steps that bring the right partners together. Mutations in many genes that encode cell surface molecules known to engage in interactions with other cell surface molecules, result in adult brain wiring defects. However, the interpretation of an ‘attractive’ or ‘repulsive’ guidance mechanism is only one aspect of the remarkable variety of molecular mechanisms that contribute to spatiotemporal positioning during development [6,9]. The adult phenotype may reveal little about the transient or dynamic nature of developmental events that occurred at a specific time and place. In addition, earlier defects can change the developmental growth program dramatically and mask later functions.
Amongst developmental growth processes, the identification of neuronal partners during brain wiring is particularly challenging to study in an in vivo context. This difficulty is further exacerbated if the molecular and cellular mechanisms are dynamic and transient and only observable in living brain tissue. As a consequence, it remains largely unknown to what extent spatiotemporal positioning facilitates or determines synaptic partnerships in vivo. In the following sections, we review recent progress in our understanding of the remarkable, and often surprising, molecular and cellular mechanisms that encode positional strategies during developmental growth of synaptic connectivity.
Pre-specification, post-specification and synaptic promiscuity
Early proposals for molecular mechanisms of connectivity focused on precise matchmaking, i.e. key-and-lock mechanisms for neuronal connections based on Sperry’s notion of chemical tags of specificity: ‘a kind of chemical code with matching values between the retinal and tectal maps.’ [10]. In its strict form, molecular matchmaking pre-determines synaptic partners and thus prevents the neuron from forming synapses promiscuously with incorrect partners. (Fig. 1A). However, many neurons have the ability to form synapses with incorrect partners, including themselves [9,11], as also shown in several recent examples below. Notably, activity-dependent synaptic pruning initially requires excessive and therefore somewhat promiscuous synapse formation for the development of precise connectivity through synaptic pruning. We call this post-specification of connectivity, which is now known to occur as either activity-dependent or -independent synapse elimination [1,12,13] (Fig. 1B). By contrast, synaptic pre-specification occurs when only certain neurons get to contact each other at the same time and place during the developmental period when they are competent to form synaptic connections (Fig. 1C). A theoretical neuron that is only exposed to correct partners at the time it is competent to make synapses, could do so promiscuously without sacrificing specificity.
Figure 1. Mechanisms that contribute to correct synaptic partnerships.
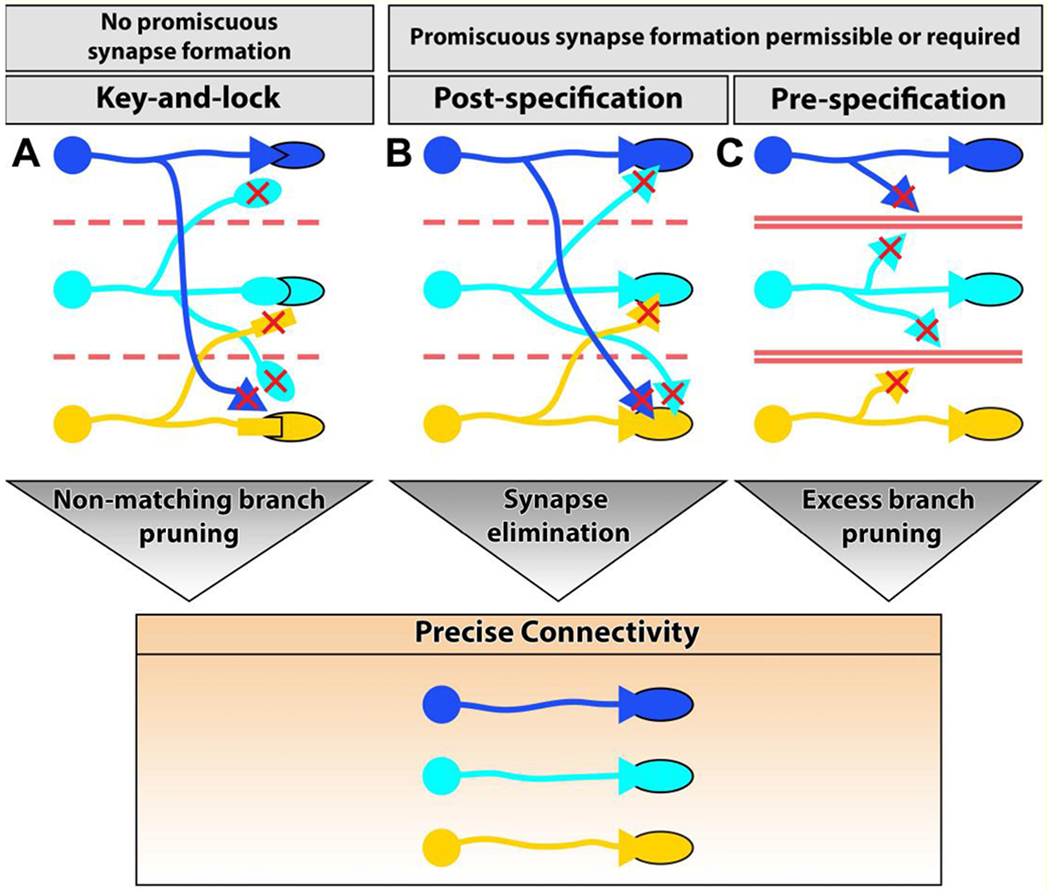
The following processes can contribute to precise connectivity during developmental growth. (A) Key-and-lock recognition, or molecular matchmaking, determines precise partners and precludes unspecific synaptic promiscuity. (B) Post-specification: neurons initially form exuberant or promiscuous synapses. Activity-dependent or activity-independent fine-tuning eliminates incorrect synapses in a typically competitive developmental process. (C) Pre-specification: neurons that have the capacity to form unspecific synapses can be restricted in their encounters of potential partners in time and space, ensuring precise connectivity.
Birth order
Brain development requires the coordinated neuronal differentiation in a temporally regulated manner [14,15]. Furthermore, development of neural circuits is intricately linked to the timing of the neuronal birth order [16–19]. The birth order can contribute to the temporal organization of development by enabling successive neurite outgrowth, leading to successive target area innervation and thereby spatial segregation of subsequent synapse formation (Fig. 2A). For example, during motor circuit development in mice the timing of neuronal birth leads to spatial segregation of antagonistic extensor-flexor pre-motor neurons and their synaptic partners [20]. In that study, the change in the spatial positioning was shown to change the synaptic partner choice without changing the cell-fate or expression of known cell-type specific cell surface receptors. In the vertebrate visual system, the difference in the neuronal birth order can lead to differential occupancy of the target field and therefore altered target selection [21]. Here, early born neurons extended axons over a large area, while late born neurons are constrained to occupy a significantly smaller target field with a reduced need for synaptic pruning. These studies demonstrate that temporal coordination of neuronal specification can affect the regulation of synaptic partner choice by either spatial segregation of afferent axons with their appropriate partner cell neurites or by making promiscuous synapses to be refined later (pre- and post-specification, respectively; Fig. 1B, C).
Figure 2. Temporal and spatial coordination of cell body and axon positioning.
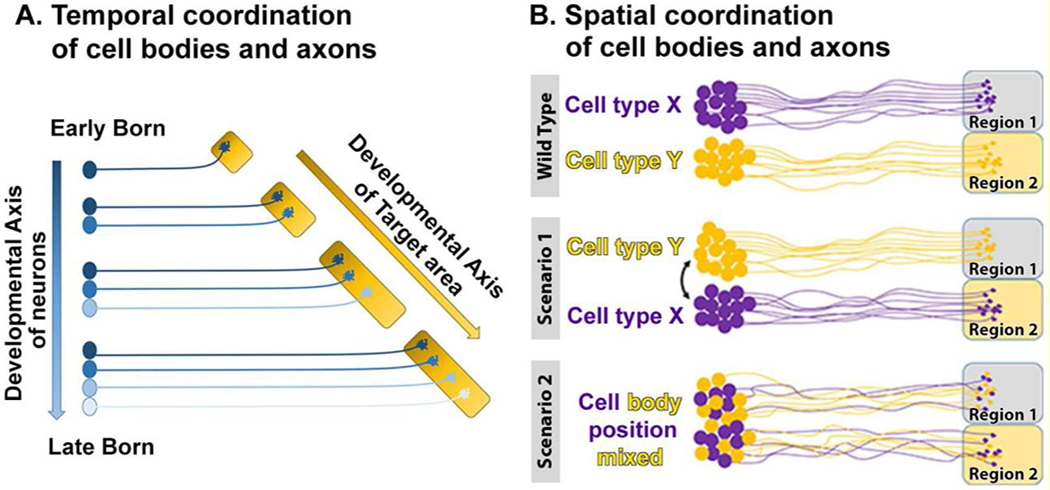
(A) The temporal order of cell differentiation leads to differences in the temporal and spatial positioning of cell bodies. Such temporal differences are reflected in spatial segregation of axons in the target field. (B) Cell body positioning can play critical roles for axon targeting. In some cases, depicted as Scenario 1 and Scenario 2, swapping cell body positions can pre-determine altered connectivity. If synapse formation is sufficiently promiscuous, synapses may form in the incorrect target areas. If synapse formation requires a specific molecular match, then mis-targeted axon terminals should not form synapses in incorrect target areas.
In the Drosophila visual system, a birth order-dependent temporal sequence of axon growth in the brain leads to positional segregation of two types of photoreceptor axons, called R8 and R7. This early segregation of positions is sufficient to bring R7 axons in significant overlap with neurites of their main post-synaptic partner cells, called Dm8 [22]. The study showed that changing the position of R8 axons, without affecting their cell fate, is sufficient to bring R8 axon terminals in sufficient proximity with the R7 target cells Dm8 to induce synapse formation between these incorrect partners. Similarly, the highly stereotypic connections of T4/T5 (major neurons in the motion vision circuit in Drosophila) are formed in a temporal order that corresponds to the birth order and a coordinated differentiation program of these neurons [16,17,23]*.
Finally, in the Drosophila navigation circuit, the temporal sequence of neurogenesis has recently been shown to regulate axon targeting of columnar neurons [18]*. This study showed that the four classes of columnar neurons in the Drosophila central complex each differentiate during a tight temporal window. Neurons born at the same time connect to the same region in the central complex, whereas neurons born at different times target to different regions. These recent examples from both vertebrates and invertebrates highlight how genetically identical neurons that are born at different times, can encounter different partner neurons during development, leading to different connectivity.
Cell Body Position
Closely related to the time of birth is the resulting cell body position. No two neurons can occupy the exact same space, and their relative positions can pre-determine restrictions for connectivity (Fig. 2B). Following birth, many neurons migrate to different positions that set up zones required for the spatiotemporal matching of neurogenesis and connectivity, as elegantly shown in the fly visual system [24].
In mouse cortex the correct migration of cortical neurons is a prerequisite for structural cortex folding [25]. Recent work revealed direct coupling of the migratory routes of developing cortical interneurons to the axon targeting program in a dynamic fashion [26]. In this study, the migratory routes of interneurons were cell-intrinsically altered through conditional deletion of the Mafb-a gene. The mutant cells with altered migratory routes exhibited significantly altered axonal arborizations compared to mutant cells that had migrated normally. In a separate study, knock-down of the microtubule binding protein Dcx was utilized to characterize the positional effect following altered migration of cortical neurons [27]. Interestingly, neurons that are ectopically positioned due to Dcx knock-down form synapses in ectopic positions, most likely with non-cognate synaptic partners. Hence, here as elsewhere, neurons reveal a principal capacity to establish synaptic contacts with available partners at the time they are competent form synapses.
Cellular positioning, which is a prerequisite for proper connectivity, has been studied in quantitative detail for motor neuron cell bodies that are organized into clusters called ‘pools’ in the spinal cord. Several type 1 and type 2 classical cadherins function in the spatiotemporally precise arrangement of these motor pools [28,29]*. Interestingly, these studies did not reveal an obvious ‘Cadherin code’ for motor pool organization, but suggested that partially redundant Cadherin functions temporally separate segregation phases. In all cases highlighted here, the positions of cell bodies altered the subsequent growth processes, leading to ultimate mis-wiring defects.
Pre-Target Axon-Axon interactions
Historically, the visual system holds a special place in the study of neuronal connectivity. Retinal output neurons map neighboring points in visual space as neighboring connections in both the vertebrate and invertebrate brains, a principle known as retinotopy [30]. Remarkably, the spatial organizations of axons along their length, i.e. their ‘neighborliness’ (Fig. 3A), was shown early on to be preserved in flies [31] and cichlid fishes [32], and more recently, based on whole brain connectomics, in zebrafish [33]. In the fly visual system, a complicated wiring principle called ‘neural superposition’ further pools only those retinal axons in distinct synaptic units that carry the same visual information [34]. In the vertebrate olfactory system, olfactory sensory neuron axons pre-sort before reaching their corresponding glomeruli and establish topographic order in the anterior-posterior axis of the olfactory bulb [35]. During retinocollicular wiring in the mouse visual system, nasal retinal ganglion cells project to the caudal part of the superior colliculus, whereas temporal retinal ganglion cells project to the rostral side; this leads to a precise topographic map and target-independent, inter-axonal interactions are a necessary part of this process [36]. A more recent study utilized elegant live imaging experiments to characterize axon pre-sorting in Xenopus explants [37]**. This study characterized how axons from the same (homotypic) side fasciculate, while heterotypic interactions with axons from the other side result in ‘tip-toe-tracking’. Both processes can be directly related to filopodial dynamics that are defective in the cyfip2 mutant. Finally, axon-axon interactions have also recently been shown to regulate midline binary choices of retinal ganglion cells in mice [38].
Figure 3. Axon-axon and dendrite-dendrite interactions create patterns that restrict neuronal encounters in time and space.
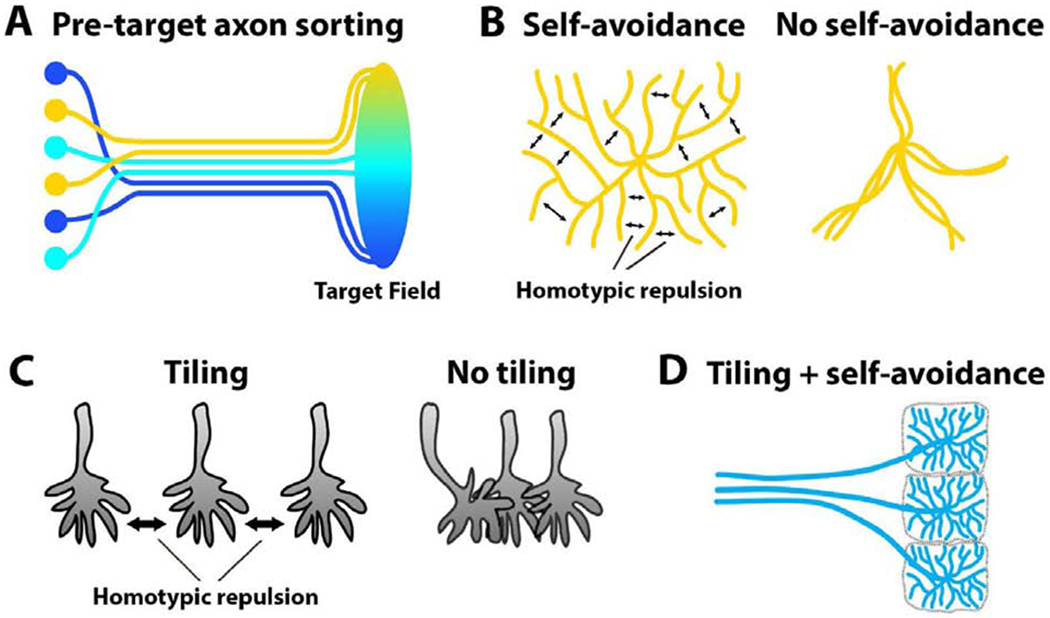
(A) Inter-axonal interactions facilitate selective fasciculation and topographic sorting among axons before they reach their target field. (B) Homotypic repulsion between sister processes causes arborizations to spread (left). Loss of self-avoidance results in crossing and clumping of arborizations (right). (C) Homotypic repulsion between processes results in equal spacing (tiling). Loss of repulsion causes axons to overlap and clump together. (D) Concurrent utilization of these self-repulsive and non-self-repulsive mechanisms can create patterns that restrict and facilitate specific neuronal encounters.
In addition to axon sorting through inter-axonal interactions, topographic order among axons can be established by intermediate secondary structures before axons reach their final target regions. For example, representation of mouse facial whiskers is transferred to the neocortex by thalamo-cortical axons. During development, these axons are already topographically ordered after passing through the basal ganglia primordium [39]. These examples highlight how early axonal patterning serves as important input for downstream developmental processes that lead to correct connectivity.
Branch patterning in the target region
Axonal and dendritic projections of many neurons branch out in their respective target areas in search of neuronal partners. A key discovery was the role of self-avoidance for the spreading of branches, as reviewed previously [40,41]. In the absence of self-avoidance, branches clump together and thereby reduce the target area for synaptic partnerships (Fig. 3B). In Drosophila, the Down syndrome cell adhesion molecule 1 (Dscam1) gene can produce 38,016 transmembrane proteins through non-deterministic alternative splicing and this variability is required as random differentiation marks for ‘self’ versus ‘non-self’. Similar to Drosophila Dscam1, the vertebrate clustered proto-cadherin (Pcdh) cell surface proteins are required for self-avoidance, with an analogous function for self/non-self-discrimination in mouse retinal starburst amacrine cells [41,42]. In a recent study, it has been shown that Pcdh diversity is also required for the mouse olfactory neural circuit assembly [43]*. In the complete loss of all Pcdh genes, olfactory sensory neuron axons were clumped and distorted which led to the formation of abnormal protoglomeruli and thus mis-wiring.
While self-avoidance requires recognition of’self and blindness with respect to ‘non-self, tiling is based on repulsion between ‘non-self branches (Fig. 3C). The same classes of molecules mediating repulsion have been implicated in self-avoidance and tiling [6,44,45]. In addition, repulsion through mutual inhibition has previously been shown to pattern the spatial positioning of axons [46]. In a recent study, it has been shown that regular tiling of radial glial cells in the neocortex is essential for the laminar and columnar organization of neurons in cerebral cortex [47] Once axons find themselves in an incorrect position of a laminar structure, synapse formation may happen between incorrect partners, as shown in the Drosophila central complex [48] Repulsion-based mechanisms can thereby pre-pattern axonal and dendritic positions for presumptive synaptic partnerships (Fig. 3D), and changes of these patterns have downstream effects on synaptic connectivity.
Synaptic partner selection strategies
What happens when pre- and postsynaptic branches meet? Molecular interactions precede synapse formation [1], and they do so either promiscuously or, to exclude incorrect partnerships, with specificity. It has long been known that the number of synapses can be a function of overlap between dendritic and axonal arbors of two potential synaptic partners, a principle known as ‘Peter’s rule’ [49] (Fig. 4A). Specificity can be ‘sharpened’ several-fold above predictions from Peter’s rule through adhesive biasing, as shown in the vertebrate visual system for a specific synaptic amacrine cell type and a retinal ganglion cell type that both express the homophilic adhesion molecule sidekick2 [50]. Another type of retinal ganglion cells, the ‘ONα’ type, are in contact with several bipolar cell types, yet 70% of its synapses are with B6 bipolar cells. When B6 cells are ablated, synapses form with other bipolar cell types that normally have few or no synapses with the ONα retinal ganglion cells [51]. Finally, in the hippocampus, Schaffer collateral synapses with parvalbumin-positive interneurons match predictions by Peter’s rule, while Schaffer collateral synapses with pyramidal cells exhibit increased specificity [52]. Hence, axon-dendritic overlap can critically contribute to synapse formation without being by itself sufficient for synaptic specificity.
Figure 4. Positional and dynamic properties of axo-dendritic contacts restrict synapse formation.
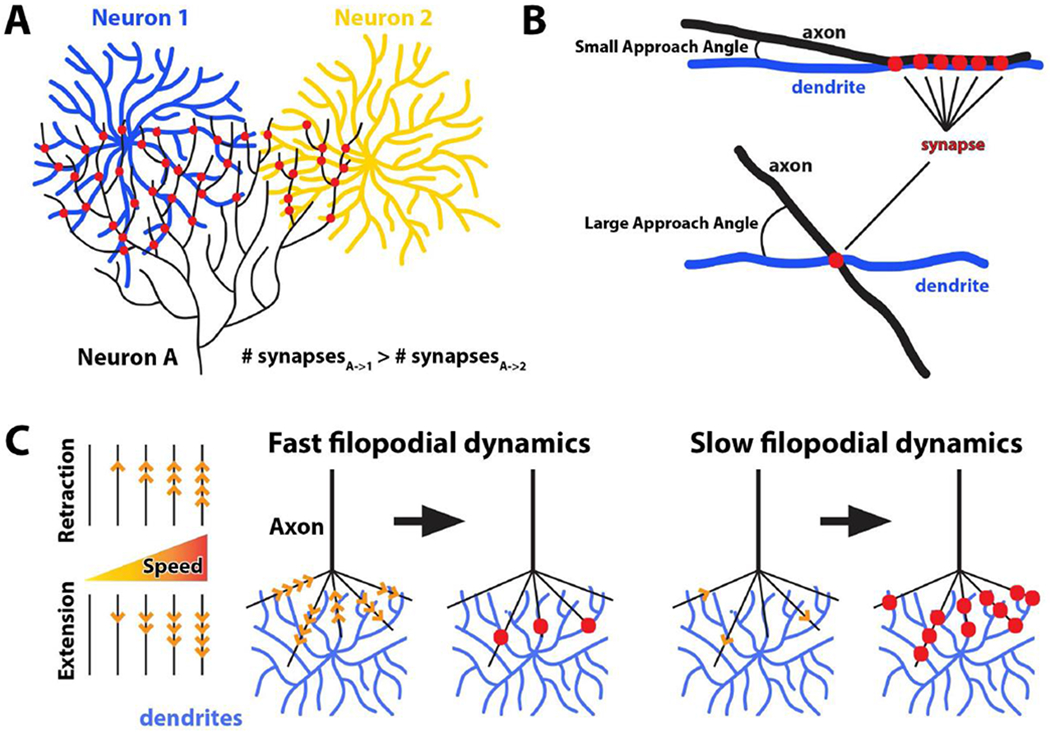
(A) Overlap between axonal and dendritic arbors influences the number of synapses between based on Peter’s rule. If no other mechanisms contributed to synaptic specificity, neuron A (black) should form more synapses with neuron 1 (blue) than neuron 2 (yellow) due to larger overlap. (B) Approach angles of axons and dendrites were recently shown to be a determinant of synaptic specificity. (C) Kinetics of axonal filopodia can restrict synaptic partner choice by modulating number of synapses. In this case, fast and destabilized filopodia more often fail to establish contacts, while slower, stable filopodia may increase the probability for synapse formation.
Being at the right time and place based on axon-dendritic overlap may not be enough to ensure that neurons actually meet each other. A recent study in the spinal sensory-motor reflex circuit found that axon-dendritic overlap (akin to Peter’s rule) was insufficient to explain the connection specificity [7]**. Instead, the authors found that, remarkably, specific approach angles of axons and dendrites served as a determinant of connection specificity (Fig. 4B).
The idea of axon-dendritic overlap as a determinant for synapse-specific contacts has mostly been studied in fixed preparation. Recent evidence suggests that the kinetics, i.e. speed and stability, of axon-dendritic interactions may serve to further restrict synaptic partner choice. In the Drosophila visual system, photoreceptor axon terminal dynamics can be dialed up and down through modulation of autophagy, leading to more synapses for slower, stable filopodia and fewer synapses for faster, destablized filopodia [8,53]. Remarkably, the increased synapse formation also lead to the recruitment of incorrect synaptic partners whose dendritic arborizations are available in the target area. Hence, both interaction angles and speed can quantitatively restrict to what extent neurons meet each other.
Concluding Remarks
Neurons want to make synapses. If the correct partners are not available, many neurons have the capacity to make promiscuous synapses with incorrect partners, including with themselves. Developmental growth brings partners together in a slow process during which earlier steps serve as necessary basis for subsequent developmental steps. Ever more precise analyses of molecular and cellular mechanisms in this process reveal developmental steps that would not have been knowable from analyses of developmental outcomes alone. More developmental steps increase the opportunities for the growth program to restrict what neurons encounter each other at the time they express specific surface molecules required to turn encounters into synapses. The recent discoveries of an increasing number of positional strategies are a direct consequence of spatiotemporally higher-resolved analyses. These highlights illuminate only a few of the ways how the growth program controls neuronal encounters. Each highlight reflects an incomplete snapshot of a process that must occur in the context of cell-intrinsic properties, tissue properties and molecular interactions. Only together, these properties and mechanisms create positional effects and constitute the ‘instruction’ for directional growth and synapse formation.
Highlights.
A series of developmental steps restricts neuronal contacts during brain wiring
Restricting contacts in time and space allows for more promiscuous synapse formation
Positional strategies are dynamic processes in time and space
Directional instructions are syntheses of molecular, cellular and tissue properties
Acknowledgements
We would like to thank Bassem Hassan, Mathias Wernet and Ridvan Kiral for critical reading of this manuscript. We further thank all members of the Hiesinger, Wernet and Hassan labs in the Division for Neurobiology at the Freie Universitat Berlin for their support and helpful discussions. This work was supported by the NIH (RO1EY018884) and the German Research Foundation (DFG, SFB 958, SFB186) and FU Berlin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sudhof TC: Towards an Understanding of Synapse Formation. Neuron 2018, 100:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sando R, Jiang X, Sudhof TC: Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 2019, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart MP, Hobert O: Neurexin controls plasticity of a mature, sexually dimorphic neuron. Nature 2018, 553:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong W, Mosca TJ, Luo L: Teneurins instruct synaptic partner matching in an olfactory map. Nature 2012, 484:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apostolo N, de Wit J: Compartmentalized distributions of neuronal and glial cell-surface proteins pattern the synaptic network. Curr Opin Neurobiol 2019, 57:126–133. [DOI] [PubMed] [Google Scholar]
- 6.Petrovic M, Schmucker D: Axonal wiring in neural development: Target-independent mechanisms help to establish precision and complexity. Bioessays 2015, 37:996–1004. [DOI] [PubMed] [Google Scholar]
- 7.**.Balaskas N, Abbott LF, Jessell TM, Ng D: Positional Strategies for Connection Specificity and Synaptic Organization in Spinal Sensory-Motor Circuits. Neuron 2019, 102:1143–1156 e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]; In the spinal sensory-motor reflex circuit, approach angles of axons and dendrites are a determinant of connection specificity. Synaptic clustering at small approach angles is independent of the motor pool identity.
- 8.Kiral FR, Linneweber GA, Georgiev SV, Hassan BA, von Kleist M, Hiesinger PR: Autophagy-dependent filopodial kinetics restrict synaptic partner choice during <em>Drosophila</em> brain wiring. 2019:762179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan BA, Hiesinger PR: Beyond Molecular Codes: Simple Rules to Wire Complex Brains. Cell 2015, 163:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperry RW: Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proceedings of the National Academy of Sciences 1963, 50:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Loss H, Glaser EM: Autapses in neocortex cerebri: synapses between a pyramidial cell’s axon and its own dendrites. Brain Research 1972:355–360. [DOI] [PubMed] [Google Scholar]
- 12.Neniskyte U, Gross CT: Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci 2017, 18:658–670. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman OJ, McGuirt AF, Tang G, Sulzer D: Roles for neuronal and glial autophagy in synaptic pruning during development. Neurobiol Dis 2019, 122:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holguera I, Desplan C: Neuronal specification in space and time. Science 2018, 362:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi AM, Fernandes VM, Desplan C: Timing temporal transitions during brain development. Curr Opin Neurobiol 2017, 42:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.*.Mora N, Oliva C, Fiers M, Ejsmont R, Soldano A, Zhang TT, Yan J, Claeys A, De Geest N, Hassan BA: A Temporal Transcriptional Switch Governs Stem Cell Division, Neuronal Numbers, and Maintenance of Differentiation. Dev Cell 2018, 45:53–66 e55. [DOI] [PubMed] [Google Scholar]; This study shows the temporal co-ordination of transcription factors that regulate the generation of sufficient numbers of T4/T5 cells during the development of Drosophila motion vision circuit. The temporal regulation of the differentiation program is critical for subsequent circuit development.
- 17.*.Pinto-Teixeira F, Koo C, Rossi AM, Neriec N, Bertet C, Li X, Del-Valle-Rodriguez A, Desplan C: Development of Concurrent Retinotopic Maps in the Fly Motion Detection Circuit. Cell 2018, 173:485–498 e411. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes the temporal coordination between the differentiation of T4/T5 cells and targeting of their axons and dendrites in the target field. Using temporal labelling of early vs. late born T4/T5 cells, the study demonstrates synergistic development of motion vision circuit in Drosophila.
- 18.*.Sullivan LF, Warren TL, Doe CQ: Temporal identity establishes columnar neuron morphology, connectivity, and function in a Drosophila navigation circuit. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies the temporal origins of four types of Drosophila central complex neurons. Neurons born during the same temporal window exhibit the same connectivity patterns. Transient expression of the transcription factor Pax6/’eyeless is critical for temporally coordinated targeting.
- 19.Mi D, Li Z, Lim L, Li M, Moissidis M, Yang Y, Gao T, Hu TX, Pratt T, Price DJ, et al. : Early emergence of cortical interneuron diversity in the mouse embryo. Science 2018, 360:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripodi M, Stepien AE, Arber S: Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature 2011, 479:61–66. [DOI] [PubMed] [Google Scholar]
- 21.Osterhout JA, El-Danaf RN, Nguyen PL, Huberman AD: Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Rep 2014, 8:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni A, Ertekin D, Lee CH, Hummel T: Birth order dependent growth cone segregation determines synaptic layer identity in the Drosophila visual system. Elife 2016, 5:e13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.*.Apitz H, Salecker I: Spatio-temporal relays control layer identity of direction-selective neuron subtypes in Drosophila. Nat Commun 2018, 9:2295. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes the sequential cell fate choices made during the development of motion vision circuit in the Drosophila. The sequential actions of Wingless, Decapentaplegic and Notch signaling control cell fate choices of T4/T5 cells in a spatiotemporally coordinated manner.
- 24.Apitz H, Salecker I: A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system. Nat Neurosci 2015, 18:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Toro D, Ruff T, Cederfjall E, Villalba A, Seyit-Bremer G, Borrell V, Klein R: Regulation of Cerebral Cortex Folding by Controlling Neuronal Migration via FLRT Adhesion Molecules. Cell 2017, 169:621–635 e616. [DOI] [PubMed] [Google Scholar]
- 26.Lim L, Pakan JMP, Selten MM, Marques-Smith A, Llorca A, Bae SE, Rochefort NL, Marin O: Optimization of interneuron function by direct coupling of cell migration and axonal targeting. Nat Neurosci 2018, 21:920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martineau FS, Sahu S, Plantier V, Buhler E, Schaller F, Fournier L, Chazal G, Kawasaki H, Represa A, Watrin F, et al. : Correct Laminar Positioning in the Neocortex Influences Proper Dendritic and Synaptic Development. Cereb Cortex 2018, 28:2976–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewitz C, Duan X, Zampieri N: Organization of motor pools depends on the combined function of N-cadherin and type II cadherins. Development 2019, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.*.Dewitz C, Pimpinella S, Hackel P, Akalin A, Jessell TM, Zampieri N: Nuclear Organization in the Spinal Cord Depends on Motor Neuron Lamination Orchestrated by Catenin and Afadin Function. Cell Rep 2018, 22:1681–1694. [DOI] [PubMed] [Google Scholar]; This study shows that a double knock-out and a triple knock-out of two groups of type II cadherins do not affect motor pool segregation. The findings argue against a simple ‘Cadherin code’ and reveal coordinated spatiotemporal segregation of motor pools.
- 30.Kolodkin AL, Hiesinger PR: Wiring visual systems: common and divergent mechanisms and principles. Curr Opin Neurobiol 2017, 42:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braitenberg V: Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res 1967, 3:271–298. [DOI] [PubMed] [Google Scholar]
- 32.Scholes JH: Nerve fibre topography in the retinal projection to the tectum. Nature 1979, 278:620–624. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand DGC, Cicconet M, Torres RM, Choi W, Quan TM, Moon J, Wetzel AW, Scott Champion A, Graham BJ, Randlett O, et al. : Whole-brain serial-section electron microscopy in larval zebrafish. Nature 2017, 545:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langen M, Agi E, Altschuler DJ, Wu LF, Altschuler SJ, Hiesinger PR: The Developmental Rules of Neural Superposition in Drosophila. Cell 2015, 162:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, Sakano H: Pre-target axon sorting establishes the neural map topography. Science 2009, 325:585–590. [DOI] [PubMed] [Google Scholar]
- 36.Suetterlin P, Drescher U: Target-independent ephrina/EphA-mediated axon-axon repulsion as a novel element in retinocollicular mapping. Neuron 2014, 84:740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.**.Cioni JM, Wong HH, Bressan D, Kodama L, Harris WA, Holt CE: Axon-Axon Interactions Regulate Topographic Optic Tract Sorting via CYFIP2-Dependent WAVE Complex Function. Neuron 2018, 97:1078–1093 e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mutational analysis of CYFIP2 reveals a specific role for axon pre-sorting of retinal ganglion cells. Axons from the same side (homotypic) fasciculate, while heterotypic interactions with axons from the ‘other’ side result in ‘tip-toe-tracking’ based on differential filopodial dynamics.
- 38.Peng J, Fabre PJ, Dolique T, Swikert SM, Kermasson L, Shimogori T, Charron F: Sonic Hedgehog Is a Remotely Produced Cue that Controls Axon Guidance Trans-axonally at a Midline Choice Point. Neuron 2018, 97:326–340 e324. [DOI] [PubMed] [Google Scholar]
- 39.Lokmane L, Proville R, Narboux-Neme N, Gyory I, Keita M, Mailhes C, Lena C, Gaspar P, Grosschedl R, Garel S: Sensory map transfer to the neocortex relies on pretarget ordering of thalamic axons. Curr Biol 2013, 23:810–816. [DOI] [PubMed] [Google Scholar]
- 40.Kise Y, Schmucker D: Role of self-avoidance in neuronal wiring. Curr Opin Neurobiol 2013, 23:983–989. [DOI] [PubMed] [Google Scholar]
- 41.Zipursky SL, Sanes JR: Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell 2010, 143:343–353. [DOI] [PubMed] [Google Scholar]
- 42.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR: Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 2012, 488:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.*.Mountoufaris G, Chen WV, Hirabayashi Y, O’Keeffe S, Chevee M, Nwakeze CL, Polleux F, Maniatis T: Multicluster Pcdh diversity is required for mouse olfactory neural circuit assembly. Science 2017, 356:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows how reduced diversity of Protocadherins leads to a failure of olfactory sensory neurons to correctly segregate in glomeruli. The mutant animals can smell but display odor discrimination defects, suggesting miswiring of the circuit.
- 44.Lefebvre JL: Neuronal territory formation by the atypical cadherins and clustered protocadherins. Semin Cell Dev Biol 2017, 69:111–121. [DOI] [PubMed] [Google Scholar]
- 45.Chen WV, Nwakeze CL, Denny CA, O’Keeffe S, Rieger MA, Mountoufaris G, Kirner A, Dougherty JD, Hen R, Wu Q, et al. : Pcdhalphac2 is required for axonal tiling and assembly of serotonergic circuitries in mice. Science 2017, 356:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langen M, Koch M, Yan J, De Geest N, Erfurth ML, Pfeiffer BD, Schmucker D, Moreau Y, Hassan BA: Mutual inhibition among postmitotic neurons regulates robustness of brain wiring in Drosophila. Elife 2013, 2:e00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa N, Plestant C, Yabuno-Nakagawa K, Li J, Lee J, Huang CW, Lee A, Krupa O, Adhikari A, Thompson S, et al. : Memo1-Mediated Tiling of Radial Glial Cells Facilitates Cerebral Cortical Development. Neuron 2019, 103:836–852 e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie X, Tabuchi M, Brown MP, Mitchell SP, Wu MN, Kolodkin AL: The laminar organization of the Drosophila ellipsoid body is semaphorin-dependent and prevents the formation of ectopic synaptic connections. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters A, Feldman ML: The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. I. General description. J Neurocytol 1976, 5:63–84. [DOI] [PubMed] [Google Scholar]
- 50.Krishnaswamy A, Yamagata M, Duan X, Hong YK, Sanes JR: Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature 2015, 524:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tien NW, Soto F, Kerschensteiner D: Homeostatic Plasticity Shapes Cell-Type-Specific Wiring in the Retina. Neuron 2017, 94:656–665 e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon O, Feng L, Druckmann S, Kim J: Schaffer Collateral Inputs to CA1 Excitatory and Inhibitory Neurons Follow Different Connectivity Rules. J Neurosci 2018, 38:5140–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozel MN, Kulkarni A, Hasan A, Brummer J, Moldenhauer M, Daumann IM, Wolfenberg H, Dercksen VJ, Kiral FR, Weiser M, et al. : Serial Synapse Formation through Filopodial Competition for Synaptic Seeding Factors. Dev Cell 2019, 50:447–461 e448. [DOI] [PMC free article] [PubMed] [Google Scholar]


