Abstract
Two purines, caffeine and urate, have been associated with a reduced risk of idiopathic Parkinson’s disease (PD) in multiple cohorts and populations. The Harvard Biomarkers Study (HBS) is a longitudinal study designed to accelerate the discovery and validation of molecular diagnostic and progression markers of early-stage PD. To investigate whether these ‘reduced risk’ factors are associated with PD within this cohort, we conducted a cross-sectional, case-control study in 566 subjects consisting of idiopathic PD patients and healthy controls. Caffeine intake as assessed by a validated questionnaire was significantly lower in idiopathic PD patients compared to healthy controls in males (mean difference −125 mg/day, p <0.001) but not in females (mean difference −30 mg/day, p = 0.29). A strong inverse association was also observed with plasma urate levels both in males (mean difference −0.46 mg/dL, p = 0.017) and females (mean difference −0.45mg/dL, p = 0.001). Both analyses stratified for sex and adjusted for age, body mass index, and either urate level or caffeine consumption, respectively. These results highlight the robustness of caffeine intake and urate as factors inversely associated with idiopathic PD.
Keywords: Caffeine, uric acid, biomarker, Parkinson’s disease
INTRODUCTION
An increasing number of genes and environmental factors play an important role in onset and progression of Parkinson’s disease (PD) [1]. Large-scale epidemiological studies have identified several such PD-associated risk factors associated with higher or lower disease incidence and faster or slower disease progression [2, 3]. Caffeine and urate are both purines and well-established inverse risk factors linked to reduced PD risk, and both possess neuroprotective properties via adenosine receptor antagonist and antioxidant actions, respectively [4]. Clinically well-characterized cohorts are essential to identify and validate gene-environment interactions. Here we have analyzed the association of lower caffeine intake and plasma urate in the Harvard Biomarkers Study (HBS), one such well-characterized longitudinal case-control study.
METHODS
Study population
The present study includes 369 cases with idiopathic PD and 197 healthy controls with available plasma urate values from the full Harvard Biomarkers Study cohort. Diagnosis of PD was made by Board-certified neurologists with fellowship-training in movement disorders as described before [5].
Urate measurements
Urate was measured in plasma samples collected at each participant’s initial HBS visit using routine colorimetric testing.
Assessment of caffeine intake
Caffeine intake was assessed at each participant’s initial HBS visit using a semi-quantitative questionnaire. The questionnaire queried participants’ usual consumption of caffeinated and decaffeinated coffee, tea, and soft drinks during the previous 12 months in standard volumes (cups for coffee and tea and cans for soft drinks) with 9 possible frequencies ranging from never to 6 or more per day. Mean daily caffeine consumption was calculated using the following estimated caffeine content: 137 mg per 8 oz cup of caffeinated coffee, 47 mg per 8 oz cup of caffeinated tea, and 46 mg per 12 oz can of caffeinated soda, based on U.S. Department of Agriculture food composition sources as described elsewhere [6].
Standard protocol approvals and patient consents
Informed consent was obtained from all participants. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Massachusetts General Hospital.
Statistical analysis
Idiopathic PD and control subjects were compared by two-sample t-test or Fisher’s exact test both overall and stratified by sex. In addition to unadjusted analyses by t-test, associations of caffeine consumption and urate level with disease status were tested by multiple linear regression of caffeine consumption or urate levels or multiple logistic regression of disease status, in each case stratifying by sex and adjusting for age body mass index (BMI) and either urate level or caffeine consumption, respectively, given their known associations with PD and shared purine structure. Age and BMI were modeled as cubic B-splines with a single knot at their respective means. As covariates, caffeine consumption and urate levels were also modeled as cubic B-splines. As focal predictors in the logistic regressions, quintiles of caffeine consumption and urate levels were used to provide unstructured estimates of their pattern of association with disease status. Models with quintiles of both caffeine and urate were too sparse to estimate. Analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC). Significance was declared for two-tailed p <0.05.
RESULTS
A total of 566 subjects were included in our analysis. An overview of population characteristics is shown in Table 1. There was a higher proportion of men among PD cases compared to healthy controls (HC, 64% vs. 38%, p <0.001). Caffeine consumption was lower overall in PD (mean ± SD: 155 ± 161 mg/day) compared to HC (230 ± 199 mg/day). Urate levels were also lower overall in PD cases (4.56 ± 1.26 mg/dL) compared to HC (4.77 ± 1.29 mg/dL). Stratifying by sex and adjusting for age, BMI, and urate level, caffeine consumption was lower among PD vs. HC men (mean difference −125 mg/day, 95% CI −172 to −78 mg/day, p <0.001) but not among PD vs. HC women (mean difference −30 mg/day, 95% CI −86 to 26 mg/day, p = 0.29). Stratifying by sex and adjusting for age, BMI, and caffeine consumption, urate levels were lower both among PD vs. HC men (mean difference −0.46 mg/dL, 95% CI −0.83 to −0.08 mg/dL, p = 0.017) and among PD vs. HC women (mean difference −0.45 mg/dL, 95% CI −0.73 to −0.18 mg/dL, p = 0.001).
Table 1.
Baseline characteristics of healthy controls (HC) and Parkinson’s disease patients (PD) in the Harvard Biomarkers Study
| HC | PD | p value | ||
|---|---|---|---|---|
| OVERALL | Sample Size | 197 | 369 | |
| Age | 67.3 ± 10.3 | 66.3 ± 9.6 | 0.21 | |
| Urate (mg/dL) | 4.77 ± 1.29 | 4.56 ± 1.26 | 0.06 | |
| Caffeine (mg/day) | 230 ± 199 | 155 ± 161 | <0.001 | |
| BMI | 26.9 ± 5.4 | 26.4 ± 4.8 | 0.34 | |
| MALES | Sample Size | 75 | 236 | |
| Age | 70.3 ± 9.5 | 66.0 ± 10.0 | 0.001 | |
| Urate (mg/dL) | 5.34 ± 1.24 | 4.91 ± 1.18 | 0.007 | |
| Caffeine (mg/day) | 268 ± 205 | 165 ± 166 | <0.001 | |
| BMI | 27.0 ± 3.6 | 26.8 ± 4.6 | 0.65 | |
| FEMALES | Sample Size | 122 | 133 | |
| Age | 65.5 ± 10.4 | 66.7 ± 8.9 | 0.34 | |
| Urate (mg/dL) | 4.41 ± 1.19 | 3.93 ± 1.16 | 0.001 | |
| Caffeine (mg/day) | 206 ± 193 | 138 ± 151 | 0.002 | |
| BMI | 26.7 ± 6.2 | 25.8 ± 5.1 | 0.12 |
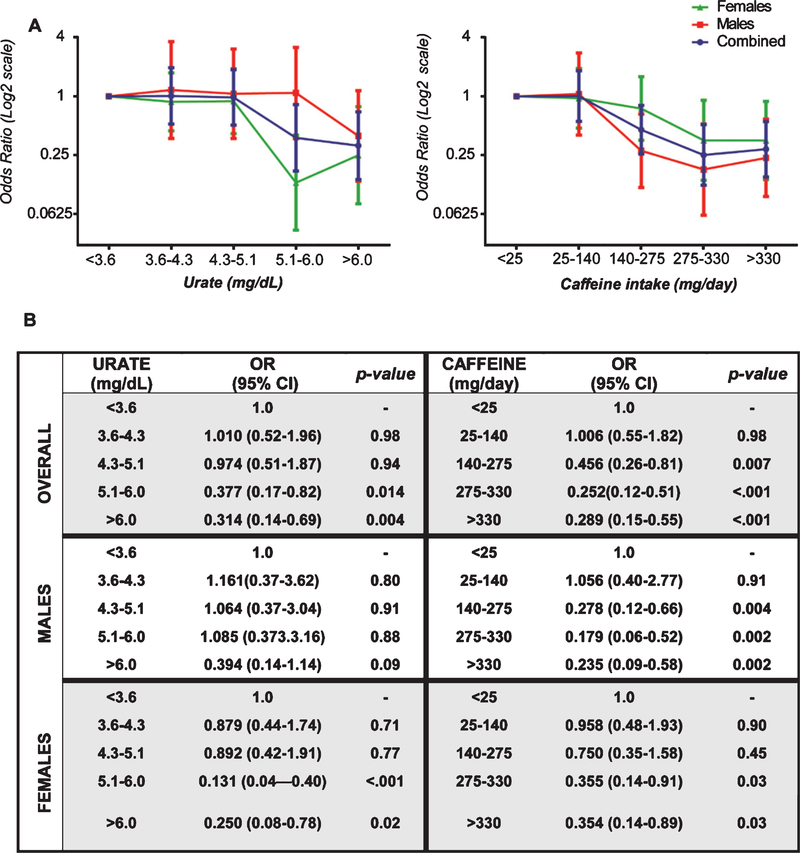
The odds of having PD decreased significantly with increasing caffeine consumption in a concentration-dependent manner across quintiles of caffeine consumption, adjusting for age, sex, BMI and plasma urate. Compared with the lowest caffeine consumption quintile, the prevalence of PD was 71% lower in the highest quintile (p <0.001 for trend across all quintiles) (Fig. 1).
Fig. 1.
Adjusted odds ratio (OR) of PD in each quintile of urate concentration or quintile of caffeine consumption overall as well as stratified by sex shown in (A) with underlying data presented in tabular format (B). OR is plotted in the logarithmic scale in the panel A. Error bars represent 95% confidence intervals (CI).
Similarly, the odds of having PD decreased significantly with increasing urate in a concentration-dependent manner across quintiles of plasma urate, adjusting for age, sex, BMI and caffeine consumption. Compared with the lowest urate quintile, the prevalence of PD was 69% lower in the highest quintile (p <0.001 for trend across all quintiles) (Fig. 1).
DISCUSSION
Higher caffeine consumption and higher plasma urate concentration have been consistently associated with reduced risk of idiopathic PD in multiple cohorts and populations [7, 8]. The current findings validate the association of these well-established inverse risk factors in a cross-sectional study of the clinically well-characterized group of 369 cases and 197 controls nested within the HBS cohort. Gender stratified analysis were consistent with the overall results. Interestingly, an equally large association between urate and PD risk was observed among women, which contrasts with most studies of the association between urate and idiopathic PD that stratified by sex [9, 10]. The biological mechanisms underlying such sex specificity remain unclear.
Several emerging biomarkers of PD risk/progression (for e.g., SNCA SNP/transcripts, GBA mutation types, and plasma Vit D3) have been identified using HBS [5, 11–16]. The replication of purine associations with PD in the HBS further illustrate its utility as a longitudinal case-control cohort well-suited for deep analysis of relationships between dietary factors, genes, established and novel biomarkers, and clinical phenotypes of PD. The findings also highlight the strength and consistency of these purines’ inverse associations with PD, regardless of whether either purine is simply a marker of reduced PD risk (e.g., with reduced caffeine intake potentially reflecting a reduced propensity to form or maintain a caffeine habit) or is a protective mediator of reduced risk warranting its targeting in therapeutic trials [17, 18]. Although the closure of the SURE-PD3 trial [19] for futility argues against urate elevation as a disease-modifying strategy for people with clinically manifest idiopathic PD, it does not diminish urate’s well established utility as a prominent PD biomarker, predictive of both reduced risk and slower progression of idiopathic PD. Accordingly, measurement of serum urate levels in randomized clinical trials targeting PD progression may increase their power or reduce their sample size and cost. In addition, the reproducible association of low urate levels with increased risk of idiopathic PD supports the generalizability of findings in the HBS.
This study has several strengths and limitations. The majority of the PD cases enrolled in HBS were confirmed by neurology board-certified, movement disorders fellowship-trained neurologists with annual reassessments. Controls were comparable to the PD cases in being drawn from the same source population. Relevant confounding factors (age, sex, body mass index, caffeine consumption and urate levels) were examined and adjusted for as indicated. Collection of questionnaire data and biosamples was standardized and biomarkers analyses were performed by staff blinded to diagnosis. However, our cohort represents patients and controls receiving care at a single institution, Harvard Medical School, thus may subject to selection bias and may not accurately represent the general US population.
ACKNOWLEDGMENTS
The authors thank all the study participants and their families for their support and participation in the HBS. This study was funded by NIH (grants U01NS090259 and R01NS110879), Michael J. Fox Foundation for Parkinson’s Research (MJFF; Grant# 9330), and Farmer Family Foundation Initiative for Parkinson’s Disease Research (FFFIPDR). C.R.S’s work was supported by NIH grants U01NS095736, U01NS100603, R01AG057331, and R01NS115144 and by the MJFF. HBS was made possible by the Harvard NeuroDiscovery Center with additional support from the APDA Center for Advanced Parkinson Research of Brigham & Women’s Hospital and the Massachusetts Alzheimer’s Disease Research Center (NIH grant P50AG005134). We also thank the HBS Investigators: Yuliya Kuras, Karbi Choudhury, Aleksandar Videnovic, Nutan Sharma, Vikram Khurana, Claudio Meleo De Gusmao, Reisa Sperling; Massachusetts General Hospital: Alice W. Flaherty, Deborah Blacker, Anne-Marie Wills, Steven E. Arnold, Ann L. Hunt, Nicte I. Mejia, Anand Viswanathan, Mark W. Albers, Maria Allora-Palli, David Hsu, Alexandra Kimball, Scott McGinnis, John Becker, Randy Buckner, Thomas Byrne, Maura Copeland, Bradford Dickerson, Matthew Frosch, Theresa Gomez-Isla, Steven Greenberg, Julius Hedden, Elizabeth Hedley-Whyte, Keith Johnson, Raymond Kelleher, Aaron Koenig, Maria Marquis-Sayagues, Gad Marshall, Sergi Martinez-Ramirez, Donald McLaren, Olivia Okereke, Elena Ratti, Christopher William, Koene Van Dij, Shuko Takeda, Anat Stemmer-Rachaminov, Jessica Kloppenburg, Catherine Munro, Rachel Schmid, Sarah Wigman, Sara Wlodarcsyk; Data Coordination: Brigham and Women’s Hospital: Thomas Yi; Biobank Management Staff: Brigham and Women’s Hospital: Idil Tuncali.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1].Delamarre A, Meissner WG (2017) Epidemiology, environmental risk factors and genetics of Parkinson’s disease. Presse Med 46(2 Pt 1), 175–181. [DOI] [PubMed] [Google Scholar]
- [2].Marras C, Canning CG, Goldman SM (2019) Environment, lifestyle, and Parkinson’s disease: Implications for prevention in the next decade. Mov Disord 34, 801–811. [DOI] [PubMed] [Google Scholar]
- [3].Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol 15, 1257–1272. [DOI] [PubMed] [Google Scholar]
- [4].Morelli M, Carta AR, Kachroo A, Schwarzschild MA (2010) Pathophysiological roles for purines: Adenosine, caffeine and urate. Prog Brain Res 183, 183–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ding H, Sarokhan AK, Roderick SS, Bakshi R, Maher NE, Ashourian P, Kan CG, Chang S, Santarlasci A, Swords KE, Ravina BM, Hayes MT, Sohur US, Wills AM, Flaherty AW, Unni VK, Hung AY, Selkoe DJ, Schwarzschild MA, Schlossmacher MG, Sudarsky LR, Growdon JH, Ivinson AJ, Hyman BT, Scherzer CR (2011) Association of SNCA with Parkinson: Replication in the Harvard NeuroDiscovery Center Biomarker Study. Mov Disord 26, 2283–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ascherio A, Zhang SM, Hernán MA, Kawachi I, Colditz GA, Speizer FE, Willett WC (2001) Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol 50, 56–63. [DOI] [PubMed] [Google Scholar]
- [7].Kieburtz K, Wunderle KB (2013) Parkinson’s disease: Evidence for environmental risk factors. Mov Disord 28, 8–13. [DOI] [PubMed] [Google Scholar]
- [8].Bakshi R, Logan R, Schwarzschild MA (2015) Purines in Parkinson’s: Adenosine A2A receptors and urate as targets for neuroprotection. In The Adenosinergic System: A Non-Dopaminergic Target in Parkinson’s Disease, Current Topics in Neurotoxicity, 10, Morelli M, Simola N, Wardas, eds. Springer International Publishing, Switzerland, pp. 101–126. [Google Scholar]
- [9].Shen L, Ji HF (2013) Low uric acid levels in patients with Parkinson’s disease: Evidence from meta-analysis. BMJ Open 3, e003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gao X, O’Reilly EJ, Schwarzschild MA, Ascherio A (2016) Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurolog 86, 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grünblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wüllner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR; Global PD Gene Expression (GPEX) Consortium (2010) PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med 2, 52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ding H, Dhima K, Lockhart KC, Locascio JJ, Hoesing AN, Duong K, Trisini-Lipsanopoulos A, Hayes MT, Sohur US, Wills AM, Mollenhauer B, Flaherty AW, Hung AY, Mejia N, Khurana V, Gomperts SN, Selkoe DJ, Schwarzschild MA, Schlossmacher MG, Hyman BT, Sudarsky LR, Growdon JH, Scherzer CR (2013) Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 81, 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Locascio JJ, Eberly S, Liao Z, Liu G, Hoesing AN, Duong K, Trisini-Lipsanopoulos A, Dhima K, Hung AY, Flaherty AW, Schwarzschild MA, Hayes MT, Wills AM, Shivraj Sohur U, Mejia NI, Selkoe DJ, Oakes D, Shoulson I, Dong X, Marek K, Zheng B, Ivinson A, Hyman BT, Growdon JH, Sudarsky LR, Schlossmacher MG, Ravina B, Scherzer CR (2015) Association between alpha-synuclein blood transcripts and early, neuroimaging-supported Parkinson’s disease. Brain 138(Pt 9), 2659–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, Elbaz A, Brice A, Ravina B, van Hilten JJ, Cormier-Dequaire F, Corvol JC, Barker RA, Heutink P, Marinus J, Williams-Gray CH, Scherzer CR; International Genetics of Parkinson Disease Progression (IGPP) Consortium (2016) Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann Neurol 80, 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu G, Locascio JJ, Corvol JC, Boot B, Liao Z, Page K, Franco D, Burke K, Jansen IE, Trisini-Lipsanopoulos A, Winder-Rhodes S, Tanner CM, Lang AE, Eberly S, Elbaz A, Brice A, Mangone G, Ravina B, Shoulson I, Cormier-Dequaire F, Heutink P, van Hilten JJ, Barker RA, Williams-Gray CH, Marinus J, Scherzer CR; HBS; CamPaIGN; PICNICS; PROPARK; PSG; DIGPD; PDBP (2017) Prediction of cognition in Parkinson’s disease with a clinical-genetic score: A longitudinal analysis of nine cohorts. Lancet Neurol 16, 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong X, Liao Z, Gritsch D, Hadzhiev Y, Bai Y, Locascio JJ, Guennewig B, Liu G, Blauwendraat C, Wang T, Adler CH, Hedreen JC, Faull RLM, Frosch MP, Nelson PT, Rizzu P, Cooper AA, Heutink P, Beach TG, Mattick JS, Müller F, Scherzer CR (2018) Enhancers active in dopamine neurons are a primary link between genetic variation and neuropsychiatric disease. Nat Neurosci 21, 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Parkinson Study Group SURE-PD Investigators, Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jaglin J, Ahmed A, Russell DS, Cotto C, Goudreau JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaida J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K (2013) Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: A randomized clinical trial. JAMA Neurol 71, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sokol LL, Young MJ, Espay AJ, Postuma RB (2016) Cautionary optimism: Caffeine and Parkinson’s disease risk. J Clin Mov Disord 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Study of Urate Elevation in Parkinson’s Disease, Phase 3 (SURE-PD3) https://clinicaltrials.gov/ct2/show/NCT02642393 retrieved February 2nd, 2020.