Abstract
Tubulin, the building block of microtubules, is subject to chemically diverse and evolutionarily conserved post-translational modifications that mark microtubules for specific functions in the cell. Here we describe in vitro methods for generating homogenous acetylated, glutamylated or tyrosinated tubulin and microtubules using recombinantly expressed and purified modification enzymes. The generation of differentially modified microtubules now enables a mechanistic dissection of the effects of tubulin post-translational modifications on the dynamics and mechanical properties of microtubules as well as the behavior of motors and microtubule associated proteins.
Keywords: microtubule, tubulin post-translational modifications, tubulin acetylation, tubulin glutamylation, tubulin tyrosination, tubulin tyrosine ligase (TTL), tubulin tyrosine ligase-like (TTLL), tubulin acetyltransferase (α-TAT), motors
Introduction
Microtubules are dynamic polymers essential for cell division, intracellular transport, and morphogenesis (Howard & Hyman, 2003; Nogales, 2000). The building block of microtubules is the αβ-tubulin heterodimer. Humans have six α-tubulin (α1A, α1B, α1C, α3A, α4A, and α8) and seven β-tubulin isoforms (βI, βII, βIII, βIVa, βIVb, βV, and βVI) (Sullivan, 1988). Multiple α- and β-tubulins are typically expressed in a cell, giving rise to isotypically diverse microtubules (Miller, Xiao, Burd, Horwitz, Angeletti, & Verdier-Pinard, 2010). Moreover, the complexity of microtubule arrays is further modulated by post-translational modifications. Tubulin is subject to several chemically-diverse and evolutionarily-conserved post-translational modifications: 1) cyclical removal and addition of the α-tubulin C-terminal tyrosine (resulting in “Glu-tubulin”) (Barra, Rodriguez, Arce, & Caputto, 1973), 2) irreversible removal of the penultimate glutamate of α-tubulin (resulting in “Δ2-tubulin”) (Paturle-Lafanechere, Edde, Denoulet, Van Dorsselaer, Mazarguil, Le Caer, et al., 1991), 3) acetylation of α-tubulin (L’Hernault & Rosenbaum, 1983, 1985), 4) poly-glutamylation and 5) poly-glycylation of α- and β-tubulin (Alexander, Hunt, Lee, Shabanowitz, Michel, Berlin, et al., 1991; Edde, Rossier, Le Caer, Desbruyeres, Gros, & Denoulet, 1990; Redeker, Levilliers, Schmitter, Le Caer, Rossier, Adoutte, et al., 1994; Redeker, Melki, Prome, Le Caer, & Rossier, 1992; Rudiger, Plessman, Kloppel, Wehland, & Weber, 1992).
Most post-translational modifications occur on the unstructured negatively charged tubulin C-terminal tails (Figure 1) (Nogales, Wolf, & Downing, 1998; Sullivan, 1988). Tubulin tails decorate the microtubule exterior and can interact with motors and microtubule associated proteins (MAPs) and modulate their activities (Garnham & Roll-Mecak, 2012; Janke & Bulinkski, 2011; Wloga & Gaertig, 2010). Cytoplasmic linker protein-170 (CLIP-170), a plus end microtubule tracking protein, preferentially binds tyrosinated tubulin (Bieling, Kandels-Lewis, Telley, van Dijk, Janke, & Surrey, 2008) and the microtubule severing enzyme spastin preferentially severs poly-glutamylated microtubules (Lacroix, van Dijk, Gold, Guizetti, Aldrian-Herrada, Rogowski, et al., 2010; Roll-Mecak & McNally, 2010; Roll-Mecak & Vale, 2008). Glutamylation also increases synaptic vesicle transport by kinesin-2 and targets MAP2 to dendritic microtubules (Ikegami, Heier, Taruishi, Takagi, Mukai, Shimma, et al., 2007). Acetylation of lysine 40 on α-tubulin is unique among tubulin modifications as it occurs inside the microtubule lumen (Nogales, Whittaker, Milligan, & Downing, 1999; Soppina, Herbstman, Skiniotis, & Verhey, 2012), close to the inter-protofilament interface where it can affect microtubule stability (Cueva, Hsin, Huang, & Goodman, 2012; Topalidou, Keller, Kalebic, Nguyen, Somhegyi, Politi, et al., 2012).
Figure 1:
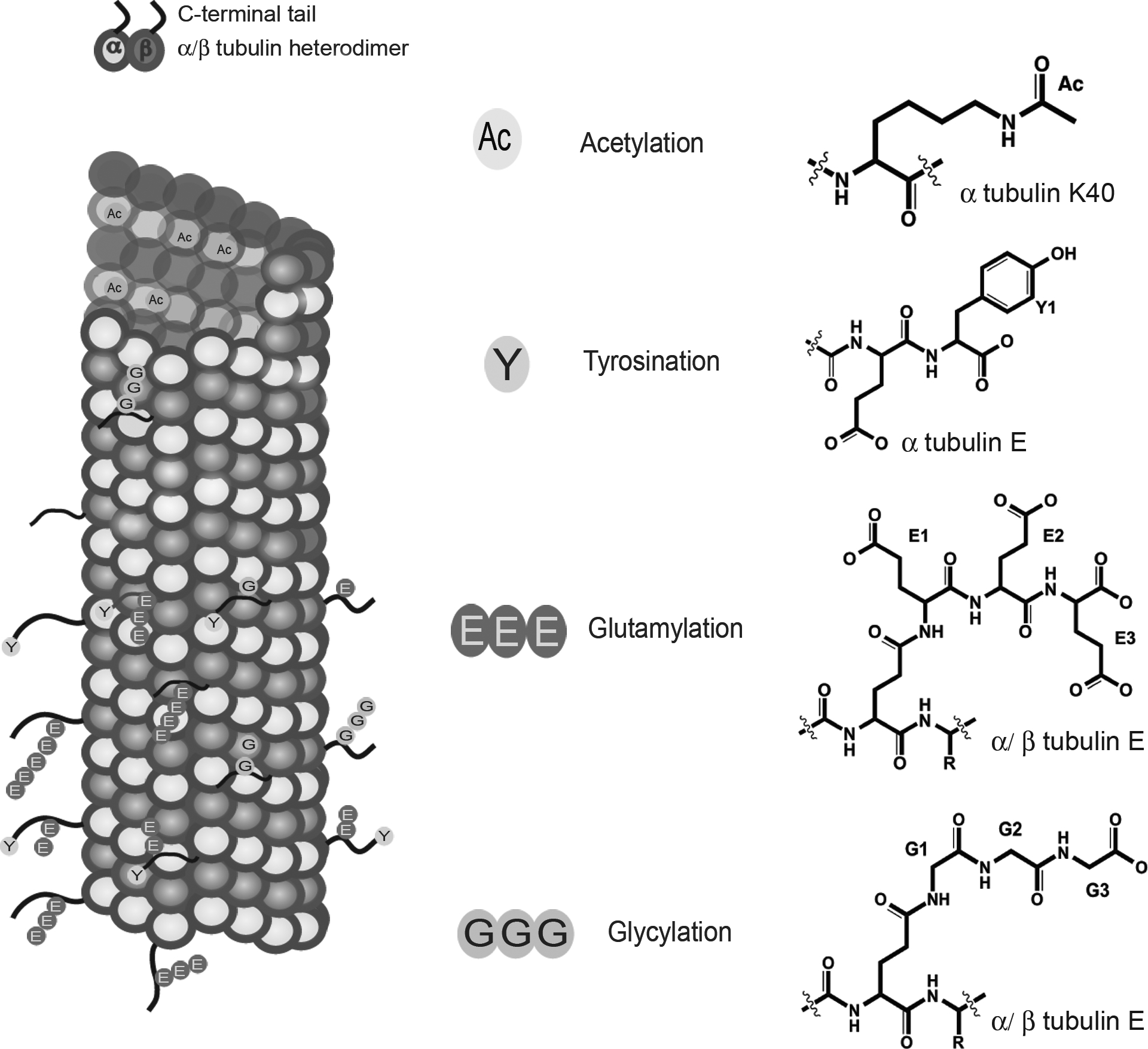
(Left) Schematic representation of a microtubule (α-tubulin, green; β-tubulin, blue). The unstructured C-terminal tails are shown in red. Tyrosination (cyan), glutamylation (red), and glycylation (brown) occur on the unstructured C-terminal tails. Acetylation on α-tubulin Lys40 (orange) occurs in the lumen. (Right) Chemical structures of various tubulin modifications. The elongated Glu chain is thought to be linear and not branched (Redeker, Le Caer, Rossier, & Prome, 1991). Note: Glycylation is not discussed in this chapter.
Tubulin post-translational modifications have been known for several decades and studies using modification specific antibodies revealed the differential localization of post-translationally modified microtubules in the cell as well as their markedly different stabilities. However, a mechanistic understanding of their effect on microtubule biophysical properties as well as the behavior of microtubule regulators has been lacking. This is partly due to the difficulty in obtaining unmodified and modified tubulin that carries only one specific modification. Tubulin has traditionally been purified from brain tissue through repeated cycles of polymerization and depolymerization (Weisenberg, 1972). This approach cannot easily be applied to other sources with lower tubulin concentrations than brain tissue, since it is hard to reach critical tubulin concentrations for robust polymerization. As a consequence, brain has been the de facto source for tubulin purification for several decades. However, brain tubulin is a heterogeneous mixture of isoforms and contains abundant post-translational modifications such as poly-glutamylation, detyrosination, and acetylation (Sullivan, 1988). Moreover, modification levels vary from preparation to preparation depending on how the brain tissue was harvested and stored prior to tubulin isolation. In order to investigate the effects of individual modifications on microtubule behavior as well as their effect on the recruitment and activity of cellular effectors, it is necessary to prepare unmodified homogeneous tubulin that can be modified “at will” with a single type of modification. This necessitates: (1) preparation of biochemical quantities of unmodified or “naïve” tubulin, (2) preparations of active tubulin modification enzymes, and (3) development of protocols for the controlled modification of naïve tubulin using these enzyme preparations.
The challenge in purifying milligram amounts of unmodified tubulin from various sources was recently overcome by the development of an affinity-based purification that uses the tubulin-binding TOG domains from MAP215 crosslinked to solid support (Widlund, Podolski, Reber, Alper, Storch, Hyman, et al., 2012). Moreover, we now have an almost complete catalog of tubulin post-translational modification enzymes, making it possible for the first time to undertake a systematic dissection of the roles of post-translational modifications in modulating microtubule functions (Garnham & Roll-Mecak, 2012).
Here we describe protocols for obtaining differentially-modified tubulin and microtubules using recombinantly-expressed tubulin modification enzymes. The selectively modified microtubules obtained using the protocols described here can be used in biochemical and biophysical assays to evaluate the effects of individual post-translational modifications on microtubule dynamics and the behavior of motors and MAPs. We focus here on three chemically-distinct post-translational modifications: acetylation, poly-glutamylation and tyrosination. Tubulin acetyltransferase (α-TAT) acetylates α-tubulin on Lys 40 in the microtubule lumen (Akella, Wloga, Kim, Starostina, Lyons-Abbott, Morrissette, et al., 2010; Shida, Cueva, Xu, Goodman, & Nachury, 2010). Tubulin tyrosine ligase-like 7 (TTLL7), the most abundant tubulin poly-glutamylase in neurons, adds glutamate chains to the tubulin C-terminal tails (Ikegami, Mukai, Tsuchida, Heier, Macgregor, & Setou, 2006; van Dijk, Rogowski, Miro, Lacroix, Edde, & Janke, 2007). Tubulin tyrosine ligase (TTL) catalyzes the re-addition of the genomically encoded α-tubulin C-terminal tyrosine (Raybin & Flavin, 1977; Schroder, Wehland, & Weber, 1985).
1. Purification and characterization of unmodified microtubules
tsA201 cells are HEK293 derivatives with low levels of tubulin post-translational modifications and thus an excellent source of unmodified tubulin (Miller et al., 2010). The tubulin used in the protocols described here was purified from tsA201 cells using a His-TOG1 (Slep & Vale, 2007) column by adapting a recently published protocol (Widlund et al., 2012).
The tubulin isolated using this affinity purification method is highly pure as evaluated by SDS-PAGE and reverse phase liquid chromatography mass spectrometry (LC-MS) (Figure 2A). To analyze the purified tubulin via LC-MS, mix 2 μl of 5 μM tubulin with 10 μl of 0.1% trifluoroacetic (TFA) acid and centrifuge at 16,000 xg for 10 min at 4°C. Load 12 μl of the sample onto a Zorbax 300SB-C18 column (50 mm x 2.1 mm) (Agilent) attached in-line with an Agilent 6224 electrospray ionization time-of-flight LC-MS. Use a 0–70% acetonitrile gradient in 0.05% TFA at a 0.2 ml/min flow rate. The data can be analyzed using the Agilent MassHunter Workstation platform. The LC-MS analyses show the presence of one α- and three β-tubulin isoforms (α1B, βI, βIII, and βIVB) (Figure 2A) and the absence of post-translational modifications. The purified tubulin polymerizes robustly into microtubules using standard polymerization protocols (section 3a). Negative stain electron microscopy shows no aggregates or intermediate polymerization products (Figure 2A).
Figure 2:
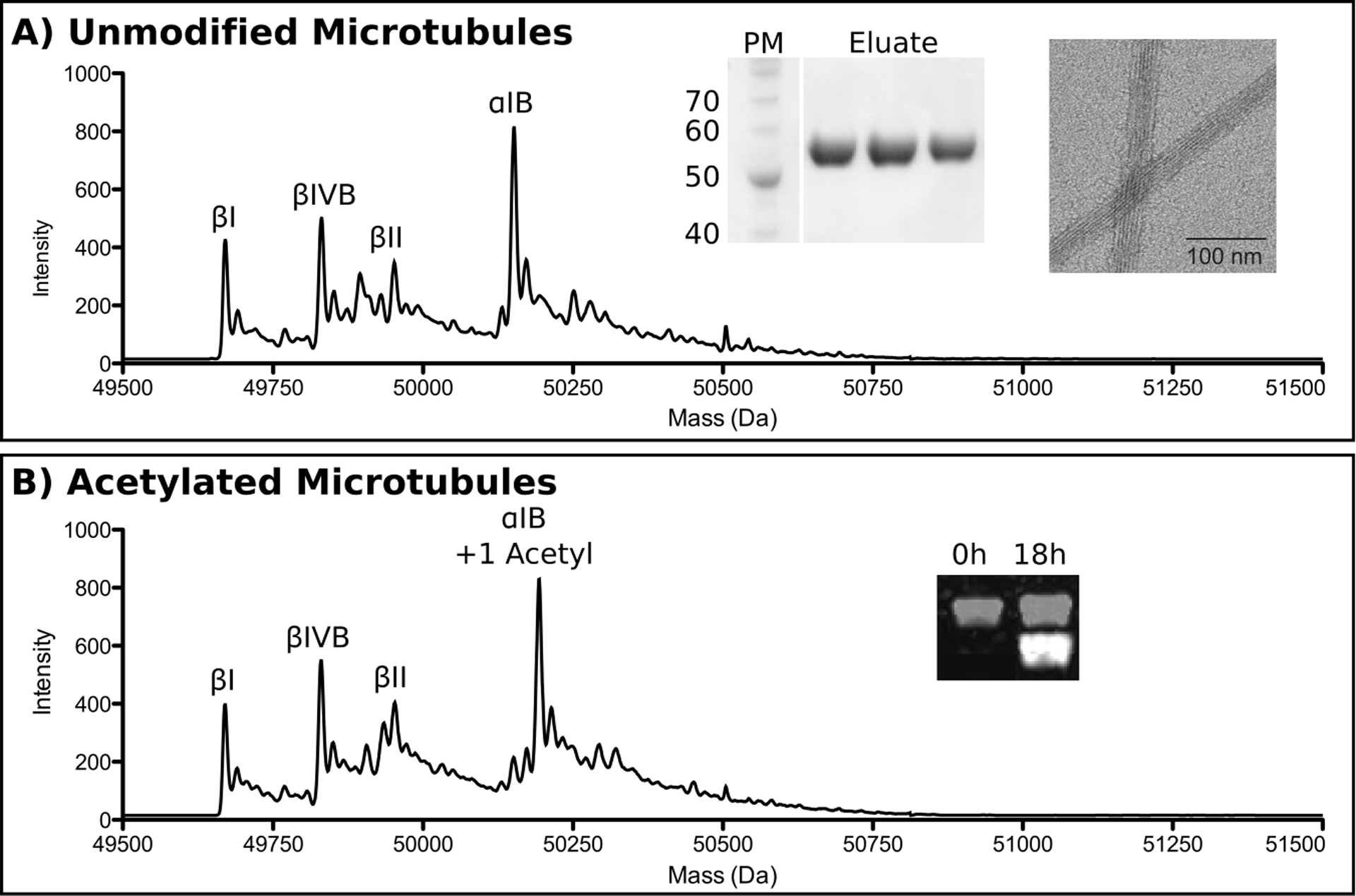
(A) Reverse phase LC-MS, SDS-PAGE, and negative stain electron microscopy analysis of unmodified microtubules. Individual tubulin isoforms are labeled in the spectrum. PM, protein markers. Eluate indicates elution fractions from the TOG affinity column. (B) Reverse phase LC-MS and Western blot analysis of unmodified microtubules acetylated by α-TAT. Tubulin isoforms labeled as in A. Inset shows progression of acetylation monitored by Western blot using antibodies specific for acetylated tubulin. α-tubulin, grey; acetylated tubulin, white. The two channels are offset for clarity.
2. Purification of tubulin modification enzymes: α-TAT, TTLL7, and TTL
Required Reagents
Tobacco etch virus protease (TEV). This can be expressed and purified according to published protocols (Kapust, Tözsér, Fox, Anderson, Cherry, Copeland, et al., 2001) or purchased from Sigma Aldrich.
Required Equipment
Microfluidizer for cell disruption (we use the C3 Homogenizer from Avestin)
AKTA purifier (GE Healthcare)
2a. Expression and purification of α-TAT
Solutions required for α-TAT Purification
10X phosphate buffered saline (PBS)
α-TAT resuspension buffer: 1X PBS supplemented with 10 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM dithiothreitol (DTT), protease inhibitor cocktail (Roche Applied Science)
α-TAT GST-A buffer: 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 10 mM MgCl2, 5 mM DTT
α-TAT GST-B buffer: 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10 mM MgCl2, 5 mM DTT
α-TAT ion exchange buffer A: 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 2 mM DTT
α-TAT ion exchange buffer B: 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 2 mM DTT, 2 M NaCl
α-TAT gel filtration buffer: 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 5 mM MgCl2, 2 mM tris(2-carboxyethyl)phosphine (TCEP)
Express Mus musculus α-TAT (residues 1–196) in E. coli Rosetta2(DE3)pLysS as an N-terminal cleavable GST fusion protein. Induce expression overnight (O/N) at 16°C with 0.35 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Pellet cells via centrifugation and resuspend in α-TAT resuspension buffer. Lyse cells by microfluidization (three passes at 12,000 psi) while keeping the sample cold. Perform all the following steps at 4°C. Supplement the lysate with 0.4 M NaCl and spin for 45 min at 31,000 xg to pellet cellular debris. Pass lysate supernatant over a gravity flow GST column equilibrated in α-TAT GST-A buffer. Wash resin with 10 column volumes (CVs) of α-TAT GST-A buffer. Resuspend GST resin with 1 column volume (CV) of α-TAT GST-B buffer and rock slurry O/N following addition of TEV protease at a 1:50 molar ratio of protease:α-TAT. Load GST slurry flow-through onto a Q-sepharose column equilibrated in 5% α-TAT ion exchange buffer B and collect flow-through. (Note: α-TAT does not bind Q-sepharose resin. This is a substractive purification step). Load Q-sepharose flowthrough onto an S75 gel filtration column (GE Healthcare) equilibrated in α-TAT gel filtration buffer. If the Q-sepharose flowthrough is too dilute, first concentrate using an Amicon concentrator with a 5-kDa cutoff (Millipore). Pool fractions containing α-TAT and determine protein concentration using 280 nm absorbance. Concentrate to 4 mg/mL and flash freeze in α-TAT gel filtration buffer supplemented with 15% glycerol.
2b. Expression and Purification of the Glu-ligase TTLL7
Solutions Required for TTLL7 Purification
TTLL7 resuspension buffer: 50 mM Tris-HCl (pH 7.4), 200 mM NaCl, 10 mM MgCl2, 1 mM PMSF
TTLL7 GST-A buffer: 50 mM Tris-HCl (pH 7.4), 500 mM NaCl, 10 mM MgCl2, 2 mM DTT
TTLL7 GST-B buffer: 50 mM Tris-HCl (pH 7.4), 500 mM NaCl, 10 mM MgCl2, 2 mM DTT, 20 mM reduced glutathione, 20 mM Tris-HCl (pH 8.8)
TTLL7 ion exchange buffer A: 50 mM HEPES (pH 7.0), 10 mM MgCl2, 2 mM DTT
TTLL7 ion Exchange buffer B: 50 mM HEPES (pH 7.0), 10 mM MgCl2, 2 mM DTT, 2 M NaCl
TTLL7 gel filtration buffer: 20 mM HEPES (pH 7.0), 150 mM NaCl, 10 mM MgCl2, 1 mM DTT
Express Xenopus tropicalis TTLL7 (residues 1–560) in E. coli Rosetta2(DE3)pLysS as a cleavable N-terminal GST fusion protein. Induce expression with 0.5 mM IPTG at 16°C and harvest after 16 hr. Pellet cells and resuspend in cold TTLL7 resuspension buffer. Lyse cells using a microfluidizer (3 passes at 12,000 psi). Perform the following steps at 4°C. Spin lysate for 45 min at 31,000 xg to pellet cellular debris. Run lysate supernatant over a GST column equilibrated in TTLL7 GST-A buffer. Wash column with 10 CVs of TTLL7 GST-A buffer and elute with 2 CVs of TTLL7 GST-B buffer. Pool TTLL7 containing fractions and load onto a heparin sepharose-6 column (GE Healthcare) equilibrated in 25% TTLL7 ion exchange buffer B. Wash the column with 2 CVs of 25% TTLL7 ion exchange buffer B and elute with a 25–50% gradient over 10 CVs. Pool fractions containing TTLL7, dilute 1:1 with TTLL7 ion exchange buffer A and digest O/N with TEV protease at a 1:50 protease:TTLL7 molar ratio. Following TEV digestion, load TTLL7 onto the heparin column equilibrated in 12.5% TTLL7 ion exchange buffer B, wash with 2 CVs of 12.5% TTLL7 ion exchange buffer B, and elute with a 12.5%−50% gradient over 8 CVs. Pool fractions containing TTLL7 and load onto a Superdex 75 gel filtration column equilibrated in TTLL7 gel filtration buffer. Pool TTLL7 containing fractions and determine concentration from absorbance at 280 nm. If needed, concentrate TTLL7 using a 30-kDa cutoff Amicon concentrator (Millipore). Protein can be flash frozen and stored at −80 °C following 15% glycerol addition.
2c. Expression and Purification of TTL
Solutions Required for TTL Purification
TTL resuspension buffer: 1X PBS, 10 mM MgCl2, 1 mM PMSF, 5 mM DTT
TTL GST-A buffer: 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 10 mM MgCl2, 5 mM DTT
TTL GST-B buffer: 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10 mM MgCl2, 5 mM DTT
TTL ion exchange buffer A: 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 2 mM DTT
TTL ion exchange buffer B: 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 2 mM DTT, 2 M NaCl
TTL hydrophobic column buffer A: 20 mM Hepes (pH 7.0), 10 mM MgCl2, 5 mM DTT, 1 M Ammonium sulfate
TTL hydrophobic column buffer B: 20 mM Hepes (pH 7.0), 10 mM MgCl2, 5 mM DTT
Express full-length Mus musculus TTL in E. coli Rosetta2(DE3)pLysS as a cleavable N-terminal GST fusion. Follow the α-TAT purification protocol up to and including the Q-sepharose step. Bring TTL Q-sepharose flow-through to 1 M ammonium sulfate and load onto a Phenyl-sepharose column (GE healthcare) equilibrated in TTL hydrophobic column buffer A. Elute the protein using a 0–100% TTL hydrophobic column buffer B gradient over 10 CVs. Pool TTL containing fractions and determine protein concentration using 280 nm absorbance. If required, concentrate TTL using a 10-kDa cutoff Amicon concentrator. TTL can be flash frozen and stored at −80 °C following addition of 15% glycerol.
3. Generation of differentially modified tubulin and microtubules
The following section describes the in vitro enzymatic modification of microtubules and tubulin using the purified enzymes obtained using protocols described in the previous section. α-TAT and TTLL7 act preferentially on microtubules, while TTL only tyrosinates monomeric tubulin effectively. Thus, we use microtubules as substrates for the acetylation and glutamylation reaction and monomeric tubulin for the tyrosination reaction. Monomeric tubulin can also be acetylated and glutamylated by α-TAT and TTLL7, respectively, but with lower efficiency.
Required Equipment
Optima MAX-XP table top ultracentrifuge (Beckman Coulter)
electrospray ionization time-of-flight LC-MS (we use an Agilent 6224)
3a. Preparation of Taxol-Stabilized Microtubules
Required Reagents and Solutions
Taxol stock: 10 mM in DMSO, stored at −20 °C
5X BRB80: 400 mM PIPES (pH 6.8), 5 mM MgCl2, 5 mM EGTA. Store in the dark at 4°C.
2x Polymerization Buffer: 20% (v:v) DMSO, 2 mM GTP, 2 mM MgCl2, 2 mM EGTA
Glycerol Cushion: 60% glycerol in 1X BRB80 supplemented with 10 μM taxol
BRB80-DT: 1X BRB80 supplemented with 1 mM DTT and 10 μM taxol
Thaw frozen tubulin in 37°C water bath and immediately place on ice. Pre-clear the tubulin to remove aggregates via ultracentrifugation at 436,000 xg for 10 min at 4°C (Note: It is important to pre-chill the rotor as well as the centrifuge tubes). Remove the supernatant and place on ice. The pellet contains small amounts of tubulin aggregates. Re-measure the concentration of tubulin in the supernatant by Bradford assay. Mix tubulin 1:1 (v/v) with 2X Polymerization Buffer. Mix thoroughly and incubate in a 37°C water bath for 30–60 min. Supplement polymerization reaction with 5 μM taxol and incubate in a 37°C water bath for 15 min. Pre-warm glycerol cushion and BRB80-DT at 37°C. Overlay polymerization reaction on glycerol cushion (80 μl Glycerol Cushion: 100 μl polymerization reaction). Pellet microtubules by ultracentrifugation using pre-warmed rotor at 109,000 xg for 10 min at 30°C. Discard supernatant. Wash pellet and walls of the ultracentrifuge tube with BRB80-DT. This step removes any unpolymerized tubulin on the tube walls. Resuspend microtubule pellet in 1X BRB80-DT (Note: Cut the pipette tip when mixing microtubules to minimize shearing). Measure tubulin concentration in 6 M guanidine hydrochloride using an extinction coefficient of 115,000 M−1.
3b. Generation of acetylated microtubules using α-TAT
This protocol generates 100% acetylated tubulin or microtubules using recombinant α-TAT.
Required Solutions
Acetyl-coA stock: 100 mM in water. Store at −20°C.
Acetylation buffer: 1XBRB80 supplemented with 250 mM KCl, 1 mM DTT, 5 μM taxol, 100 μM Acetyl coA
Incubate microtubules with α-TAT at a 1:1 molar ratio in acetylation buffer at room temperature (RT) O/N. The extent of post-translational modification can be monitored by reverse phase LC-MS as well as Western blot using a tubulin acetylation specific antibody (below). Reverse phase LC-MS analysis (described in section 1) shows complete acetylation of α1B tubulin by α-TAT, indicated by the +42 Da mass shift observed in the mass spectra ((50,193 Da (acetylated) versus 50,151 Da (non-modified)) (Figure 2B). No additional species with a mass shift are visible underscoring the specificity of the modification. The acetylation reaction can also be performed with non-taxol stabilized microtubules at 37°C in the presence of 20% glycerol and 1 mM GTP (Kormendi, Szyk, Piszczek, & Roll-Mecak, 2012).
Detection of tubulin acetylation by Western blot
We use infrared dye (IRDye®) conjugated secondary antibodies (Li-Cor) to detect tubulin post-translational modifications on Western blots. Two different secondary IRDye antibodies are used simultaneously: one secondary antibody detects the tubulin antibody while the other detects the antibody specific for the tubulin post-translational modification.
Required Reagents and Solutions
1X PBS
1X PBS-T; 0.1% (v:v) Tween-20 in 1X PBS
Blocking agent: 4% milk powder in 1X PBS
Dilution Buffer: 4% milk powder in 1X PBS-T
Primary antibody 1 solution: mouse 6–11B (recognizes acetylated tubulin) (Sigma Aldrich) diluted 1:10,000 in Dilution Buffer
Primary antibody 2 solution: rabbit E-19R (recognizes α tubulin N-terminus) (Santa Cruz Biotech) diluted 1:1,000 in Dilution Buffer
Secondary antibody 1: IR Dye 680LT goat (polyclonal) anti-mouse IgG (Li-Cor)
Secondary antibody 2: IR Dye 800LT goat (polyclonal) anti-rabbit IgG (Li-Cor)
Required Equipment
Odyssey CLx (Li-Cor)
iBlot 7-minute Blotting System (Life Technologies) (Note: Traditional transferring methods such as wet and semi-wet transfers will work as well)
Separate 125 ng each of naïve and acetylated tubulin on SDS-PAGE and transfer onto nitrocellulose membrane. All subsequent steps require rocking of the membrane. First, block O/N in blocking agent at 4°C or for 2 hr at RT. Perform all subsequent steps at RT. Incubate membrane in primary antibody 1 solution for 1 hr. Wash twice with Dilution Buffer then incubate membrane in primary antibody 2 solution for 1 hr. Wash 5 times with 1X PBS-T for 5 min. Incubate with secondary antibody 1 and secondary antibody 2 simultaneously (both diluted 1:18,000 in dilution buffer) for 1 hr (Note: protect the blot from light as the IR Antibodies are light sensitive). Wash blot 5 times with 1X PBS-T for 5 min, then twice with 1X PBS. Image using Li-Cor Odyssey CLx (Li-Cor). The acetylated tubulin produces a strong signal in the 680 nm channel (white) that is not observed at the 0 hr time point (Figure 2B). The strong 800 nm signal (grey) represents total tubulin loaded. The anti-acetylation tubulin antibody can be calibrated once using a tubulin sample that is 100% acetylated.
If desired, α-TAT can be removed after the acetylation reaction by a high salt wash. Pellet the acetylated microtubules by ultracentrifugation for 10 min at 109,000 xg. Re-suspend pellet in 100 μl of BRB80-DT supplemented with 350 mM NaCl and incubate at 37°C for 10 min (Note: It is important to keep buffers warm in order not to depolymerize microtubules). Overlay the resuspended pellet on 100 μl glycerol cushion (1X BRB80, 350 mM NaCl, 60% glycerol and 10 μM taxol). Pellet microtubules by ultracentrifugation at 109,000 xg for 15 min, 30°C. Remove supernatant and wash pellet with 200 μl of BRB80-DT supplemented with 350 mM NaCl followed by 200 μl of BRB80-DT buffer. Re-suspend pellet in BRB80-DT to desired volume and concentration. Removal of the enzyme can be verified by SDS-PAGE or reverse phase LC-MS.
3c. Generation of poly-glutamylated microtubules using TTLL7
The following protocol describes the poly-glutamylation of unmodified microtubules with recombinantly expressed TTLL7. Varying the incubation time with TTLL7 controls the length of poly-glutamate chains added to α- and β-tubulin.
Required Solutions
Glutamate stock: 100 mM in water, pH adjusted to 7.0, stored at −20°C.
Adenosine triphosphate (ATP) stock: 100 mM in water, pH adjusted to 7.0, stored at −20°C.
Glutamylation Buffer: 20 mM HEPES (pH 7.0), 50 mM NaCl, 5 mM MgCl2, 1 mM ATP, 1 mM glutamate, 1 mM DTT.
Use taxol-stabilized microtubules as the substrate for TTLL7. If addition of glutamate chains primarily on β-tubulin is desired, incubate TTLL7 with unmodified microtubules at 1:10 molar ratio of enzyme:substrate for 1 hr at RT in glutamylation buffer. Reverse-phase LC-MS analysis of the reaction reveals multiple mass increments of 129 Da, indicating the addition of glutamate residues. Closer inspection reveals chains consisting of 12, 3, and 2 glutamates attached to βIVB, βI, and βIII tubulin respectively, while α1B tubulin is mono-glutamylated only sparingly (Figure 3, top panel). Polyglutamate chains of increasing length can be added to both α- and β-tubulin by increasing the incubation time. Incubation for 6 hr produces chains of up to 4, 17, and 22 glutamates on α1B, βI and βIVB respectively (Figure 3B), while an 18 h incubation generates chains up to 10, 28, and 35 glutamates on α1B, βI, and βIVB, respectively (Figure 3C). No unmodified βIII tubulin remains after 6 hr, however the poly-glutamylated moieties are not visible in the spectrum because of low signal.
Figure 3:
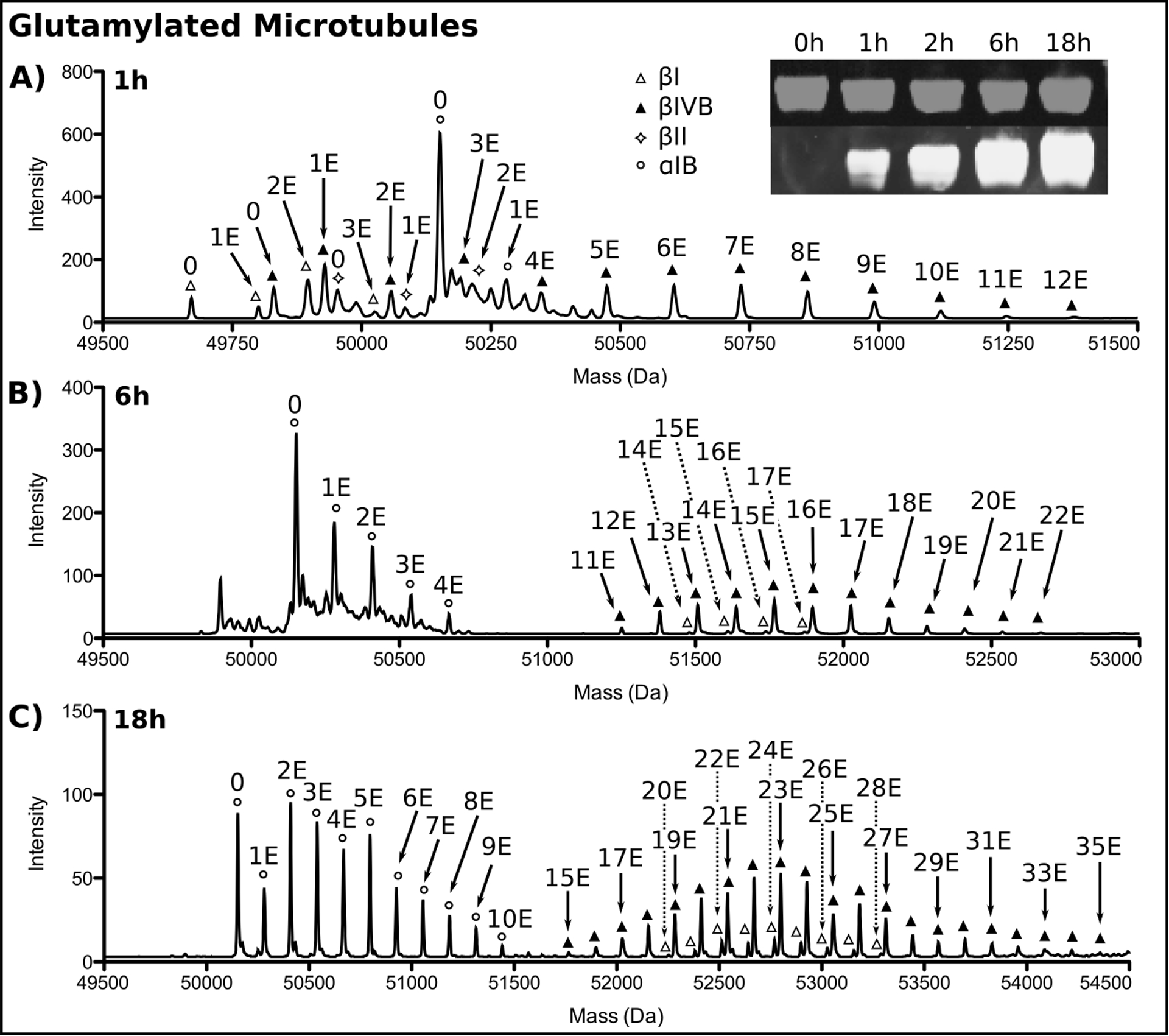
Reverse phase LC-MS and Western blot analysis of unmodified microtubules glutamylated by TTLL7. (A), 1 h, (B), 6 h, (C), 18 h incubation. Tubulin isoforms are indicated by symbols. The number of glutamates added to each isoform by TTLL7 is indicated. Only every other glutamate added to βI and βIVB at 18 h is labeled for clarity. Inset shows the progression of tubulin glutamylation monitored by Western blot at the indicated time points. α-tubulin, grey, glutamylated tubulin, white. The two channels are offset for clarity.
The extent of glutamylation can also be monitored by Western blot using the monoclonal GT335 antibody (Adipogen, 1:2000 dilution) that recognizes the branch point created during the addition of the first glutamate residue of a growing glutamate chain (Figure 1) (Wolff, 1992). Follow the protocol detailed in section 3b. Figure 3 shows the signal in the 680 nm channel (grey) intensifies over the course of the reaction, indicating increasing levels of glutamylation. The 800 nm channel (white) monitors total tubulin loaded.
If desired, TTLL7 can be removed after the glutamylation reaction by a high salt wash (see protocol described in section 3b for the removal of α-TAT from modified microtubules).
3d. Generation of tyrosinated tubulin using TTL
This protocol generates 100% tyrosinated tubulin using recombinant TTL.
TTL acts preferentially on the tubulin monomer and is inefficient at modifying tubulin already incorporated into microtubules (Raybin & Flavin, 1975; Szyk, Deaconescu, Piszczek, & Roll-Mecak, 2011). Thus, in order to generate tyrosinated microtubules, monomeric tubulin is first tyrosinated and then polymerized into microtubules. Mammalian brain tubulin contains high levels of several detyrosinated α–tubulin isoforms (Gundersen, Kalnoski, & Bulinski, 1984) (Figure 4A). LC-MS analysis of porcine brain tubulin (Cytoskeleton) identified detyrosinated α1A and α1B tubulin, both present in multiple glutamylation states, with up to three glutamates attached to each (Figure 4A) (Redeker, 2010). The unmodified tubulin isolated from tsA201 cells is 100% tyrosinated (Figure 1A), thus this protocol is applicable to brain tubulin or other tubulin preparations that have high levels of detyrosinated tubulin.
Figure 4:
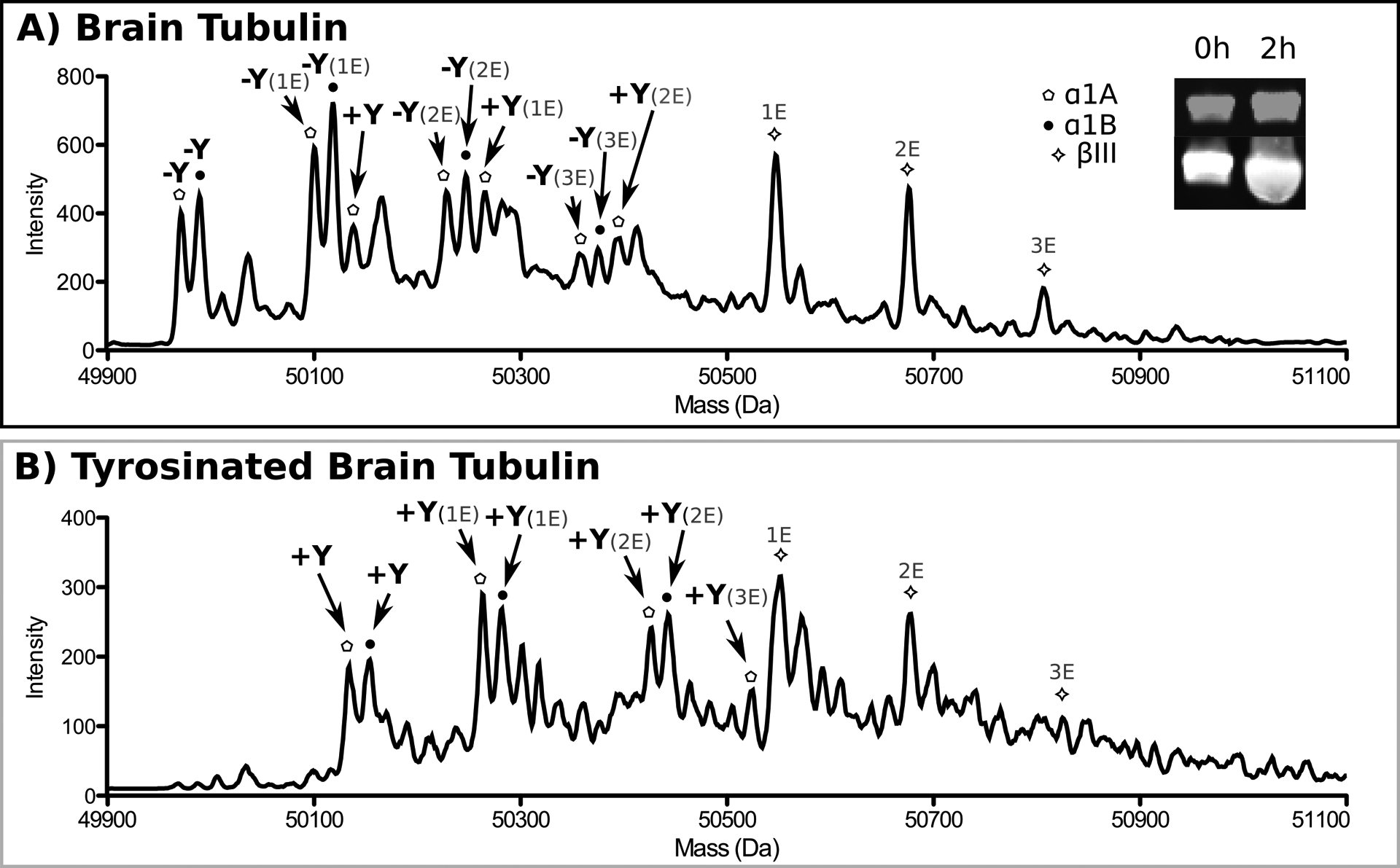
(A) Reverse phase LC-MS analysis of porcine brain tubulin. Tubulin isoforms are indicated by their respective symbols. Additional α- and β-tubulin isoforms are present, however they elute at different time points and are not shown. The tyrosination status of each isoform is indicated in bold black font, while glutamylation is indicated in grey. The inset shows the progression of tyrosination by Western blot using antibodies specific for tyrosinated tubulin. α-tubulin, grey, tyrosinated tubulin, white. The two channels are offset for clarity. (B) Reverse phase LC-MS analysis of porcine brain tubulin tyrosinated by TTL. Isoforms labeled as in A.
Required Solutions
Tyrosine stock: 30 mM in water, stored at −20°C.
ATP stock: 100 mM in water, pH adjusted to 7.0, stored at −20°C.
Tyrosination buffer: 1X BRB80, 1 mM DTT, 0.3 mM Tyr, 2 mM ATP
Incubate TTL with tubulin at a 1:50 ratio for 2 hr at RT in tyrosination buffer. Reverse phase LC-MS analysis reveals all detyrosinated α-tubulin isoforms are converted to the tyrosinated form after 2 hr (Figure 4B). The extent of tubulin tyrosination can also be monitored by Western blot with a monoclonal antibody specific for tyrosinated tubulin (TUB-1A2, Sigma; 1:1000 dilution) and following the protocol in section 2a. Figure 4 shows the signal in the 680 nm channel (grey) intensifies over the course of the reaction, indicating increasing levels of tyrosination. The signal in the 800 nm channel (grey) represents total tubulin. The anti-tyrosinated tubulin antibody can be calibrated using a tubulin sample that is 100% tyrosinated.
If removal of TTL from the reaction is desired, cycle the tubulin once to remove TTL as well as any non-polymerization competent tubulin and aggregates (see below).
Tubulin Cycling
Supplement the tyrosinated tubulin with 1 mM GTP and 33% (v:v) glycerol. Allow tubulin to polymerize for 40 min at 37 °C. Layer polymerized tubulin on a 60% glycerol cushion in 1X BRB80. Pellet the microtubules by ultra-centrifugation using a pre-warmed rotor at 109,000 xg for 10 min at 37 °C. Aspirate supernatant and cushion. Rinse microtubule pellet with 1X BRB80. Incubate pellet on ice for 5 min. Resuspend pellet in 1X BRB80 supplemented with 1 mM DTT. The volume of buffer is chosen based on the desired final tubulin concentration. Incubate on ice for 30 min, intermittently pipetting up and down. Centrifuge sample at 109,000 xg for 10 min at 4 °C to remove aggregates and microtubules that did not depolymerize. Collect supernatant and freeze in small aliquots for future use in microtubule dynamic assays or to generate taxol-stabilized microtubules (as described in section 3a). For example, one could use differentially tyrosinated tubulin to investigate the role of tyrosination on the kinetics of association to the microtubule plus end of tracking proteins (Bieling, Kandels-Lewis, Telley, van Dijk, Janke, & Surrey, 2008).
4. Conclusion
Here we describe the purification of three recombinant tubulin-modifying enzymes - α-TAT, TTLL7, and TTL - as well as protocols for enzymatic modification of naïve and brain tubulin using these enzymes to produce differentially modified microtubules. These different “flavors” of microtubules can be used in a wide range of biochemical and biophysical assays to systematically dissect the specific effects of acetylation, tyrosination, and glutamylation on microtubule dynamics as well as cellular effectors. For example, does one modification change the time a motor remains associated with the microtubule? Are motors specialized for different tubulin modifications? Does a post-translational modification bias a motor towards one microtubule over another at a junction? Lastly, it has been known for a long time that acetylated and poly-glutamylated microtubules have increased stabilities in cells; however, it is not yet clear whether this increased stability is due to these modifications or is an indirect effect of regulators recruited to these microtubules. The ability to make unmodified and homogenously modified microtubules finally enables the investigation of the direct effects of post-translational modifications on microtubule dynamics as well as the identification through proteomic approaches of microtubule regulators that are differentially recruited to post-translationally modified microtubules.
References
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, et al. (2010). MEC-17 is an alpha-tubulin acetyltransferase. Nature, 467(7312), 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, et al. (1991). Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci U S A, 88(11), 4685–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra HS, Rodriguez JA, Arce CA, & Caputto R (1973). A soluble preparation from rat brain that incorporates into its own proteins (14C)arginine by a ribonuclease-sensitive system and (14C)tyrosine by a ribonuclease-insensitive system. J Neurochem, 20(1), 97–108. [DOI] [PubMed] [Google Scholar]
- Bieling P, Kandels-Lewis S, Telley IA, van Dijk J, Janke C, & Surrey T (2008). CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J Cell Biol, 183(7), 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva JG, Hsin J, Huang KC, & Goodman MB (2012). Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr Biol, 22(12), 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, & Denoulet P (1990). Posttranslational glutamylation of alpha-tubulin. Science, 247(4938), 83–85. [DOI] [PubMed] [Google Scholar]
- Garnham CP, & Roll-Mecak A (2012). The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton (Hoboken), 69(7), 442–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, & Bulinski JC (1984). Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell, 38(3), 779–789. [DOI] [PubMed] [Google Scholar]
- Howard J , & Hyman AA (2003). Dynamics and mechanics of the microtubule plus end. Nature, 422, 753–758. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Heier RL, Taruishi M, Takagi H, Mukai M, Shimma S, et al. (2007). Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc Natl Acad Sci U S A, 104(9), 3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, & Setou M (2006). TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J Biol Chem, 281(41), 30707–30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, & Bulinski JC (2011). Post-translational regulations of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol, 12, 773–786. [DOI] [PubMed] [Google Scholar]
- Kapust RB, Tözsér J, Fox JD, Anderson DE, Cherry S, Copeland TD, et al. (2001). Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng., 14(12), 993–1000. [DOI] [PubMed] [Google Scholar]
- Kormendi V, Szyk A , Piszczek G, & Roll-Mecak A (2012). Crystal structures of tubulin acetyltransferase reveal a conserved catalytic core and the plasticity of the essential N terminus. J Biol Chem, 287(50), 41569–41575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault SW, & Rosenbaum JL (1983). Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol, 97(1), 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault SW, & Rosenbaum JL (1985). Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry, 24(2), 473–478. [DOI] [PubMed] [Google Scholar]
- Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, et al. (2010). Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol, 189(6), 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Xiao H, Burd B, Horwitz SB, Angeletti RH, & Verdier-Pinard P (2010). Methods in tubulin proteomics. Methods Cell Biol, 95, 105–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E (2000). Structural insights into microtubule function. Annu Rev Biochem(69), 277. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M , Milligan RA , & Downing KH (1999). High-Resolution Model of Microtubule. Cell, 96, 79–88. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, & Downing KH (1998). Structure of the alpha beta tubulin dimer by electron crystallography. Nature, 391(6663), 199–203. [DOI] [PubMed] [Google Scholar]
- Paturle-Lafanechere L, Edde B, Denoulet P, Van Dorsselaer A, Mazarguil H, Le Caer JP, et al. (1991). Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry, 30(43), 10523–10528. [DOI] [PubMed] [Google Scholar]
- Raybin D, & Flavin M (1975). An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun, 65(3), 1088–1095. [DOI] [PubMed] [Google Scholar]
- Raybin D, & Flavin M (1977). Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry, 16(10), 2189–2194. [DOI] [PubMed] [Google Scholar]
- Redeker V (2010). Mass spectrometry analysis of C-terminal posttranslational modifications of tubulins. Methods Cell Biol, 95, 77–103. [DOI] [PubMed] [Google Scholar]
- Redeker V, Le Caer JP, Rossier J, & Prome JC (1991). Structure of the polyglutamyl side chain posttranslationally added to alpha-tubulin. J Biol Chem, 266(34), 23461–23466. [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, et al. (1994). Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science, 266(5191), 1688–1691. [DOI] [PubMed] [Google Scholar]
- Redeker V, Melki R, Prome D, Le Caer JP, & Rossier J (1992). Structure of tubulin C-terminal domain obtained by subtilisin treatment. The major alpha and beta tubulin isotypes from pig brain are glutamylated. FEBS Lett, 313(2), 185–192. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, & McNally FJ (2010). Microtubule-severing enzymes. Curr Opin Cell Biol, 22(1), 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, & Vale RD (2008). Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature, 451(7176), 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger M, Plessman U, Kloppel KD, Wehland J, & Weber K (1992). Class II tubulin, the major brain beta tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett, 308(1), 101–105. [DOI] [PubMed] [Google Scholar]
- Schroder HC, Wehland J, & Weber K (1985). Purification of brain tubulin-tyrosine ligase by biochemical and immunological methods. J Cell Biol, 100(1), 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida T, Cueva JG, Xu Z, Goodman MB, & Nachury MV (2010). The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A, 107(50), 21517–21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep KC , & Vale RD . (2007). Structural Basis of Microtubule Plus End Tracking by XMAP215, CLIP-170, and EB1. Mol Cell, 27(6), 976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V, Herbstman J , Skiniotis G , & Verhey KJ. (2012). Luminal Localization of a-tubulin K40 Acetylation by Cryo-EM Analysis of Fab-Labeled Microtubules. PLos One, 7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KF (1988). Structure and utilization of tubulin isotypes. Annu Rev Cell Biol, 4, 687–716. [DOI] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Piszczek G, & Roll-Mecak A (2011). Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat Struct Mol Biol, 18(11), 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalidou I, Keller C , Kalebic N, Nguyen KC., Somhegyi H , Politi KA, et al. (2012). Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol, 22(17), 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk J, Rogowski K, Miro J, Lacroix B, Edde B, & Janke C (2007). A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell, 26(3), 437–448. [DOI] [PubMed] [Google Scholar]
- Weisenberg RC. (1972). Microtubule formation in vitro in solutions containing low calcium concentrations. Scine(177), 1104–1105. [DOI] [PubMed] [Google Scholar]
- Widlund PO, Podolski M , Reber S , Alper J , Storch M, Hyman AA , et al. (2012). One-step purification of assembly-competent tubulin from diverse eukaryotic sources. Mol Biol Cell, 23, 4393–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, & Gaertig J (2010). Post-translational modifications of microtubules. J Cell Sci, 123(Pt 20), 3447–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]


