Abstract
Background
Colorectal cancer (CRC) screening with fecal immunochemical testing (FIT) can reduce CRC mortality. Effectiveness of FIT may be compromised when patients do not adhere to a regular schedule. However, having no standard measure of repeat FIT presents challenges for assessing effectiveness across populations and settings. We compared three measures of repeat FIT in a large, integrated healthcare system in Dallas, TX.
Methods
We identified 18,257 patients age-eligible (50–60 years) for FIT in January 1 – December 31, 2010 and followed over four rounds of screening. Measures included: 1) repeat FIT in prior screeners, or completion of FIT within 9–15 months of the previous; 2); Yes-No patterns, whereby patients were assigned yes or no in 9–15 month windows; and 3) proportion of time covered, or the amount of time patients were up-to-date with screening relative to time eligible.
Results
Repeat FIT varied by measure. Using a prior screeners measure, 15.8% of patients with a normal FIT in Round 1 completed repeat FIT in Round 2. Repeat FIT was notably higher (52.3%) using proportion of time covered. The most common Yes-No pattern was YNNN or “one-and-done,” and only 9.4% of patients completed two consecutive FITs across all rounds (YYNN).
Conclusions
Different measures of repeat FIT yielded a range of estimates, making comparison across studies difficult. Researchers should weigh the advantages and disadvantages of each measure and select the most appropriate to their research question.
Impact
Our study highlights the need for future research of repeat FIT measures that best approximate screening effectiveness.
Keywords: colorectal cancer, screening, healthcare delivery, measurement, adherence
Introduction
Colorectal cancer (CRC) screening is endorsed as an effective preventive health service1, 2 because it reduces morbidity and mortality from CRC.3 Regular screening lowers CRC mortality by detecting cancers at an earlier stage,4, 5 and it may also decrease incidence by removing precancerous polyps.6, 7 Most professional organizations recommend average-risk screening begin at age 50 years with colonoscopy every 10 years, sigmoidoscopy every 5 years, CT colonography every 5 years, annual guaiac-based fecal occult blood test (gFOBT) or fecal immunochemical test (FIT), or FIT-DNA every 1 or 3 years.3, 8, 9 In the U.S., about 60% of age-eligible adults are up-to-date with CRC screening,10 and prevalence differs by race/ethnicity, sex, educational attainment, insurance status, and geographic region.11–14
Although most adults in the U.S. complete screening with colonoscopy,11, 15 FIT plays a central role in screening programs of large, managed care plans,16 underserved or rural populations,17, 18 and healthcare systems transitioning to population health outreach.19 Success of FIT-based strategies depends on several provider- and patient-level factors,20, 21 including coordinated efforts to track and report test results, optimizing test performance, timely follow-up of abnormal results, and patient adherence to recommended tests and intervals.
Most studies measure patient adherence to one-time FIT,22 but adherence is a more complex process that includes completing an initial FIT and repeat screening or diagnostic testing, depending on test findings.23 For example, performance and quality measures, such as the Healthcare Effectiveness Data and Information Set (HEDIS®), report completion of FIT in the last year, ignoring adherence to repeat FIT in subsequent rounds or follow-up with colonoscopy. Measuring adherence to repeat FIT can be particularly challenging because patients may complete screening earlier or later than the recommended interval; “crossover” to colonoscopy (by preference or for diagnostic evaluation) and therefore not need a repeat FIT; or become ineligible for FIT as a screening test after an abnormal result or “red flag” symptoms (e.g., hematochezia, iron deficiency anemia).24 Further, there is no standard measure of repeat FIT, making it difficult to assess test effectiveness across patient populations and healthcare systems. New measures are needed to better address these challenges.
Herein, we compare three measures of repeat FIT in a large population of screening age-eligible patients receiving care in an integrated system.
Materials and Methods
Setting
We conducted a retrospective cohort study at Parkland Health & Hospital System, Dallas County’s safety-net healthcare system. Parkland is a vertically integrated health system, including an 880-bed inpatient hospital, 12 community-based primary care clinics, and outpatient specialty clinics. Since 2009, Parkland has used a comprehensive electronic health record (EHR) to integrate care across inpatient and outpatient settings. Parkland provides low-cost primary and specialty care to under- and uninsured residents of Dallas County through a sliding-fee program funded by county tax dollars. Both FIT kits and colonoscopy are provided to patients at a reduced cost (e.g., $25 co-payment for colonoscopy). Screening is offered opportunistically to adult patients of eligible screening age (≥ 50 years); most average-risk patients receive FIT kits (vs. colonoscopy) during in-person, primary care clinic visits.25
Study population
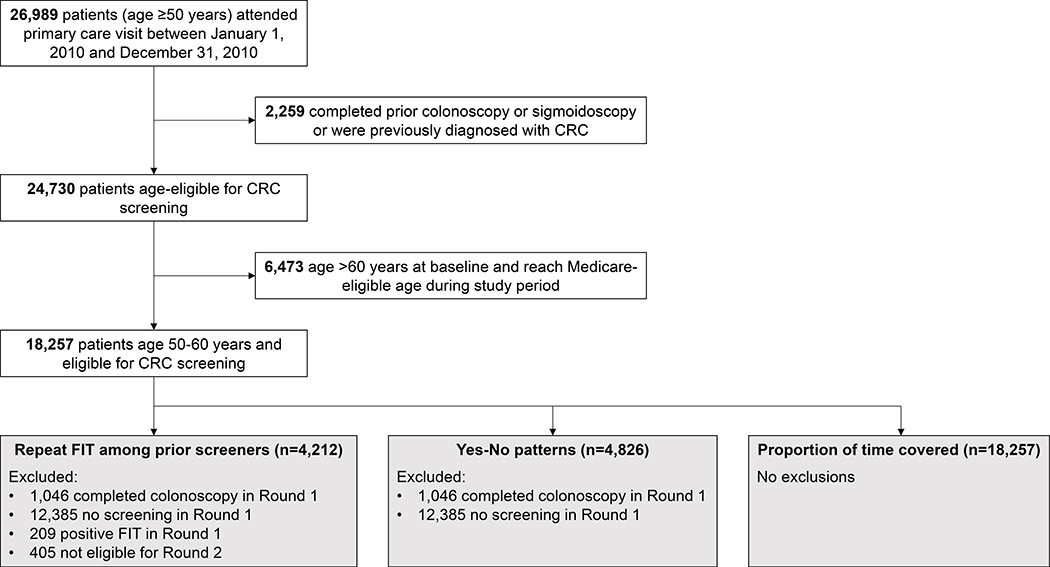
We defined the study population using EHR data to identify patients (age ≥50 years) who attended a primary care visit between January 1, 2010 and December 31, 2010 (n=26,989). We excluded patients who received a prior colonoscopy or sigmoidoscopy or were previously diagnosed with CRC (n=2,259). Because patients who reach Medicare-eligible age may opt to receive care outside the health system, and therefore, the EHR may no longer capture screening, we further excluded patients aged >60 years at baseline (n=6,573). The final study population comprised 18,257 patients (age 50–60 years) age-eligible for screening (Figure 1). We followed these patients through September 30, 2014 over four approximate 12-month intervals or “rounds” of screening.
Figure 1.
Study flow diagram
Using EHR data, we described demographic characteristics of the study population, including age at baseline, sex, race/ethnicity (non-Hispanic white, non-Hispanic back, Hispanic, and other), comorbidity score (0, 1, or ≥2, using the Charlson comorbidity index26), and insurance (Medicare, Medicaid, charity care, and commercial or other), as well as screening completion in Round 1 (FIT, colonoscopy, or no screening).
Measures
We compared three measures of repeat FIT, summarized below and with additional detail presented in Table 1.
Table 1:
Measures of repeat fecal immunochemical test for colorectal cancer screening
| Measure | Definition | Denominator | Example | Advantages | Disadvantages | Relevant research questions |
|---|---|---|---|---|---|---|
| Repeat FIT in prior screeners | Completion of a subsequent FIT between 9 and 15 months of previous, normal FIT | Previously completed FIT with normal result; exclude patients crossed over to endoscopy, diagnosed with CRC, moved away from healthcare system, or died after prior FIT | For a patient who completed a FIT with normal result on July 1, 2010, any FIT performed between April 1, 2011 and October 1, 2011 | Denominator uses all available information to determine eligibility | Estimates of repeat FIT in later rounds restricted to self-selected sample previously completed FIT | Does repeat FIT vary by screening in a prior round? Time to rescreening or next test |
| Yes-No patterns | Completion of subsequent FIT in 9–15 month windows of index FIT, across all screening rounds | Previously completed FIT (normal or abnormal result) | A patient who completed an index FIT and subsequent FIT at month 23 has a pattern of YNYN | Captures intermittent screening (e.g., YNYN); readily identifies drop off (e.g., YNNN or YYNN) | Overwhelming number of patterns given amount of follow-up; does not assign effectiveness to each pattern (e.g., YYNN vs. YNYN) | What proportion of patients complete FIT in all rounds? How frequently do patients adhere to a biennial schedule? |
| Proportion of time covered | Number of days up-to-date with screening divided by number of days eligible for screening; time assigned to screening tests based on type, quality, and findings | All eligible for FIT (no prior colorectal cancer, colectomy, colonoscopy in 10 years, sigmoidoscopy in 5 years) | Two normal FITs completed 9 months apart provides 21 months coverage | Captures all screening tests and recommended intervals; does not penalize patients for tests completed just outside the recommended interval; provides measure of program effectiveness37 | Requires detailed endoscopy and pathology reports; cumbersome to calculate; assigns different coverage time for identical behaviors; sensitive to amount of follow-up | For how much time are patients adherent to screening? How long do patients remain adherent to FIT? |
1. Repeat FIT in prior screeners
For the subset of patients completing a normal FIT in Round 1 and eligible for screening in Round 2 (n=4,212, Figure 1), we defined repeat FIT as completion of a subsequent FIT between 9 and 15 months of the previous test. We included a 3-month grace period around the guideline recommend 12-month interval to allow for some flexibility in patient scheduling. For example, if a patient completed a FIT on July 1, 2010, we counted any FIT completed between April 1, 2011 and October 1, 2011 as repeat FIT. Patients with abnormal FIT results or who completed colonoscopy, and therefore, were ineligible for a subsequent FIT, were excluded from the denominator of the subsequent screening round. We conducted a sensitivity analysis defining repeat FIT as completion of a subsequent FIT between 9 and 18 months of the previous test.
2. Yes-No patterns
We measured repeat FIT by quantifying patterns of FIT in 9–15 month windows, whereby patients were assigned YES or NO in each window. For example, a patient who completed a FIT in Round 1 and Round 3 was assigned a pattern of YNYN. To limit the number of possible patterns, we focused on patients completing any FIT in Round 1 (n=4,826, Figure 1). We used the date of FIT in Round 1 to determine the appropriate 9–15-month window, resetting the window with each subsequent FIT completed. We created additional patterns that accounted for positive FIT and censored patients thereafter. For example, a patient who completed a normal FIT in Round 1, no FIT in Round 2, and a positive FIT in Round 3 was assigned a pattern of YNYpos. In a sensitivity analysis, we measured repeat FIT in 6–18 month windows.
3. Proportion of time covered
Among all screen-eligible patients (n=18,257, Figure 1), we used proportion of time covered (PTC) to measure repeat FIT as the number of days patients were up-to-date with screening relative to the number of days they were eligible.27 PTC is frequently used in medication adherence research,28 and we previously demonstrated how this approach can be used to measure adherence to CRC screening.27 We assigned 12 months’ coverage to normal FIT and 6 months to positive FIT. For example, a normal FIT followed by a positive FIT completed 15 months later would provide 18 months. If patients had overlapping FITs, we adjusted the end date of the previous test, rather than sum all time covered by FIT (e.g., two normal FITs completed 9 months apart would provide 21 months). For patients who “crossed over” to colonoscopy after FIT, we assigned coverage time based on exam quality and results (e.g., 10 years for a normal exam, 5 years for one or two small adenomas). To facilitate comparison with the other approaches, we also used PTC to measure repeat FIT among patients completing any FIT in Round 1 (n=4,826, Figure 1).
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). The study was approved by the Institutional Review Board at UT Southwestern Medical Center.
Results
We identified 18,257 patients (age 50–60 years) who attended a primary care visit and were age-eligible for screening (Figure 1). Mean age was 54.9 years. As shown in Table 2, most patients were women (62.4%), non-Hispanic black (40.5%) or Hispanic (34.9%), and had no comorbid conditions (53.8%). In Round 1, 4,826 (26.4%) patients completed FIT, 1,046 (5.7%) completed colonoscopy, and 12,385 (67.8%) did not complete screening.
Table 2.
Characteristics of 18,257 patients age-eligible (50–60 years) for colorectal cancer screening between January 1, 2010 and December 31, 2010, Parkland Health & Hospital System, Dallas, TX
| n | (%) | |
|---|---|---|
| Age at baseline, mean (SD) | 54.9 (3.12) | |
| 50–54 | 9,632 | (52.8) |
| 55–60 | 8,625 | (47.2) |
| Sex | ||
| Male | 6,867 | (37.6) |
| Female | 11,390 | (62.4) |
| Race/ethnicity | ||
| Non-Hispanic white | 3,307 | (18.1) |
| Non-Hispanic black | 7,394 | (40.5) |
| Hispanic | 6,362 | (34.9) |
| Other | 1,194 | (6.5) |
| Insurance | ||
| Medicaid | 2,093 | (11.5) |
| Medicare | 1,844 | (10.1) |
| Charity care | 13,315 | (72.9) |
| Commercial or other | 1,005 | (5.5) |
| Comorbidity score | ||
| 0 | 9,826 | (53.8) |
| 1 | 5,069 | (27.8) |
| 2+ | 3,362 | (18.4) |
| Screening in Round 1 | ||
| FIT | 4,826 | (26.4) |
| Colonoscopy | 1,046 | (5.7) |
| No screening | 12,385 | (67.8) |
NOTE: Round 1: January 1, 2010 – December 31, 2010
Prevalence of repeat FIT varied by the measure used. For example, using the prior screeners measure, 15.8% of patients who completed a normal FIT in Round 1 completed repeat FIT in Round 2; yet, using Yes-No patterns, 9.4% of patients completed two consecutive FITs over four rounds of screening (YYNN). With the PTC measure, repeat FIT was notably higher (52.3%) among those completing a FIT in Round 1.
Repeat FIT among prior screeners
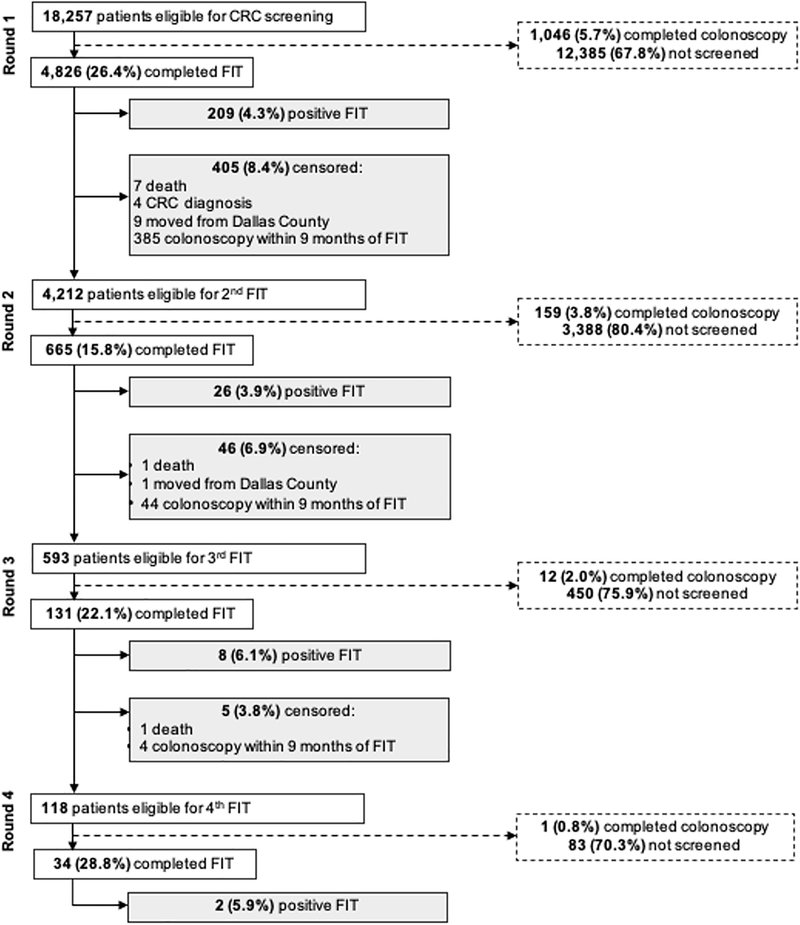
Among the subset of patients with a normal FIT in Round 1 (n=4,212), repeat FIT ranged from 15.8 – 28.8% in Round 2–4 (Figure 2). Only 665 (15.8%), 131 (3.1%), and 34 (0.8%) completed two, three, and four consecutive FITs, respectively. In a sensitivity analysis defining repeat screening as a subsequent FIT completed within 9–18 months (vs. 9–15 months in primary analysis) of the index FIT, we observed a slightly higher proportion of patients completing repeat FIT in each round (range 25.0 – 38.1%).
Figure 2.
Repeat FIT among prior screeners with normal FIT in Round 1 (n=4,212)
Yes-No patterns
The most common yes-no pattern was “one and done” (YNNN, 66.7%), followed by YYNN (9.4%), shown in Table 3a. Only 37 patients (0.7%) completed four, consecutive FITs across the screening rounds (either YYYY or YYYYpos). Among those with negative FIT (n=4,539), 70.9% completed 1 of 4, 23.6% completed 2 of 4, 4.7% completed 3 of 4, and 0.7% completed 4 of 4 FITs (Table 3b). There were no appreciable changes in the direction or magnitude of results in a sensitivity analysis using 6–18 month windows.
Table 3a.
Yes-No patterns of repeat FIT across four rounds of colorectal cancer screening (n=4,826)
| Round 1 (n=4,826) | Round 2 (n=697) | Round 3 (n=494) | Round 4 (n=515) | As % of total (n=4,826) |
|---|---|---|---|---|
| Y | N | N | N | 66.7 (3,220) |
| Y | N | N | Y | 6.8 (329) |
| Y | N | Y | Y | 0.9 (44) |
| Y | N | Y | N | 6.0 (290) |
| Y | Y | N | N | 9.4 (454) |
| Y | Y | N | Y | 1.5 (74) |
| Y | Y | Y | N | 1.9 (94) |
| Y | Y | Y | Y | 0.7 (34) |
| Y pos | 4.3 (209) | |||
| Y | N | N | Y pos | 0.4 (18) |
| Y | N | Y pos | 0.3 (14) | |
| Y | Y pos | 0.5 (25) | ||
| Y | Y | Y pos | 0.2 (8) | |
| Y | Y | Y | Y pos | 0.1 (3) |
| Y | Y | N | Y pos | 0.1 (5) |
| Y | N | Y | Y pos | 0.1 (5) |
NOTE: Y, FIT completed in round; N, FIT not completed in round; Ypos, FIT completed in round with positive result.
Table 3b.
Consolidated Yes-No patterns among patients with negative FIT only (n=4,539)
| Consolidated pattern | Corresponding Yes-No pattern(s) | As % of total (n=4,539) |
|---|---|---|
| 1 of 4 | YNNN | 70.9 (3,220) |
| 2 of 4 | YYNN, YNYN, YNNY | 23.6 (1,073) |
| 3 of 4 | YYYN, YYNY, YNYY | 4.7 (212) |
| 4 of 4 | YYYY | 0.7 (34) |
Proportion of time covered
Mean PTC was 29.1% (95% CI: 28.6—29.5%) among all FIT-eligible patients (n=18,257) and 52.3% (95% CI: 51.6 – 53.1%) among those completing a FIT in Round 1 (n=4,826). As we previously reported,27 common screening patterns were only one or two FITs during the study period. Average time to first FIT was 11.3 months from cohort entry, and 17.9 months from first to second FIT.
Discussion
Long-term effectiveness of FIT screening may be compromised when patients do not adhere to a regular schedule,24 yet having no standard measure of repeat FIT presents challenges for assessing effectiveness across patient populations and healthcare systems. To address these challenges, we compared three measures of repeat FIT, and results varied widely by the measure used. Each measure has advantages and disadvantages, and further research is needed to determine which measure best approximates patient adherence and screening effectiveness.
Prevalence of repeat FIT varied depending upon measure approach used. For example, when defined as the proportion of Round 1 participants completing FIT in Round 2, about 15% of patients completed repeat screening. Repeat FIT was lower when measured using Yes-No patterns across the four screening rounds – only 10% of patients completed FIT in two consecutive rounds, and even fewer completed four tests (<1%). Prevalence appeared higher when measured as the proportion of time covered across the four screening rounds. Variability in repeat FIT is important to consider when comparing results across studies, which often use different approaches for measuring adherence. A recent systematic review similarly observed variability in the proportion of patients completing repeat FIT depending upon how the outcome was defined – 75% of prior screeners completed repeat FIT, but repeat FIT was much lower (45%) when defined as completing two, consecutive rounds.29
Our findings also highlight how the choice of denominator impacts estimates of repeat FIT. As Chubak et al. described,30 there are generally three denominators used in studies of screening adherence: 1) the entire population; 2) patients meeting eligibility for screening; and 3) patients who have been previously screened. Confusion surrounding these denominators can make it difficult to determine prevalence and compare estimates across studies. We used measures focused on patients who were age-eligible, as well as those who had been previously screened with FIT, and we noted differences in prevalence of repeat FIT between the two. For example, using a PTC, repeat FIT was much higher when restricted to the denominator of patients completing a FIT in Round 1 vs. all patients eligible for FIT. Many European studies of screening outreach programs31–33 include a mix of denominators (i.e., combine newly eligible and prior screeners), conflating results and overestimating adherence to repeat FIT. Similarly, quality measures and performance standards, such as HEDIS®34 or ACO Quality Measures,35 report FIT as a cross-sectional measure, which may also overestimate adherence.
Moving forward, we recommend researchers weigh the advantages and disadvantages of each measure of repeat FIT (Table 1) and select the measure most appropriate to their research question. The measure should also be oriented to clinically relevant time points in the screening process. For example, PTC may be better suited to answer, “For how much time are patients adherent to FIT?” PTC provides an estimate of total time spent adherent to FIT as a function of time eligible, and programmatic or individual success can be compared using a mean or median PTC. Values near 25% or 50% may inform interventions to minimize coverage gaps among patients intermittingly screened. Yes-No patterns may be useful in addressing questions such as, “What proportion of patients complete FIT in all rounds?” Patterns of YYYY show the proportion who remain adherent to FIT over four rounds of screening, while patterns of YYNN or YNYN can identify patients who engage in screening inconsistently
Strengths and limitations
Few studies have been able to measure repeat FIT because it requires long periods of observation in a relatively closed health system. An important strength of our study is that, rather than measure repeat FIT using a shifting denominator of all-comers in each screening round, we followed the same group of patients across four rounds. Patients received care in an integrated health system, and because Parkland is the only option for primary and specialty care for uninsured and low-income patients in Dallas County, it is unlikely that patients received screening outside the system. We used data from a comprehensive EHR, ensuring efficient, accurate, and uniform capture of screening data elements using standard coding systems. However, these robust data may not be routinely availability in other settings, and researchers may additionally weigh data quality and availability when selecting measures of repeat FIT. Our findings are limited to a safety-net healthcare system, and prevalence of repeat FIT was much lower compared to insured populations.36 Although estimates of repeat FIT may differ across settings and populations, we expect the magnitude of difference between measures to be similar.
We address many of the challenges of measuring adherence to repeat FIT, but some challenges remain. First, the optimal strategy to account for positive FIT is not clear. Simply limiting analyses to only those with normal results may induce a selection bias because there are likely differences between patients with positive vs. normal FIT. We addressed this challenge differently with each measure: excluded from denominator of subsequent round (“prior screeners”), created separate (Yes-No patterns), and assigned coverage by test result (PTC). Similarly, patients may experience other events, including symptoms, colonoscopy, cancer diagnosis, or death, between screening rounds and that may make them ineligible for a subsequent FIT. The PTC approach accounts for crossover from FIT to colonoscopy and censors patients at cancer diagnosis or death, but the other measures do not. Censoring at these events may overestimate adherence, and one proposed solution is to weight observations according to the number of rounds each patient is screen-eligible.30 Finally, these measures do not illustrate reasons for repeat FIT, for example, providers not ordering vs. patients not completing the test.
Conclusion
In summary, different measures of repeat FIT yielded a range of estimates. Our study highlights several next steps to address in future research: 1) understanding if and how measures of repeat FIT are associated with clinical outcomes, such as stage of diagnosis and mortality; 2) identifying measures that best approximate screening effectiveness; and 3) establishing a gold standard to facilitate comparison across populations and health systems.
Acknowledgements
Grant support: Research reported in this publication was supported by the National Cancer Institute at the National Institutes of Health under award numbers U54 CA163308 and UM1 CA222035 (CS Skinner, EA Halm) and the Cancer Prevention and Research Institute of Texas under award number PP160075 (AG Singal).
Abbreviations
- CRC
colorectal cancer
- EHR
electronic health record
- FIT
fecal immunochemical test
- gFOBT
guaiac-based fecal occult blood test
- HEDIS
Healthcare Effectiveness Data and Information Set
- PTC
proportion of time covered
Footnotes
Conflicts of Interest: Dr. Singal has served as a member of scientific advisory boards with Exact Sciences. None of the authors have any relevant conflicts of interest.
Financial Information
The sponsor had no role in: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med 2006;31:52–61. [DOI] [PubMed] [Google Scholar]
- 2.Maciosek MV, Solberg LI, Coffield AB, et al. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med 2006;31:80–9. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Elmunzer BJ, Hayward RA, Schoenfeld PS, et al. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 9.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872–85. [DOI] [PubMed] [Google Scholar]
- 10.White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde CN, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev 2011;20:1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQueen A, Vernon SW, Meissner HI, et al. Are there gender differences in colorectal cancer test use prevalence and correlates? Cancer Epidemiol Biomarkers Prev 2006;15:782–91. [DOI] [PubMed] [Google Scholar]
- 13.Sabatino SA, White MC, Thompson TD, et al. Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep 2015;64:464–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro JA, Klabunde CN, Thompson TD, et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2012;21:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology 2004;127:1670–7. [DOI] [PubMed] [Google Scholar]
- 16.Jensen CD, Corley DA, Quinn VP, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer 2016;122:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013;173:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin TR, Jamieson L, Burley DA, et al. Organized colorectal cancer screening in integrated health care systems. Epidemiologic reviews 2011;33:101–110. [DOI] [PubMed] [Google Scholar]
- 20.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217–1237.e3. [DOI] [PubMed] [Google Scholar]
- 21.Cusumano VT, May FP. Making FIT Count: Maximizing Appropriate Use of the Fecal Immunochemical Test for Colorectal Cancer Screening Programs. Journal of General Internal Medicine 2020:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlow WE, Beaber EF, Geller BM, et al. Evaluating screening participation, follow-up, and outcomes for breast, cervical, and colorectal cancer in the PROSPR consortium. JNCI: Journal of the National Cancer Institute 2020;112:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev 2014;23:1147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. Jama 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner CS, Ahn C, Singal AG, et al. Outcomes associated with use of the Cancer Risk Intake System among primary care safety-net patients identified as needing colorectal cancer screening. Prev Med Rep 2019;16:101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CC, Sigel BM, Yang E, et al. Adherence to colorectal cancer screening measured as the proportion of time covered. Gastrointest Endosc 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value in Health 2007;10:3–12. [DOI] [PubMed] [Google Scholar]
- 29.Murphy CC, Sen A, Watson B, et al. A Systematic Review of Repeat Fecal Occult Blood Tests for Colorectal Cancer Screening. Cancer Epidemiol Biomarkers Prev 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chubak J, Hubbard R. Defining and measuring adherence to cancer screening. J Med Screen 2016;23:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steele RJ, Kostourou I, McClements P, et al. Effect of repeated invitations on uptake of colorectal cancer screening using faecal occult blood testing: analysis of prevalence and incidence screening. Bmj 2010;341:c5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steele RJ, Kostourou I, McClements P, et al. Effect of gender, age and deprivation on key performance indicators in a FOBT-based colorectal screening programme. J Med Screen 2010;17:68–74. [DOI] [PubMed] [Google Scholar]
- 33.Grazzini G, Ciatto S, Cislaghi C, et al. Cost evaluation in a colorectal cancer screening programme by faecal occult blood test in the District of Florence. J Med Screen 2008;15:175–81. [DOI] [PubMed] [Google Scholar]
- 34.Sarfaty M, Myers RE. The effect of HEDIS measurement of colorectal cancer screening on insurance plans in Pennsylvania. The American journal of managed care 2008;14:277–282. [PubMed] [Google Scholar]
- 35.McWilliams JM, Landon BE, Chernew ME. Performance in Year 1 of Pioneer Accountable Care Organizations. N Engl J Med 2015;373:777. [DOI] [PubMed] [Google Scholar]
- 36.Singal AG, Corley DA, Kamineni A, et al. Patterns and predictors of repeat fecal immunochemical and occult blood test screening in four large health care systems in the United States. Am J Gastroenterol 2018;113:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson JC, Robertson DJ. Monitoring compliance with colorectal cancer screening: Do we have it covered? Gastrointest Endosc 2018;88:332–334. [DOI] [PubMed] [Google Scholar]