Abstract
The achievement of proper bone mass and architecture, and their maintenance throughout life requires the concerted actions of osteoblasts, the bone forming cells, and osteoclasts, the bone resorbing cells. The differentiation and activity of osteoblasts and osteoclasts are regulated by molecules produced by matrix-embedded osteocytes, as well as by cross-talk between osteoblasts and osteoclasts through secreted factors. In addition, it is likely that direct contact between osteoblast and osteoclast precursors, and the contact of these cells with osteocytes and cells in the bone marrow, also modulate bone cell differentiation and function. With the advancement of molecular and genetic tools, our comprehension of the intracellular signals activated in bone cells has evolved significantly, from early suggestions that osteoblasts and osteoclasts have common precursors and that osteocytes are inert cells in the bone matrix, to the very sophisticated understanding of a network of receptors, ligands, intracellular kinases/phosphatases, transcription factors, and cell-specific genes that are known today. These advances have allowed the design and FDA-approval of new therapies to preserve and increase bone mass and strength in a wide variety of pathological conditions, improving bone health from early childhood to the elderly. We have summarized here the current knowledge on selected intracellular signal pathways activated in osteoblasts, osteocytes, and osteoclasts.
Keywords: osteoblast, osteoclast, osteocyte, bone, differentiation, apoptosis
2. INTRODUCTION
The maintenance of bone mass throughout life is attained by the regulated actions of osteoblasts, the bone forming cells, and osteoclasts, the bone resorbing cells (Figure 1) (Bellido et al., 2014). Osteoblasts are cuboidal cells present on the bone surface and are derived from mesenchymal stem cells (MSCs). Osteoblasts produce and secrete components of the bone matrix and regulate the deposit of hydroxyapatite, the mineral component of the mature bone. Osteoclasts are multinucleated cells derived from hematopoietic precursors also present on the bone surface. Osteoclasts produce enzymes that work in an acid environment and are able to dissolve bone mineral and proteins such as collagen molecules, removing bone and allowing for new bone to be formed. At the same time, osteoclastic bone resorption results in the release into the circulation of factors such as TGFβ, which are stored in bone and can now act on target cells and tissues.
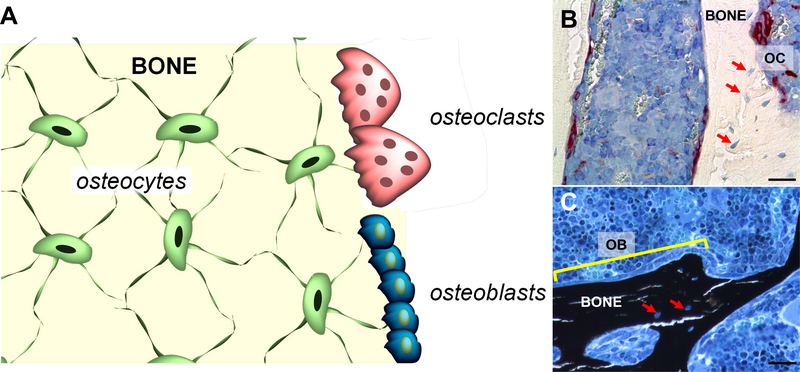
Figure 1.
A. Schematic representation of bone containing osteocytes embedded in the matrix, and osteoblasts and osteoclasts on the surface. B and C. Histologic sections of distal femur stained for B. TRAP (osteoclasts, OC, red) and counterstained with Toloduine blue and C. von Kossa (mineralized bone, black) and counterstained with McNeal. A row of osteoblasts (OB) is indicated by the yellow line. Red arrows point at osteocytes within the bone matrix. Bone sections were prepared and stained at the ICMH Histology and Histomorphometry Core, Indianapolis, IN, USA. Scale bars indicate 25μm.
Osteoblasts and osteoclast differentiation and activity are under the control of osteocytes, former osteoblasts embedded in the mineralized bone matrix (Bonewald, 2011). Over the past decade, our understanding of osteocyte biology has expanded and it is now clear that these cells are producers and targets of extracellular molecules that modulate cell activity and survival. In addition, all bone cells, as well as cells present in the bone marrow and in other tissues, produce factors that trigger intracellular signaling and modulate bone formation and resorption. Thus, a well-controlled network of cytokines and growth factors participate in the regulation of bone homeostasis. In addition to this paracrine/autocrine regulation, the generation, proliferation and survival of bone cells is regulated by systemic hormones, including parathyroid hormone, sex steroids (both androgens and estrogens) and glucocorticoids, reviewed elsewhere (Zuo and Wan, 2017, Wein and Kronenberg, 2018, Hachemi et al., 2018). In this chapter, we will describe the signaling pathways triggered in osteoblasts, osteocytes, and osteoclasts by selected regulatory molecules, as well as by direct cell-to-cell contact. The intracellular signaling mechanisms activated by additional bone-acting stimuli, as well as the biological consequences of these signaling pathways have been recently described in (Plotkin and Bivi, 2014, Verlinden et al., 2016, Sims, 2016, Strazzulla and Cronstein, 2016, Grafe et al., 2018, Lisowska et al., 2018, Thompson et al., 2012).
3. OSTEOBLASTS AND OSTEOCYTES
Osteoblasts, which originate from MSC precursors, are the cells responsible for the synthesis of bone matrix proteins to form osteoid, and for matrix mineralization, to form mineralized, mature bone (Figure 1) (Bellido et al., 2014). Under the influence of locally produced molecules, the expression of lineage-specific transcription factors is stimulated and MSC undergo differentiation (Bradley et al., 2018). These transcription factors include osterix, and Runx2 (Bellido et al., 2014) and deletion of these genes in mice results in defective osteoblast differentiation and lack of mineralized bone (Otto et al., 1997, Nakashima et al., 2002).
As osteoblast become surrounded by the bone matrix, they undergo changes in their morphology and the pattern of gene expression, transitioning into osteocytes, which are the most abundant and long-lived cells in bone. In spite the difference in morphology and gene expression pattern, most of the extracellular signaling molecules affect both osteoblasts and osteocytes, albeit with different biological consequences. We will describe the signaling pathways activated in both cell types, indicating when these pathways lead to different biological outcomes in osteoblast and osteocytes (Figure 2).
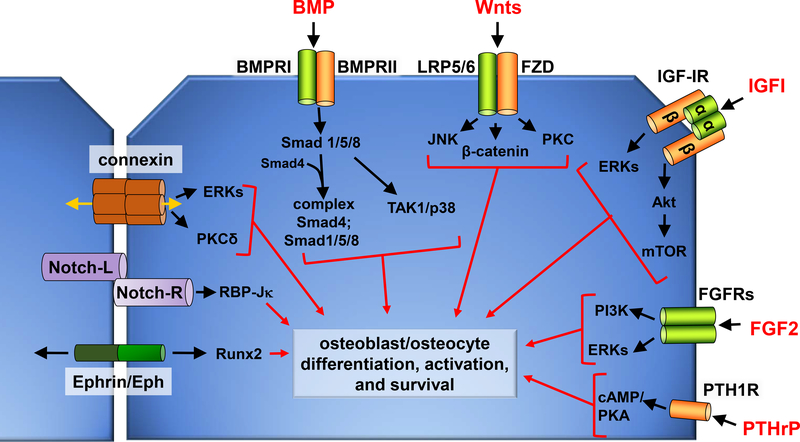
Figure 2.
Schematic representation of two neighboring osteoblastic cells, showing extracellular ligands and their corresponding receptors, and cell-to-cell signaling molecules. Selected downstream signaling molecules and the biological outcomes are also included. Notch-L: Notch ligand; Notch-R: Notch receptor.
3.1. Bone morphometric proteins
Bone morphogenetic protein (BMPs) are growth factors that belong to the transforming growth factor (TGF) β superfamily of proteins originally identified as inducers of ectopic bone formation (Katagiri and Watabe, 2016) and are now known to exert a potent osteogenic effect by promoting osteoblast differentiation and function. BMPs are synthesized as precursors, which are later cleaved and become active. Structurally, most of the BMPs exist as covalently-bound homodimers, although heterodimers have also been found.
BMPs bind to the BMP receptor (BMPR) type I (ALK-1–7) alone or in combination with a type II receptor (BMPR-II, ACTR-IIA, or ACTR-IIB). Type I and II receptors differ in cellular expression and ability to bind particular ligands, but all share kinase activity. Downstream of the receptors, BMPs phosphorylate Smad proteins. The so-called BMP-specific proteins include Smad1, Smad5 and Smad8, known as receptor-regulated Smads or R-Smads. R-Smad, in turn, bind to Smad4 and this complex translocates to the nucleus, where it can bind to transcription factors either stimulating or inhibiting gene transcription. In addition, Smad6 and 7 (inhibitory Smads or I-Smads) compete with R-Smad for Smad4 binding, therefore preventing Smad1/5/8 nuclear translocation and regulation of BMP-associated genes. The induction of gene transcription downstream of BMP2 requires connexin (Cx) 43 expression, and deletion of Cx43 from calvaria osteoblastic cells inhibits osteoblast differentiation induced by BMP-2 in vitro (Hashida et al., 2014). This effect of Cx43 requires direct cell-to-cell communication and cannot be restored by a Cx43 mutant that lacks the ability to form gap junction channels (see below for further description of connexins).
One of the transcription factors regulated by Smads downstream of BMP action is Runt-related transcription factor 2 (Runx2), which is required for osteoblast differentiation (Komori, 2006). Activation of the BMP/Smad signaling pathway results in the transcription of proteins involved in osteoblast differentiation and functions, such as osteocalcin, collagen alpha-1(I) chain, and alkaline phosphatase. In addition to activating Smads (the canonical BMP signaling pathway), BMPs also activate the non-canonical MAPKKK 7/TGF-β-activated kinase 1 (TAK1)-p38 pathway, independent of Smads (Gomez-Puerto et al., 2018), a pathway required for bone and tooth development (Xu et al., 2008).
In addition to the intracellular regulators, BMP activity is regulated by extracellular antagonists and potentiators (Katagiri and Watabe, 2016). Antagonists include chordin, follistatin, gremlin, and noggin, among others, which sequester BMPs in the extracellular space or block ligand-receptor binding. BMPs potentiators include heparin, heparan-sulfate and dextran-sulfate, which bind to BMPs and potentiate the pro-osteoblastic effect of BMP-2, −4 and −7 (Takada et al., 2003, Irie et al., 2003, Ruppert et al., 1996). Other BMP potentiators have been described, although their role in osteoblastic cells is not known (Katagiri and Watabe, 2016).
The importance of BMP signaling for skeletal development, bone strength, and fracture repair has been demonstrated by human mutations in the ligands, receptors, antagonists, and intracellular signaling molecules (Wu et al., 2016). Further, animal models with both loss- and gain-of-function mutations have been developed, allowing for a better understanding of the role of the pathway and the consequences of its manipulation for human diseases.
3.2. Wnt-induced signaling
Wnts are a family of secrete glycoproteins originally described in Drosophila, as the Wingless (Wg) gene that is involved in wing development and in mice, as the Int-1 gene that is involved in breast cancer development (Nusse and Varmus, 1992, Nusse et al., 1991). Wnts induce intracellular signaling by binding to members of the frizzled (FZD) family of receptors, which comprise 10 members (Schulte, 2015). FZDs are seven transmembrane proteins, with a structure similar to G protein-coupled receptors. In addition, all members of the FZD family contain a cysteine-rich domain in the amino terminus that constitute the ligand-binding site, and a cytoplasmic tail that interacts with intracellular molecules. Wnts also interact with members of the low density lipoprotein receptor protein (LRP) family, which includes LRP4, LRP5 and LRP6. While LRP5 and 6 have been shown to mediate the activation of the Wnt signaling pathway, LRP4 binds to and facilitates the action of the Wnt antagonist sclerostin, the product of the Sost gene (Williams, 2017).
Through its binding to FZD receptors, Wnt ligands can activate four different signaling pathways: the canonical Wnt/β-catenin pathway, the non-canonical planar cell polarity (PCP) pathway, the Wnt-Ca2+, and the protein kinase A pathways (Baron and Kneissel, 2013). It has been shown that members of the Wnt family of proteins can activate one particular pathway in a cell context-dependent manner. Nevertheless, activation of both canonical and non-canonical Wnt signaling result from Wnt proteins binding to FZD-LRP5/6 co-receptor complex, followed by phosphorylation and activation of Disheveled (Dsh).
Activation of canonical Wnt signaling leads to the stabilization of β-catenin, followed by its translocation to the nucleus, where β-catenin binds to the transcription factor LEF and increases the transcription of Wnt target genes. In osteoblastic cells, osteoprotegerin (OPG) is one of the most studied Wnt target gene. OPG is involved in osteoclast differentiation and counteracts the pro-osteoclastogenic effects of receptor activator of NFκB ligand (RANKL), resulting in decreased osteoclast differentiation (see below for more details on osteoclastogenesis). Consistent with this, decreased bone mass induced by targeted deletion of the gene encoding β-catenin in osteoblasts and osteocytes results from enhanced bone resorption due to low OPG levels.
The role of Wnts in bone has been elucidated through understanding the genetic basis of human diseases associated with the pathway, and confirmed through genetic studies in which the different components of the signaling pathways have been modified either in cells of the osteoblastic lineage or in the germline (Baron and Kneissel, 2013). For example, both gain- and loss-of-function mutations have been described for LRP5, leading to high bone mass phenotypes and osteoporosis pseudoglioma, respectively (Williams, 2017). In addition, high bone mass phenotypes have also been described in individuals with loss of function mutations of the LRP4 gene (Leupin et al., 2011). Further, absence of sclerostin due to null mutations, or reduced expression of the protein due to absence of a regulatory region of the gene result in sclerosteosis or van Buchem’s disease, respectively, conditions with high bone mass (Williams, 2017).
In addition, mutations in Wnt10b result in split hand/foot malformation 6 (Kantaputra et al., 2018, Zmuda et al., 2009) and deletion of this Wnt in mice leads to decreased bone marrow mesenchymal progenitors, low bone mass, and reduced expression of osteoblastic differentiation markers (Stevens et al., 2010). Further, osteoblast-derived Wnt16 suppresses osteoclast formation by increasing OPG levels (Moverare-Skrtic et al., 2014, Alam et al., 2016), and SNPs associated with loss of Wnt16 function are linked to low BMD and osteoporotic fractures (Koller et al., 2013).
The associated of certain Wnts with particular FZD receptors lead to the phosphorylation of Dsh, followed by the activation of the PCP pathway, downstream of the small GTPases Rho and Rac, and the MAPK/c-Jun N-terminal kinase (JNK). The PCP pathway is involved in cytoskeletal organization, cell migration, and tissue pattering, and ultimately, in tissue morphogenesis. In bone, PCP is involved in the control of limb shape and dimensions. Thus, activation of PCP downstream of Wnt5a results in the transcription of the gene Vangl2, and absence of Vangl2 leads to deficient limb bud development in mice. In addition, individuals with loss-of-function mutations in the Wnt5a gene or its co-receptor ROR2 exhibit brachydactyly type B and Robinow syndrome, conditions associated with shortening of long bones, and abnormal bone shape, among other abnormalities.
Further studies showed that the non-canonical members of the Wnt family of proteins Wnt4, Wnt5a, Wnt7b, and Wnt11 increase MSCs differentiation into the osteoblastic lineage. For example, Wnt5a stimulates osteogenic differentiation and inhibits adipogenesis in human adipose tissue-derived MSCs through activation of the Rho-associated protein kinase ROCK. In addition, the expression of Wnt5a and its co-receptor ROR2 are increased by mechanical stimulation, leading to RhoA activation, which is required for upregulation of Runx2 and the commitment of precursor cells to the osteogenic lineage induced by mechanical stimulation. As discussed later in this review, Wnt5a also plays a direct role in osteoclast differentiation.
3.2.1. Inhibitors of Wnt Signaling
Activation of the Wnt signaling pathway is controlled by the regulation of expression and secretion of Wnt proteins, as well as by a network of inhibitors that act at different levels of the signaling cascade (Plotkin and Bivi, 2014, Lerner and Ohlsson, 2015). In bone, human mutations and animal experiments have demonstrated the importance of the inhibitors in the regulation of bone cell differentiation and function and, ultimately, bone mass and strength. Further, pharmacologic approaches are being developed to take advantage of the anabolic effect of Wnt signaling in bone, by blocking the Wnt antagonist, leading to increased bone mass (Baron and Gori, 2018).
3.2.1.1. Dickkopf
The Dickkopf (Dkk) proteins are members of a family of cysteine-rich domain containing proteins (Baron and Kneissel, 2013). Dkk1 and Dkk2 inhibit Wnt signaling by binding to LRP5/6 and Kremen (another Wnt inhibitor, see below). The expression of Dkk1, the most studied member of the family, is hormonally regulated, and it is also controlled by growth factors and mechanical stimulation. Dkk1 expression is required for limb morphogenesis and in its absence the head does not develop in Dkk1 knockout mice (Huang et al., 2018). On the other hand, deletion of one Dkk1 allele results to high bone mass due to increased osteoblast number and activity, whereas Dkk1 overexpression results in reduced osteoblast number and activity and low bone mass. Consistent with an inhibitory role of Dkk1 on osteoblast function, high bone mass-associated LRP5 mutations render LRP5 proteins resistant to Dkk1-mediated inhibition of Wnt signaling. Dkk2 and Dkk4 have also been shown to inhibit canonical Wnt signaling in the presence of Kremen.
3.2.1.2. Kremen
The two members of this family of proteins, Kremen 1 and 2 are co-receptors for Dkk1, enhancing Dkk1-induced Wnt signaling inhibition (Baron and Kneissel, 2013, Plotkin and Bivi, 2014). Kremen 1 is widely expressed whereas Kremen 2 is predominantly expressed in bone cells. Kremen 2-deficient mice exhibit an age-dependent high bone mass phenotype, which can be detected at 2 years of age, and Kremen 1 and 2 double knockout mice exhibit increased Wnt signaling, bone mass, and bone formation. Conversely, transgenic mice overexpressing Kremen 2 exhibit reduced osteoblastic Wnt signaling and differentiation and activity and low bone mass.
3.2.1.3. Sclerostin
The product of the Sost gene, sclerostin, is expressed in osteocytes in bone and was originally described as an inhibitor of BMP due to its homology with known BMP antagonists (Baron and Kneissel, 2013, Plotkin and Bivi, 2014). It is now known that sclerostin binds with higher affinity to LRP5/6 and that LRP4 is required for the inhibition of Wnt signaling by sclerostin. As a consequence of its Wnt inhibitory effects, the absence of the Sost gene, reduced sclerostin levels or prevention of sclerostin association with LRP5/6 results in high bone mass. More recently, it has been shown that sclerostin can affect osteoclast differentiation indirectly, by stimulating RANKL expression in osteocytic cells in vitro and RANKL/OPG levels in vivo (Tu et al., 2015, Wijenayaka et al., 2011). However, whether this action of sclerostin depends on its binding to LRPs or on the regulation of Wnt signaling pathway, and the intracellular signaling molecules involved in RANKL/OPG regulation in osteoblastic cells remains unknown.
3.2.1.4. Secreted Frizzled-Related Proteins
Secreted FZD-related proteins (sFRPs) are soluble proteins that bind to Wnts and prevent their association with the FZD receptors on the cell surface, inhibiting intracellular Wnt signaling (Baron and Kneissel, 2013, Plotkin and Bivi, 2014). Among the five members of the family of proteins, sFRP-1 was shown to be expressed in bone and to inhibit osteoblast differentiation and induce osteoblast and osteocyte apoptosis. Consistent with this, deletion of Sfrp1 results in high bone mass whereas its overexpression decreased bone mass in transgenic mice. sFRP4 also inhibits bone formation by preventing osteoblast proliferation, and its overexpression in bone or liver lead to elevated sFRP4 circulating levels and low bone mass. Consistent with a role of the protein in bone, SFRP4 polymorphisms have been associated with changes in bone mass in the hip and spine in humans.
3.2.1.5. Wise
Wise is another LRP5/6 binding protein that prevents Wnt signaling (Baron and Kneissel, 2013, Plotkin and Bivi, 2014). It has also been shown to associate with LRP4, and mutations of both Wise and LRP4 genes lead to similar abnormal teeth, suggesting that the two molecules interact during tooth development. The absence of Wise results in the activation of Wnt signaling and the modulation of the fibroblast growth factor (FGF) and sonic hedgehog pathways.
3.3. Insulin-Like Growth Factor
Insulin-like growth factor (IGF) I and II, named based on their structural similarity with insulin, and their insulin-like properties, are produced mainly in the liver and also in bone and adipose tissue (Yakar et al., 2018). The two forms of the ligand share high homology. IGFI is expressed both during embryonic development and after birth, whereas IGFII is expressed in the embryo but is downregulated after birth. IGFs bind to and activate two receptors, IGF-IR and IGF-IIR, and can also bind to the insulin receptor, albeit with low affinity. IGF-IR is a tetramers formed by two extracellular ligand binding α subunits and two transmembrane β subunits, which have tyrosine kinase activity. IGF-IR activation results in the phosphorylation of adaptor insulin receptor substrate (IRS) proteins or of Shc (Bikle, 2008), followed by activation of the growth factor receptor binding protein 2 (Grb2)/Sos/Ras/Raf/MAPK extracellular signal–regulated kinase kinase (MEK), resulting in extracellular signal-regulated kinase ERK activation. In addition, activation of the IGF-IR also results in the activation of PI3K, followed by phosphorylation and activation of Akt, and of the mammalian target of rapamycin (mTOR), eukaryotic translation initiation factor 4E (eIF4E), and p70S6 kinase (S6K). In general, activation of this receptor leads to cell proliferation and survival and has been associated with cancer cell metastatic potential. The IGF-IIR, also known as cation-independent mannose-6-phosphate receptor, has no intrinsic kinase activity and binds to IGF-II with high affinity, inducing IGF-II lysosomal degradation. IGF-IR is expressed in bone during development and in adults, whereas mRNA expression for IGF-IIR has been shown in embryos but not adult rat bones (Bikle et al., 1994). The activity of IGFs is modulated by the IGF binding proteins (IGFBP) 1–6, which regulate IGF bioavailability and can either enhance or reduce IGF effects (Bunn and Fowlkes, 2003). IGFBPs also exert direct effects on cell function, independent of IGFs. IGFs and IGFBPs form a complex with the acid-labile subunit (ALS), which is required for IGF/IGFBP circulation and IGF-I function (Boisclair et al., 2001).
Both IGF-I and II are produced locally by osteoblastic cells and stored in the bone matrix as an inactive form bound to IGFBPs (Kasukawa et al., 2004). During bone resorption by osteoclasts, IGFs are released from the bone matrix and can act as signals to recruit osteoblast precursors to the newly eroded bone surface. Osteoblasts also produce several IGBPs, with a pattern that depends on the stage of osteoblast differentiation. The role of IGFs on osteoblast activity has been extensively studied in vitro and in vivo, using genetically modified mice in which the levels of the ligands and/or the receptors were manipulated globally or in a cell-specific manner (Yakar et al., 2018). In particular, it has been shown that IGFs induce osteoblast proliferation and differentiation and inhibit apoptosis (Bikle, 2008). The importance of IGF on bone development has been demonstrated by the profound skeletal defects observed in mice lacking IGF-I or IGF-IR, with impaired growth and delayed ossification, and reduced bone mass and osteoblast number and surface. The growth deficiency and delayed ossification observed in IGF-IR-deficient mice are reproduced in mice lacking Akt1/Akt2, a kinase activated by IGFs, demonstrating the importance of this signal transduction pathway for bone growth (Peng, 2003). In osteoblastic cells, IGF-I has been shown to activate the PI3K/PKD/Akt and Ras/Raf/MAPK pathways, both leading to activation of p70S6, which mediate the proliferative and anti-apoptotic actions of IGF-I (Govoni, 2012). A pathway activated by IGF-I via Giβγ leading to osteoblastic cell proliferation and survival independently of p70SK activation has also been proposed (Grey et al., 2003).
3.4. Parathyroid Hormone-Related Peptide
Parathyroid hormone-related peptide (PTHrP) is a locally produced parathyroid hormone (PTH) analog expressed by osteoblasts and osteocytes (Schluter, 1999). Both proteins share high homology at the first 34 aminoacids and bind to the PTH receptor 1 (PTH1R) expressed in osteoblasts and osteocytes. However, they differ in pattern of expression and exhibit different functions; and while PTHrP deletion is lethal due to defective rib bone formation, PTH null mice show a milder phenotype and survive after birth. PTHrP is required for the commitment of osteoblast precursors to the osteoblastic lineage and for osteoblast maturation, and through actions on osteoblastic cells, on osteoclast differentiation via increases in RANKL expression.
Upon binding to the PTH1R, PTHrP induces the phosphorylation of the receptor and the activation of GTP-binding proteins. In osteoblasts, PTHrP binding to PTH1R results in the activation of the Gsα subunit of the G protein leading to cAMP/PKA signaling. PKA, in turn, phosphorylates and activates transcription factors, including CREB, c-Fos, c-Jun, and the osteoblastic transcription factor Runx2.
PTHrP can also activate of the Gq subunit downstream of the PTH1R. This results in activation of the 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase [phospholipase C (PLC)] and PI3K/diacylglycerol pathways, which increase intracellular Ca2+ and stimulate PKC activity. Further, PTHrP can either activate or inhibit the MAPK ERK1/2 signaling pathway, depending on whether cells are undergoing proliferation or differentiation, respectively.
The activity of PTHrP is regulated by metalloproteinases that cleave the protein generating the PTHrP1–17 peptide, which can stimulate calcium signaling but cannot activate cAMP. In addition, a PTHrP12–48 proteolytic product regulates the bone marrow niche and acts as a suppressor of osteoclastogenesis. A PTHrP107–139 peptide is also found in vivo and displays anti-resorptive and pro-osteoblastic properties when administered to animal models that lack sex steroids, have excess glucocorticoids, or have diabetes (Esbrit et al., 2016, Lozano et al., 2011).
Another way of regulating PTHrP signaling is through internalization of the receptor leading to desensitization of the cells to PTHrP (as well as PTH) effects. The internalization occurs upon ligand binding, by the recruitment of the scaffolding proteins β-arrestin-1 and −2. Binding to β-arrestins reduce the affinity of PTH1R for Gsα and suppresses cAMP-mediated responses. Receptors can be later recycled to the cell surface or targeted for degradation.
PTHrP also exert nuclear actions in osteoblastic cells, independent of PTH1R. Thus, PTHrP translocate to the nucleus where it can regulate apoptosis and cell proliferation by altering the pattern of gene expression (Garcia-Martin et al., 2014).
3.5. Fibroblast Growth Factors
Fibroblast growth factors (FGFs) are a family of proteins that exert both paracrine and endocrine functions (Beenken and Mohammadi, 2009). FGFs bind to the extracellular matrix until activated by proteases, when they are released to activate FGF receptors (FGFRs). Four FGFRs (1–4) have been described, all members of the tyrosine kinase receptor family, and FGFR1–3 are expressed in osteoblastic cells (Ornitz and Marie, 2015). Binding of FGFs to the receptors results in the activation of the intracellular signaling molecules PLC-γ1, MAPK, and PI3K/Akt (Yun et al., 2010). In addition to direct actions of FGFs through their receptors, the growth factors affect bone cells indirectly, through the up-regulation of other local factors, such as TGFβ, IGF-I and VEGF (Ornitz and Marie, 2015).
Genetic studies in mice showed differing effects of different FGFR in osteoblastic cells. Thus, FGFR2 deletion in osteochondroprogenitors results in shorter axial and appendicular skeleton and reduced bone formation, whereas FGFR1 deletion in osteoprogenitors and differentiated osteoblasts results in increased bone mass, suggesting that the receptor mediates inhibitory actions on osteoblastic cells (Yu et al., 2003, Jacob et al., 2006).
Regarding FGFR ligands, FGF2 is required for proper osteoblast differentiation and activity, and its deletion results in decreased bone mass in mice (Yu, 2003). The mechanism of action of FGF2 and the consequence of overexpression of the growth factor depend on the molecular weight isoform, with the low molecular weight inducing activation of the WNT/β-catenin signaling pathway and increased bone formation (Xiao et al., 2009), and the high molecular isoform inducing the activation of the FGF23/FGFR/MAPK signaling pathway and leading to reduced bone formation in vitro (Xiao et al., 2012). In vitro studies demonstrated that the pro-osteoblastogenic effects of FGF2 are potentiated by expression of Cx43 (Hebert and Stains, 2013, Lima et al., 2009, Niger et al., 2012, Niger et al., 2010, Niger et al., 2013) (see below). In addition, FGF2 promotes osteoblast survival through the activation of the PI3K signaling pathway (Park et al., 2009).
Other members of the FGF family of proteins with action on bone cells are FGF18, which is expressed in osteoblastic cells and induces osteogenic differentiation through activation of the FGFR1/2 and the MAPK and PI3K signaling pathways in vitro (Hamidouche et al., 2010), and FGF21, which is produced in pancreas, liver, and adipose tissue and decreases osteoblast surface, and bone mass and strength (Charoenphandhu et al., 2017).
Bone, and in particular, mature osteoblasts and osteocytes, are the main source of FGF23, a growth factor that reduces vitamin D levels and regulates phosphate homeostasis through inhibition of renal phosphate reabsorption (Yoshiko et al., 2007). FGF23 synthesis is, in turn, regulated by phosphate levels, vitamin D and PTH (Clinkenbeard and White, 2016). Activation of the FGFR by FGF23 requires the expression of the co-receptor Klotho, expressed in kidney (in the distal convoluted tubule) and the parathyroid gland (Clinkenbeard and White, 2016, Komaba and Lanske, 2018). More recently, it was shown that Klotho is also expressed in bone and, in particular, in osteoblasts and osteocytes (Rhee et al., 2011). Further studies showed that absence of Klotho from osteocytes results in increased bone mass and osteoblast activity in mice, whereas Klotho overexpression inhibits osteoblastic cell differentiation in vitro (Komaba and Lanske, 2018). The mechanism for the actions of FGF23 on osteoblastic cells is not completely understood, but it is believed to be mediated by induction of the expression of the Wnt/β-catenin inhibitor Dkk1, resulting on the inhibition of the bone anabolic Wnt pathway. In addition, FGF23 regulates its own synthesis through Klotho in osteoblastic cells. The effects of FGF23 on osteoblastic cells via FGFR1/Klotho stimulation result in the activation of ERKs, leading to modulation of gene expression.
4. OSTEOCLAST SIGNALING MECHANISMS
Osteoclasts are the predominant bone-degrading cell in the body (Figure 1). The highly motile nature of osteoclasts, combined with their resorptive capacity, can result in extensive degradation of cortical bone, cancellous bone, alveolar bone, and tooth dentin. The molecular analysis of human forms of osteopetrosis caused by defects in osteoclast resorption, and the prevalence of low bone mass osteoporosis especially in the elderly which is due to excessive osteoclast activity, highlight the importance of understanding osteoclast biology for human disease. The catabolic effects of osteoclasts must therefore be tightly controlled, which occurs in part through the regulation of osteoclast formation and cell death, as well as the expression and secretion of bone degrading chemical and biological factors. The birth, life, and death of osteoclasts is regulated by a complex series of signaling molecules and pathways, many of which are known, while others still remain to be determined.
4.1. Osteoclast Morphology and Function
4.1.1. Regulation of Osteoclast Resorption by Proton Pump and Chloride Channel
Upon attachment to bone, osteoclasts change their morphology to become polarized multinucleated cells, which enables them to form two specialized surface domains, known as the sealing zone and the ruffled border membrane. The sealing zone surrounds the ruffled border and provides the machinery to enable attachment to the extracellular matrix. The ruffled border, on the other hand, is a highly convoluted membrane domain through which the hydrolytic enzymes and factors necessary for bone resorption are secreted (Baron et al., 1985, de Vernejoul et al., 1988, Baron et al., 1988, Blair et al., 1989, Vaananen et al., 2000, Bruzzaniti and Baron, 2006).
The ruffled border contains an extensive amount of enfolded membrane, which is formed from the directed fusion of intracellular vesicles with the apical domain of the osteoclasts. The ruffled border membrane provides a large surface area through which bone dissolution and endocytosis of bone degradation products can occur. The degradation products of collagen and other matrix components are endocytosed at the ruffled border, transported through the cell to be degraded or secreted, or are released into the extracellular space during cell migration.
During bone resorption, the osteoclast subcellular lacunar compartment is acidified through the combined actions of the vacuolar ATPase (proton pump) (Scimeca et al., 2000, Wu et al., 2009, Lee et al., 2006, Feng et al., 2009, Yang et al., 2012), in conjunction with chloride ions secreted by the chloride channel CIC-7 (CLCN7) (Kasper et al., 2005, Kornak et al., 2001). Numerous mutations of the vacuolar ATPase or CIC-7 are known, which cause dysfunction in osteoclast resorption, leading to osteopetrosis in mice (Kornak et al., 2001) and humans (Cleiren et al., 2001, Waguespack et al., 2003). The targeted delivery of protons and chloride ions to the osteoclast resorption lacuna lowers the pH and dissolves hydroxyapatite while a mixture of proteases, including matrix metalloproteases such as MMP9 (Kim et al., 2016, Sundaram et al., 2007) and MMP14 (Sato et al., 1997) as well as tartrate-resistant acid phosphatase (TRAP) (Marks and Grolman, 1987, Allen et al., 1989) and cathepsin K (Troen, 2004, Gelb et al., 1996, Lazner et al., 1999, Gowen et al., 1999), a cysteine protease expressed predominately in osteoclasts, digest the organic bone matrix.
The extrusion of protons by the vATPase and chloride ions through the CIC-7 alters intracellular membrane polarity. Therefore, proton generation in mature osteoclasts is controlled by carbonic anhydrase II, an enzyme that catalyzes the interconversion of carbon dioxide and water to form protons and carbonic acid. In addition, electroneutrality within the osteoclast is maintained by the actions of CIC-7. These channels function as Cl-/H+ antiporters such that for every two chloride ions that are released into the resorption lacuna, one hydrogen ion flows in. The actions of CIC-7 also require OSTM1 (osteopetrosis-associated transmembrane protein 1) which is a transmembrane protein also localized to lysosomes which is thought to function as a β subunit of CIC-7, controlling its intracellular localization and protein stability (Chalhoub et al., 2003, Lange et al., 2006, Maranda et al., 2008, Pangrazio et al., 2006).
4.1.2. Small GTP-Binding Proteins
Several small GTP-binding proteins are known to be expressed in osteoclasts and play a role in targeting the acid transporting vesicles to the ruffled border membrane (Mulari et al., 2003b, Mulari et al., 2003a, Sun et al., 2005, Itzstein et al., 2011). For example, Rab5c is known to be associated with early recycling endosomes, while Rab11b is associated with cytosolic (perinuclear) recycling compartments and is thought to regulate turnover of the ruffled border membrane, and osteoclast motility. In addition, the GTP-binding proteins, Rab7 and Rab9, which are known to be associated with the late endosomes in other cells, were found to localize at the ruffled border membrane, suggesting that this specialized plasma membrane domain may behave similar to a late endosomal compartment. Several lysosomal proteins are also expressed at the ruffled border membrane and in the resorption lacuna, suggesting a lysosomal origin for these vesicles (Ferron et al., 2013, Lacombe et al., 2013). Indeed, the resorption lacuna acts much like an extracellular lysosome, providing the acidic environment as well as the proteases required for the degradation of the organic matrix of bone, mostly composed of type I collagen.
4.1.3. Osteoclast Attachment and Migration
Osteoclast attachment is mediated though integrins, which bind to arginyl-glycyl-aspartic acid (RGD) peptides at eroded bone surfaces (Shankar et al., 1995, Fisher et al., 1993, Horton et al., 1991, Helfrich et al., 1992). The integrins, as well as host of other cytoskeletal proteins, and filamentous actin, are localized to the sealing zone of osteoclasts. Osteoclasts resorption is linked with their motility, which is in part facilitated through the rapid polymerization and depolymerization of actin, as well as the assembly/disassembly of signaling molecules at adhesion “hot spots” in the sealing zone known as podosomes (Jurdic et al., 2006). Podosomes are not just found in osteoclasts, but also in highly migratory cells such as macrophages and v-src transformed cancer cells (Spinardi et al., 2004, Linder and Aepfelbacher, 2003, Destaing et al., 2003). Structurally, podosomes contain a central actin core surrounded by actin regulatory proteins, integrins, and the integrin-associated proteins such as talin, cortactin, dynamin (McNiven et al., 2004, Ochoa et al., 2000). In addition, they contain a large number of signaling molecules such as Src, Pyk2, talin, cortactin, and others. Unlike focal adhesions, which are relatively more stable structures, podosome clusters assemble and disassemble within minutes in a process involving the rapid polymerization and depolymerization of the central actin core (McNiven et al., 2004, Ochoa et al., 2000). Thus, podosome turnover and targeted integrin engagement/disengagement in the sealing zone enables osteoclasts to rapidly migrate and, in the process, digest significant amounts of bone during their short life spans. Indeed, in studies published as far back as 1984, it was reported that rabbit osteoclasts in culture display an average resorption rate of 165 μm3/h (Ali et al., 1984, Boyde et al., 1984).
4.2. Osteoclast Differentiation
Osteoclasts are derived from the hematopoietic, myeloid lineage. Many of the extracellular signals that drive osteoclast proliferation, differentiation, and fusion are secreted by the mesenchymal-derived osteoblasts and osteocytes. The osteoclast differentiation process can be recapitulated in vitro by the addition of two primary cytokines, macrophage colony-stimulating factor (M-CSF) and RANKL (Figure 3) (Kong et al., 1999, Wiktor-Jedrzejczak et al., 1990), which are secreted by osteoblasts and osteocytes, as discussed previously in this review.
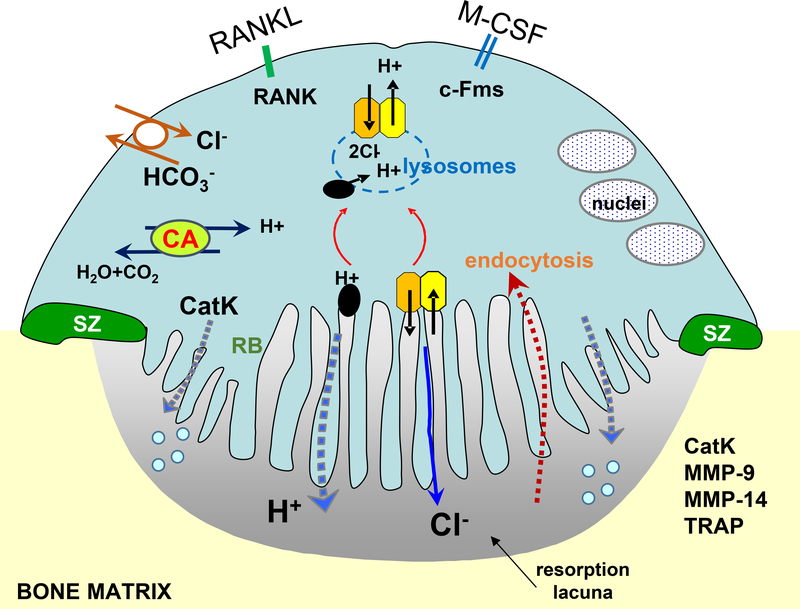
Figure 3.
Schematic representation of a resorbing osteoclast showing the sealing (SZ), ruffled border (RB) membrane, nuclei and resorption lacuna. Mature osteoclasts are polarized with the apical domain directed towards the bone surface. Protons generated by carbonic anhydrase (CA) are transported via the proton pump (black elipse) to the ruffled border membrane. A coupled basolateral bicarbonate/chloride exchanger (solid white circle) maintains electroneutrality, avoiding changes in pH and/or membrane polarization of the cell. Secretion of protons via the proton pump (black circles) and chloride ions via ClC-7 (orange) in complex with Ostm1 (yellow) acidifies the resorption lacuna. Bone dissolution occurs through the actions of secreted cathepsin K (CatK), matrix metalloproteases and TRAP. Bone degradation products are released into the bone microenvironment during cell migration, or are internalized via recycling vesicles into the cell and are secreted, or degraded via the lysosomes (blue dashed ring). RANKL binding to RANK receptor and M-CSF binding to the c-Fms receptor are also indicated.
4.2.1. M-CSF/c-FMS
The critical role of M-CSF is demonstrated from deletions/mutations studies in mice (op/op) and rats (tl/tl) which have a point mutation in the csf1 gene and express non-functional M-CSF protein (Felix et al., 1990, Marks et al., 1992). As a result, osteoclasts are absent in these mice, leading to severe osteopetrosis. The predominant role of M-CSF is in supporting the proliferation and survival of osteoclasts. Binding of M-CSF to its receptor c-Fms (or CSFR-1R) induces phosphorylation at key tyrosine sites in the cytoplasmic tail of c-Fms, which recruits the tyrosine kinase c-Src (Figure 3). The c-Fms/c-Src protein complex then recruits the phosphatidylinositol 3-kinase (PI3K) and the E3 ubiquitin ligase, c-Cbl, in turn leading to the activation of the Akt pathway, and subsequently to the Grb2-mediated activation of ERK (Horne et al., 2005, Yokouchi et al., 2001, Sanjay et al., 2001, Lee and States, 2000, Xiong et al., 2011). Therefore, through a complex series of steps, M-CSF binding to c-Fms, increases osteoclast precursor proliferation and survival, ultimately through the ERK and PI3K/Akt pathways.
4.2.2. Signaling by the RANK Receptor
RANKL (also known as OPGL, ODF and TRANCE) binds to its cognate receptor, RANK (Figure 3). As with c-Fms, genetic mouse models have demonstrated the critical role of the RANKL-RANK system in osteoclast formation, and mice deficient in either the ligand or the receptor develop osteopetrosis (Walsh and Choi, 2003, Kong et al., 1999, Hanada et al., 2010, Odgren et al., 2003). The binding of RANKL to its receptor, RANK, on osteoclasts induces the association of RANK with the TRAF family members, including TRAF2 and TRAF6. Among these, TRAF6, has been most strongly associated with osteoclast formation and function and mice lacking TRAF6 develop osteoclast-deficient osteopetrosis (Naito et al., 1999, Lomaga et al., 1999). Downstream effectors of the RANK-TRAF6 complex in osteoclasts, include NF-κB, Akt, and Nuclear Factor of Activated T-cells cytoplasmic 1 (NFATc1). Further, the activation of PI3K/Akt may be dependent on Src kinase activity, since in the absence of active c-Src, RANKL-mediated Akt activation is prevented and PI3K inhibitors block osteoclast formation. In addition to activating the PI3K/Akt pathway, TRAF6 binding to RANK, recruits TGF-β-activated kinase (TAK) 1 and the TAK-1-binding protein (TAB) 2, and this protein complex activates the MAPK pathways including ERK, JNK, and p38, promoting pre-osteoclast proliferation (Moon et al., 2012, Sugatani et al., 2003, Wong et al., 1999).
In contrast to the pro-osteoclastic effects of RANK-RANKL activation, an alternative receptor for RANKL in osteoclasts has recently been described which leads to the inhibition of osteoclast development. The leucine-rich repeat-containing G-protein coupled receptor 4 (LGR4, also known as GPR48) is a suppressor of osteoclast formation (Styrkarsdottir et al., 2013, Yi et al., 2013, Luo et al., 2016, Zhu et al., 2016, Jin and Yang, 2016, Zhou et al., 2017). RANKL binding to LGR4 activates Gαq, calcium signaling, and GSK3-β which lead to the inhibition of NFATc1 in the nucleus, blocking osteoclast differentiation. In support of the critical role of LRG4 in osteoclastogenesis, deletion of LRG4 in all tissues or specifically in monocytes leads to osteoporosis in mice, due to increases in osteoclast number and activity. Further, it was demonstrated that the soluble extracellular domain of LGR4 can bind RANKL, inhibiting osteoclast differentiation and increasing bone mass in several mouse models. Therefore, LGR4 acts as a second receptor for RANKL that negatively regulates osteoclast formation and activity.
4.2.3. Osteoclast Transcription Factors
Signaling through the c-Fms and RANK receptors leads to the activation of important osteoclasts transcription factors, including microphthalmia-induced transcription factor (MITF) and NFATc1 (Meadows et al., 2007). These transcription factors drive the expression of several genes necessary for osteoclast resorption. For example, in response to RANKL-RANK binding, MITF upregulates TRAP and cathepsin K, which are required for the ability of mature osteoclasts to degrade the bone matrix. MITF is also known to regulate the expression of ClC-7 and Ostm1.
RANKL also activates NF-κB through two distinct pathways, known as the classical pathway or the alternative pathways. NF-κB consists of five members, including p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2). Activation of NF-κB can have both positive and negative effects on osteoclast activity (Boyce et al., 2015). While TRAF6 activates only the classical NF-κB pathway, TRAF2 and TRAF5 activate both pathways (Hauer et al., 2005, Yi et al., 2014, Mizukami et al., 2002). When activated by RANKL, NF-κB translocates to the nucleus and binds its target genes, leading to the upregulation of c-Fos. Subsequently, c-Fos binds to NFATc1. Thus, NF-kB positively regulates osteoclast differentiation and function by activating c-Fos and NFATc1, as well as by inhibiting specific NFATc1 repressor proteins. Conversely, NF-kB can inhibit osteoclast formation and function through TRAF3 and P110, and the suppressors of c-Fos/NFATc1 signaling, IRF8, and RBP-J. NFATc1 is also regulated by calcium/calmodulin and it is thought that RANKL-mediated phosphorylation of specialized motifs known as immunoreceptor tyrosine-based activation motifs (ITAMs). Phosphorylation of ITAMs result in the activation of Syk and PLCγ, which mobilizes intracellular calcium and, in turn activates calcineurin, a calmodulin-dependent phosphatase that directly dephosphorylates serine residues in NFATc1. Two specific tyrosine kinases, BTK and Tec, may provide the molecular link between ITAM and RANK receptor signaling leading to osteoclastogenesis (Shinohara et al., 2008, Pitcher and van Oers, 2003).
It is important to note that osteoclast differentiation can also be stimulated independently of RANKL, through tumor necrosis factor alpha (TNFα), and M-CSF is a common factor required for both RANKL and TNFα-induced osteoclast differentiation (Kobayashi et al., 2000). Osteoclast differentiation induced by TNFα occurs via the TNF receptor (TNFR) 1 and 2 which are expressed on osteoclast precursors. TNFR1 and TNFR2 also use TRAF2 as a common signal transducer, through which they promote NFATc1 activation and osteoclast differentiation (Kanazawa and Kudo, 2005). However, osteoclasts induced by TNFα formed resorption pits only in the presence of interleukin 1 alpha (IL-1α), and therefore play an important role in bone resorption associated with inflammatory bone diseases.
4.2.4. Osteoclast Precursor Fusion Proteins
The formation of a mature multinucleated osteoclasts requires the fusion of mononuclear precursor cells. Two signaling proteins have been identified which regulate osteoclast fusion and multinucleation, namely DC-STAMP (dendritic-cell specific transmembrane protein) and OC-STAMP (osteoclast stimulatory transmembrane protein) (Kukita et al., 2004, Vignery, 2005, Yagi et al., 2005, Miyamoto, 2006, Yagi et al., 2007, Yang et al., 2008, Mensah et al., 2010). DC-STAMP is expressed as a dimer on the cell surface of both human and murine osteoclast progenitors. It was discovered that mice lacking DC-STAMP form mononuclear osteoclast precursors that express TRAP, a key enzyme required for bone resorption, but these mononuclear precursors do not fuse and the DC-STAMP-deficient mice exhibit defects in osteoclast number and activity, leading to osteosclerosis. Conversely, DC-STAMP overexpression increases the formation of mature multinucleated osteoclasts. Although RANKL does not directly bind DC-STAMP it can modulate the expression of DC-STAMP in osteoclast precursors. In recent studies, it was reported that DC-STAMP contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail, and that DC-STAMP regulates osteoclast differentiation through the NFACTc1 signaling (Chiu et al., 2017).
The second osteoclast “fusogenic” protein, OC-STAMP, is also induced by exposure to RANKL (Kim et al., 2011, Yang et al., 2008). Unlike DC-STAMP, OC-STAMP appears to only be expressed in osteoclast lineage cells. In murine osteoclast progenitors, inhibition of OC-STAMP activity through the use of a neutralizing antibody or siRNA-mediated knockdown results in a marked, reduction in the number of mature multinucleated osteoclasts, whereas overexpression of OC-STAMP significantly increases the number of mature osteoclasts. Similar to mice lacking DC-STAMP, mice in which OC-STAMP is blocked contain TRAP-positive mononuclear cells, but few mature multinucleated osteoclasts (Kim et al., 2011, Yang et al., 2008).
4.3. Osteoclast Survival and Apoptosis
To prevent dysregulated bone resorption, osteoclast number is regulated at the level of cell differentiation and by limiting osteoclast life span via programed cell death, or apoptosis. Osteoclast survival is controlled by the same factors that are required for osteoclast activity, including the Ras-ERK pathway, and the PI3K/Akt signaling pathways. The mTOR protein is a target of PI3K activity and activation of this kinase is required for the anti-apoptotic actions of M-CSF, RANKL and TNFα in osteoclasts. Activation of the small GTPase Rac1, and the upstream effector, Vav3, are also involved in M-CSF induced cell survival.
The shortening of the osteoclast life span controls the extent and depth of osteoclast resorption activity. Osteoclast apoptosis is accelerated in the absence of supporting osteoblasts or bone marrow cells, and in conditions leading to low levels of RANKL, M-CSF or IL-1. Detachment of osteoclasts also induces apoptosis, presumably because integrins become disengaged from RGD peptides in the bone matrix.
The ability of osteoclasts to sense calcium in the bone microenvironment occurs through the calcium sensing receptor, CaSR. Activation of the CaSR in osteoclasts in response to calcium induces the PLC-mediated remodeling of their cytoskeleton, leading to decreased osteoclast bone resorbing activity (Mentaverri et al., 2006, Bennett et al., 2001). The PI3K/Akt pathway is also implicated in the response of osteoclasts to extracellular calcium (Bennett et al., 2001, Boudot et al., 2010). Thus, at high concentration of calcium, which mimics the microenvironment of demineralized bone, the activated CaSR drives osteoclast apoptosis (Lorget et al., 2000). Interestingly, at low concentrations of calcium in culture, osteoblasts produce more RANKL, thus driving osteoclastogenesis (Takeyama et al., 2000).
Currently, the majority of drug agents available to protect against bone loss are the anti-resorptive therapies (Stepan et al., 2003). These anti-osteoclastic therapies include estrogen and the selective estrogen receptor modulator (SERM) raloxifene, which both decrease osteoclast life span and promote apoptosis. Estrogens act via a complex signaling mechanism to inhibit the activation of T cells, decreasing their secretion of RANKL and TNFα. Estrogen also inhibits osteoclast differentiation and decreases life span, by regulating the expression of Fas ligand (FasL) in osteoblasts (Krum et al., 2008), which binds the death receptor Fas in osteoclasts (Nakamura et al., 2007a, Nakamura et al., 2007b), accelerating osteoclast cell death. Finally, the anti-resorptive amino-bisphosphonates also decrease bone remodeling by decreasing osteoclast activity and by inducing osteoclast apoptosis (Dunford et al., 2006, Tella and Gallagher, 2014, Tsubaki et al., 2014).
4.3.1. Bcl-2 Proteins and Osteoclast Apoptosis
Apoptosis occurs through either the extrinsic (death receptor) pathway or the intrinsic (mitochondria) pathway (Hengartner, 2000). In the extrinsic pathway, activation of a TNFR induces apoptosis via the activation of caspase 8, an aspartate-specific cysteine protease. However, in the intrinsic pathway, cytochrome c release from the mitochondria is regulated by different Bcl-2 family members which are either anti-apoptotic or pro-apoptotic in action. For example, in mature osteoclasts, cytokine withdrawal leads to reduced expression of anti-apoptotic Bcl-2 and rapid apoptosis (Tanaka et al., 2010). Removal of RANKL signaling also leads to the activation of Bid-induced and caspase-3 induced osteoclast apoptosis (Vaira et al., 2008). M-CSF withdrawal also decreases Bcl-2 through inactivation of the transcription factor, MITF (Tanaka et al., 2010). Osteoclast apoptosis is also regulated by increased expression of the pro-apoptotic protein, Bim, a BH3-domain-only member of the Bcl-2 family proteins (Wakeyama et al., 2007). In the absence of Bim, mice have decreased osteoclast activity, despite increased cell survival (Tanaka et al., 2010). Bim expression, and therefore apoptosis, is kept in check through the c-Cbl-mediated ubiquitin/protease degradation pathway that is regulated by caspase-3, such that in the absence of caspase-3, the degradation of Bim is suppressed. The expression of Bim is also down-regulated by IL-3 signaling through the Raf/ERK and/or PI3K/mTOR pathways. In contrast, upregulation of the anti-apoptotic protein, Bcl-XL, which decreases Src, and inhibits the cleavage of procaspase-9, initiating osteoclast apoptosis.
4.3.2. FasL-Induced Osteoclast Apoptosis
Osteoclast apoptosis is also believed to be regulated in part through the osteoblast-secreted factor FasL (or CD95L) (Kovacic et al., 2010). FasL is a membrane-bound protein belonging to the TNF family proteins. FasL expression in osteoblasts is regulated by estrogens, and MMP3-induced FASL cleavage in osteoblasts leads to the secretion of FasL, and subsequently to the osteoblast-mediate apoptosis of osteoclasts (Garcia et al., 2013, Krum et al., 2008). FasL binds the prototypical TNF family death receptor, Fas (FS-7-associated surface antigen; CD95; APO1; TNFR superfamily member 6, TNFRSF6) (Wu et al., 2003). Fas, is widely expressed in numerous cells, including macrophages and osteoclast progenitors. After Fas activation the adaptor protein FADD (Fas associated death domain) binds Fas, which leads to the recruitment of procaspase 8 and 10, forming a death-inducing signaling complex (DISC). The DISC complex then activates caspase 8, and consequently caspase 3 in the cytosol, promoting apoptosis. Although Fas is the main receptor of FasL, the decoy receptor 3 (DcR3), another member of the TNFR superfamily, also acts as a decoy receptor for FasL (Sheikh and Fornace, 2000, Yang et al., 2004). DcR3 is a secreted protein and competitively binds and inhibits several TNF family proteins, not just FasL. It was reported that DcR3 acts as a ligand to induce the differentiation of macrophages into osteoclasts. DcR3 also suppresses RANKL-induced osteoclast formation via the NF-kB and NFATc1 pathway (Cheng et al., 2013), thus functioning as a regulator of osteoclast apoptosis.
4.4. Osteoblast to Osteoclast Signaling
In addition to the well-known pro-osteoclastic factors, such as RANKL, M-CSF and Il-1, osteoblasts secrete factors that decrease osteoclast formation and activity. It has long been established that RANKL-mediated osteoclast formation is opposed by OPG, which is expressed by osteoblasts and osteocytes. OPG acts a soluble decoy receptor for RANKL, effectively reducing the local level of RANKL, and consequently, decreasing osteoclast differentiation and cell number. Moreover, in the presence of OPG, increased rates of apoptosis of osteoclasts and osteoclast precursors has been observed which is due to activation of the classic FasL/Fas apoptosis pathway (Wang et al., 2015, Kovacic et al., 2007).
As discussed previously in this review, osteoblasts produce several Wnt family proteins. Wnt5a binds to two different receptor complexes in osteoclast precursors. Wnt5a binds to the FZD and LRP5/6 signaling complex and activates β-catenin in osteoclasts to promote osteoclastogenesis (Yamashita et al., 2012, Glass et al., 2005). On the other hand, Wnt5a binding to FZD and Ror2 (receptor tyrosine kinase-like orphan receptor 2) upregulates RANK expression in osteoclasts, which increases RANKL-mediated osteoclast formation (Maeda et al., 2012).
Osteoblast to osteoclast signaling also occurs through the semaphorins family proteins. Semaphorin 3A (Sema3A) binds to a specific receptor complex on osteoclast precursors that is composed of neurophilin-1 (Nrp1) and plexin-A1, and activation of this complex inhibits osteoclast differentiation (Hayashi et al., 2012, Li et al., 2017). It has also been shown that another semaphorin, Sema6C/6D, binds to osteoclast precursor to stimulate ITAMs and DNAX-activating protein 12 (DAP12), which enhances RANK signaling and osteoclastogenesis (Takegahara et al., 2006, Kang et al., 2018, Koga et al., 2004). Of note, mice lacking the ITAM adaptor proteins, FcRγ (Fc receptor common gamma subunit) and DAP12 (DNAX-activating protein 12), exhibit defective osteoclast differentiation, leading to severe osteopetrosis. In osteoclast precursors, the FcRγ and DAP12 proteins can also activate calcium signaling through PLCγ which is also important for regulating osteoclast survival (Bennett et al., 2001).
5. REGULATION OF BONE CELL DIFFERENTIATION AND FUNCTION THROUGH CELL-TO-CELL CONTACT
5.1. Notch Signaling
The Notch signaling pathway is activated by direct contact of a cell expressing the Notch ligands Delta-like proteins (Delta1, 3 and 4) and Jagged (1 and 2) and a cell expressing the receptors known as Notch proteins (Notch 1–4) (Artavanis-Tsakonas et al., 1995). Both ligands and receptors are transmembrane protein and their binding leads to activation of intracellular signaling in the cell expressing the receptor (unidirectional). Osteoblasts and osteocytes (as well as osteoclasts) express both component of the Notch signaling system (Canalis, 2018b). As with other molecules affecting osteoblastic cell differentiation and function, the relevance of Notch signaling for osteoblasts and osteocytes biology was put in evidence by the establishment of the link of human mutations with bone diseases (Canalis, 2018a, Zanotti and Canalis, 2012).
Upon ligand-receptor binding, the canonical Notch signaling pathway is activated, resulting in the proteolytic cleavage of the Notch receptor. This cleavage occurs in two steps, the first one as a result of the activity of the metalloproteinase ADAM17/TACE, which removes the extracellular domain of the receptor. The second step is carried out by the γ-secretase complex that is formed by presenilin-1 and −2, nicastrin, PEN-2, and Aph-1, resulting in the release of the Notch intracellular domain or NICD. The NICD then translocates to the nucleus where it forms a complex with the transcription factors recombining binding protein suppressor of hairless (RBP-Jκ) and mastermind-like (MAML). This trimeric complex recruits co-activators and promotes the transcription of Notch target genes. As for BMP and Wnt signaling, a non-canonical Notch signaling pathway independent of RBP-Jκ has been described, although its role in bone cell is not known (Canalis, 2018b).
Activation of Notch signaling has profound effects on osteoblastogenesis, which depend on the stage of maturation of the cells of the mesenchymal/osteoblastic lineage. Thus, transgenic mice expressing the NICD (which acts as a constitutive active Notch receptor) in osteoblast precursors exhibit high bone mass and increased osteoblast number and proliferation, but impaired osteoblast maturation (Engin et al., 2008, Canalis et al., 2013b). As a results of this altered osteoblastogenesis, immature cells accumulate and produce disorganized, woven bone with accumulation of non-mineralized bone (osteoid). Further, NICD overexpression earlier in the osteoblast differentiation pathway results in reduced bone mass, associated with reduced osteoblast differentiation (Zanotti et al., 2008). On the other hand, NICD overexpression in osteocytes results in increased cancellous bone mass and cortical thickness associated with decreased osteoclast number and bone formation in cancellous bone and no change in osteoclasts but increased osteoblasts in cortical bone (Canalis et al., 2013a). The cellular changes are associated with a decrease in the RANKL/OPG ratio, which favors inhibition of osteoclast differentiation, and a decrease in the Wnt antagonist sclerostin and the consequent increase in Wnt/β-catenin signaling, which favors osteoblast differentiation. These pieces of evidence highlight the cross-talk between different signaling pathways in bone cells. Further in vitro studies showed that the Notch/Wnt signaling cross-talk is also involved in the osteoblast-osteocyte transition (Shao et al., 2018). However, in this case it was shown that Notch activation inhibits Wnt/β-catenin signaling, which in turn results in the terminal differentiation of osteocytes. Conversely, activation of Wnt/β-catenin in osteocytes results in activation of Notch signaling (Tu et al., 2015).
Further studies show that double deletion of presenilin-1 and −2 (the enzymes that participate in the release of NICD) or of the Notch receptors-1 and −2 from MSCs in mice results in high bone mass (Hilton et al., 2008). However, presenilin1/2-deficient mice undergo accelerated age-dependent bone loss by a combination of decreased bone formation and increased resorption. The increased resorption occurs indirectly, through increased RANKL/OPG ratio in cells of the osteoblastic lineage.
On the other hand, removal of presenilin-1 and −2 in mature osteoblasts results in decreased bone mass (Engin et al., 2008). However, the phenotype was not due to decreased osteoblast differentiation/activity, as bone formation parameters did not differ between knockout and control mice. Instead, osteoclast number/surface and eroded surface were increased in the mice lacking the presinilins indicating an indirect effect of Notch signaling in osteoblastic cells on osteoclastogenesis.
Osteocytic Notch1/2 deletion results in increased bone mass in both cancellous and cortical bone compartments, due to mechanisms that are both sex- and age-dependent (Canalis et al., 2013a). In addition, the same authors showed that conditional overexpression of Notch ICD in osteocytes results in inhibition of bone formation and resorption, leading to higher bone mass in a low remodeling state (Canalis et al., 2013a, Canalis et al., 2013b).
In addition to the indirect effect on osteoclasts through the control of the RANKL/OPG system, modulation of Notch signaling in osteoclast precursors directly regulates osteoclastogenesis (Canalis, 2018b). Studies have shown that deletion of the canonical Notch signaling transcription factor RBP-Jk in osteoclast precursors enhances osteoclast differentiation. However, the effect of activation of Notch in these precursors depends on the receptor and while Notch1 inhibits osteoclastogenesis, Notch2 activates it. The effect of Notch2 is associated with the interaction of NICD2 (the fragment of the Notch2 released upon receptor-ligand interaction) with NF-κB, resulting in NFATc1 transcription and osteoclast differentiation. In addition, a study showed that Notch1 or Notch3 deletion in bone marrow macrophages enhanced osteoclast differentiation and overexpression of the Notch ligand Jagged1 inhibited osteoclast formation in vitro (Bai et al., 2008). On the other hand, another study showed that silencing of Notch2, but not Notch1, inhibits osteoclastogenesis (Fukushima et al., 2008). The reason for the discrepancy between the two studies is not clear, although it is possible that the difference stems from the diverse methods used to remove Notch expression (silencing versus Cre-LoxP system/in vivo global gene deletion).
5.2. Ephrin-Ephrin Receptor System
The Ephrin-Ephrin receptor (Eph receptor) system mediates direct cell-to-cell communication through the interaction of the transmembrane proteins Ephrin with the Eph receptor (Cayuso et al., 2015). Unlike the unidirectional Notch signaling, signaling cascades are activated in cells expressing both Ephrin and Eph receptor, constituting a bidirectional signaling system. Two families of Ephrins have been described, ephrinA1–6 and ephrinB1–3 proteins, which bind to EphA1–10 and EphB1–6 receptors, respectively (Committee, 1997). Ephrin ligands are bound to the cell membranes through a glycosylphosphatidylinositol tail (ephrinA) or through a transmembrane domain (ephrinB). The receptors contain intracellular tyrosine kinase domains, which became activated upon ligand binding. The expression of Ephrins and Eph receptors has been demonstrated in osteoblasts and osteoclasts, with osteoblasts expressing ephrinA and B ligands and EphA and EphB receptors, and osteoclasts expressing only ephrinsA2, B1, and B2, but not EphB or EphA1, EphA2, or EphA4 receptors (Zhao et al., 2006). Osteocytes also express ephrinB2 and EphB4 (Wang et al., 2014, Sims and Vrahnas, 2014) and ephrinB1 and B2 (Sims and Martin, 2014). Activation of the Ephrin/Eph receptor system have been proposed to regulate the coupling of bone formation and resorption by triggering signaling in both cells expressing Ephrins and Eph receptors (Lindsey et al., 2018). In particular, in vitro studies showed that osteoclast ephrinB2 binds to osteoblast EphB4 resulting in stimulation of osteoblast differentiation by activation of genes such as Runx2 (forward signaling) and inhibition of osteoclast differentiation through decreased expression of the osteoclastic transcription factors c-Fos and NFATc1 (reverse signaling) (Zhao et al., 2006). However, while the existence of this osteoblast-osteoclast interaction has been demonstrated in vitro, it is not clear whether it would exist in vivo, because osteoblasts and osteoclasts are not normally located in close proximity on the bone surface to allow for direct cell-to-cell contact (Sims and Martin, 2014). On the other hand, a more likely scenario has been proposed, in which ephrinB2/EphB4 is required for osteoblast differentiation and regulates osteoblast-osteoclast precursor interactions. Thus, blockade of ephrinB2/EphB4 interaction results in decreased osteoblast differentiation in the presence of PTH, and in increased osteoclast differentiation in vitro and in vivo. In addition to this interaction between two different cell types (heterotypic), the role of Eprhin/Eph interaction in osteoblastic cells (homotypic) was shown in mice lacking ephrinB1, which exhibit decreased osteoblast differentiation and activity (Tonna and Sims, 2014). This phenotype results from the lack of interaction between ephrinB1 and EphB2 in osteoblastic cells, with reduced expression of the transcription factor osterix and of alkaline phosphatase. Conversely, stimulation of ephrinB1 reverse signaling by EphB2 results in increased osterix expression and alkaline phosphatase activity. Similarly, homotypic eprhinB2/EphB4 interaction is required for late stage osteoblast differentiation and blockade of this interaction results in the accumulation of osteoblasts and osteoid, but decreased mineralization. In addition, blockade of eprhinB2/EphB4 interaction leads to increased RANKL expression and osteoclast number in PTH-treated mice.
5.3. Connexins and Intracellular Signaling
Connexins are four transmembrane-spanning proteins that associate in hexamers known as connexons or hemichannels (Beyer et al., 1990). Hemichannels present in neighboring cells align to form gap junction channels that allow the communication between the two cells. Hemichannels can also be found in unopposed cell membranes, where they mediate the interaction of the cell with the extracellular medium (Goodenough and Paul, 2003). In bone cells, hemichannels are involved in the transduction of mechanical, pharmacologic and hormonal stimuli into intracellular signaling (Plotkin and Stains, 2015, Plotkin et al., 2017). In addition to their classic function as channel forming proteins, connexins are also mediators of intracellular signaling, mainly through interaction of the cytoplasmic connexin C-terminus region with structural proteins and enzymes, such as kinases and phosphatases (Leithe et al., 2018). In osteoblastic cells, the interactions between connexins and intracellular signaling molecules is required for prostaglandin release and anti-apoptosis induced by mechanical stimulation, differentiation induced by fibroblast growth factor 2, and cell survival induced by bisphosphonates and parathyroid hormone (Plotkin and Stains, 2015).
The expression and function of connexins in osteoblasts and osteocytes has been extensively studied (Plotkin and Stains, 2015). In particular, Cx43, Cx37, Cx45, and Cx46 are expressed in osteoblastic cells, being Cx43 the most highly expressed and best studied connexin in bone. In vitro and in vivo studies showed that Cx43 deletion reduces osteoblastogenesis whereas its overexpression leads to increased osteoblast differentiation in vitro. Thus, overexpression of Cx43 in osteoblastic cells increases ERK signaling through a mechanism that requires direct cell-to-cell contact and intercellular communication (Stains and Civitelli, 2005, Niger et al., 2012), whereas expression of Cx45, which acts has partial dominant negative effects on Cx43 actions, reduces ERK and Akt activation in Cx43-expressing cells (Stains and Civitelli, 2005). Activation of ERK/PI3K pathways by Cx43 results in increased Sp1 phosphorylation and its recruitment to the promoter of osteoblastic genes such as osteocalcin and osterix, increasing their expression and osteoblast differentiation (Stains and Civitelli, 2005, Niger et al., 2011, Stains et al., 2003).
Further studies demonstrated that increased Cx43 levels in osteoblastic cells in vitro enhances the activation of protein kinase C delta (PKCδ) induced by FGF2 (Lima et al., 2009, Niger et al., 2012), through direct binding of PKCδ with Cx43 C-terminal domain (Niger et al., 2010, Hebert and Stains, 2013). In addition, Cx43 increases the transcriptional activity of the master regulator of osteoblastogenesis Runx2 through activation of inositol polyphosphate/PKCδ signaling in osteoblastic cells (Niger et al., 2013, Niger et al., 2012, Lima et al., 2009).
In addition to its role on osteoblast differentiation and FGF2 actions, Cx43 also mediate the anti-apoptotic effects of bisphosphonates and PTH (Plotkin and Bellido, 2013). In the case of bisphosphonates, addition of the drug to cultured osteocytic cells results in opening of Cx43 hemichannel, and the activation of a signaling cascade that involves the formation of a complex comprising β-arrestin, Scr, MEK and ERKs (Plotkin et al., 2002, Plotkin et al., 2005). This leads to the phosphorylation of the cytoplasmic ERK target p90RSK, which in turn phosphorylates C/EBPβ and BAD, resulting in cell survival. This anti-apoptotic signaling pathway does not required nuclear actions of ERKs or ERK-mediated gene transcription and, instead, it is blocked by forced ERK nuclear accumulation.
Cx43 expression is also required for the anti-apoptotic effect of PTH on osteoblastic cells in vitro (Plotkin and Bellido, 2013). As with bisphosphonates, hemichannel but not gap junction channel activity are required for the effect of the hormone. Cx43 associates with β-arrestin through the phosphorylated serine 368 in the C-terminus domain of the connexin (Bivi et al., 2011). Cx43-β-arrestin association release the PTH1R from its binding to β-arrestin, allowing for cAMP accumulation, protein kinase A (PKA) activation and downstream gene transcription.
Cx43 also mediates the activation of intracellular signaling induced by mechanical stimulation in osteocytic cells (Plotkin and Bellido, 2013). In this case, in vitro studies showed that Cx43 C-terminus domain interacts with α5β1 integrin, leading to opening of Cx43 hemichannels in osteocytes subjected to fluid flow shear stress (Batra et al., 2012, Batra et al., 2014). Opened Cx43 hemichannels mediate the release of autocrine/paracrine factors such as ATP and prostaglandin E2 (PGE2) (Cherian et al., 2005, Genetos et al., 2007, Siller-Jackson et al., 2008). PGE2, in turn has been shown to mediate the survival effect of mechanical stimulation in osteocytes (Kitase et al., 2010). The protective effect of the prostaglandin is mediated by its interaction with EP2 and EP4 receptors, resulting in the activation of the cAMP/PKA and the Wnt/β-catenin pathways.
Reduced Cx43 levels in osteoblastic cells indirectly affects osteoclast differentiation (Watkins et al., 2011, Zhang et al., 2011, Bivi et al., 2012). Thus, deletion of Cx43 from osteoblast precursors, mature osteoblasts, or osteocytes in mice results in increased RANKL/OPG ratio and high osteoclast numbers in vivo. Cx43 is also expressed in osteoclastic cells from normal mice (Ilvesaro et al., 2000) in osteoclasts from patients with Paget’s disease of the bone and giant cell tumors of the bone, although the role of the connexin in these conditions has not been established (Schilling et al., 2008). On the other hand, in vitro studies showed that Cx43 blockade inhibits osteoclast fusion and function (Ilvesaro et al., 2000, Schilling et al., 2008, Ilvesaro et al., 2001), although whether Cx43 is also required for osteoclast differentiation and function in vivo remains to be determined. On the other hand, Cx37, another connexin member of the same family of gap junction proteins as Cx43, which is expressed in osteoclasts, is involved in osteoclast differentiation in mice in vivo (Pacheco-Costa et al., 2014). Thus, mice with global deletion of Cx37 exhibit increased bone mass due to reduced osteoclast differentiation. The impaired osteoclastogenesis in these mice is associated with accumulation of osteoclast precursors and activation of the Notch signaling pathway.
6. CONCLUDING REMARKS
In this chapter, we described several of the signaling pathways triggered in osteoblasts, osteocytes, and osteoclasts by selected regulatory molecules, as well as by direct cell-to-cell contact. Through these interrelated signaling pathways, the generation, viability and function of osteoblasts, osteocytes and osteoclasts is controlled. Appropriate differentiation and activity of each of the bone cells is required for the maintenance of bone mass, architecture, and strength. Therefore, understanding the interrelated signaling stimuli that regulate these cells, and the intracellular signaling pathways activated, will allow for better design of strategies to improve bone mass in conditions associated with low bone mass and increased bone fragility.
7. ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health R01-AR053643 to LIP, and ICMH pilot grant funding to LIP and AB, and NIAMS R01-AR060332 to AB. AB was also supported in part by the Indiana Clinical and Translational Sciences Institutes of Health, in part by grant number ULITR001108 from the National Center for Advancing Translational Sciences, Clinical and Translational Science Award.
9. REFERENCES
- ALAM I, ALKHOULI M, GERARD-O’RILEY RL, WRIGHT WB, ACTON D, GRAY AK, PATEL B, REILLY AM, LIM KE, ROBLING AG & ECONS MJ 2016. Osteoblast-Specific Overexpression of Human WNT16 Increases Both Cortical and Trabecular Bone Mass and Structure in Mice. Endocrinology, 157, 722–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALI NN, BOYDE A & JONES SJ 1984. Motility and resorption: osteoclastic activity in vitro. Anat Embryol (Berl), 170, 51–6. [DOI] [PubMed] [Google Scholar]
- ALLEN SH, NUTTLEMAN PR, KETCHAM CM & ROBERTS RM 1989. Purification and characterization of human bone tartrate-resistant acid phosphatase. J Bone Miner Res, 4, 47–55. [DOI] [PubMed] [Google Scholar]
- ARTAVANIS-TSAKONAS S, MATSUNO K & FORTINI ME 1995. Notch signaling. Science, 268, 225–32. [DOI] [PubMed] [Google Scholar]
- BAI S, KOPAN R, ZOU W, HILTON MJ, ONG CT, LONG F, ROSS FP & TEITELBAUM SL 2008. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J. Biol. Chem, 283, 6509–6518. [DOI] [PubMed] [Google Scholar]
- BARON R & GORI F 2018. Targeting WNT signaling in the treatment of osteoporosis. Curr Opin Pharmacol, 40, 134–141. [DOI] [PubMed] [Google Scholar]
- BARON R & KNEISSEL M 2013. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med, 19, 179–192. [DOI] [PubMed] [Google Scholar]
- BARON R, NEFF L, BROWN W, COURTOY PJ, LOUVARD D & FARQUHAR MG 1988. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J.Cell Biol, 106, 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON R, NEFF L, LOUVARD D & COURTOY PJ 1985. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J.Cell Biol, 101, 2210–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATRA N, BURRA S, SILLER-JACKSON AJ, GU S, XIA X, WEBER GF, DESIMONE D, BONEWALD LF, LAFER EM, SPRAGUE E, SCHWARTZ MA & JIANG JX 2012. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. U. S. A, 109, 3359–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATRA N, RIQUELME MA, BURRA S, REKHA K, GU S & JIANG JX 2014. Direct Regulation of Osteocytic Connexin 43 Hemichannels through AKT Kinase Activated by Mechanical Stimulation. J. Biol. Chem, 289, 10582–10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEENKEN A & MOHAMMADI M 2009. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov, 8, 235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLIDO T, PLOTKIN LI & BRUZZANITI A 2014. Bone cells In: D B. & M A (eds.) Basic and Applied Bone Biology. First ed.: Elsevier. [Google Scholar]
- BENNETT BD, ALVAREZ U & HRUSKA KA 2001. Receptor-operated osteoclast calcium sensing. Endocrinology, 142, 1968–74. [DOI] [PubMed] [Google Scholar]
- BEYER EC, PAUL DL & GOODENOUGH DA 1990. Connexin family of gap junction proteins. J. Membr. Biol, 116, 187–194. [DOI] [PubMed] [Google Scholar]
- BIKLE DD 2008. Integrins, insulin like growth factors, and the skeletal response to load. Osteoporos. Int, 19, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIKLE DD, HARRIS J, HALLORAN BP, ROBERTS CT, LEROITH D & MOREY-HOLTON E 1994. Expression of the genes for insulin-like growth factors and their receptors in bone during skeletal growth. Am J Physiol, 267, E278–86. [DOI] [PubMed] [Google Scholar]
- BIVI N, CONDON KW, ALLEN MR, FARLOW N, PASSERI G, BRUN L, RHEE Y, BELLIDO T & PLOTKIN LI 2012. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J. Bone Min. Res, 27, 374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIVI N, LEZCANO V, ROMANELLO M, BELLIDO T & PLOTKIN LI 2011. Connexin43 interacts with barrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J. Cell. Biochem, 112, 2920–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAIR HC, TEITELBAUM SL, GHISELLI R & GLUCK S 1989. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science, 245, 855–7. [DOI] [PubMed] [Google Scholar]
- BOISCLAIR YR, RHOADS RP, UEKI I, WANG J & OOI GT 2001. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol, 170, 63–70. [DOI] [PubMed] [Google Scholar]
- BONEWALD LF 2011. The Amazing Osteocyte. J. Bone Miner. Res, 26, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUDOT C, SAIDAK Z, BOULANOUAR AK, PETIT L, GOUILLEUX F, MASSY Z, BRAZIER M, MENTAVERRI R & KAMEL S 2010. Implication of the calcium sensing receptor and the Phosphoinositide 3-kinase/Akt pathway in the extracellular calcium-mediated migration of RAW 264.7 osteoclast precursor cells. Bone, 46, 1416–23. [DOI] [PubMed] [Google Scholar]
- BOYCE BF, XIU Y, LI J, XING L & YAO Z 2015. NF-kappaB-Mediated Regulation of Osteoclastogenesis. Endocrinol Metab (Seoul), 30, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDE A, ALI NN & JONES SJ 1984. Resorption of dentine by isolated osteoclasts in vitro. Br Dent J, 156, 216–20. [DOI] [PubMed] [Google Scholar]
- BRADLEY EW, WESTENDORF JJ, VAN WIJNEN AJ & DUDAKOVIC A 2018. Osteoblasts. Function, Development, and Regulation In: BILEZIKIAN J., BOUILLON R, CLEMENS T. & COMPSTON J. (eds.) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism,. 9th ed.: Wiley. [Google Scholar]
- BRUZZANITI A & BARON R 2006. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord, 7, 123–39. [DOI] [PubMed] [Google Scholar]
- BUNN RC & FOWLKES JL 2003. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol. Metab, 14, 176–181. [DOI] [PubMed] [Google Scholar]
- CANALIS E 2018a. Clinical and experimental aspects of notch receptor signaling: Hajdu-Cheney syndrome and related disorders. Metabolism, 80, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANALIS E 2018b. Notch in skeletal physiology and disease. Osteoporos Int, 29, 2611–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANALIS E, ADAMS DJ, BOSKEY A, PARKER K, KRANZ L & ZANOTTI S 2013a. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem, 288, 25614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANALIS E, PARKER K, FENG JQ & ZANOTTI S 2013b. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology, 154, 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAYUSO J, XU Q & WILKINSON DG 2015. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev Biol, 401, 122–31. [DOI] [PubMed] [Google Scholar]
- CHALHOUB N, BENACHENHOU N, RAJAPUROHITAM V, PATA M, FERRON M, FRATTINI A, VILLA A & VACHER J 2003. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med, 9, 399–406. [DOI] [PubMed] [Google Scholar]
- CHAROENPHANDHU N, SUNTORNSARATOON P, KRISHNAMRA N, SA-NGUANMOO P, TANAJAK P, WANG X, LIANG G, LI X, JIANG C, CHATTIPAKORN N. & CHATTIPAKORN S. 2017. Fibroblast growth factor-21 restores insulin sensitivity but induces aberrant bone microstructure in obese insulin-resistant rats. J Bone Miner Metab, 35, 142–149. [DOI] [PubMed] [Google Scholar]
- CHENG CP, SHEU MJ, SYTWU HK & CHANG DM 2013. Decoy receptor 3 suppresses RANKL-induced osteoclastogenesis via down-regulating NFATc1 and enhancing cell apoptosis. Rheumatology (Oxford), 52, 609–22. [DOI] [PubMed] [Google Scholar]
- CHERIAN PP, SILLER-JACKSON AJ, GU S, WANG X, BONEWALD LF, SPRAGUE E & JIANG JX 2005. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell, 16, 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIU YH, SCHWARZ E, LI D, XU Y, SHEU TR, LI J, DE MESY BENTLEY KL, FENG C, WANG B, WANG JC, ALBERTORIO-SAEZ L, WOOD R, KIM M, WANG W & RITCHLIN CT 2017. Dendritic Cell-Specific Transmembrane Protein (DC-STAMP) Regulates Osteoclast Differentiation via the Ca(2+) /NFATc1 Axis. J Cell Physiol, 232, 2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEIREN E, BENICHOU O, VAN HUL E, GRAM J, BOLLERSLEV J, SINGER FR, BEAVERSON K, ALEDO A, WHYTE MP, YONEYAMA T, DEVERNEJOUL MC & VAN HUL W 2001. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet, 10, 2861–7. [DOI] [PubMed] [Google Scholar]
- CLINKENBEARD EL & WHITE KE 2016. Systemic Control of Bone Homeostasis by FGF23 Signaling. Curr Mol Biol Rep, 2, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMITTEE, E. N. 1997. Unified nomenclature for Eph family receptors and their ligands, the ephrins Eph Nomenclature Committee; Cell, 90, 403–4. [DOI] [PubMed] [Google Scholar]
- DE VERNEJOUL MC, HOROWITZ M, DEMIGNON J, NEFF L & BARON R 1988. Bone resorption by isolated chick osteoclasts in culture is stimulated by murine spleen cell supernatant fluids (osteoclast-activating factor) and inhibited by calcitonin and prostaglandin E2. J.Bone Miner.Res, 3, 69–80. [DOI] [PubMed] [Google Scholar]
- DESTAING O, SALTEL F, GEMINARD JC, JURDIC P & BARD F 2003. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol.Biol.Cell, 14, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNFORD JE, ROGERS MJ, EBETINO FH, PHIPPS RJ & COXON FP 2006. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. J Bone Miner Res, 21, 684–94. [DOI] [PubMed] [Google Scholar]
- ENGIN F, YAO Z, YANG T, ZHOU G, BERTIN T, JIANG MM, CHEN Y, WANG L, ZHENG H, SUTTON RE, BOYCE BF & LEE B 2008. Dimorphic effects of Notch signaling in bone homeostasis. Nat. Med, 14, 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESBRIT P, HERRERA S, PORTAL-NUNEZ S, NOGUES X & DIEZ-PEREZ A 2016. Parathyroid Hormone-Related Protein Analogs as Osteoporosis Therapies. Calcif. Tissue Int, 98, 359–369. [DOI] [PubMed] [Google Scholar]
- FELIX R, CECCHINI MG & FLEISCH H 1990. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology, 127, 2592–4. [DOI] [PubMed] [Google Scholar]
- FENG S, DENG L, CHEN W, SHAO J, XU G & LI YP 2009. Atp6v1c1 is an essential component of the osteoclast proton pump and in F-actin ring formation in osteoclasts. Biochem J, 417, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRON M, SETTEMBRE C, SHIMAZU J, LACOMBE J, KATO S, RAWLINGS DJ, BALLABIO A & KARSENTY G 2013. A RANKL-PKCbeta-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev, 27, 955–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER JE, CAULFIELD MP, SATO M, QUARTUCCIO HA, GOULD RJ, GARSKY VM, RODAN GA & ROSENBLATT M 1993. Inhibition of osteoclastic bone resorption in vivo by echistatin, an "arginyl-glycyl-aspartyl" (RGD)-containing protein. Endocrinology, 132, 1411–1413. [DOI] [PubMed] [Google Scholar]
- FUKUSHIMA H, NAKAO A, OKAMOTO F, SHIN M, KAJIYA H, SAKANO S, BIGAS A, JIMI E & OKABE K 2008. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol. Cell Biol, 28, 6402–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-MARTIN A, ARDURA JA, MAYCAS M, LOZANO D, LOPEZ-HERRADON A, PORTAL-NUNEZ S, GARCIA-OCANA A & ESBRIT P 2014. Functional roles of the nuclear localization signal of parathyroid hormone-related protein (PTHrP) in osteoblastic cells. Mol Endocrinol, 28, 925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA AJ, TOM C, GUEMES M, POLANCO G, MAYORGA ME, WEND K, MIRANDA-CARBONI GA & KRUM SA 2013. ERalpha signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J Bone Miner Res, 28, 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELB BD, SHI GP, CHAPMAN HA & DESNICK RJ 1996. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science, 273, 1236–1238. [DOI] [PubMed] [Google Scholar]
- GENETOS DC, KEPHART CJ, ZHANG Y, YELLOWLEY CE & DONAHUE HJ 2007. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell Physiol, 212, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASS DA 2ND, BIALEK P., AHN JD, STARBUCK M, PATEL MS, CLEVERS H, TAKETO MM, LONG F, MCMAHON AP, LANG RA & KARSENTY G 2005. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell, 8, 751–64. [DOI] [PubMed] [Google Scholar]
- GOMEZ-PUERTO MC, IYENGAR PV, GARCIA DE VINUESA A, TEN DIJKE P & SANCHEZ-DUFFHUES G 2018. Bone morphogenetic protein receptor signal transduction in human diseases. J Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODENOUGH DA & PAUL DL 2003. Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol Cell Biol, 4, 285–294. [DOI] [PubMed] [Google Scholar]
- GOVONI KE 2012. Insulin-like growth factor-I molecular pathways in osteoblasts: potential targets for pharmacological manipulation. Curr Mol Pharmacol, 5, 143–52. [PubMed] [Google Scholar]
- GOWEN M, LAZNER F, DODDS R, KAPADIA R, FEILD J, TAVARIA M, BERTONCELLO I, DRAKE F, ZAVARSELK S, TELLIS I, HERTZOG P, DEBOUCK C & KOLA I 1999. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J.Bone Miner.Res, 14, 1654–1663. [DOI] [PubMed] [Google Scholar]
- GRAFE I, ALEXANDER S, PETERSON JR, SNIDER TN, LEVI B, LEE B & MISHINA Y 2018. TGF-beta Family Signaling in Mesenchymal Differentiation. Cold Spring Harb Perspect Biol, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREY A, CHEN Q, XU X, CALLON K & CORNISH J 2003. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology, 144, 4886–4893. [DOI] [PubMed] [Google Scholar]
- HACHEMI Y, RAPP AE, PICKE AK, WEIDINGER G, IGNATIUS A & TUCKERMANN J 2018. Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J Mol Endocrinol, 61, R75–R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMIDOUCHE Z, FROMIGUE O, NUBER U, VAUDIN P, PAGES JC, EBERT R, JAKOB F, MIRAOUI H & MARIE PJ 2010. Autocrine fibroblast growth factor 18 mediates dexamethasone-induced osteogenic differentiation of murine mesenchymal stem cells. J Cell Physiol, 224, 509–15. [DOI] [PubMed] [Google Scholar]
- HANADA R, HANADA T & PENNINGER JM 2010. Physiology and pathophysiology of the RANKL/RANK system. Biol Chem, 391, 1365–70. [DOI] [PubMed] [Google Scholar]
- HASHIDA Y, NAKAHAMA K, SHIMIZU K, AKIYAMA M, HARADA K & MORITA I 2014. Communication-dependent mineralization of osteoblasts via gap junctions. Bone, 61, 19–26. [DOI] [PubMed] [Google Scholar]
- HAUER J, PUSCHNER S, RAMAKRISHNAN P, SIMON U, BONGERS M, FEDERLE C & ENGELMANN H 2005. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A, 102, 2874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI M, NAKASHIMA T, TANIGUCHI M, KODAMA T, KUMANOGOH A & TAKAYANAGI H 2012. Osteoprotection by semaphorin 3A. Nature, 485, 69–74. [DOI] [PubMed] [Google Scholar]
- HEBERT C & STAINS JP 2013. An intact connexin43 is required to enhance signaling and gene expression in osteoblast-like cells. J. Cell Biochem, 114, 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELFRICH MH, NESBITT SA, DOREY EL & HORTON MA 1992. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J.Bone Miner.Res, 7, 335–343. [DOI] [PubMed] [Google Scholar]
- HENGARTNER MO 2000. The biochemistry of apoptosis. Nature, 407, 770–6. [DOI] [PubMed] [Google Scholar]
- HILTON MJ, TU X, WU X, BAI S, ZHAO H, KOBAYASHI T, KRONENBERG HM, TEITELBAUM SL, ROSS FP, KOPAN R & LONG F 2008. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat. Med, 14, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORNE WC, SANJAY A, BRUZZANITI A & BARON R 2005. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev, 208, 106–25. [DOI] [PubMed] [Google Scholar]
- HORTON MA, TAYLOR ML, ARNETT TR & HELFRICH MH 1991. Arg-Gly-Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp.Cell Res, 195, 368–375. [DOI] [PubMed] [Google Scholar]
- HUANG Y, LIU L & LIU A 2018. Dickkopf-1: Current knowledge and related diseases. Life Sci, 209, 249–254. [DOI] [PubMed] [Google Scholar]
- ILVESARO J, TAVI P & TUUKKANEN J 2001. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC. Musculoskelet. Disord, 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILVESARO J, VÄÄNÄNEN K & TUUKKANEN J 2000. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J. Bone Min. Res, 15, 919–926. [DOI] [PubMed] [Google Scholar]
- IRIE A, HABUCHI H, KIMATA K & SANAI Y 2003. Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem Biophys Res Commun, 308, 858–65. [DOI] [PubMed] [Google Scholar]
- ITZSTEIN C, COXON FP & ROGERS MJ 2011. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases, 2, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB AL, SMITH C, PARTANEN J & ORNITZ DM 2006. Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev Biol, 296, 315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN Y & YANG Y 2016. LGR4: A new receptor for a stronger bone. Sci China Life Sci, 59, 735–6. [DOI] [PubMed] [Google Scholar]
- JURDIC P, SALTEL F, CHABADEL A & DESTAING O 2006. Podosome and sealing zone: Specificity of the osteoclast model. Eur.J.Cell Biol, 85, 195–202. [DOI] [PubMed] [Google Scholar]
- KANAZAWA K & KUDO A 2005. TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J Bone Miner Res, 20, 840–7. [DOI] [PubMed] [Google Scholar]
- KANG S, NAKANISHI Y, KIOI Y, OKUZAKI D, KIMURA T, TAKAMATSU H, KOYAMA S, NOJIMA S, NISHIDE M, HAYAMA Y, KINEHARA Y, KATO Y, NAKATANI T, SHIMOGORI T, TAKAGI J, TOYOFUKU T & KUMANOGOH A 2018. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat Immunol, 19, 561–570. [DOI] [PubMed] [Google Scholar]
- KANTAPUTRA PN, KAPOOR S, VERMA P, INTACHAI W & KETUDAT CAIRNS JR 2018. Split hand-foot malformation and a novel WNT10B mutation. Eur J Med Genet, 61, 372–375. [DOI] [PubMed] [Google Scholar]
- KASPER D, PLANELLS-CASES R, FUHRMANN JC, SCHEEL O, ZEITZ O, RUETHER K, SCHMITT A, POET M, STEINFELD R, SCHWEIZER M, KORNAK U & JENTSCH TJ 2005. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. Embo j, 24, 1079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASUKAWA Y, MIYAKOSHI N & MOHAN S 2004. The anabolic effects of GH/IGF system on bone. Curr. Pharm. Des, 10, 2577–2592. [DOI] [PubMed] [Google Scholar]
- KATAGIRI T & WATABE T 2016. Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM K, PUNJ V, KIM JM, LEE S, ULMER TS, LU W, RICE JC & AN W 2016. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev, 30, 208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM MH, PARK M, BAEK SH, KIM HJ & KIM SH 2011. Molecules and signaling pathways involved in the expression of OC-STAMP during osteoclastogenesis. Amino Acids, 40, 1447–59. [DOI] [PubMed] [Google Scholar]
- KITASE Y, BARRAGAN L, JIANG JX, JOHNSON ML & BONEWALD LF 2010. Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J. Bone Miner. Res, 25, 2657–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI K, TAKAHASHI N, JIMI E, UDAGAWA N, TAKAMI M, KOTAKE S, NAKAGAWA N, KINOSAKI M, YAMAGUCHI K, SHIMA N, YASUDA H, MORINAGA T, HIGASHIO K, MARTIN TJ & SUDA T 2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med, 191, 275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGA T, INUI M, INOUE K, KIM S, SUEMATSU A, KOBAYASHI E, IWATA T, OHNISHI H, MATOZAKI T, KODAMA T, TANIGUCHI T, TAKAYANAGI H & TAKAI T 2004. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature, 428, 758–763. [DOI] [PubMed] [Google Scholar]
- KOLLER DL, ZHENG HF, KARASIK D, YERGES-ARMSTRONG L, LIU CT, MCGUIGAN F, KEMP JP, GIROUX S, LAI D, EDENBERG HJ, PEACOCK M, CZERWINSKI SA, CHOH AC, MCMAHON G, ST POURCAIN B, TIMPSON NJ, LAWLOR DA, EVANS DM, TOWNE B, BLANGERO J, CARLESS MA, KAMMERER C, GOLTZMAN D, KOVACS CS, PRIOR JC, SPECTOR TD, ROUSSEAU F, TOBIAS JH, AKESSON K, ECONS MJ, MITCHELL BD, RICHARDS JB, KIEL DP & FOROUD T 2013. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J Bone Miner Res, 28, 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMABA H & LANSKE B 2018. Role of Klotho in bone and implication for CKD. Curr Opin Nephrol Hypertens, 27, 298–304. [DOI] [PubMed] [Google Scholar]
- KOMORI T 2006. Regulation of osteoblast differentiation by transcription factors. J. Cell Biochem. [DOI] [PubMed] [Google Scholar]
- KONG YY, YOSHIDA H, SAROSI I, TAN HL, TIMMS E, CAPPARELLI C, MORONY S, OLIVEIRA-DOS-SANTOS AJ, VAN G, ITIE A, KHOO W, WAKEHAM A, DUNSTAN CR, LACEY DL, MAK TW, BOYLE WJ & PENNINGER JM 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature, 397, 315–23. [DOI] [PubMed] [Google Scholar]
- KORNAK U, KASPER D, BOSL MR, KAISER E, SCHWEIZER M, SCHULZ A, FRIEDRICH W, DELLING G & JENTSCH TJ 2001. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell, 104, 205–215. [DOI] [PubMed] [Google Scholar]
- KOVACIC N, GRCEVIC D, KATAVIC V, LUKIC IK & MARUSIC A 2010. Targeting Fas in osteoresorptive disorders. Expert Opin Ther Targets, 14, 1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACIC N, LUKIC IK, GRCEVIC D, KATAVIC V, CROUCHER P & MARUSIC A 2007. The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol, 178, 3379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUM SA, MIRANDA-CARBONI GA, HAUSCHKA PV, CARROLL JS, LANE TF, FREEDMAN LP & BROWN M 2008. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J, 27, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUKITA T, WADA N, KUKITA A, KAKIMOTO T, SANDRA F, TOH K, NAGATA K, IIJIMA T, HORIUCHI M, MATSUSAKI H, HIESHIMA K, YOSHIE O & NOMIYAMA H 2004. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med, 200, 941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACOMBE J, KARSENTY G & FERRON M 2013. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle, 12, 2744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGE PF, WARTOSCH L, JENTSCH TJ & FUHRMANN JC 2006. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature, 440, 220–3. [DOI] [PubMed] [Google Scholar]
- LAZNER F, GOWEN M & KOLA I 1999. An animal model for pycnodysostosis: the role of cathepsin K in bone remodelling. Mol.Med.Today, 5, 413–414. [DOI] [PubMed] [Google Scholar]
- LEE AW & STATES DJ 2000. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol, 20, 6779–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE SH, RHO J, JEONG D, SUL JY, KIM T, KIM N, KANG JS, MIYAMOTO T, SUDA T, LEE SK, PIGNOLO RJ, KOCZON-JAREMKO B, LORENZO J & CHOI Y 2006. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med, 12, 1403–9. [DOI] [PubMed] [Google Scholar]
- LEITHE E, MESNIL M & AASEN T 2018. The connexin 43 C-terminus: A tail of many tales. Biochim Biophys Acta Biomembr, 1860, 48–64. [DOI] [PubMed] [Google Scholar]
- LERNER UH & OHLSSON C 2015. The WNT system: background and its role in bone. J Intern Med, 277, 630–49. [DOI] [PubMed] [Google Scholar]
- LEUPIN O, PITERS E, HALLEUX C, HU S, KRAMER I, MORVAN F, BOUWMEESTER T, SCHIRLE M, BUENO-LOZANO M, FUENTES FJ, ITIN PH, BOUDIN E, DE FF, JENNES K, BRANNETTI B, CHARARA N, EBERSBACH H, GEISSE S, LU CX, BAUER A, VAN HW & KNEISSEL M 2011. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J. Biol. Chem, 286, 19489–19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z, HAO J, DUAN X, WU N, ZHOU Z, YANG F, LI J, ZHAO Z & HUANG S 2017. The Role of Semaphorin 3 A in Bone Remodeling. Front Cell Neurosci, 11, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIMA F, NIGER C, HEBERT C & STAINS JP 2009. Connexin43 Potentiates Osteoblast Responsiveness to Fibroblast Growth Factor 2 via a Protein Kinase C-Delta/Runx2-dependent Mechanism. Mol. Biol. Cell, 20, 2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDER S & AEPFELBACHER M 2003. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol, 13, 376–385. [DOI] [PubMed] [Google Scholar]
- LINDSEY RC, RUNDLE CH & MOHAN S 2018. Role of IGF1 and EFN-EPH signaling in skeletal metabolism. J Mol Endocrinol, 61, T87–T102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISOWSKA B, KOSSON D & DOMARACKA K 2018. Lights and shadows of NSAIDs in bone healing: the role of prostaglandins in bone metabolism. Drug Des Devel Ther, 12, 1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMAGA MA, YEH WC, SAROSI I, DUNCAN GS, FURLONGER C, HO A, MORONY S, CAPPARELLI C, VAN G, KAUFMAN S, VAN DER HEIDEN A, ITIE A, WAKEHAM A, KHOO W, SASAKI T, CAO Z, PENNINGER JM, PAIGE CJ, LACEY DL, DUNSTAN CR, BOYLE WJ, GOEDDEL DV & MAK TW 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev, 13, 1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORGET F, KAMEL S, MENTAVERRI R, WATTEL A, NAASSILA M, MAAMER M & BRAZIER M 2000. High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem Biophys Res Commun, 268, 899–903. [DOI] [PubMed] [Google Scholar]
- LOZANO D, FERNANDEZ-DE-CASTRO L, PORTAL-NUNEZ S, LOPEZ-HERRADON A, DAPIA S, GOMEZ-BARRENA E & ESBRIT P 2011. The C-terminal fragment of parathyroid hormone-related peptide promotes bone formation in diabetic mice with low-turnover osteopaenia. Br. J. Pharmacol, 162, 1424–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO J, YANG Z, MA Y, YUE Z, LIN H, QU G, HUANG J, DAI W, LI C, ZHENG C, XU L, CHEN H, WANG J, LI D, SIWKO S, PENNINGER JM, NING G, XIAO J & LIU M 2016. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med, 22, 539–46. [DOI] [PubMed] [Google Scholar]
- MAEDA K, KOBAYASHI Y, UDAGAWA N, UEHARA S, ISHIHARA A, MIZOGUCHI T, KIKUCHI Y, TAKADA I, KATO S, KANI S, NISHITA M, MARUMO K, MARTIN TJ, MINAMI Y & TAKAHASHI N 2012. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med, 18, 405–12. [DOI] [PubMed] [Google Scholar]
- MARANDA B, CHABOT G, DECARIE JC, PATA M, AZEDDINE B, MOREAU A & VACHER J 2008. Clinical and cellular manifestations of OSTM1-related infantile osteopetrosis. J Bone Miner Res, 23, 296–300. [DOI] [PubMed] [Google Scholar]
- MARKS SC JR. & GROLMAN ML 1987. Tartrate-resistant acid phosphatase in mononuclear and multinuclear cells during the bone resorption of tooth eruption. J Histochem Cytochem, 35, 1227–30. [DOI] [PubMed] [Google Scholar]
- MARKS SC JR., WOJTOWICZ A, SZPERL M, URBANOWSKA E, MACKAY CA, WIKTOR-JEDRZEJCZAK W, STANLEY ER & AUKERMAN SL 1992. Administration of colony stimulating factor-1 corrects some macrophage, dental, and skeletal defects in an osteopetrotic mutation (toothless, tl) in the rat. Bone, 13, 89–93. [DOI] [PubMed] [Google Scholar]
- MCNIVEN MA, BALDASSARRE M & BUCCIONE R 2004. The role of dynamin in the assembly and function of podosomes and invadopodia. Front Biosci, 9, 1944–53. [DOI] [PubMed] [Google Scholar]
- MEADOWS NA, SHARMA SM, FAULKNER GJ, OSTROWSKI MC, HUME DA & CASSADY AI 2007. The expression of Clcn7 and Ostm1 in osteoclasts is coregulated by microphthalmia transcription factor. J Biol Chem, 282, 1891–904. [DOI] [PubMed] [Google Scholar]
- MENSAH KA, RITCHLIN CT & SCHWARZ EM 2010. RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens. J Cell Physiol, 223, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENTAVERRI R, YANO S, CHATTOPADHYAY N, PETIT L, KIFOR O, KAMEL S, TERWILLIGER EF, BRAZIER M & BROWN EM 2006. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. Faseb j, 20, 2562–4. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO T 2006. The dendritic cell-specific transmembrane protein DC-STAMP is essential for osteoclast fusion and osteoclast bone-resorbing activity. Mod Rheumatol, 16, 341–2. [DOI] [PubMed] [Google Scholar]
- MIZUKAMI J, TAKAESU G, AKATSUKA H, SAKURAI H, NINOMIYA-TSUJI J, MATSUMOTO K & SAKURAI N 2002. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol, 22, 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOON JB, KIM JH, KIM K, YOUN BU, KO A, LEE SY & KIM N 2012. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J Immunol, 188, 163–9. [DOI] [PubMed] [Google Scholar]
- MOVERARE-SKRTIC S, HENNING P, LIU X, NAGANO K, SAITO H, BORJESSON AE, SJOGREN K, WINDAHL SH, FARMAN H, KINDLUND B, ENGDAHL C, KOSKELA A, ZHANG FP, ERIKSSON EE, ZAMAN F, HAMMARSTEDT A, ISAKSSON H, BALLY M, KASSEM A, LINDHOLM C, SANDBERG O, ASPENBERG P, SAVENDAHL L, FENG JQ, TUCKERMANN J, TUUKKANEN J, POUTANEN M, BARON R, LERNER UH, GORI F & OHLSSON C 2014. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat. Med, 20, 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULARI M, VAARANIEMI J & VAANANEN HK 2003a. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc Res Tech, 61, 496–503. [DOI] [PubMed] [Google Scholar]
- MULARI MT, ZHAO H, LAKKAKORPI PT & VAANANEN HK 2003b. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic, 4, 113–25. [DOI] [PubMed] [Google Scholar]
- NAITO A, AZUMA S, TANAKA S, MIYAZAKI T, TAKAKI S, TAKATSU K, NAKAO K, NAKAMURA K, KATSUKI M, YAMAMOTO T & INOUE J 1999. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells, 4, 353–362. [DOI] [PubMed] [Google Scholar]
- NAKAMURA A, LY C, CIPETIC M, SIMS NA, VIEUSSEUX J, KARTSOGIANNIS V, BOURALEXIS S, SALEH H, ZHOU H, PRICE JT, MARTIN TJ, NG KW, GILLESPIE MT & QUINN JM 2007a. Osteoclast inhibitory lectin (OCIL) inhibits osteoblast differentiation and function in vitro. Bone, 40, 305–15. [DOI] [PubMed] [Google Scholar]
- NAKAMURA T, IMAI Y, MATSUMOTO T, SATO S, TAKEUCHI K, IGARASHI K, HARADA Y, AZUMA Y, KRUST A, YAMAMOTO Y, NISHINA H, TAKEDA S, TAKAYANAGI H, METZGER D, KANNO J, TAKAOKA K, MARTIN TJ, CHAMBON P & KATO S 2007b. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell, 130, 811–23. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA K, ZHOU X, KUNKEL G, ZHANG Z, DENG JM, BEHRINGER RR & DE CROMBRUGGHE B 2002. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell, 108, 17–29. [DOI] [PubMed] [Google Scholar]
- NIGER C, BUO AM, HEBERT C, DUGGAN BT, WILLIAMS MS & STAINS JP 2012. ERK Acts in Parallel to PKC delta to Mediate the Connexin43-dependent Potentiation of Runx2 Activity by FGF2 in MC3T3 Osteoblasts. Am. J. Physiol Cell Physiol, 302, C1035–C1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGER C, HEBERT C & STAINS JP 2010. Interaction of connexin43 and protein kinase C-delta during FGF2 signaling. BMC. Biochem, 11, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGER C, LIMA F, YOO DJ, GUPTA RR, BUO AM, HEBERT C & STAINS JP 2011. The transcriptional activity of osterix requires the recruitment of Sp1 to the osteocalcin proximal promoter. Bone, 49, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGER C, LUCIOTTI MA, BUO AM, HEBERT C, MA V & STAINS JP 2013. The regulation of runt-related transcription factor 2 by fibroblast growth factor-2 and connexin43 requires the inositol polyphosphate/protein kinase Cdelta cascade. J. Bone Miner. Res, 28, 1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUSSE R, BROWN A, PAPKOFF J, SCAMBLER P, SHACKLEFORD G, MCMAHON A, MOON R & VARMUS H 1991. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell, 64, 231. [DOI] [PubMed] [Google Scholar]
- NUSSE R & VARMUS HE 1992. Wnt genes. Cell, 69, 1073–87. [DOI] [PubMed] [Google Scholar]
- OCHOA GC, SLEPNEV VI, NEFF L, RINGSTAD N, TAKEI K, DANIELL L, KIM W, CAO H, MCNIVEN M, BARON R & DE CAMILLI P 2000. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol, 150, 377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODGREN PR, KIM N, MACKAY CA, MASON-SAVAS A, CHOI Y & MARKS SC JR. 2003. The role of RANKL (TRANCE/TNFSF11), a tumor necrosis factor family member, in skeletal development: effects of gene knockout and transgenic rescue. Connect Tissue Res, 44 Suppl 1, 264–71. [PubMed] [Google Scholar]
- ORNITZ DM & MARIE PJ 2015. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev, 29, 1463–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTO F, THORNELL AP, CROMPTON T, DENZEL A, GILMOUR KC, ROSEWELL IR, STAMP GW, BEDDINGTON RS, MUNDLOS S, OLSEN BR, SELBY PB & OWEN MJ 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development [see comments]. Cell, 89, 765–771. [DOI] [PubMed] [Google Scholar]
- PACHECO-COSTA R, HASSAN I, REGINATO RD, DAVIS HM, BRUZZANITI A, ALLEN MR & PLOTKIN LI 2014. High Bone Mass in Mice Lacking Cx37 Due to Defective Osteoclast Differentiation. J. Biol. Chem, 289, 8508–8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANGRAZIO A, POLIANI PL, MEGARBANE A, LEFRANC G, LANINO E, DI ROCCO M, RUCCI F, LUCCHINI F, RAVANINI M, FACCHETTI F, ABINUN M, VEZZONI P, VILLA A & FRATTINI A 2006. Mutations in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J Bone Miner Res, 21, 1098–105. [DOI] [PubMed] [Google Scholar]
- PARK SJ, KIM SH, CHOI HS, RHEE Y & LIM SK 2009. Fibroblast growth factor 2-induced cytoplasmic asparaginyl-tRNA synthetase promotes survival of osteoblasts by regulating anti-apoptotic PI3K/Akt signaling. Bone, 45, 994–1003. [DOI] [PubMed] [Google Scholar]
- PITCHER LA & VAN OERS NS 2003. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol, 24, 554–60. [DOI] [PubMed] [Google Scholar]
- PLOTKIN LI, AGUIRRE JI, KOUSTENI S, MANOLAGAS SC & BELLIDO T 2005. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J. Biol. Chem, 280, 7317–7325. [DOI] [PubMed] [Google Scholar]
- PLOTKIN LI & BELLIDO T 2013. Beyond gap junctions: Connexin43 and bone cell signaling. Bone, 52, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOTKIN LI & BIVI N 2014. Local regulation of bone cell function In: D B. & M A (eds.) Basic and Applied Bone Biology. First ed.: Elsevier. [Google Scholar]
- PLOTKIN LI, DAVIS HM, CISTERNA BA & SAEZ JC 2017. Connexins and Pannexins in Bone and Skeletal Muscle. Curr. Osteoporos. Rep, 15, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOTKIN LI, MANOLAGAS SC & BELLIDO T 2002. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem, 277, 8648–8657. [DOI] [PubMed] [Google Scholar]
- PLOTKIN LI & STAINS JP 2015. Connexins and pannexins in the skeleton: gap junctions, hemichannels and more. Cell Mol. Life Sci, 72, 2853–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHEE Y, BIVI N, FARROW EG, LEZCANO V, PLOTKIN LI, WHITE KE & BELLIDO T 2011. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone, 49, 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPPERT R, HOFFMANN E & SEBALD W 1996. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem, 237, 295–302. [DOI] [PubMed] [Google Scholar]
- SANJAY A, HOUGHTON A, NEFF L, DIDOMENICO E, BARDELAY C, ANTOINE E, LEVY J, GAILIT J, BOWTELL D, HORNE WC & BARON R 2001. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J.Cell Biol, 152, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO T, DEL CARMEN OM, HOU P, HEEGAARD AM, KUMEGAWA M, FOGED NT & DELAISSE JM 1997. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J.Cell Sci, 110 ( Pt 5), 589–596. [DOI] [PubMed] [Google Scholar]
- SCHILLING AF, FILKE S, LANGE T, GEBAUER M, BRINK S, BARANOWSKY A, ZUSTIN J & AMLING M 2008. Gap junctional communication in human osteoclasts in vitro and in vivo. J. Cell Mol. Med, 12, 2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLUTER KD 1999. PTH and PTHrP: Similar Structures but Different Functions. News Physiol Sci, 14, 243–249. [DOI] [PubMed] [Google Scholar]
- SCHULTE G 2015. Frizzleds and WNT/beta-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics. Eur J Pharmacol, 763, 191–5. [DOI] [PubMed] [Google Scholar]
- SCIMECA JC, FRANCHI A, TROJANI C, PARRINELLO H, GROSGEORGE J, ROBERT C, JAILLON O, POIRIER C, GAUDRAY P & CARLE GF 2000. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone, 26, 207–213. [DOI] [PubMed] [Google Scholar]
- SHANKAR G, GADEK TR, BURDICK DJ, DAVISON I, MASON WT & HORTON MA 1995. Structural determinants of calcium signaling by RGD peptides in rat osteoclasts: integrin-dependent and -independent actions. Exp.Cell Res, 219, 364–371. [DOI] [PubMed] [Google Scholar]
- SHAO J, ZHOU Y & XIAO Y 2018. The regulatory roles of Notch in osteocyte differentiation via the crosstalk with canonical Wnt pathways during the transition of osteoblasts to osteocytes. Bone, 108, 165–178. [DOI] [PubMed] [Google Scholar]
- SHEIKH MS & FORNACE AJ JR. 2000. Death and decoy receptors and p53-mediated apoptosis. Leukemia, 14, 1509–13. [DOI] [PubMed] [Google Scholar]
- SHINOHARA M, KOGA T, OKAMOTO K, SAKAGUCHI S, ARAI K, YASUDA H, TAKAI T, KODAMA T, MORIO T, GEHA RS, KITAMURA D, KUROSAKI T, ELLMEIER W & TAKAYANAGI H 2008. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell, 132, 794–806. [DOI] [PubMed] [Google Scholar]
- SILLER-JACKSON AJ, BURRA S, GU S, XIA X, BONEWALD LF, SPRAGUE E & JIANG JX 2008. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J. Biol. Chem, 283, 26374–26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMS NA 2016. Cell-specific paracrine actions of IL-6 family cytokines from bone, marrow and muscle that control bone formation and resorption. Int J Biochem Cell Biol, 79, 14–23. [DOI] [PubMed] [Google Scholar]
- SIMS NA & MARTIN TJ 2014. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey. Rep, 3, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMS NA & VRAHNAS C 2014. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch. Biochem. Biophys. [DOI] [PubMed] [Google Scholar]
- SPINARDI L, RIETDORF J, NITSCH L, BONO M, TACCHETTI C, WAY M & MARCHISIO PC 2004. A dynamic podosome-like structure of epithelial cells. Exp.Cell Res, 295, 360–374. [DOI] [PubMed] [Google Scholar]
- STAINS JP & CIVITELLI R 2005. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol. Biol. Cell, 16, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAINS JP, LECANDA F, SCREEN J, TOWLER DA & CIVITELLI R 2003. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin - response elements in osteoblast promoters. J. Biol. Chem, 278, 24377–24387. [DOI] [PubMed] [Google Scholar]
- STEPAN JJ, ALENFELD F, BOIVIN G, FEYEN JH & LAKATOS P 2003. Mechanisms of action of antiresorptive therapies of postmenopausal osteoporosis. Endocr Regul, 37, 225–38. [PubMed] [Google Scholar]
- STEVENS JR, MIRANDA-CARBONI GA, SINGER MA, BRUGGER SM, LYONS KM & LANE TF 2010. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res, 25, 2138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAZZULLA LC & CRONSTEIN BN 2016. Regulation of bone and cartilage by adenosine signaling. Purinergic Signal, 12, 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STYRKARSDOTTIR U, THORLEIFSSON G, SULEM P, GUDBJARTSSON DF, SIGURDSSON A, JONASDOTTIR A, JONASDOTTIR A, ODDSSON A, HELGASON A, MAGNUSSON OT, WALTERS GB, FRIGGE ML, HELGADOTTIR HT, JOHANNSDOTTIR H, BERGSTEINSDOTTIR K, OGMUNDSDOTTIR MH, CENTER JR, NGUYEN TV, EISMAN JA, CHRISTIANSEN C, STEINGRIMSSON E, JONASSON JG, TRYGGVADOTTIR L, EYJOLFSSON GI, THEODORS A, JONSSON T, INGVARSSON T, OLAFSSON I, RAFNAR T, KONG A, SIGURDSSON G, MASSON G, THORSTEINSDOTTIR U & STEFANSSON K 2013. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature, 497, 517–20. [DOI] [PubMed] [Google Scholar]
- SUGATANI T, ALVAREZ U & HRUSKA KA 2003. PTEN regulates RANKL- and osteopontin-stimulated signal transduction during osteoclast differentiation and cell motility. J Biol Chem, 278, 5001–8. [DOI] [PubMed] [Google Scholar]
- SUN Y, BUKI KG, ETTALA O, VAARANIEMI JP & VAANANEN HK 2005. Possible role of direct Rac1-Rab7 interaction in ruffled border formation of osteoclasts. J Biol Chem, 280, 32356–61. [DOI] [PubMed] [Google Scholar]
- SUNDARAM K, NISHIMURA R, SENN J, YOUSSEF RF, LONDON SD & REDDY SV 2007. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp Cell Res, 313, 168–78. [DOI] [PubMed] [Google Scholar]
- TAKADA T, KATAGIRI T, IFUKU M, MORIMURA N, KOBAYASHI M, HASEGAWA K, OGAMO A & KAMIJO R 2003. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem, 278, 43229–35. [DOI] [PubMed] [Google Scholar]
- TAKEGAHARA N, TAKAMATSU H, TOYOFUKU T, TSUJIMURA T, OKUNO T, YUKAWA K, MIZUI M, YAMAMOTO M, PRASAD DV, SUZUKI K, ISHII M, TERAI K, MORIYA M, NAKATSUJI Y, SAKODA S, SATO S, AKIRA S, TAKEDA K, INUI M, TAKAI T, IKAWA M, OKABE M, KUMANOGOH A & KIKUTANI H 2006. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol, 8, 615–22. [DOI] [PubMed] [Google Scholar]
- TAKEYAMA S, YOSHIMURA Y, SHIRAI Y, DEYAMA Y, HASEGAWA T, YAWAKA Y, KIKUIRI T, MATSUMOTO A & FUKUDA H 2000. Low calcium environment effects osteoprotegerin ligand/osteoclast differentiation factor. Biochem Biophys Res Commun, 276, 524–9. [DOI] [PubMed] [Google Scholar]
- TANAKA S, WAKEYAMA H, AKIYAMA T, TAKAHASHI K, AMANO H, NAKAYAMA KI & NAKAMURA K 2010. Regulation of osteoclast apoptosis by Bcl-2 family protein Bim and Caspase-3. Adv Exp Med Biol, 658, 111–6. [DOI] [PubMed] [Google Scholar]
- TELLA SH & GALLAGHER JC 2014. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol, 142, 155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON WR, RUBIN CT & RUBIN J 2012. Mechanical regulation of signaling pathways in bone. Gene, 503, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONNA S & SIMS NA 2014. Talking among ourselves: paracrine control of bone formation within the osteoblast lineage. Calcif Tissue Int, 94, 35–45. [DOI] [PubMed] [Google Scholar]
- TROEN B 2004. The role of cathepsin K in normal bone resorption. Drug News Perspect, 17, 19–28. [DOI] [PubMed] [Google Scholar]
- TSUBAKI M, KOMAI M, ITOH T, IMANO M, SAKAMOTO K, SHIMAOKA H, TAKEDA T, OGAWA N, MASHIMO K, FUJIWARA D, MUKAI J, SAKAGUCHI K, SATOU T & NISHIDA S 2014. Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation. J Biomed Sci, 21, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TU X, DELGADO-CALLE J, CONDON KW, MAYCAS M, ZHANG H, CARLESSO N, TAKETO MM, BURR DB, PLOTKIN LI & BELLIDO T 2015. Osteocytes mediate the anabolic actions of canonical Wnt/b-catenin signaling in bone. Proc. Natl. Acad. Sci U. S. A, 112, E478–E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAANANEN HK, ZHAO H, MULARI M & HALLEEN JM 2000. The cell biology of osteoclast function. J Cell Sci, 113 ( Pt 3), 377–81. [DOI] [PubMed] [Google Scholar]
- VAIRA S, ALHAWAGRI M, ANWISYE I, KITAURA H, FACCIO R & NOVACK DV 2008. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest, 118, 2088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERLINDEN L, VANDERSCHUEREN D & VERSTUYF A 2016. Semaphorin signaling in bone. Mol Cell Endocrinol, 432, 66–74. [DOI] [PubMed] [Google Scholar]
- VIGNERY A 2005. Macrophage fusion: the making of osteoclasts and giant cells. J Exp Med, 202, 337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGUESPACK SG, KOLLER DL, WHITE KE, FISHBURN T, CARN G, BUCKWALTER KA, JOHNSON M, KOCISKO M, EVANS WE, FOROUD T & ECONS MJ 2003. Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res, 18, 1513–8. [DOI] [PubMed] [Google Scholar]
- WAKEYAMA H, AKIYAMA T, TAKAHASHI K, AMANO H, KADONO Y, NAKAMURA M, OSHIMA Y, ITABE H, NAKAYAMA KI, NAKAYAMA K, NAKAMURA K & TANAKA S 2007. Negative feedback loop in the Bim-caspase-3 axis regulating apoptosis and activity of osteoclasts. J Bone Miner Res, 22, 1631–9. [DOI] [PubMed] [Google Scholar]
- WALSH MC & CHOI Y 2003. Biology of the TRANCE axis. Cytokine Growth Factor Rev, 14, 251–63. [DOI] [PubMed] [Google Scholar]
- WANG L, LIU S, ZHAO Y, LIU D, LIU Y, CHEN C, KARRAY S, SHI S & JIN Y 2015. Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ, 22, 1654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, MENENDEZ A, FONG C, ELALIEH HZ, CHANG W & BIKLE DD 2014. Ephrin B2/EphB4 mediates the actions of IGF-I signaling in regulating endochondral bone formation. J Bone Miner Res, 29, 1900–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS M, GRIMSTON SK, NORRIS JY, GUILLOTIN B, SHAW A, BENIASH E & CIVITELLI R 2011. Osteoblast Connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol. Biol. Cell, 22, 1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIN MN & KRONENBERG HM 2018. Regulation of Bone Remodeling by Parathyroid Hormone. Cold Spring Harb. Perspect. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIJENAYAKA AR, KOGAWA M, LIM HP, BONEWALD LF, FINDLAY DM & ATKINS GJ 2011. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS. ONE, 6, e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIKTOR-JEDRZEJCZAK W, BARTOCCI A, FERRANTE AW JR., AHMED-ANSARI A, SELL KW, POLLARD JW & STANLEY ER 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A, 87, 4828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS BO 2017. LRP5: From bedside to bench to bone. Bone, 102, 26–30. [DOI] [PubMed] [Google Scholar]
- WONG BR, BESSER D, KIM N, ARRON JR, VOLOGODSKAIA M, HANAFUSA H & CHOI Y 1999. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell, 4, 1041–9. [DOI] [PubMed] [Google Scholar]
- WU H, XU G & LI YP 2009. Atp6v0d2 is an essential component of the osteoclast-specific proton pump that mediates extracellular acidification in bone resorption. J Bone Miner Res, 24, 871–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU M, CHEN G & LI YP 2016. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res, 4, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU X, MCKENNA MA, FENG X, NAGY TR & MCDONALD JM 2003. Osteoclast apoptosis: the role of Fas in vivo and in vitro. Endocrinology, 144, 5545–55. [DOI] [PubMed] [Google Scholar]
- XIAO L, ESLIGER A & HURLEY MM 2012. Nuclear FGF2 isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. J. Bone Miner. Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIAO L, LIU P, LI X, DOETSCHMAN T, COFFIN JD, DRISSI H & HURLEY MM 2009. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J Biol Chem, 284, 3170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG Y, SONG D, CAI Y, YU W, YEUNG YG & STANLEY ER 2011. A CSF-1 receptor phosphotyrosine 559 signaling pathway regulates receptor ubiquitination and tyrosine phosphorylation. J Biol Chem, 286, 952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU X, HAN J, ITO Y, BRINGAS P JR., DENG C & CHAI Y 2008. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell, 15, 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI M, MIYAMOTO T, SAWATANI Y, IWAMOTO K, HOSOGANE N, FUJITA N, MORITA K, NINOMIYA K, SUZUKI T, MIYAMOTO K, OIKE Y, TAKEYA M, TOYAMA Y & SUDA T 2005. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med, 202, 345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI M, NINOMIYA K, FUJITA N, SUZUKI T, IWASAKI R, MORITA K, HOSOGANE N, MATSUO K, TOYAMA Y, SUDA T & MIYAMOTO T 2007. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J Bone Miner Res, 22, 992–1001. [DOI] [PubMed] [Google Scholar]
- YAKAR S, WERNER H & ROSEN CJ 2018. Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol, 61, T115–T137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMASHITA T, TAKAHASHI N & UDAGAWA N 2012. New roles of osteoblasts involved in osteoclast differentiation. World J Orthop, 3, 175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG CR, WANG JH, HSIEH SL, WANG SM, HSU TL & LIN WW 2004. Decoy receptor 3 (DcR3) induces osteoclast formation from monocyte/macrophage lineage precursor cells. Cell Death Differ, 11 Suppl 1, S97–107. [DOI] [PubMed] [Google Scholar]
- YANG DQ, FENG S, CHEN W, ZHAO H, PAULSON C & LI YP 2012. V-ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast-mediated bone resorption. J Bone Miner Res, 27, 1695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG M, BIRNBAUM MJ, MACKAY CA, MASON-SAVAS A, THOMPSON B & ODGREN PR 2008. Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J Cell Physiol, 215, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YI J, XIONG W, GONG X, BELLISTER S, ELLIS LM & LIU Q 2013. Analysis of LGR4 receptor distribution in human and mouse tissues. PLoS One, 8, e78144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YI Z, LIN WW, STUNZ LL & BISHOP GA 2014. Roles for TNF-receptor associated factor 3 (TRAF3) in lymphocyte functions. Cytokine Growth Factor Rev, 25, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOUCHI M, KONDO T, SANJAY A, HOUGHTON A, YOSHIMURA A, KOMIYA S, ZHANG H & BARON R 2001. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. Journal of Biological Chemistry, 276, 35185–35193. [DOI] [PubMed] [Google Scholar]
- YOSHIKO Y, WANG H, MINAMIZAKI T, IJUIN C, YAMAMOTO R, SUEMUNE S, KOZAI K, TANNE K, AUBIN JE & MAEDA N 2007. Mineralized tissue cells are a principal source of FGF23. Bone, 40, 1565–1573. [DOI] [PubMed] [Google Scholar]
- YU K, XU J, LIU Z, SOSIC D, SHAO J, OLSON EN, TOWLER DA & ORNITZ DM 2003. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development, 130, 3063–3074. [DOI] [PubMed] [Google Scholar]
- YUN YR, WON JE, JEON E, LEE S, KANG W, JO H, JANG JH, SHIN US & KIM HW 2010. Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng, 2010, 218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZANOTTI S & CANALIS E 2012. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif. Tissue Int, 90, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZANOTTI S, SMERDEL-RAMOYA A, STADMEYER L, DURANT D, RADTKE F & CANALIS E 2008. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology, 149, 3890–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y, PAUL EM, SATHYENDRA V, DAVIDSON A, BRONSON S, SRINIVASAN S, GROSS TS & DONAHUE HJ 2011. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE, 6, e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO C, IRIE N, TAKADA Y, SHIMODA K, MIYAMOTO T, NISHIWAKI T, SUDA T & MATSUO K 2006. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab, 4, 111–121. [DOI] [PubMed] [Google Scholar]
- ZHOU M, GUO S, YUAN L, ZHANG Y, ZHANG M, CHEN H, LU M, YANG J & MA J 2017. Blockade of LGR4 inhibits proliferation and odonto/osteogenic differentiation of stem cells from apical papillae. J Mol Histol, 48, 389–401. [DOI] [PubMed] [Google Scholar]
- ZHU C, ZHENG XF, YANG YH, LI B, WANG YR, JIANG SD & JIANG LS 2016. LGR4 acts as a key receptor for R-spondin 2 to promote osteogenesis through Wnt signaling pathway. Cell Signal, 28, 989–1000. [DOI] [PubMed] [Google Scholar]
- ZMUDA JM, YERGES LM, KAMMERER CM, CAULEY JA, WANG X, NESTLERODE CS, WHEELER VW, PATRICK AL, BUNKER CH, MOFFETT SP & FERRELL RE 2009. Association analysis of WNT10B with bone mass and structure among individuals of African ancestry. J Bone Miner Res, 24, 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUO H & WAN Y 2017. Nuclear Receptors in Skeletal Homeostasis. Curr Top Dev Biol, 125, 71–107. [DOI] [PubMed] [Google Scholar]