Abstract
Hyperammonia due to ornithine transcarbamylase deficiency (OTCD) can cause a range of deficiencies in domains of executive function and working memory. Only a few fMRI studies have focused on neuroimaging data in a population with OTCD. Yet, there is a need for monitoring the disease progression and neurocognitive function in this population. In this study, we used a non-invasive neuroimaging technique, functional Near Infrared Spectroscopy (fNIRS), to examine the hemodynamics of prefrontal cortex (PFC) based on neural activation in an OTCD population. Using fNIRS, we measured the activation in PFC of the participants while performing the Stroop task. Behavioral assessment such as reaction time and correct response were recorded. We investigated the difference in behavioral measures as well as brain activation in left and right PFC in patients with OTCD and controls. Results revealed a distinction in left PFC activation between controls and patients with OTCD, where control subjects showed higher task related activation increase. Subjects with OTCD also exhibited bilateral increase in PFC activation. There was no significant difference in response time or correct response between the two groups. Our findings suggest the alterations in neurocognitive function of PFC in OTCD compared to the controls despite the behavioral profiles exhibiting no such differences. This is a first study using fNIRS to examine a neurocognitive function in OTCD population and can provide a novel insight into the screening of OTCD progression and examining neurocognitive changes.
Keywords: Metabolic Disorder, Urea Cycle Disorder, Functional Near Infrared Spectroscopy, executive function, prefrontal cortex, Cerebral Hemodynamics, Brain Activation, Stroop Task
1. Introduction:
Urea Cycle Disorders (UCD) are caused by deficiencies in any of the six enzymes or two transport proteins that are involved in the synthesis and removal of nitrogen from the blood [1]. The most common cause of UCD is due to mutation in the ornithine transcarbamylase mitochondrial enzyme that results in ornithine transcarbamylase deficiency (OTCD). Deficient protein metabolism in OTCD can result in episodes of hyperammonemia (HA) with acute elevations of ammonia causing substantial injury to the brain’s white matter [2]. OTCD has been associated with an altered neurochemical profile [3] and neurocognitive profile in an array of subdomains based in the prefrontal cortex (PFC), such as working memory, executive cognition and attention [4]. Such deficits contribute significantly to disabilities in OTCD despite normal global IQ in many patients [5] [4] and may be seen (albeit to lesser degrees) in female carriers of OTCD.
Although further studies are needed to understand the pathophysiologic mechanisms of cognitive dysfunction in OTCD, evidence from clinical neuroimaging studies have demonstrated a plausible neurologic model. Using fMRI, Gropman et. al. investigated the neurocognitive impairment in OTCD subjects while performing an N-back working memory task [6]. The N-back task is a measure of working memory capacity that is frequently used in cognitive neuroscience. This task includes showing a sequence of stimuli such as letters, and the participants are asked to respond whenever the current stimuli is the same as the one presented N-trials ago. The study found that neurologic insult associated with hyperammonemia impacted the neurocognitive function of the OTCD patients compared to age and gender matched controls. Subjects with OTCD showed greater task-related increase in Blood Oxygen Level (BOLD) signal in the right dorsolateral PFC (DLPFC) compared to control subjects. BOLD imaging is a method used in functional magnetic resonance imaging (fMRI) to observe the functional changes in brain”
The ability to inhibit cognitive interference between different stimuli during short intervals and selective attention are main elements of executive function. The Stroop task [7] is one of the most widely used executive function tasks that evokes PFC activation. Numerous functional neuroimaging studies have shown the involvement of PFC during execution of the Stroop task in healthy controls [8] as well as in those with dyslexia [9] and traumatic brain injury [10, 11].
Given the altered executive function in patients with OTCD, the Stroop task can be used to investigate the underlying neurological function in the PFC. Here, we use functional Near Infrared Spectroscopy (fNIRS) to measure the brain activation in the PFC of patients with OTCD and controls. fNIRS is a non-invasive, portable, and well-established technology that uses the light in near infrared region to measure regional changes in oxygenation in cortical regions and can be used to quantify the brain function during execution of functional tasks. fNIRS measurements are similar to that of fMRI BOLD signal. Compared to fMRI, fNIRS is less susceptible to the subject’s movement and measurement can be done in real-life situations without restricting subject’s movement or a need to be situated inside the scanner. fNIRS has been used in several clinical settings [12, 13] such as stroke rehabilitation [14, 15], pain research in patients with lower back pain [16]. It also has been shown to be a useful tool in other conditions and disorder such as language delay [17], Anxiety Disorder [18, 19], Autism Spectrum Disorder [20, 21], and Alzheimer’s Disease [22, 23]. Hence, fNIRS becomes a modality of choice for populations with neurodevelopmental disorders or young cohorts who cannot perform the task inside the scanner, as well as those with claustrophobia or contraindications to undergo MRI.
In this study, we investigated the activation of the PFC, as measured by fNIRS, during performance of color and word Stroop tasks in subjects with OTCD and a gender-matched control group. We hypothesized that the level of PFC activation would be distinct in control subjects vs. patients with OTCD. Specifically, these differences would be elucidated based on the level of task difficulty (cognitive load) and lateralization of PFC.
2. Material and Methods
2.1. Subjects
35 subjects (OTCD=18: Male=2, Control=17: Male=7, mean age=28.74±14.95) were recruited for this IRB approved study. All subjects signed informed consent to participate in the study. Inclusion criteria included diagnosis of OTCD, IQ of at least 70 and medical stability at the time of the study. Controls had to be disease free, without history of a brain injury. Further details about the study cohorts are shown in table 1.
Table 1.
Subject Demographics in final sample
| Original Sample | Final Sample | Age (mean±STD) | Gender | Diagnosis | |
|---|---|---|---|---|---|
| Control Group | 17 | 15 | 29.73±16.48 | Female=5 | - |
| OTCD Group | 18 | 17 | 25.35±13.15 | Female=15 | OTCD (Asymptomatic=2) |
2.2. Task
Color and word Stroop tasks were designed in E-prime (Psychology Software Tools, Inc) to evoke the Stroop Interference effect as an inhibitory executive function in the PFC region. To elucidate the Stroop effect, congruent and incongruent conditions are presented. In the congruent condition, which is less demanding, participants rely on responding to the stimuli, where the stimuli word matches the stimuli color. In contrast, incongruent condition requires participants to use the cognitive control to response to the color of the stimuli while the stimuli word does not match with its color thereby creating the Stroop interference.
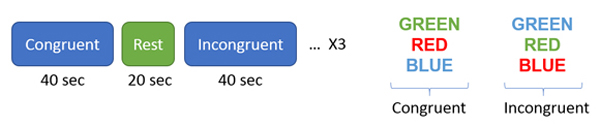
During the congruent condition, the word with the matching color (e.g. word “Green” in green color) were displayed on the screen one at the time, and while during incongruent condition the word was displayed with non-matching color (e.g. word “Green” in color blue) (see figure 1). For the congruent conditions, participants were instructed to click on the corresponding red, green and blue using the matched color buttons on an external keypad. For incongruent conditions, participants read the words but clicked on the corresponding colored bottom that matched with the color but not the word. Each condition was 40 seconds long and was repeated three times. The stimulus was shown on the screen for 1300 ms with the interstimulus duration of 350 ms.
Figure 1.
Stroop Paradigm Task design. Blocks of congruent and incongruent were displayed on the screen with the rest blocks in between, where during congruent condition the color and word were matching while in incongruent condition the color and words were different.
2.3. Functional Near Infrared Spectroscopy (fNIRS)
fNIRS is a non-invasive, portable, and well-established technology that uses the light in the near infrared region (700–900 nm) to measure regional changes in oxygenation in cortical regions. In the near infrared region, oxy-hemoglobin and deoxy-hemoglobin are the two main components of the blood that absorb the light. The light is delivered from the location of source and backscattered light that passes through cortical regions is detected by the photodetector (figure 2). The intensity of the backscattered light contains information regarding the changes in oxy- and deoxy-hemoglobin.
Figure 2.
A diagram showing NIR light (photons) passing through cortical region from location of source, while the backscattered light is detected at the detector site.
2.4. Data Analysis
NIRS raw intensity data at wavelengths of 730 and 850 nm was obtained using NIRS1000 imager (fNIRS Devices LLC) with a sensor containing 4 sources and 8 detectors with channel separation of 2.5 cm. Cobi Studio Software [24] was used for raw data acquisition. The sensor was placed on the subjects’ forehead prior to the task onset. The middle of the sensor was placed on the Fpz 10 based on 10–20 international system (figure 3).
Figure 3.
Schematic of fNIRS headband sensor containing 4 sources and 10 detectors with total of 16 channel combination
fNIRSOFT [25] was used for data pre-processing. Low pass filter (Hamming, order 20, 0.1 Hz) was applied to remove the high frequency oscillations due to respiration and heart rate [17, 24–27] followed by median filter to remove the sharp spikes due to possible motion artifact. The Sliding-Window Motion Artifact Rejection (SMAR) algorithm [28–30] was used to identify and remove the channels with bad skin contacts. From here, the intensity data was converted to the changes in HbO and Hb using modified Beer-Lambert Law. The 10 second prior to start of the task was used as a baseline to calculate the changes in HbO and Hb signals. During baseline, subjects were instructed to relax as much as possible.
To account for the variation in skull thickness due to age, we used differential path-length factor based on each subjects’ age [17, 31]. The signals were subsequently detrended for each channel to remove slow drifts [32] and Correlation Based Signal Improvement algorithm [33] was applied to ensure the negative correlation of the oxy- and deoxy-hemoglobin signals.
Changes in HbO was used as a measure of brain activation as indicated in numerous literatures as it has been shown to be a better correlated of BOLD fMRI signal and have a better signal to noise ratio compared to HbR and has been commonly used in NIRS studies [33–39]. The HbO signal was averaged over blocks of each trials, separately. We then used the mean HbO from the left and right channels to account for changes in activation in left and right PFC. For further analysis, and to emphasize on Stroop interference demand, signal from incongruent condition (interference and attention) were subtracted from congruent (involved in attention) trials to account for changes related to Stroop effect, referred to as Stroop interference activation, to minimize the activation due to attentional mechanism. A total of 3 subjects (1 OTCD and 2 Controls) were excluded due to inability to complete the task, sleepiness/lack of attention, and excessive motion.
2.4. Statistical Analysis:
We used SPSS (IBM Corp, SPSS Statistic 22) to perform statistical analysis of the fNIRS data, behavioral data, and age. Multivariate Analysis of Variance was performed to investigate the effect of diagnosis (OTCD vs Control) and gender (Male vs Female) as between subject factor on accuracy, reaction time, and activation, while controlling for age. Two-tailed partial correlation was performed to find the correlations between reaction time and accuracy and activation, while controlling for age in the model.
3. Results:
3.1. Reaction time and Accuracy
While controlling for age, we observed no significant interaction between task, diagnosis and gender on either reaction time or task accuracy (figure 4). There was no significant main effect of diagnosis on the reaction time (F(1,23)=0.03 p=0.86). There was a close to significant main effect of task (F(1,23)=4.19, p=0.052), where the reaction time during incongruent condition was slower compared to congruent condition (mean difference 124.24 (95% CI, 78.22 to 170.26), p<0.001).
Figure 4.
a) Bar graph of reaction time during performance of congruent and incongruent task based on diagnosis. There was no significant difference between OTCD and control subjects based on the reaction time. b) Bar graph of correct response during performance of congruent and incongruent task in control and OTCD group
Test of between subject effects revealed no significant effect of task, diagnosis or gender on accuracy based on the correct response. Across controls, data showed significant negative correlation between correct response and reaction time during congruent condition (r=−0.63, p=0.026) where subjects with higher accuracy tended to have faster reaction time. In controls, the accuracy of congruent condition was predictive of the accuracy during incongruent condition (r=0.64, p=0.025). Such trend was not observed in subjects with OTCD (r=−0.06, p=0.81). In subjects with OTCD the reaction time during congruent task was positively correlated with that of incongruent condition (r=0.72, p=0.004). Such correlation was not found in the control group (r=0.21, p=0.52).
3.2. Task related activation
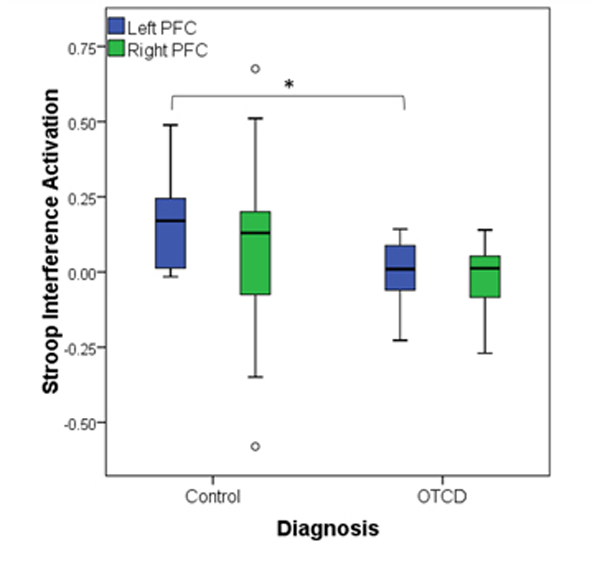
We conducted multivariate analysis to examine the effect of gender and diagnosis on Stroop interference activation from left and right PFC, while controlling for age. There was no significant interaction effect between gender and diagnosis on the Stroop activation level (F(2,26)=0.41, p=0.66) nor there was the main effect of age (F(2,26)=1.6, p=0.19). Follow up univariate two-way ANOVA revealed the significant between-subject effect of diagnosis on Left PFC (F(1,27)=6.15,p=0.02), where control subjects showed higher level of activation (mean difference 0.15 (95% CI, 0.027 to 0.28), p=0.022) compared to patients with OTCD (figure 5). There was no between subject effects in right PFC (F(1,27)=0.2,p=0.65). Figure 6 shows the cortical activation map in controls and subjects with OTCD.
Figure 5.
Activation level in Left and Right prefrontal cortex (PFC) in controls and subjects with OTCD, where control group showed higher activation in Left PFC compared to the OTCD group.
Figure 6.
Map of activation in prefrontal cortex (PFC) in a) control subjects and b) subjects with OTCD, where control group showed higher Stroop Interference activation compared to OTCD subjects in left PFC, while no activation difference was observed in right PFC.
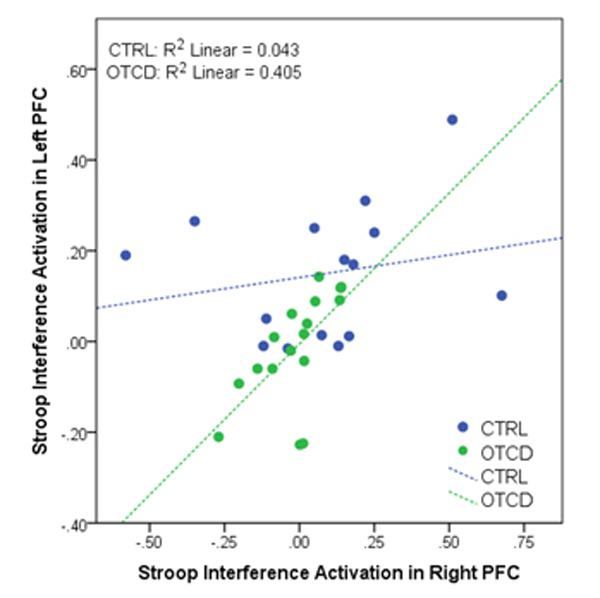
While controlling for age, within subjects with OTCD, there was a significant correlation between Stroop activation in left and right PFC, where patients with OTCD showed bilateral activation increase (r=0.62, p=0.01). Such correlation was not observed in control subjects (r=0.23, p=0.42) (figure 7).
Figure 7.
Correlation between left and right prefrontal cortex (PFC) activation in control and subjects with OTCD, where compared to controls, subjects with OTCD showed bilateral increase in level of activation.
We further found a significant positive correlation between activation in left PFC and reaction time in control group, where activation in PFC increased as subjects had faster reaction time (r=0.59, p=0.04). Such trend however was not observed in the OTCD group (r=−0.28, p=0.33).
4. Discussion:
In this fNIRS study we investigated the PFC activation during Stroop interference effect in controls and subjects with OTCD. We found significant lateralized differences in PFC activation based on Stroop effect, where control subjects showed higher level of activation in left DLPFC compared to those with OTCD. Although the difference between control and OTCD subjects was not shown in behavioral measures such as correct response and reaction time, neuroimaging assessment based on fNIRS showed lateralized differences between the two cohorts. The results also indicate that changes in level of activation in subjects with OTCD are distributed bilaterally.
This study demonstrates the feasibility of using fNIRS to assess the brain activation in subjects with OTCD. Importantly, similar to previous fMRI studies, [6], although we did not observe group differences in task accuracy or reaction time, there were differences in underlying cognitive function in the PFC region. This can suggest that divergence between the groups might not be reflected based on behavioral differences, but may instead be related to prefrontal inefficiency in subjects with OTCD.
The role of PFC in execution of the Stroop task has been shown previously and Stroop interference effect can lead to increase in hemodynamic response in this region. Study by Ven drell et. al has shown the involvement of right PFC in sustained attention during the Stroop task [40]. Other fMRI and fNIRS studies have reported the activation of left PFC in response to Stroop interference during performance of the incongruent condition in healthy control [41] [42] and healthy controls compared to those with traumatic brain injuries [43]. Study by Yanagisawa et. al. [8] also showed an improvement in cognitive performance during incongruent condition in left PFC after moderate exercise.
Based on our results, the difference in Stroop interference activation between controls and subjects with OTCD was prominent in left PFC region, where control subjects exhibited higher task-related activation increase during the more challenging condition. This difference was observed in both symptomatic and asymptomatic subjects with OTCD. In line with previous studies [44], it has been shown that asymptomatic participants, when cognitively challenged, can demonstrate cognitive deficits in the domain of executive function. The lower activation during the performance of a demanding functional task can be related to neural inefficiency in subjects with OTCD. It has been shown previously that the BOLD signal can increase with cognitive load until the task demand overcomes the individual cognitive capacity [45] [46]. This pattern of inefficiency during performance of working memory task in subjects with OTCD has been shown in previous fMRI study [6].
In line with the above studies, during Stroop interference task, subjects with OTCD could not keep up with the higher task demand causing the decrease in brain activation compared to controls in the left PFC region, responsible for regulation of executive function and interference effect [47]. The result showed prominently lateralized higher activation in the left DLPFC in controls compared to subjects with OTCD, indicating the pattern of neural inefficiency when subjects are faced with challenging interference effect.
We also observed the simultaneous bilateral increase in right and left PFC in subjects with OTCD, whereas this pattern of activation was not observed in controls. This diffuse over-recruitment bilateral activation could be due to inefficiency in cognitive function during the more demanding task to overcome the cognitive challenges. Such results could be related to the neural efficiency hypothesis where task performance can be related to cognitive abilities and neural efficiency [48]. The neural recruitment of PFC might be less efficient in the corresponding PFC regions in subjects with OTCD. Such bilateral PFC activation during cognitive performance has been elucidated as a compensatory mechanism in response to decline in brain function [49, 50].
It has been shown previously that hyperammonia is the main factor affecting the neurocognitive function and neurological injury in OTCD group due to alteration in neurotransmitters and cellular volume [51]. It should be noted that other factors such as age of onset and duration and number of hyperammonenic episodes can also affect the severity and degree of cognitive dysfunction in OTCD group. However, often that information is not known as patients may have had subclinical presentations or symptoms attributed to other conditions (abdominal migraine, flu), especially in female carriers and late onset males.
There are several limitations in our studies. First, the NIRS sensor used in this study has been designed for placement on the forehead to minimize changes in signal due to the presence of hair. Therefore, any observed differences are limited and cannot include more extensive neural regions. Further studies on effects of neural efficiency and brain activation should also include measure of verbal and non-verbal IQ to clarify the relationship between activation, and verbal and non-verbal abilities. Limitation on sample size of the study is mostly due to the rareness of OTCD, which makes it difficult to recruit patients regularly. Furthermore, we recruited high functioning subjects with IQ≥70 and at the baseline health to be able to complete the task, which further limited the sample size of this study.
Due to the rare nature of OTCD and limitation on patient availability and access, it was not possible to image the subjects during a hyperammonenic episode or measure the plasma ammonia at the time of the imaging. In the future, including measurements during acute decompensation and severe hyperammonia and at different ammonia levels would allow for a better understanding of these effects on the neurocognitive assessment using fNIRS. Further repeated and longitudinal follow-up studies from early stages and including factors such as treatment options, medications and dietary restriction can clarify the changes in neurocognitive function and the effect of these factors on improvement or decline of cognitive function. Validation of this study in pediatric cohorts can be a critical point in early diagnosis and detecting changes in underlying neurocognitive function at the early stages. In the future, we intend to expand this study to examine hemodynamic features at a younger age ranges to identify the possible neurocognitive differences at earlier time points.
5. Conclusion
In this study we examined the executive function of PFC and its lateralization in controls and subjects with OTCD during performance of the Stroop task. The results of this study suggest the underlying cognitive capacity differences between subjects with OTCD, both symptomatic and asymptomatic, and controls in recruitment of PFC regions, despite the similarities in behavioral domain. We have shown that fNIRS can be used as a non-invasive and wearable imaging technique to distinguish the pattern of cortical neural activation in subjects with OTCD. These preliminary results can have implications toward understanding the compensatory function and lateralization of PFC in OTCD patients. In the future, fNIRS can be used in longitudinal studies to investigate the features of hemodynamic response and its deviations in OTCD while emphasizing domains of executive function, working memory and attention.
Acknowledgements:
We thank the patients and families for their participation and the National Urea Cycle Foundation (NUCDF), in particular, Ms. Cynthia Le Mons for her enthusiastic support and recruitment efforts for this study.
Funding Source
This research was supported by NICHD intramural program and the O’Malley Family Foundation.
Contributor Information
Afrouz Anderson, NIH, National Institute of Child Health and Human Development, Bethesda, MD 20892.
Andrea Gropman, Children’s National Medical Center, Division of Neurogenetics and Neurodevelopmental Pediatrics, Washington, DC, 20010.
Cynthia Le Mons, National Urea Cycle Disorders Foundation, Pasadena, California 91105.
Constantine Stratakis, NIH, National Institute of Child Health and Human Development, Bethesda, MD 20892.
Amir Gandjbakhche, NIH, National Institute of Child Health and Human Development, Bethesda, MD 20892.
References:
- 1.Gropman AL, Summar M, and Leonard JV, Neurological implications of urea cycle disorders. Journal of inherited metabolic disease, 2007. 30(6): p. 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gropman AL, et al. , Diffusion tensor imaging detects areas of abnormal white matter microstructure in patients with partial ornithine transcarbamylase deficiency. AJNR. American journal of neuroradiology, 2010. 31(9): p. 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gropman AL, et al. , 1H MRS identifies symptomatic and asymptomatic subjects with partial ornithine transcarbamylase deficiency. Molecular genetics and metabolism, 2008. 95(1–2): p. 2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gropman AL and Batshaw ML, Cognitive outcome in urea cycle disorders. Molecular genetics and metabolism, 2004. 81: p. 58–62. [DOI] [PubMed] [Google Scholar]
- 5.Gyato K, et al. , Metabolic and neuropsychological phenotype in women heterozygous for ornithine transcarbamylase deficiency. Annals of Neurology, 2004. 55(1): p. 80–86.14705115 [Google Scholar]
- 6.Gropman AL, et al. , Altered neural activation in ornithine transcarbamylase deficiency during executive cognition: an fMRI study. Human Brain Mapping, 2013. 34(4): p. 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroop JR, Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 1935. 18(6): p. 643–662. [Google Scholar]
- 8.Yanagisawa H, et al. , Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. NeuroImage, 2010. 50(4): p. 1702–1710. [DOI] [PubMed] [Google Scholar]
- 9.Jinyan S, et al. , Reduced prefrontal cortex activation in the color-word Stroop task for Chinese dyslexic children: a near-infrared spectroscopy study. Journal of Physics: Conference Series, 2011. 277(1): p. 012034. [Google Scholar]
- 10.Plenger P, et al. , fNIRS-based investigation of the Stroop task after TBI. Brain Imaging and Behavior, 2016. 10(2): p. 357–366. [DOI] [PubMed] [Google Scholar]
- 11.Leung H-C, et al. , An Event-related Functional MRI Study of the Stroop Color Word Interference Task. Cerebral Cortex, 2000. 10(6): p. 552–560. [DOI] [PubMed] [Google Scholar]
- 12.Scheeren TWL, Schober P, and Schwarte LA, Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. Journal of clinical monitoring and computing, 2012. 26(4): p. 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, et al. , Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. The Cochrane database of systematic reviews, 2018. 1(1): p. CD010947-CD010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, et al. , Brain Activation and Gait Alteration During Cognitive and Motor Dual Task Walking in Stroke—A Functional Near-Infrared Spectroscopy Study. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 2018. 26(12): p. 2416–2423. [DOI] [PubMed] [Google Scholar]
- 15.Lo C-C, et al. , Near Infrared Spectroscopy Study of Cortical Excitability During Electrical Stimulation-Assisted Cycling for Neurorehabilitation of Stroke Patients. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 2018. PP: p. 1–1. [DOI] [PubMed] [Google Scholar]
- 16.Vrana A, et al. , Cortical Sensorimotor Processing of Painful Pressure in Patients with Chronic Lower Back Pain—An Optical Neuroimaging Study using fNIRS. Frontiers in Human Neuroscience, 2016. 10(578). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson AA, et al. , Prefrontal Hemodynamics in Toddlers at Rest: A Pilot Study of Developmental Variability. Frontiers in Neuroscience, 2017. 11(300). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng Y-L, et al. , A Functional Near-Infrared Spectroscopy Study of State Anxiety and Auditory Working Memory Load. Frontiers in Human Neuroscience, 2018. 12: p. 313–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuscan L-A, et al. , Exploring frontal asymmetry using functional near-infrared spectroscopy: a preliminary study of the effects of social anxiety during interaction and performance tasks. Brain Imaging and Behavior, 2013. 7(2): p. 140–153. [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga R, et al. , Usefulness of near-infrared spectroscopy to detect brain dysfunction in children with autism spectrum disorder when inferring the mental state of others. Psychiatry and Clinical Neurosciences, 2013. 67(4): p. 203–209. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F and Roeyers H, Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies. International Journal of Psychophysiology, 2019. 137: p. 41–53. [DOI] [PubMed] [Google Scholar]
- 22.Li R, et al. , Early Detection of Alzheimer’s Disease Using Non-invasive Near-Infrared Spectroscopy. Frontiers in aging neuroscience, 2018. 10: p. 366–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger FG, et al. , Brain activation in frontotemporal and Alzheimer’s dementia: a functional near-infrared spectroscopy study. Alzheimer’s Research & Therapy, 2016. 8(1): p. 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayaz H, et al. , Using MazeSuite and Functional Near Infrared Spectroscopy to Study Learning in Spatial Navigation. 2011(56): p. e3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayaz H, Functional Near Infrared Spectroscopy based Brain Computer Interface, 2010, Drexel University, : Philadelphia, PA. [Google Scholar]
- 26.Anderson AA, et al. , Exploring the role of task performance and learning style on prefrontal hemodynamics during a working memory task. PLoS One, 2018. 13(6): p. e0198257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dashtestani H, et al. , The role of prefrontal cortex in a moral judgment task using functional near-infrared spectroscopy. Brain and Behavior, 2018. 8(11): p. e01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayaz H, et al. , Sliding-window motion artifact rejection for Functional Near-Infrared Spectroscopy. Conf Proc IEEE Eng Med Biol Soc, 2010. 2010: p. 6567–70. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Ayaz H, and Shewokis PA, Mental workload classification with concurrent electroencephalography and functional near-infrared spectroscopy. Brain-Computer Interfaces, 2017. 4(3): p. 175–185. [Google Scholar]
- 30.Gentili RJ, et al. , Functional near-infrared spectroscopy-based correlates of prefrontal cortical dynamics during a cognitive-motor executive adaptation task. Frontiers in Human Neuroscience, 2013. 7: p. 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholkmann F and Wolf M, General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J Biomed Opt, 2013. 18(10): p. 105004. [DOI] [PubMed] [Google Scholar]
- 32.Dashtestani H, et al. , Canonical correlation analysis of brain prefrontal activity measured by functional near infra-red spectroscopy (fNIRS) during a moral judgment task. Behavioural Brain Research, 2019. 359: p. 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui X, Bray S, and Reiss AL, Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage, 2010. 49(4): p. 3039–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strangman G, et al. , A Quantitative Comparison of Simultaneous BOLD fMRI and NIRS Recordings during Functional Brain Activation. NeuroImage, 2002. 17(2): p. 719–731. [PubMed] [Google Scholar]
- 35.Greve D, et al. BOLD physiological noise reduction using spatio-spectral-temporal correlations with NIRS in Proc Intl Soc Mag Reson Med. 2009. Honolulu, HI. [Google Scholar]
- 36.Kawano M, et al. , Correlation between frontal lobe oxy-hemoglobin and severity of depression assessed using near-infrared spectroscopy. J Affect Disord, 2016. 205: p. 154–158. [DOI] [PubMed] [Google Scholar]
- 37.Sato H, et al. , A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. NeuroImage, 2013. 83: p. 158–73. [DOI] [PubMed] [Google Scholar]
- 38.Tong Y, Lindsey KP, and de BFB, Partitioning of physiological noise signals in the brain with concurrent near-infrared spectroscopy and fMRI. J Cereb Blood Flow Metab, 2011. 31(12): p. 2352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue Y, et al. , Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PLoS One, 2013. 8(9): p. e75058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vendrell P, et al. , The role of prefrontal regions in the Stroop task. Neuropsychologia, 1995. 33(3): p. 341–352. [DOI] [PubMed] [Google Scholar]
- 41.Schroeter ML, et al. , Near-infrared spectroscopy can detect brain activity during a color–word matching Stroop task in an event-related design. Human Brain Mapping, 2002. 17(1): p. 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlis AC, et al. , Multi-channel near-infrared spectroscopy detects specific inferior-frontal activation during incongruent Stroop trials. Biological Psychology, 2005. 69(3): p. 315–331. [DOI] [PubMed] [Google Scholar]
- 43.Yennu A, et al. , Prefrontal responses to Stroop tasks in subjects with post-traumatic stress disorder assessed by functional near infrared spectroscopy. Scientific Reports, 2016. 6: p. 30157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprouse C, et al. , Investigating neurological deficits in carriers and affected patients with ornithine transcarbamylase deficiency. Molecular genetics and metabolism, 2014. 113(1–2): p. 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callicott Joseph H., et al. , Complexity of Prefrontal Cortical Dysfunction in Schizophrenia: More Than Up or Down. American Journal of Psychiatry, 2003. 160(12): p. 2209–2215. [DOI] [PubMed] [Google Scholar]
- 46.Mattay VS, et al. , Neurophysiological correlates of age-related changes in working memory capacity. Neuroscience Letters, 2006. 392(1): p. 32–37. [DOI] [PubMed] [Google Scholar]
- 47.Vanderhasselt M-A, De Raedt R, and Baeken C, Dorsolateral prefrontal cortex and Stroop performance: Tackling the lateralization. Psychonomic Bulletin & Review, 2009. 16(3): p. 609612. [DOI] [PubMed] [Google Scholar]
- 48.Dunst B, et al. , Neural efficiency as a function of task demands. Intelligence, 2014. 42(100): p. 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vermeij A, et al. , An exploratory study of the effects of spatial working-memory load on prefrontal activation in low- and high-performing elderly. Frontiers in Aging Neuroscience, 2014. 6(303). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajah MN and D’Esposito M, Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain, 2005. 128(9): p. 1964–1983. [DOI] [PubMed] [Google Scholar]
- 51.Waisbren SE, et al. , Improving long term outcomes in urea cycle disorders-report from the Urea Cycle Disorders Consortium. Journal of inherited metabolic disease, 2016. 39(4): p. 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]