Abstract
Background/Objectives:
Clinical seizures following acute ischemic stroke (AIS) appear to contribute to worse neurologic outcomes. However, the effect of electrographic epileptiform abnormalities (EAs) more broadly is less clear. Here we evaluate the impact of epileptiform abnormalities (EAs), including electrographic seizures, periodic and rhythmic patterns, on outcomes in patients with AIS.
Methods:
This is a retrospective study of all patients with AIS aged ≥18 years who underwent at least 18 hours of continuous EEG (cEEG) monitoring at a single center between 2012 and 2017. EAs were classified according to American Clinical Neurophysiology Society (ACNS) nomenclature, and included seizures, periodic and rhythmic patterns. EA burden for each 24 hour epoch was defined using the following cutoffs: EA presence, maximum daily burden <10% vs. >10%, maximum daily burden <50% vs. >50%, and maximum daily burden using categories from ACNS nomenclature (“rare” <1%; “occasional” 1–9%; “frequent” 10–49%; “abundant” 50–89%; “continuous” >90%). Maximum EA frequency for each epoch was dichotomized into ≥ 1.5 Hz vs. < 1.5 Hz. Poor neurologic outcome was defined as a modified Rankin Scale score (mRS) of 4–6 (vs. 0–3 as good outcome) at hospital discharge.
Results:
143 patients met study inclusion criteria. 67 patients (46.9%) had EAs. 124 patients (86.7%) had poor outcome. On univariate analysis, presence of EAs (OR=3.87 [1.27–11.71], p=0.024), maximum daily burden >10% (OR=12.34 [2.34–210], p=0.001) and > 50% (OR= 8.26 [1.34–122], p=0.035) were associated with worse outcomes. On multivariate analysis, after adjusting for clinical covariates (age, gender, NIHSS, APACHE II, stroke location, stroke treatment, hemorrhagic transformation, Charlson comorbidity index, history of epilepsy), EA presence (OR=5.78 [1.36–24.56], p=0.017), maximum daily burden > 10% (OR=23.69 [2.43–230.7], p=0.006), and maximum daily burden >50% (OR=9.34 [1.01–86.72], p=0.049) were associated with worse outcomes. After adjusting for covariates, we also found a dose-dependent association between increasing EA burden and increasing probability of poor outcomes (OR 1.89 [1.18–3.03] p = 0.009). We did not find an independent association between EA frequency and outcomes (OR: 4.43 [.98–20.03] p=0.053). However, the combined effect of increasing EA burden and frequency ≥ 1.5 Hz (EA burden * frequency) was significantly associated with worse outcomes (OR 1.64 [1.03–2.63] p=0.039].
Conclusion:
Electrographic seizures and periodic and rhythmic patterns in patients with AIS are associated with worse outcomes in a dose dependent manner. Future studies are needed to assess whether treatment of this EEG activity can improve outcomes.
Keywords: ischemic stroke, seizures, epileptiform abnormalities, continuous EEG, outcomes
Introduction
Clinical seizures occur in up to 9% of patients with acute ischemic stroke (AIS) (1), and are associated with increased hospital mortality and worse functional outcomes (2,3). Cortical location and higher stroke severity are risk factors for clinical seizures (2–6). In addition to clinical seizures, electrographic seizures and non-convulsive status epilepticus are reported in 3–9% of patients with AIS undergoing continuous EEG monitoring (1,7,8).
Epileptiform abnormalities (EAs), including not only electrographic seizures but also periodic and rhythmic patterns have been shown to be associated with worse functional outcomes in patients with hemorrhagic stroke (9,10). In these patients, EAs show a dose-dependent relation with outcomes, with a higher burden being associated with worse outcomes (10–12). A metabolic supply-demand mismatch mechanism is hypothesized to underly this apparent effect of EAs on neurologic outcomes; i.e. decreased metabolic reserve of the injured brain, coupled with increased metabolic demand induced by EAs, leads to secondary brain injury (13–15).
To date no study has investigated the impact of EA burden on neurologic outcomes in patients with AIS. We hypothesize that EAs seen in AIS have a negative impact on neurologic outcomes. We also hypothesize that there is a dose dependent relation between EAs and outcomes in patients with AIS, similar to that seen in hemorrhagic stroke. Our objectives here are to 1) characterize the frequency and clinical determinants of EAs in patients with AIS, 2) investigate the impact of EA burden on hospital discharge neurologic outcomes.
Materials and Methods
Study design and inclusion criteria
This is a retrospective cohort study of patients with AIS admitted to the Massachusetts General Hospital between September 2011 and February 2017. The study was conducted under a protocol approved by the Institutional Review Board. Informed consent was not required for this retrospective study. We included all patients aged ≥ 18 years diagnosed with AIS who underwent continuous EEG monitoring (cEEG) for at least 18 hours. Prior work by our group has shown that for patients at risk for seizures, the highest probability for detecting seizures is during the first few hours of recording and decreases to < 5% at 16 hours (16). We therefore chose a minimum duration of 18 hours as inclusion criteria to ensure we capture all patients that could potentially develop EAs. Presence of AIS was confirmed by clinical presentation, computed tomography and/or magnetic resonance imaging (MRI). Patients with hemorrhagic stroke were excluded from the study. Subsequent hemorrhagic transformation of AIS was not an exclusion criterion.
Clinical covariates
Demographic and clinical variables were abstracted from the electronic health record. Clinical covariates included past medical and surgical history, and the Charlson Comorbidity index (CCI). Stroke severity was defined by the National Institutes of Health Stroke Scale (NIHSS). Stroke etiology was categorized using TOAST criteria as: large atherosclerotic, embolic, lacunar, other etiologies, or undetermined (17). Stroke location was determined using imaging. Acute stroke treatment with intravenous thrombolytics or mechanical thrombectomy was recorded. In addition, we recorded development of cerebral edema and hemorrhagic transformation of ischemic stroke. Additional clinical covariates and hospital-acquired complications included: admission Acute Physiology and Chronic Health Evaluation II (APACHE II) score, duration of mechanical ventilation, and hospital acquired infections including hospital acquired pneumonia and catheter associated infections.
cEEG recording and features
Indications for cEEG monitoring included suspected nonconvulsive seizures, altered mental status and unexplained loss of consciousness. All cEEG recordings were obtained using 21 electrodes and the conventional International 10–20 system. Raw EEGs were reviewed and reported clinically by 2 clinical neurophysiologists per institutional protocol. All neurophsyiolgists are board certified in Neurology and have passed the Critical Care EEG Monitoring Research Consortium (CCEMRC) certification test. All EEG findings were reported using the American Clinical Neurophysiology Society nomenclature (ACNS) (18). The relevant EEG data was subsequently abstracted from the clinical EEG reports.
We operationally defined epileptiform abnormalities (EAs) for this study as electrographic seizures and periodic and rhythmic patterns. Periodic and rhythmic patterns were defined using ACNS nomenclature (18), including: lateralized periodic discharges (LPDs), bilateral independent periodic discharges (BIPDs), generalized periodic discharges (GPDs), lateralized rhythmic delta activity (LRDA). We excluded generalized rhythmic delta activity (GRDA) from our definition of EAs because prior studies show, at best, only weak associations with both seizures and functional outcomes (11,19,20). We also excluded sporadic epileptiform discharges from our definition of EAs, because these findings are non-continuous and thus less likely to cause metabolic stress/crisis according to the metabolic supply-demand mismatch hypothesis.
Electrographic seizures were defined as repetitive spikes, sharp waves, sharp-slow wave complexes, or rhythmic activity lasting at least 10 seconds at a frequency of 3Hz or more, or patterns with lower frequencies with evolution in frequency, morphology, or spatial extent (10).
EA burden was abstracted from the EEG reports. We recorded EA burden in each 18–24 hour epoch using ACNS terminology: continuous, >90%; abundant, 50–89%, frequent, 10–49%; occasional, 1–9%; rare <1% (18).
EA burden for each patient was quantified in 2 ways:
Presence: presence of any EAs within any epoch
Maximum daily burden: maximum burden captured within any 18–24 hour epoch
For analysis we examined the following EA cut-offs: EA presence, maximum daily burden <10% vs. >10%, maximum daily burden <50% vs. >50%, and maximum daily burden using an ordinal scale based on the ACNS nomenclature (none, rare, occasional, frequent, abundant, continuous) (18).
We also abstracted the maximum frequency of EAs from the EEG reports. For analysis, we dichotomized maximum frequency into ≥ 1.5 Hz vs. < 1.5 Hz. We used the 1.5 Hz threshold as periodic discharges above this frequency, on scalp recordings, were shown to be associated with lower brain tissue oxygenation (13) and increased risk for seizures (19).
Outcomes
Our primary objective was to assess the impact of EA burden on neurologic outcome at hospital discharge, measured by the modified Rankin Scale (mRS) (21) (mRS 0: no symptoms; mRS 1: no significant disability; mRS 2: slight disability; mRS 3: moderate disability; mRS 4: moderate severe disability; mRS 5: severe disability; mRS 6: dead). mRS was abstracted from physician and physical therapy notes at discharge. Outcomes were abstracted retrospectively and adjudicated by independent reviewers (SFZ, MT, HAN, MS, SK, ME, EB). At the time of outcome abstraction, the reviewers were blinded to the EEG findings. For analysis, we dichotomized outcomes into good (mRS 0–3) vs. poor (mRS 4–6) outcomes.
Statistical analysis
For descriptive statistics, we calculated mean, median and inter quartile ranges. Univariate analysis was performed using Fisher’s exact test for dichotomized and categorical variables, and the Mann-Whitney-U-test for continuous variables. Significance was set at 0.05, and 2-sided P values are reported. We performed multivariate logistic regression analysis to assess the relation between EAs and discharge mRS. We adjusted for baseline variables that are associated with worse neurologic outcomes based on prior studies. These included age, gender, NIHSS, stroke location (cortical vs. subcortical, and temporal vs. extra temporal), anterior vs. posterior circulation stroke, acute stroke treatment and hemorrhagic transformation (22–25). To adjust for critical illness severity and baseline comorbidities we also included the APACHE II score and Charlson Comorbidity Index (CCI), and prior history of epilepsy in our multivariate logistic regression models (22,26). Odds ratios and 95% confidence intervals (OR [95% CI]) were calculated to quantify the association of EAs with outcomes. Goodness of fit for logistic regression models was assessed using the Hosmer-Lemeshow test.
Results
Demographic and clinical variables
During the study period there were on average 836 ischemic stroke admissions per year (range 790–895/year). 143 patients met inclusion criteria. Clinical and demographic variables are summarized in Table 1. There were no missing data. The median age of the cohort was 66 years. 49.7 % of patients were female. The median NIHSS score on admission was 10. Most patients (80.4%, n=119) had cortical strokes. The most common stroke etiology was cardio-embolism (43.4%, n=64).
Table 1.
Clinical and demographic variables.
| All patients (n=143) | EAs present a (n=67) | EAs absent (n=76) | P value | |
| Age: median, (IQR) | 66 (54–77.5) | 67 (56.5–77.8) | 66 (49–77.5) | 0.37 |
| Gender: F (%) | 71 (49.7%) | 34 (50.8%) | 37 (48.7%) | 0.81 |
| Apache II: median, (IQR) | 21 (15–26) | 20 (14.3–25.8) | 21 (15–26) | 0.78 |
| NIHSS: median, (IQR) | 10 (6–17) | 10 (6.3–18.5) | 10.5 (6–16.5) | 0.97 |
| CCI: median, (IQR) | 4 (2–6.8) | 4 (3–7) | 4 (2–6) | 0.18 |
| Stroke Etiology | ||||
| Large Artery | 48 (33.6%) | 19 (28.4%) | 29 (38.2%) | 0.19 |
| Embolic | 62 (43.4%) | 30 (44.8%) | 32 (42.1%) | |
| Small vessel | 6 (4.2%) | 1 (1.5%) | 5 (6.6%) | |
| Other etiology | 12 (8.4%) | 7 (10.5%) | 5 (6.6%) | |
| Cryptogenic | 15 (10.5%) | 10 (14.9%) | 5 (6.6%) | |
| Stroke location | ||||
| Cortical | 115 (80.4%) | 56 (83.6%) | 59 (77.6%) | 0.41 |
| Subcortical | 28 (19.6%) | 11 (16.4%) | 17 (22.4%) | |
| Anterior circulation | 78 (54.6%) | 40 (59.7%) | 38 (50.0%) | 0.43 |
| Posterior circulation | 29 (20.3%) | 11 (16.4%) | 18 (23.7%) | |
| Multiple vascular territories | 36 (25.2%) | 16 (23.9%) | 20 (26.3%) | |
| Temporal | 57 (39.9%) | 33 (49.3%) | 24 (31.6%) | 0.04 |
| Extra-temporal | 86 (60.1%) | 34 (50.8%) | 52 (68.4%) | |
| Thrombolytic treatment/Stroke intervention | 16 (11.2%) | 5 (7.5%) | 11 (14.5%) | 0.29 |
| History of epilepsy | 17 (11.9%) | 11 (16.4%) | 6 (7.9%) | 0.13 |
| Clinical seizure on presentation | 23 (16.1%) | 15 (22.4%) | 8 (10.5%) | 0.07 |
| Clinical seizure during duration of hospital admission | 29 (20.3%) | 21 (31.4%) | 8 (10.5%) | 0.003 |
| cEEG duration in hours: median (IQR) | 44.8 (27.3–71.3) | 59.7 (40.4–86.5) | 39.2 (25.3 – 46.5) | <0.0001 |
| Anti-epileptic drugs | 89 (62.2%) | 56 (83.6%) | 33 (43.4%) | <0.0001 |
| Cerebral edema | 35 (24.5%) | 19 (28.4%) | 16 (21.1%) | 0.34 |
| Hemorrhagic transformation | 21 (14.7%) | 10 (14.9%) | 11 (14.5%) | 1.00 |
| Hemicraniectomy | 12 (8.4%) | 7 (10.5%) | 5 (6.6%) | 0.55 |
| Hospital acquired infections | 63 (44.1%) | 24 (35.8%) | 39 (51.3%) | 0.07 |
| Duration of MV(days): median (IQR) | 3 (0–7) | 4 (0–8) | 2 (0–6) | 0.51 |
| Length of stay (days): Median (IQR) | 14 (7.5–25) | 14 (8–26) | 14 (7–21.5) | 0.27 |
| Discharge mortality | 36 (25.2%) | 20 (29.9%) | 16 (21.1%) | 0.25 |
| Withdrawal of care | 33 (91.7%) | 19/20 (95.0%) | 14/16 (87.5%) | |
EAs excluding GRDA
APACHE II: Acute Physiology and Chronic Health Evaluation II; CCI: Charlson Comorbidity Index; cEEG: continuous electroencephalography; EAs: epileptiform abnormalities; MV: mechanical ventilation; NIHSS: National Institute of Health Stroke Scale
EA incidence and predictors
46.9% of patients (n=67) had EAs on continuous EEG recording. Table 2 summarizes the frequency and distribution of all EEG patterns seen in our patient cohort. Sporadic epileptiform discharges (56.7%, n=81) and GRDA (23.8%, n=34) were the most common findings. 16.8% of the patients (n= 24) had electrographic seizures. Among patients with EAs more than half (58.2%, n=39), had multiple overlapping pattern types (Table 2). The most common isolated patterns were LPDs (14.9%, n=10). We did not see any brief potentially ictal rhythmic or periodic discharges (B(I)RDs). The only multifocal findings we encountered were sporadic discharges. Five patients (3.5%) had multifocal sporadic discharges. Among patients with EAs, 40.3% (n=27) had a maximum daily burden of 50–89% (abundant) as the most common burden category.
Table 2.
Continuous EEG features
| Overall prevalence of EEG patterns | N (% of 143) |
|---|---|
| Electrographic seizures | 24(16.8%) |
| Lateralized periodic discharges (LPDs) | 33 (23.1%) |
| Bilateral independent periodic discharges (BIPDs) | 6 (4.2%) |
| Generalized periodic discharges (GPDs) | 17 (11.9%) |
| Lateralized rhythmic delta activity (LRDA) | 23 (16.1%) |
| Generalized rhythmic delta activity (GRDA) | 34(23.8%) |
| Sporadic discharges | 81(56.7%) |
| Isolated vs. Multiple overlapping patterns | N (% of 67) |
| Isolated electrographic seizures | 4 (6.0%) |
| Isolated LPDs | 10 (14.9%) |
| Isolated BIPDs | 2 (3.0%) |
| Isolated GPDs | 9 (13.4%) |
| Isolated LRDA | 3 (4.5%) |
| Isolated GRDA | 13 (19.4%) |
| Multiple patterns | 39 (58.2%) |
| Maximum daily burden a | N (% of 67) |
| Rare (<1%) | 4 (6.0%) |
| Occasional (1–9%) | 7 (10.4%) |
| Frequent (10–49%) | 16 (23.9%) |
| Abundant (50–89%) | 27 (40.3%) |
| Continuous (>90%) | 13 (19.4%) |
| Maximum frequency | N (% of 67) |
| ≥ 1.5 Hz | 49 (73.1%) |
| < 1.5 Hz | 18 (26.9%) |
18–24 hour epoch with the maximum daily burden of EAs.
EAs: epileptiform abnormalities
Patients with EAs were more likely to have clinical seizures during the admission (31.4% in patients with EAs vs. 10.5% in patients without; OR: 3.89 [1.61–9.32] p = 0.003). Patients with EAs were also more likely to have stroke with temporal lobe involvement (49.3% in patients with EAs vs. 31.6% in patients without; OR: 2.10 [1.07–4.14] = 0.04). Other factors such as APACHE II score, NIHSS score, presence of cerebral edema, etiology, anterior vs. posterior circulation and the development of hospital-acquired infections were not associated with a greater risk for EAs (Table 1).
Outcome Association with EA Burden: Univariate analysis
13.3% (n=19) of patients had good outcome versus 86.7% (n=124) of patients with poor outcome. The discharge mortality rate was 25.2% (n=36). Discharge mortality was similar across both groups, and majority of these patients had withdrawal of life sustaining therapies.
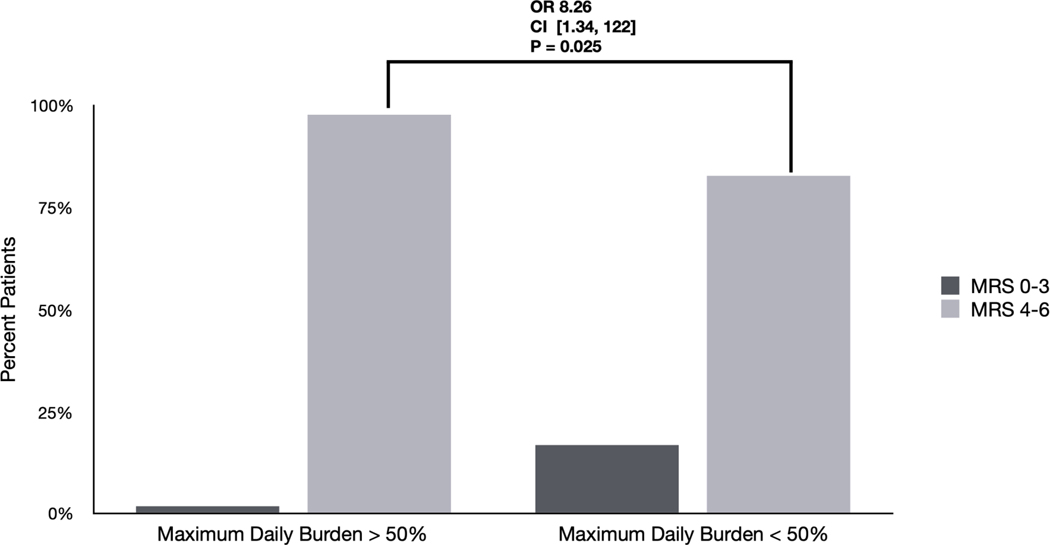
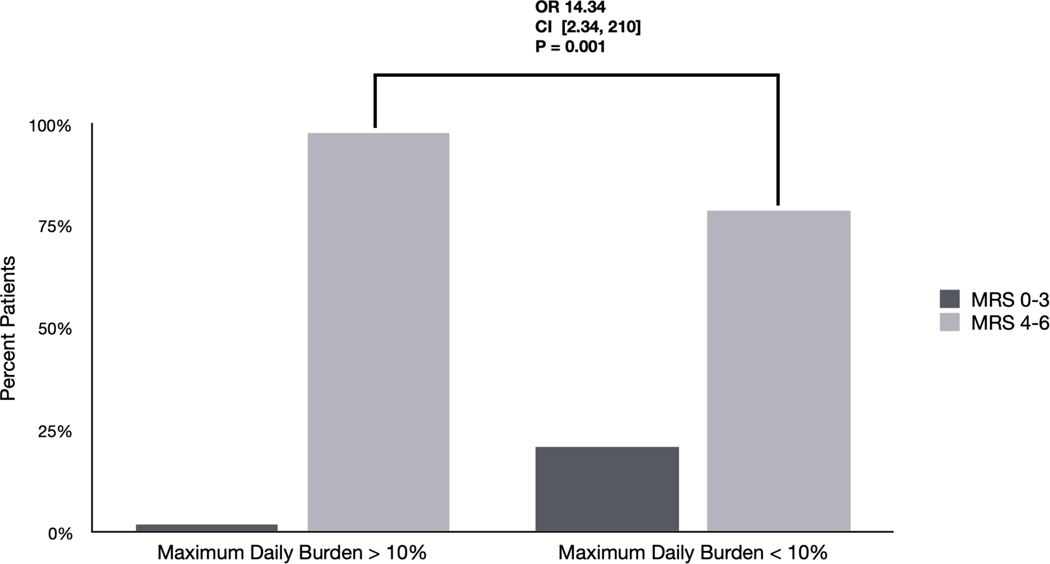
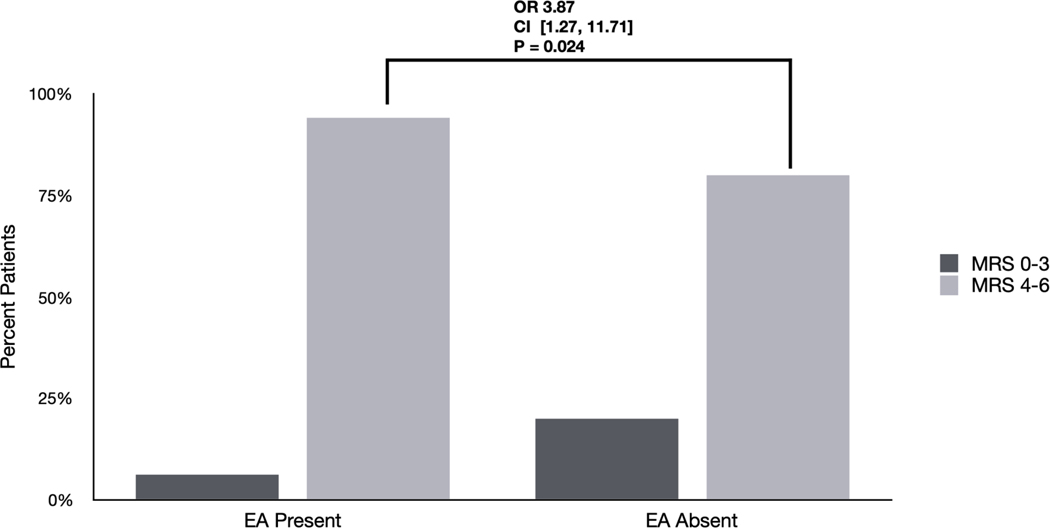
We evaluated univariate association of neurologic outcome with three different notions of EA burden; all three showed significant associations (Figure 1). First, presence of EAs was associated with worse outcome (OR 3.87 [1.27 – 11.71] p=0.024). Second and third, the maximum daily burden of EAs was associated with worse outcomes, whether using a cutoff of EA burden >10% (OR 14.34 [2.34–210] p=0.001) or a cutoff of > 50% (OR 8.23 [1.34 – 122] p=0.025).
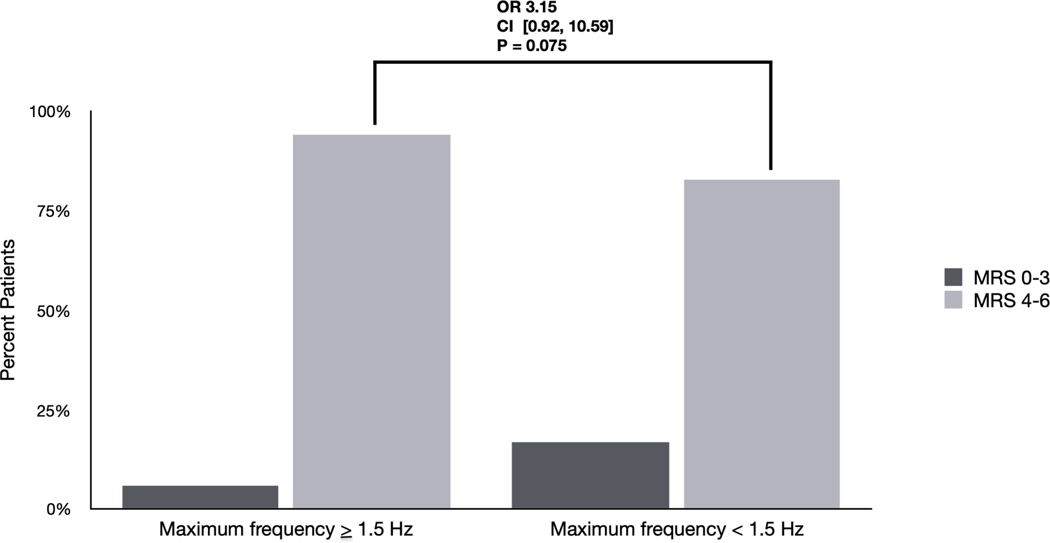
Figure 1. Differences in outcomes based on EA burden and frequency: Univariate analyses.
A) Differences in outcomes between patients with EAs based on presence vs. absence. 94% of patients with EAs had poor outcomes (mRS 4–6) compared with 80 % without EAs (OR 3.87 [1.27 – 11.71] p=0.024).
B) Differences in outcomes among patients with maximum daily burden > 10%. 98% of patients with a maximum daily burden > 10% had poor outcomes (mRS 4–6) compared with 79% of patients with maximum daily burden < 10% (OR 14.34 [2.34–210] p=0.001).
C) Differences in outcomes among patients with EAs based on maximum daily burden >50%. 98 % of patients with a maximum daily burden of >50% had poor outcomes (mRS 4–6) compared with 83% of patients with a maximum daily burden < 50% (OR 8.23 [1.34 – 122] p=0.025).
D) Differences in outcomes among patients with EAs based on maximum frequency ≥ 1.5Hz. 94 % of patients with a maximum frequency ≥ 1.5Hz had poor outcomes (mRS 4–6) compared with 83% of patients with maximum frequency < 1.5 Hz (OR 3.15 [0.92–10.59] p=0.075).
EAs: Epileptiform abnormalities
Outcome Association with EA Burden: Multivariate analysis
We created four multivariate logistic regression models for each of the following EA burden contrasts: EA presence vs absence; maximum daily burden >10% vs <10%; maximum daily burden >50% vs <50%; increasing EA burden on an ordinal scale defined by ACNS nomenclature (none, rare, occasional, frequent, abundant, continuous). The final covariates included were: age, gender, NIHSS score, APACHE II score, CCI, prior history of epilepsy, stroke location (cortical vs. subcortical, and temporal vs. extra temporal), anterior vs. posterior circulation stroke, acute stroke treatment/intervention, and hemorrhagic transformation (21–26).
After adjusting for co-variates, EA presence continued to be significantly associated with worse outcomes (OR=5.78 [1.36–24.56], p=0.017). Similarly, after adjusting for covariates, maximum daily burden > 10% was associated with poor outcomes (OR=23.69 [2.43–230.7], p=0.006), as was maximum daily burden >50% (OR=9.34 [1.01–86.72], p=0.049). Finally, after adjusting for covariates we found that increasing maximum daily burden on an ordinal scale from none to continuous, as defined by ACNS nomenclature, was also associated with worse outcomes (OR 1.89 [1.18–3.03] p = 0.009) (Figure 2).
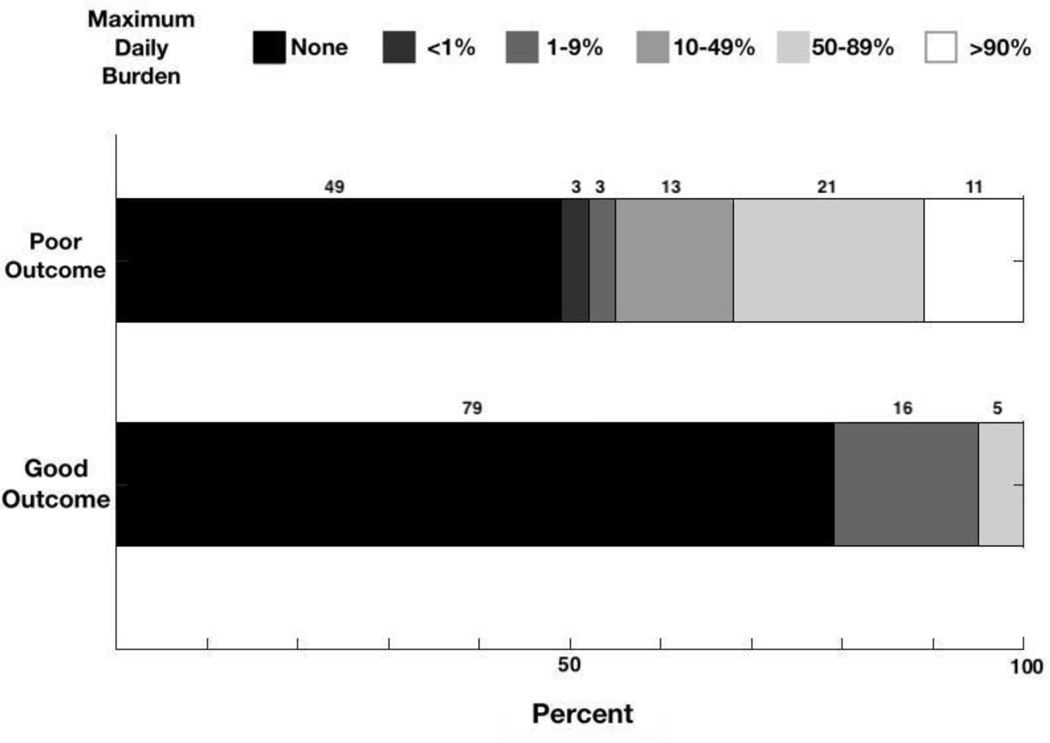
Figure 2. Association of maximum daily EA burden with neurologic outcome.
The proportion of patients within each burden group during the epoch with maximum daily burden is compared between patients with poor versus good outcome. Patients with poor outcomes tend to have higher maximum daily burden. After adjusting for covariates (age, gender, APACHE II, NIHSS, stroke treatment, cortical location, temporal vs. extratemporal stroke, anterior vs. posterior circulation, hemorrhagic transformation, CCI, history of epilepsy) we found that increasing maximum daily burden was associated with worse discharge outcome (OR 1.89 [1.18–3.03] p = 0.009).
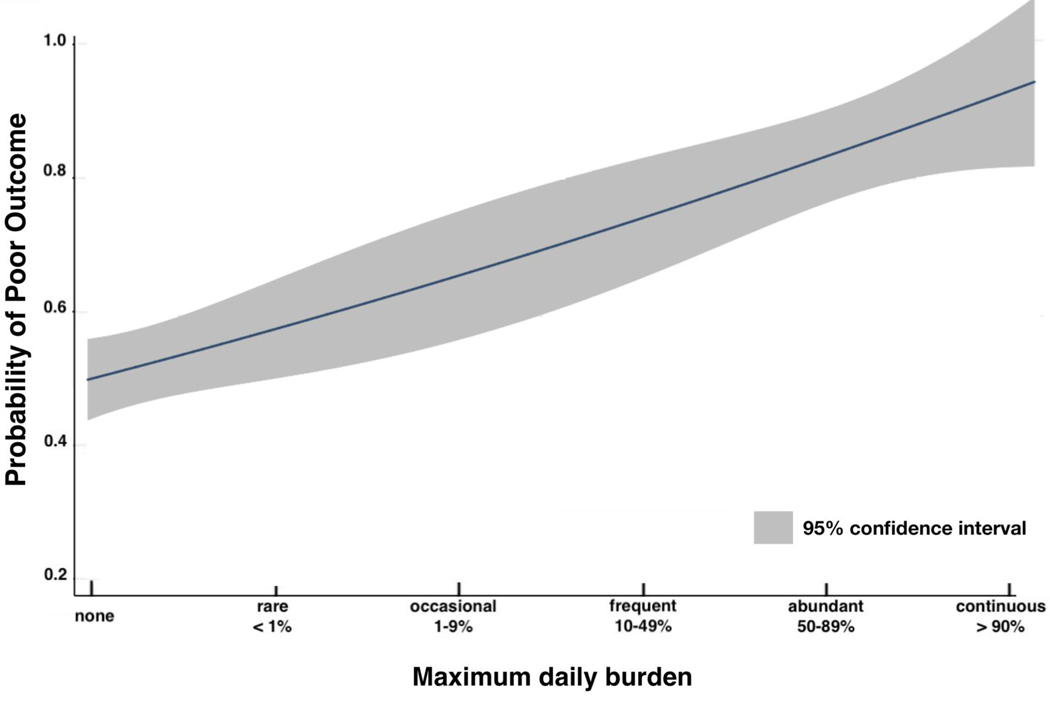
In addition, we estimated the relationship between the probability of poor outcome vs. maximum daily burden as defined by ACNS nomenclature. The plot shown in Figure 3 was created using a weighted multivariate logistic regression, where the weighting was used to account for the small proportion of poor outcomes. The results show a dose-response relationship: increasing maximum daily burden is associated with a monotonically increasing probability of poor outcomes.
Figure 3. Probability of poor outcome with increasing EA burden.
This dose-response prediction plot is obtained from the multivariate logistic regression model assessing the relation of increasing maximum daily burden defined by the ACNS nomenclature (none, rare, occasional, frequent, abundant, continuous), after adjusting for covariates (age, gender, APACHE II, NIHSS, stroke treatment, cortical location, temporal vs. extratemporal stroke, anterior vs. posterior circulation, hemorrhagic transformation, CCI, history of epilepsy). The plot was obtained after application of weights to balance outcomes during the model parameter estimation procedure given the small overall proportion of good outcomes. The probability of poor outcomes increases with increasing maximum daily burden. The shaded area represents the 95% confidence intervals of the model predictions.
Outcome Association with EA Burden excluding mortality: Subgroup analysis
Table 1. shows there was no significant difference in discharged mortality between patients with EAs and those without (OR 1.60 [0.75–3.39], p =0.25). However, almost all these patients had withdrawal of life sustaining therapies (87.5% (14/16) in patients without EAs, and 95% (19/20) in patients with EAs). We therefore did a subgroup analysis assessing the relation of EAs and discharge outcomes excluding mortality. For this subgroup analysis we dichotomized outcomes into good (mRS 0–3) vs. poor (mRS 4–5) outcomes. 91.5% (43/47) patients with EAs had poor outcomes compared with 75% (45/60) patients without EAs (OR: 3.58 [1.15–11.07], p= 0.04). After adjusting for covariates, presence of EAs continued to be significantly associated with poor outcome in this subgroup analysis (OR 6.35 [1.30–31.04] p=0.022). Increasing EA burden also continued to be significantly associated with worse outcomes (OR 1.96 [1.17–3.24] p= 0.01].
Outcome Association with EA maximum frequency and with EA burden*frequency
On univariate analysis, although patients with maximum EA frequency ≥ 1.5 Hz were more likely to have poor outcomes (93.9 % (46/49) vs. 83.0% (78/94) in patients with maximum frequency < 1.5 Hz), this association was not statistically significant (OR 3.15 [0.92–10.59] p=0.075) (Figure 1D). After adjusting for covariates, maximum frequency ≥ 1.5 Hz continued to have a non-significant association with poor outcomes (OR: 4.43 [.98–20.03] p=0.053]. Finally, we created a multivariate logistic regression to assess the combined effect of increasing EA burden and frequency ≥ 1.5 Hz (EA burden * frequency). After adjusting for covariates we found EA burden * frequency was significantly associated with worse outcomes (OR 1.64 [1.03–2.63] p=0.039].
Discussion
Our study demonstrates that EAs can be frequently seen in patients with severe AIS, particularly in patients with clinical seizures during admission and those with strokes that have temporal lobe involvement. In these patients, EAs are associated with an independent negative impact on discharge outcomes. Additionally, EAs exhibit a dose-dependent association with worse outcomes.
We found a higher overall prevalence of EAs including both seizures and other periodic and rhythmic patterns (46.9%) in our patient cohort compared to prior studies that have reported a 3–17% rate of electrographic seizures and other inter-ictal activity in patients with ischemic stroke (8, 27–30). This may be explained by the high overall NIHSS and predominantly cortical strokes in our patient cohort, both of which have both been shown to be associated with a higher risk of seizures (29,30). A higher frequency of continuous EEG utilization (as opposed to short-duration “routine” EEGs) may also explain the higher prevalence of seizures in our study. Finally, we used standardized ACNS terminology and a strict definition of electrographic seizures, compared to prior studies that used variable definitions of seizures and other periodic and rhythmic patterns.
Within our cohort, clinical seizures during hospitalization were strongly associated with a risk of EAs (OR: 3.89 [1.61–9.32] p = 0.003). The association of clinical seizures with electrographic seizures is consistent with prior studies (7). In addition, temporal lobe involvement of strokes was seen more commonly in patients with EAs (OR: 2.10 [1.07–4.14] = 0.04). This is may be explained by the epileptogenicity of the temporal lobe and hippocampal involvement (31). Within our patient cohort there was no significant difference in stroke etiology, and severity, although these have previously been described as risk factors for seizures (8,29,30). This likely results from our patients being sicker, with higher stroke scales and the majority of strokes being cortical.
We found an independent association between EAs and worse outcomes. In addition, we found that increasing EA burden in patients with AIS is associated with worse outcomes. Seizure and EA burden has similarly been associated with worse outcomes in patients with aneurysmal subarachnoid hemorrhage, and critically ill pediatric patients (10,12). In the Columbia Subarachnoid Hemorrhage Outcomes Project (SHOP), each increasing hour of seizures was associated with worse functional and cognitive outcomes at 3 months (10). We recently showed that increasing EA burden (including both seizures and periodic and rhythmic patterns) is associated with worse 3-month functional outcome in patients with aneurysmal subarachnoid hemorrhage (11). In a study of critically ill pediatric patients, a seizure burden threshold of 20% per hour (12 min) was associated with neurologic decline (12).
Interestingly, we did not find a significant association between higher EA frequency and discharge outcomes. However, we did find that the EA burden construct combing burden with frequency (EA burden * frequency) was significantly associated with worse outcomes. We have several potential explanations for these findings: 1) The main driver for association with poor outcomes is the EA burden, 2) Frequency alone may not have an association with outcomes, but higher frequencies may enhance the magnetite of association that EA burden has on outcomes, 3) Our study may be underpowered to assess impact of frequency and a larger study with more detailed frequency analysis beyond our binary categorization is warranted to better understand the association of EA frequency and its interaction with burden and outcomes.
Majority of our patients had multiple pattern types, with a small number of patients with each pattern in isolation. Given increasing recognition that these patterns lie on an ictal-interictal continuum (32), and multiple patterns can be seen in the same patients, we clustered them together. We did exclude GRDA from our definition of EAs due to its weak associations with both seizures and functional outcomes (11,19, 20). Increasing evidence in patients with acute brain injury suggests periodic and rhythmic patterns may contribute to secondary brain injury (13–15). These patterns are associated with PET hypermetabolism similar to that seen in patients with acute seizures (15). Intracranial multi-modal depth monitoring in patients with subarachnoid hemorrhage has revealed brain tissue oxygenation (PbtO2) reduction, increased cerebral blood flow, and increased cerebral perfusion pressure associated with these patterns, particularly at high frequencies (13). Similarly, patients with traumatic brain injury with periodic discharges on depth recordings have evidence of increased brain metabolism, low brain glucose and elevated microdialysis lactate/pyruvate ratios (14). Although much of the intra-cranial monitoring data is from subarachnoid hemorrhage and traumatic brain injury patients, the PET hypermetabolism associated with these patterns, was seen across multiple disease etiologies including ischemic stroke (15). This association with secondary brain injury, increased metabolic demand and blood flow, may explain the association of increasing EA burden, with worse outcomes in our cohort of patients with AIS.
Although we found EAs to be associated in a dose-dependent manner with worse outcomes in patients with AIS, our findings suggest but do not definitively demonstrate that this is a causal relationship (as opposed to an epiphenomenon), nor do our findings necessarily suggest that EAs warrant aggressive treatment. Despite limited data on the management of EAs, specifically periodic and rhythmic patterns (32), we found that majority (83%) of our patients with EAs were treated with anti-seizure drugs (ASDs). Given patients with EAs were significantly more likely to be treated with ASDs, we considered treatment to be a part of the causal pathway and excluded ASDs from the final multivariate models assessing the independent relation between EAs and outcomes. Further investigation of the relationship between EAs, ASD treatment and outcomes is necessary in order to guide appropriate treatment. This is particularly important in light of the variable ASD prescribing patterns in response to EEG findings among physicians caring for patients with acute brain injuries across institutions (33). ASDs themselves have side effects such as cognitive slowing, fatigue, gait unsteadiness, mood symptoms, headaches and drowsiness that can worsen outcomes (34–36). Additionally, some studies suggest that prophylactic ASD use in neuro-critically ill patients may worsen cognitive and functional outcomes (37,38).
Limitations of our study include its retrospective nature and its single-center study design which limit the generalizability of our findings. In addition, the majority of our patients had poor outcomes explaining the large odds ratios in our study, and we did not assess long-term outcomes. While reviewers (SFZ, MT, HAN, MS, SK, ME, EB) were blinded to the EEG findings during outcome adjudication, one of the reviewers (SFZ) was involved in cEEG interpretation during the period 2015–2016 and may have recalled cases. Although all cEEG interpreters were familiar with ACNS nomenclature, there may still be some inter-rater variability. We did not perform a detailed frequency analysis beyond binary categorization, and did not assess other ACNS modifier terms (i.e., “+F, +S, +R”) as these were not consistently reported at our center, and some of these modifiers tend to have a lower inter-rater agreement (39). In addition, our patients had higher stroke severity, and large number of patients with cortical involvement that limits the generalizability of our findings to all patients with ischemic stroke. Nevertheless, ours is the first study to perform a detailed initial analysis of the relationship between EA burden and neurologic outcomes in patients with AIS.
Conclusions
In conclusion, we found that EAs are associated with worse discharge outcomes in patients with AIS that have higher severity and cortical involvement. Current stroke guidelines provide a weak recommendation for EEG monitoring in patients with AIS (40), and our findings suggest there is a subgroup of patients in whom cEEG monitoring should be considered more often. Our findings support the need for larger multi-center prospective studies to investigate the long-term impact of EAs in patients with AIS. Future randomized studies are indicated to determine whether treatment of these EEG patterns improves outcomes.
STROBE Statement—checklist of items that should be included in reports of observational studies
| Item No. | Recommendation | Page No. | Relevant text from manuscript | |
|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study‘s design with a commonly used term in the title or the abstract | 3 | This is a retrospective study of all patients with AIS aged ≥18 years who underwent at least 18 hours of continuous EEG (cEEG) monitoring at a single center between 2012 and 2017. |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 3 | |||
| Introduction | ||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 5 | Here we characterize the frequency and clinical determinants of EAs in patients with AIS and investigate the impact of EA burden on hospital discharge neurologic outcomes. |
| Methods | ||||
| Study design | 4 | Present key elements of study design early in the paper | 5 | This is a retrospective study of patients with AIS admitted to the Massachusetts General Hospital between September 2011 and February 2017. |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 5 | This is a retrospective cohort study of patients with AIS admitted to the Massachusetts General Hospital between September 2011 and February 2017. |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-controlstudy—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
6 and 7 | We included all patients aged ≥ 18 years diagnosed with AIS who underwent continuous EEG monitoring (cEEG) for at least 18 hours. Presence of AIS was confirmed by clinical presentation, computed tomography and/or magnetic resonance imaging (MRI). Patients with hemorrhagic stroke were excluded from the study. Subsequent hemorrhagic transformation of AIS was not an exclusion criterion. Demographic and clinical variables were abstracted from the electronic health record. All cEEG recordings were obtained using 21 electrodes and the conventional International 10-20 system. Raw EEG data was reviewed and reported clinically by 2 clinical neurophysiologists per institutional protocol. EEG reports were updated at least twice daily. The relevant EEG data was subsequently abstracted from the clinical EEG reports. |
|
(b) Cohort study— For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case |
||||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 6,7, 8 | Predictors as detailed above. Our primary objective was to assess the impact of EA burden on neurologic outcome at hospital discharge, measured by the modified Rankin Scale (mRS) (20) (mRS 0: no symptoms; mRS 1: no significant disability; mRS 2: slight disability; mRS 3: moderate disability; mRS 4: moderate severe disability; mRS 5: severe disability; mRS 6: dead). mRS was abstracted from physician and physical therapy notes at discharge. For analysis, we dichotomized outcomes into good (mRS 0–3) vs. poor (mRS 4–6) outcomes. |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 6,7,8 | |
| Bias | 9 | Describe any efforts to address potential sources of bias | 11 | |
| Study size | 10 | Explain how the study size was arrived at | 6 | We included all patients aged ≥ 18 years diagnosed with AIS who underwent continuous EEG monitoring (cEEG) for at least 18 hours |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 7,8 | We recorded EA burden in each 18–24 hour epoch using ACNS terminology: continuous, >90%; abundant, 50–89%, frequent, 10–49%; occasional, 1–9%; rare <1% (17). EA burden for each patient was quantified in 2 ways: 1) Presence: presence of any EAs within any epoch 2) Maximum daily burden: maximum burden captured within any 18–24 hour epoch For analysis we examined the following EA cut-offs: EA presence, maximum daily burden <10% vs. >10%, maximum daily burden <50% vs. >50%, and maximum daily burden using an ordinal scale based on the ACNS nomenclature (none, rare, occasional, frequent, abundant, continuous) (11, 17). |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 8,9 | |
| (b) Describe any methods used to examine subgroups and interactions | ||||
| (c) Explain how missing data were addressed | 9 | There was no missing data | ||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study— If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
||||
| (e) Describe any sensitivity analyses | ||||
| Results | ||||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 9 | |
| (b) Give reasons for non-participation at each stage | ||||
| (c) Consider use of a flow diagram | ||||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 9,10 | |
| (b) Indicate number of participants with missing data for each variable of interest | ||||
| (c) Cohort study—Summarise follow-up time (eg, average and total amount) | ||||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | 10 | 13.6% (n=20) of patients had good outcome versus 86.4% (n=127) of patients with poor outcome. The discharge mortality rate was 24.5% (n=36). |
| Case-control study—Report numbers in each exposure category, or summary measures of exposure | ||||
| Cross-sectional study—Report numbers of outcome events or summary measures | ||||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted 10,11 estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 10,11 | 13.6% (n=20) of patients had good outcome versus 86.4% (n=127) of patients with poor outcome. The discharge mortality rate was 24.5% (n=36). We evaluated univariate association of neurologic outcome with three different notions of EA burden; all three showed significant associations (Figure 1). First, presence of EAs was associated with worse outcome (OR=3.14 [1.11– 8.82], p=0.029). Second and third, the maximum daily burden of EAs was associated with worse outcomes, whether using a cutoff of EA burden >10% (OR=7.32 [1.63–32.91], p=0.003) or a cutoff of > 50% (OR= 8.74 [1.13–67.55], p=0.014). Outcome Association with EA Burden: Multivariate analysis We created four multivariate logistic regression models for each of the following EA burden contrasts: EA presence vs absence; maximum daily burden >10% vs <10%; maximum daily burden >50% vs <50%; increasing EA burden on an ordinal scale defined by ACNS nomenclature (none, rare, occasional, frequent, abundant, continuous). The final covariates included were: age, gender, NIHSS score, APACHE II score, CCI, stroke location and hemorrhagic transformation (21–24). After adjusting for co-variates, EA presence continued to be significantly associated with worse outcomes (OR=3.74[1.14–12.27], p=0.029). Similarly, after adjusting for covariates, maximum daily burden > 10% was associated with poor outcomes OR=8.61 [1.74–42.55], p=0.008), as was maximum daily burden >50% (OR=8.38 [1.01–69.58], p=0.049). Finally, after adjusting for covariates we found that increasing maximum daily burden on an ordinal scale from none to continuous, as defined by ACNS nomenclature, was also associated with worse outcomes (OR 1.61 [1.11–2.35] p:0.013) (Figure 2.). In addition we estimated the relationship between the probability of poor outcome vs. maximum daily burden as defined by ACNS nomenclature. The plot shown in Figure 3 was created using a weighted multivariate logistic regression, where the weighting was used to account for the small proportion of poor outcomes. The results show a dose-response relationship: increasing maximum daily burden is associated with a monotonically increasing probability of poor outcomes. |
| (b) Report category boundaries when continuous variables were categorized | ||||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | ||
| Discussion | ||||
| Key results | 18 | Summarise key results with reference to study objectives | 13,14 | Our study demonstrates that EAs occur frequently seen in AIS, particularly in patients with clinical seizures during admission, and have an independent negative impact on discharge outcomes. Additionally, EAs exhibit a dose- dependent association with worse outcomes. |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 17 | Limitations of our study include its retrospective nature and its single-center study design which limit the generalizability of our findings. In addition, the majority of our patients had poor outcomes, and we did not assess long-term outcomes. Nevertheless, ours is the first study to perform a detailed initial analysis the relationship between EAs and neurologic outcomes in patients with AIS. |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 15–16 | In conclusion, we found that EAs are associated with worse discharge outcomes in patients with AIS. Current stroke guidelines provide a weak recommendation for EEG monitoring in patients with AIS (35). Our findings support the need for larger multi-center prospective studies to investigate the long-term impact of EAs in patients with AIS. Future randomized studies are indicated to determine whether treatment of these EEG patterns improves outcomes. |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 17 | Limitations of our study include its retrospective nature and its single-center study design which limit the generalizability of our findings. |
| Other information | ||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 2 | |
Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies.
Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Acknowledgments
Dr. Tabaeizadeh has nothing to disclose; Dr. Aboul Nour has nothing to disclose; Dr. Shoukat has nothing to disclose; Dr. Sun has nothing to disclose; Dr. Jin reports grants from Sage Therapeutics, during the conduct of the study; Dr. Javed has nothing to disclose; Dr. Kassa has nothing to disclose; Dr. Edhi has nothing to disclose; Dr. Bordbar has nothing to disclose; J. Gallagher has nothing to disclose; V Moura has nothing to disclose; M. Ghanta has nothing to disclose; YP Shao has nothing to disclose; Dr. Cole reports grants from Sage Therapeutics, during the conduct of the study; Dr. Rosenthal reports grants from Sage Therapeutics, during the conduct of the study; Dr. Westover reports grants from Sage Therapeutics, grants from NIH-NINDS 1K23NS090900, grants from NIH-NINDS1R01NS102190, during the conduct of the study; Dr. Zafar reports grants from Sage Therapeutics, grants from NIH-NINDS 1K23NS114201 during the conduct of the study.
Footnotes
The STROBE reporting checklist was used in manuscript preparation.
This manuscript complies with all instructions to authors.
The authorship requirements have been met and the final manuscript was approved by all authors.
This manuscript has not been published elsewhere and is not under consideration by another journal.
This manuscript adheres to ethical guidelines. The study was conducted under a protocol approved by the Institutional Review Board. Informed consent was not required for this retrospective study.
References
- 1.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109:506–23. [DOI] [PubMed] [Google Scholar]
- 2.Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Heal Risk Manag. 2008;4:715–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CW, Saposnik G, Fang J, Steven DA BJ. Seizures Worsen Stroke Outcome : New Evidence From a Large Sample. Epilepsy Curr. 2014;15:30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesser RP, Luders H, Dinner DSMH. Epileptic seizures due to thrombotic and embolic cerebrovascular disease in older patients. Epilepsia. 1985;26:622–30. [DOI] [PubMed] [Google Scholar]
- 5.Hornig CR, Buttner T, Hufnagel A, Schrider-Rosenstock K DW. Epileptic seizures following ischemic cerebral infarction: clinical picture, CT findings, and prognosis. EurArchPsychiatry NeurolSci. 1990;239:379–83. [DOI] [PubMed] [Google Scholar]
- 6.Burneo JG, Fang J, Saposnik G. Impact of seizures on morbidity and mortality after stroke: A Canadian multi-centre cohort study. Eur J Neurol. 2010;17:52–8. [DOI] [PubMed] [Google Scholar]
- 7.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. [DOI] [PubMed] [Google Scholar]
- 8.Belcastro V, Vidale S, Gorgone G, Pisani LR, Sironi L, Arnaboldi M, et al. Non-convulsive status epilepticus after ischemic stroke: a hospital-based stroke cohort study. J Neurol. 2014;261:2136–42. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J, Jetté N, Chum F, Green R, Schmidt M, Choi H, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65. [DOI] [PubMed] [Google Scholar]
- 10.De Marchis GM, Pugin D, Meyers E, Velasquez A, Suwatcharangkoon S, Park S, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zafar SF, Postma EN, Biswal S, Boyle EJ, Bechek S, O’Connor K, et al. Effect of epileptiform abnormality burden on neurologic outcome and antiepileptic drug management after subarachnoid hemorrhage. Clin Neurophysiol. 2018;129:2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne ET, Zhao XY, Frndova H, McBain K, Sharma R, Hutchison JS, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic Correlates of the Ictal-Interictal Continuum: FDG-PET During Continuous EEG. Neurocrit Care. 2016;24:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westover MB, Shafi MM, Bianchi MT, Moura LM, O’Rourke D, Rosenthal ES, et al. The probability of seizures during EEG monitoring in critically ill adults. Clinical Neurophysiology. 2015;126:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch LJ, Laroche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz AR, Vlachy J, Lee JW, Gilmore EJ, Ayer T, Haider HA, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181–8. [DOI] [PubMed] [Google Scholar]
- 20.Kim JA, Rosenthal ES, Biswal S, Zafar S, Shenoy A V., O’Connor KL, et al. Epileptiform abnormalities predict delayed cerebral ischemia in subarachnoid hemorrhage. Clin Neurophysiol. 2017;128:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. Journal of Neurology, Neurosurgery & Psychiatry. 1991;54:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rost NS, Bottle A, Lee JM, Randall M, Middleton S, Shaw L, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. Journal of the American Heart Association. 2016;5:e002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst M, Boers AM, Forkert ND, Berkhemer OA, Roos YB, Dippel DW, et al. Impact of ischemic lesion location on the mRS score in patients with ischemic stroke: a voxel-based approach. American Journal of Neuroradiology. 2018;39:1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommer P, Posekany A, Serles W, Marko M, Scharer S, Fertl E, et al. Is Functional Outcome Different in Posterior and Anterior Circulation Stroke?. Stroke. 2018. November;49(11):2728–32. [DOI] [PubMed] [Google Scholar]
- 25.van Kranendonk KR, Treurniet KM, Boers AMM, Berkhemer OA, van den Berg LA, Chalos V, et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg. 2019;11:464–468. [DOI] [PubMed] [Google Scholar]
- 26.Moon BH, Park SK, Jang DK, Jang KS, Kim JT, Han YM. Use of APACHE II and SAPS II to predict mortality for hemorrhagic and ischemic stroke patients. Journal of Clinical Neuroscience. 2015;22:111–5. [DOI] [PubMed] [Google Scholar]
- 27.Mecarelli O, Pro S, Randi F, Dispenza S, Correnti A, Pulitano P, et al. EEG patterns and epileptic seizures in acute phase stroke. Cerebrovasc Dis. 2011;31:191–8. [DOI] [PubMed] [Google Scholar]
- 28.Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, et al. Acute seizures after intracerebral hemorrhage: A factor in progressive midline shift and outcome. Neurology. 2003;60:1441–6. [DOI] [PubMed] [Google Scholar]
- 29.Reith J, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Seizures in acute stroke: Predictors and prognostic significance: The copenhagen stroke study. Stroke. 1997;28:1585–9. [DOI] [PubMed] [Google Scholar]
- 30.Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Cote R, et al. Background: Seizures After Stroke, A Prospective Multicenter Study. Arch Neurol 2000; 57:1617–22. [DOI] [PubMed] [Google Scholar]
- 31.Myint PK, Staufenberg EF, Sabanathan K. Post-stroke seizure and post-stroke epilepsy. Postgraduate medical journal. 2006;82:568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivaraju A, Gilmore EJ. Understanding and managing the ictal-interictal continuum in neurocritical care. Current treatment options in neurology. 2016;18:8. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez V, Ruiz AA, LaRoche S, Hirsch LJ, Parres C, Voinescu PE, et al. The use and yield of continuous EEG in critically ill patients: A comparative study of three centers. Clinical Neurophysiology. 2017;128:570–8. [DOI] [PubMed] [Google Scholar]
- 34.Brodie MJ, Richens A YA. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trail Group. Lancet. 1995;345:476–9. [DOI] [PubMed] [Google Scholar]
- 35.Perucca P, Carter J, Vahle VGF. Adverse antiepileptic drug effects Toward a clinically and neurophysiologically relevant taxonomy. Neurology. 2009;72:1223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker GA, Jacoby A, Buck D, Stalgis CMD. Quality of life of people with epilepsy: a European study. Epilepsia. 1997;38:353. [DOI] [PubMed] [Google Scholar]
- 37.Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36:583–7. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SJ, Joo YJ, Kim YB, Hong CK CJ. Effects of prophylactic antiepileptic drugs on clinical outcomes in patients with a good clinical grade suffering from aneurysmal subarachnoid hemorrhage. J cerbrovascular Endovasc neurosurgery. 2015;17:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB, Critical Care EEG Monitoring Research Consortium. Interrater agreement for critical care EEG terminology. Epilepsia. 2014;55:1366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claassen J, Taccone FS, Horn P, Holtkamp M, Stocchetti N, Oddo M. Recommendations on the use of EEG monitoring in critically ill patients: Consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–51. [DOI] [PubMed] [Google Scholar]