Abstract
Rationale and Objectives
Pathologic complete response (pCR) in patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer after HER2-targeted therapy correlates increased disease-free survival and decreased mastectomy rates. The aim of this study was to explore tumor shrinkage patterns and initial tumor enhancement with pCR in HER2-positive breast cancer.
Materials and Methods
This was an institutional review board-approved retrospective analysis of 51 HER2 positive breast cancer patients with breast MRI both pre- and post-HER2-targeted therapy. IER (initial enhancement percentage over baseline at first post-contrast imaging), pattern of tumor shrinkage, and DCE-MRI imaging features were assessed. Wilcoxon rank, Spearman correlation, Fisher’s exact and Mann-Whitney tests were used to correlate MRI imaging features with pCR. IER reader agreement was evaluated by intraclass correlation. Binary logistic regression was used to evaluate multivariate associations with pCR.
Results
56.9% (29/51) of patients had pCR at surgery. Concentric tumor shrinkage pattern was associated with pCR (p=0.001, AUC 0.778): accuracy 80.4%, specificity 96.6% and sensitivity of 59.1%. There was no association with pCR and imaging response as defined by RECIST criteria (p=0.169), pre-treatment IER (Reader 1 (R1) p=0.665, Reader 2 (R2) p=0.766), or lesion size (p=0.69). IER was associated with axillary metastases (R1 p=0.016, R2 <0.001) and ki-67 (R1 r=0.52, p=0.008, R2 r=−0.44, p=0.028).
Conclusion
The shrinkage pattern of HER2-positive tumors after targeted therapy may be associated with pCR. There was no association between IER and pCR. Future studies evaluating the correlation of shrinkage patterns to texture radiomics are of interest.
Keywords: breast cancer, magnetic resonance imaging, pathologic complete response, HER2
Introduction
The identification and early targeted treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer has increased disease-free survival and overall survival benefit (1). Pre-surgical initiation of HER2-receptor targeted monoclonal antibodies decreases tumor burden, increases pathologic complete response (pCR) and allows for minimally invasive breast cancer resection instead of mastectomy in patients who have documented decrease in index cancer size (2,3).
PCR is a well-established early prognostic factor for HER2-positive breast cancer therapy. Patients who demonstrate pCR after HER2-targeted treatment have shorter post-operative chemotherapy times, decreased local recurrence and increased survival times (4). Early identification of candidates who are not likely to achieve pCR is therefore important as these patients could benefit from changes to their initial neoadjuvant chemotherapy (NAC) regimens. Similarly, early identification of patients who are likely to achieve pCR may decrease surgical interventions; clinical trials are underway for HER2-positive and triple negative breast cancer patients in which breast cancer resection is omitted if post-treatment imaging-guided core biopsy detects no residual invasive cancer or ductal carcinoma in situ (DCIS) (5). Early imaging identification of pCR is therefore of increasing clinical importance.
Breast MRI is more accurate than ultrasound or mammography for the staging and initial evaluation of breast cancer. While breast MRI is also more accurate than ultrasound and mammography in determining response to chemotherapy, MRI imaging response as measured by RECIST criteria or more recently, by change in tumor volume (6) is less accurate than pre-treatment assessment and often underestimates residual disease (7–9). Although tumor volume change is the most accurate metric to date, it is a time-consuming and difficult assessment to make, particularly in patients with multifocal disease, extensive nonmass enhancement, or both. Multiparametric estimates of disease response including first- and second-order texture features, perfusion kinetic parameters and diffusion-weighted imaging (10–13) can improve the estimation of pCR, but require specialized equipment and may also incur additional scan time or post-processing time.
Recent studies have shown that initial enhancement on pre-chemotherapy images and patterns of tumor shrinkage on post-chemotherapy imaging may correlate with pCR at time of surgical excision (14–17). However, few studies have evaluated these factors solely in HER2-enriched breast cancers; tumor shrinkage in particular has only been demonstrated in luminal cancers (14) and not reproduced in a mixed population of breast cancers (17). The purpose of our study was therefore to explore and evaluate the association of tumor shrinkage and initial tumor enhancement with pathologic complete response in HER2-positive breast cancer.
Materials and Methods
This study was Institutional Review Board approved and Health Insurance Portability and Accountability Act compliant. As this was a retrospective review of data, requirements for informed consent were waived.
Subjects
All patients who underwent both initial and post-treatment, pre-surgical breast MRI at our institution between January 1 2010 and January 1, 2018 with HER2-positive breast cancer were evaluated for inclusion in our study. 107 consecutive patients with HER2-positive breast cancer were identified; 56 were excluded because they did not receive HER2-targeted therapy prior to surgery. The final cohort included 51 women (mean age 50.9, range 25.4–87.7) with HER2-positive invasive cancer.
Treatment
All patients underwent HER2-targeted treatment after initial diagnosis by fine needle aspiration or ultrasound-guided core biopsy. Patients were offered trastuzumab, pertuzumab, or a combination of the two. Patients who were estrogen-receptor positive were also offered taxane-based therapy. Patients completed an average of 12 weeks therapy (minimum 6 weeks, maximum 24 weeks) before follow-up MRI.
Pathologic Assessment
All cancers were biopsy-proven and pathologically assessed at our intuition by one of three dedicated breast pathologists. Pathologic verification included tumor type (ductal, lobular or otherwise) and tumor grade (by Nottingham histologic score). Estrogen receptor (ER) and progesterone (PR) status was considered positive if 1% or more of the tumor cells showed nuclear staining at immunohistochemistry analysis as per American Society of Clinical Oncology/College of American Pathologists criteria. HER2 status was considered positive if the immunohistochemistry result was 3+ (positive) or 2+ (borderline) and confirmed by fluorescence in situ hybridization testing. Ki-67 was recorded as a scale measurement from 1–100% and considered high if greater than 14% per St. Gallen criteria. (18) Axillary nodal metastasis was defined as positive if image-guided biopsy was positive for malignancy prior to therapy initiation.
There is currently no clear consensus on the definition of pCR after neoadjuvant therapy. At our institution we define pCR as ypT0/ypTis by TNM staging, which considers ductal carcinoma in situ at surgery (ypTis) as consistent with pCR, and any micro-invasive (ypT1mi) or larger cancer in the surgical bed as not consistent with pCR. Final pathology at time of surgery was noted from retrospective chart review.
MRI Acquisition
All women underwent bilateral breast DCE-MRI on a 3.0T magnet (TimTrio, Siemens Medical Solutions, Erlangen, Germany) in the prone position using a dedicated surface breast coil (7-Channel Breast Biopsy Array; InVivo Research, Gainesville, FL). The diagnostic protocol at our institution consisted of sagittal T1-weighted gradient echo, sagittal T2-weighted, sagittal T1-weighted gradient echo fat-suppressed volume-interpolated breath-hold exam (VIBE) pre- and three postcontrast acquisitions beginning 70 seconds postinjection of Gd-DTPA (Magnevist, Bayer Healthcare, Leverkusen, Germany) at 0.1 mM/kg body weight at 2 mL/s via an intravenous catheter followed by saline flush. Each VIBE acquisition time was 100 seconds, with a total DCE-MRI imaging time of 35 minutes. T1-weighted imaging parameters included: repetition time / echo time (TR/TE) = 4.74/1.79 msec, flip angle 10°, slice thickness 1.0 mm, matrix 384 × 384, field of view (FOV) 320 mm. Subtraction images were automatically generated at the workstation by subtraction of the precontrast images from the first post-contrast images on a pixel-by-pixel basis.
Data Analysis
Initial Enhancement Ratio
First post-contrast images acquired at 70 seconds postinjection were used for lesion analysis. A commercially available software (DynaCAD, InVivo) was used to measure the initial enhancement ratio (IER; % signal increase over baseline at first postcontrast acquisition). IER was evaluated by two independent readers blinded to treatment response (XX and YY 3 years’ experience each) by analyzing signal intensity changes in representative voxels on first postcontrast subtraction images. Readers used a semi-automatic segmentation technique to select a whole lesion region of interest (ROI) on a reader-selected slice of interest, excluding regions of necrosis and manually editing the generated ROI if necessary. The software then automatically selects the voxel with the highest percentage increase in signal intensity within that ROI. This value was recorded as the IER, as previously described.(15)
Concentric shrinkage
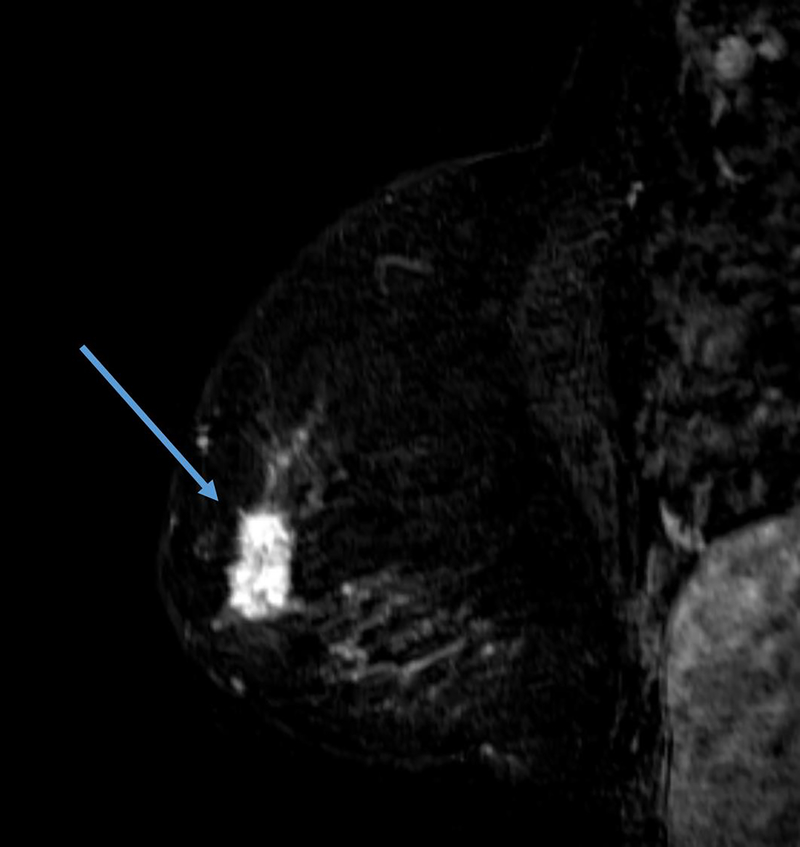
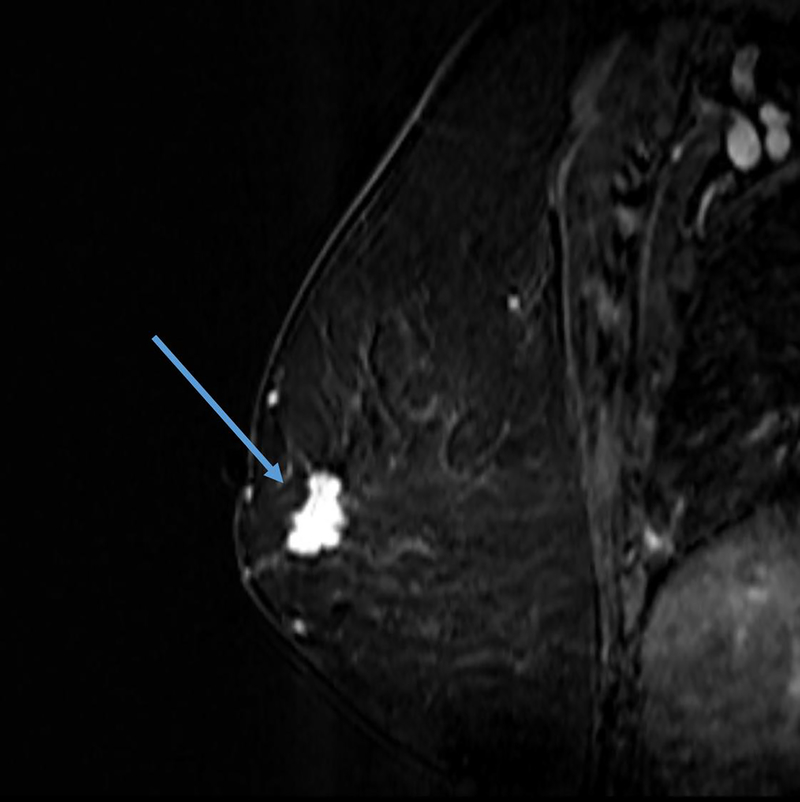
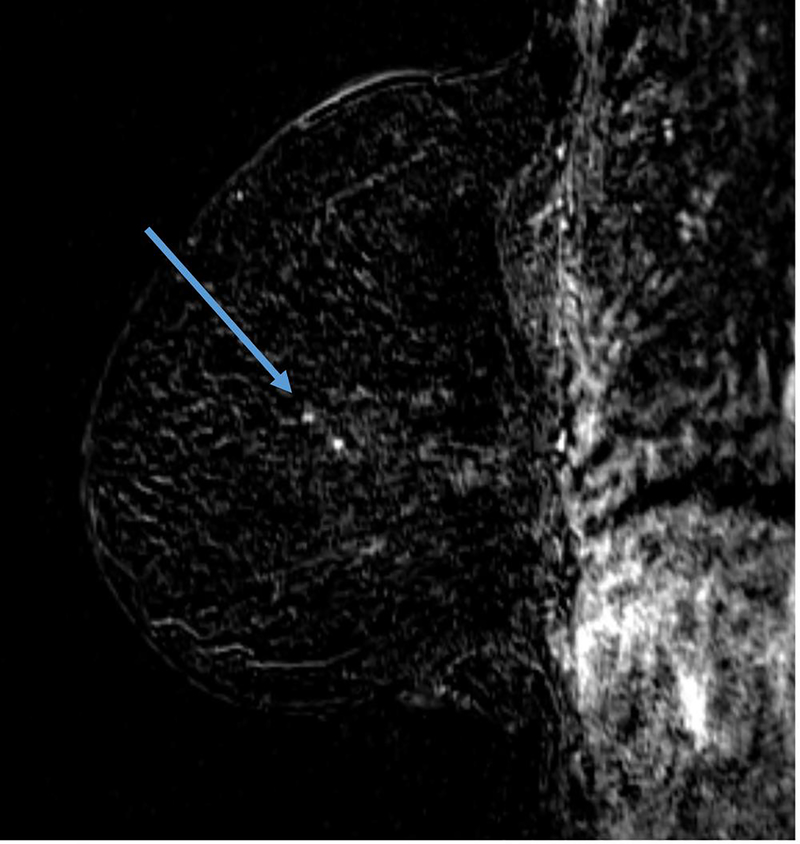
The definition of concentric shrinkage was determined according to Fukada et al who defined concentric shrinkage as a “simple concentric shrinkage” or “concentric shrinkage to small foci” and all else as “non-concentric.”(14) Similar to Fukada et al the decision was made to collapse these categories into concentric and non-concentric shrinkage due to the need for sufficient sample size (Figure 1).
Figure 1.
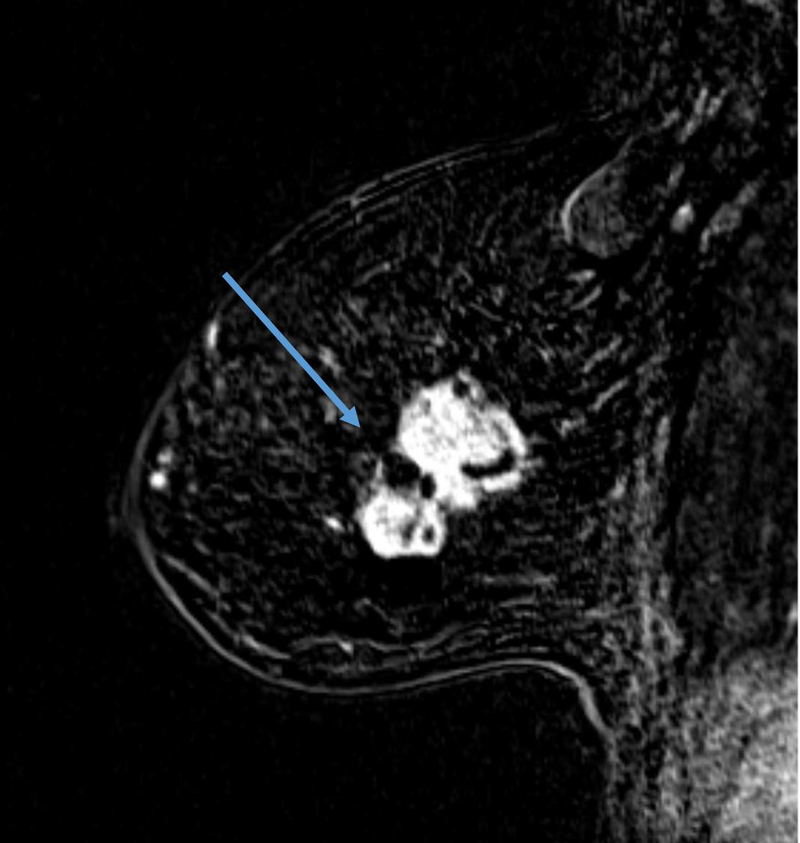
A, B. 61-year-old woman with Stage IIIA hormone receptor positive HER2-positive breast cancer. Non-concentric shrinkage was noted at post-treatment MRI after six cycles taxane-based chemotherapy and trastuzumab. There was no pCR at breast conservation therapy.
C and D. 48-year-old woman with hormone positive HER2-positive Stage II cancer. Concentric shrinkage was noted at post-treatment MRI after five cycles taxane-based chemotherapy and trastuzumab. PCR was noted at breast conservation therapy.
Background parenchymal enhancement
Contralateral and peritumoral background parenchymal enhancement (BPE) were assessed qualitatively on the first post-contrast subtraction image by the original reader of the clinical study and reassessed by a second reader (XX, 3 years’ experience). Differences were resolved by consensus. Peritumoral BPE was defined as the qualitative BPE measured within 5 mm from the tumor margin, as previously described.(19) BPE was graded on a 1–4 scale (minimal, mild, moderate, and marked) corresponding to MRI BI-RADS criteria.
Statistical Analysis
Patients with and without pCR were compared in terms of the IER measurements from each reader using an exact Mann-Whitney test. Associations involving nominal categorical factors were assessed using the chi-square or Fisher’s exact test. Bivariate associations of IER were assessed using the Spearman rank correlation.Reader agreement for IER was assessed in terms of the single rater intra-class correlation coefficient (ICC) for absolute agreement based on a two-way mixed effects model and the coefficient of reproducibility from a Bland-Altman analysis. The single rater ICC assesses the reliability of the individual measurements provided by independent readers rather than the reliability of mean values over the readers. Denoting the lower limit of a 95% confidence interval (CI) for the ICC as L, the ICC was interpreted as an indication of agreement that was poor if L≤0.5, moderate for 0.5<L<0.75, good for 0.75≤L<0.9 and excellent for L≥0.9.
For sensitivity and specificity analysis of concentric shrinkage, residual invasive cancer at surgery was considered a positive result and pCR was considered a negative result. As the hypothesis was that presence of concentric shrinkage is associated with pCR, a true positive was defined as the absence of concentric shrinkage with residual tumor at surgery and a true negative was defined as the presence of concentric shrinkage with pCR at surgery. Additional binary logistic regression analysis was performed to evaluate associations between pCR and categorical covariates. All categorical covariates were assessed for multicolinearity utilizing the variance inflation factor. Differences in all statistical tests were conducted at the two-sided 5% significance level using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient population
51 women (mean age 50.9, range 25.4–87.7) imaged between January 2011 and December 2018 had breast MRI performed at our institution and received HER2-targeted therapy. Patients who were ER/PR-positive on initial biopsy (27/51, 52.9%) also received taxane-based chemotherapy. Of these 51 women, 47 patients had both pre- and post-NAC breast MRI performed prior to surgery and 4 patients received HER2-targeted therapy after a Stage IV diagnosis (Table 1). As these four Stage IV patients had potentially resectable metastatic disease and planned to undergo primary breast surgery at our institution if metastases resolved during therapy (25%, 1/4), they were included in the data analysis. Of the 51 patients, 56.9% (29/51) had pCR at time of surgery.
Table 1:
Tumor and MRI imaging characteristics (n=51 patients). There was no significant difference between these characteristics in patients with or without pathologic complete response (all p>0.05). NME = nonmass enhancement.
| Features | N=51 |
|---|---|
| Mean tumor size | 4.32 cm (range 1.2–11.0 cm) |
| Pathology | |
| Invasive ductal carcinoma | 51/51 (100%) |
| Grade | |
| I | 0/51 (0%) |
| II | 1/51 (2.0%) |
| III | 50/51 (98.0%) |
| Stage | |
| IA | 0/51 (0%) |
| IB | 0/51 (0%) |
| IIA | 2/51 (3.9%) |
| IIB | 12/51 (23.5%) |
| IIIA | 16/51 (31.3%) |
| IIIB | 10/51 (19.6%) |
| IIIC | 7/51 (13.7%) |
| IV | 4/51 (7.8%) |
| MRI characteristics | |
| Type | |
| Mass | 43/51 (84.3%) |
| NME | 8/51 (15.7%) |
| Wash-out curve | |
| I | 1/51 (2.0%) |
| II | 1/51 (2.0%) |
| III | 49/51 (96.0%) |
| Imaging response at post-treatment MRI | |
| None | 6/51 (11.8%) |
| Partial* | 33/51 (64.7%) |
| Complete | 12/51 (23.5%) |
Partial imaging response was defined as any response as per RECIST 2.0 criteria that was not described as “complete imaging response” by the interpreting breast radiologist.
Factors associated with pCR
Concentric shrinkage was associated with pCR (p=0.001, AUC 0.778) with accuracy of 80.4%, specificity of 96.6% and sensitivity of 59.1%. The single patient who had pCR but did not have concentric shrinkage on post-treatment MRI was noted to have 2.4 cm of intermediate grade DCIS at time of breast conservation surgery with no invasive component (ypTis). Complete imaging response was also associated with pCR (p=0.048, AUC=0.627). However, imaging response as defined by RECIST criteria was not associated with pCR (p=0.064, AUC=0.596).
There was no association with pCR and, pre-treatment IER (Reader 1 (R1) p=0.665, Reader 2 (R2) p = 0.766), lesion type (p=0.726), lesion shape (p=0.391), lesion size (p= 0.69) lesion internal enhancement (p=0.136), size (p=0.403), mammographic presentation (p=0.187), positive axillary nodes (p=0.066), hormone receptor positivity (p=0.089), ki-67 (0.081), or BPE (p=0.78, Table 1).
Binary logistic regression modeling and pCR
Given the prevalence of pCR of 56.90% (29/51) and the sample size of 51, the study was appropriately powered for a binary logistic regression model of pCR with two covariates. No two or three covariate model demonstrated statistical significance of any included covariate, with the exception of concentric shrinkage. No two or three covariate model had a higher AUC or accuracy than univariate logistic regression of concentric shrinkage.
Factors associated with IER
IER was associated with the presence of axillary metastases (R1 p=0.016, R2 <0.001) and ki-67 (R1 r=0.52, p=0.008, R2 r=−0.44, p=0.028).
There was no association with pre-treatment IER and BPE (0.102–0.155), lesion type (0.21–0.754), or lesion size (0.216–0.238).
IER had good reader agreement (ICC=0.87, coefficient of reproducibility 79.5).
Factors associated with BPE
BPE did not correlate with presence of axillary metastases (0.284–0.713), ki-67 (0.839–0.948) or lesion type (0.118–0.299).
Factors associated with positive hormone receptors
Hormone-receptor negative cancers had higher ki-67% (average 55.6%, range 0–95%) than hormone-positive cancers (average 37.75%, range 10–70%, p=0.026). There was no correlation between hormone receptor status and presence of axillary metastases (p=0.30), size (p=0.761), IER (p=0.083–0.345), or BPE (p=0.195–0.439)
Discussion
This study evaluated potential predictors of pCR at mammography and MRI for HER2-positive patients and found that only the pattern of tumor shrinkage and complete imaging response was associated with pCR. Of note, shrinkage pattern was a more accurate and specific predictor of HER2-targeted therapeutic response than any other variable tested, including the presence or absence of complete imaging response on post-treatment MRI and the quantified early contrast enhancement over baseline at pre-treatment imaging (IER). Multiple logistic regression modeling involving additional covariates did not improve on the accuracy offered by tumor shrinkage alone.
Concentric shrinkage is of interest as an easily measured assessment of HER2-directed therapy, as the high specificity it offers may allow for early identification of nonresponders who could benefit from additional therapy prior to surgical managementWhile the ACRIN 6657/I-SPY trial demonstrated that change in tumor volume over time is more sensitive than RECIST criteria (6), this is difficult to assess and incorporate into real-time clinical reads. While the ongoing development of machine-learning based automatic breast lesion segmentation (20) may change this in the near future, the technology is not yet ready for widespread clinical use. Fukada et al suggested that luminal cancer concentric shrinkage may be related to a relatively homogenous cell population more likely to be suppressed by taxane-based chemotherapy (14). While these findings were not reproduced by Shin et al (17), this follow-up study included a mix of tumor types without HER2 subset analysis. HER2-enriched breast cancers, despite considerable variability in hormone-receptor expression between patients, are similar to luminal A cancers in that they are likely to begin as a relatively homogenous cell population (21,22). Retrospective pathologic studies have demonstrated that 2.7–11% of metastatic HER2 cancers demonstrated genetic heterogeneity and 8.7–18% demonstrated regional heterogeneity; patients with genetic heterogeneity were less likely to respond to trastuzumab and had shorter disease-free survival (21,22). Concentric shrinkage, therefore, may reflect a more homogenous tumor that responds better to HER2-targeted therapy. This correlates with recent radiomics breast cancer texture analyses that demonstrate as tumor heterogeneity increases, recurrence-free survival decreases (11,13).
We did not confirm any association with IER and pCR in this study. Our results do not match those of Kim et al, who recently found a strong correlation between an early enhancement ratio on pre-treatment MRI and pCR (16). Our differing results may be a result of our stricter inclusion criteria of patients who received pre-operative HER2-targeted therapy, which by definition included high-grade and later-stage cancers compared to Kim et al. Previous studies of IER have also correlated with tumor grade, presence of axillary metastases, invasive cancer and ki-67, all markers of tumor aggressiveness (15). The results of our study confirming the association of IER with ki-67 and axillary metastases but not pCR suggest that while IER does correlate to more aggressive and later stage disease, the differing values of IER within later stage cancers does not offer prognostic information about pCR. Other imaging attributes should be explored as a marker for pCR in this population.
Limitations of our study include its small sample size and its single center nature. At our institution many patients are referred from outside institutions subsequent to cancer diagnosis and as such, many of our pre-NAC MRIs are from outside institutions and not available for kinetic analysis. However, this was intended as an exploratory analysis of predictors of pCR; broader evaluation of concentric shrinkage patterns and its correlation with multiparametric imaging features, particularly radiomics, is of interest for further studies. An additional critique is that we chose to include four Stage IV patients in our analysis. These four patients presented with potentially resectable metastases at initial diagnosis. As the clinical decision was made to pursue primary breast surgery if they responded to HER2-targeted chemotherapy, we chose to include them in our study on an intent-to-treat basis and, indeed, the one patient with sufficient response did have pCR at mastectomy and has not had disease recurrence at the most recent follow-up.
In conclusion, the shrinkage pattern of HER2-positive tumors after neoadjuvant therapy may be associated with pCR, while initial tumor enhancement is not. Concentric shrinkage demonstrates high specificity for HER2-positive breast cancer pCR and is easily evaluated at the time of clinical read-out. Future studies evaluating the correlation of this finding to texture radiomics are of interest.
Table 2:
MRI imaging features and their association with pathologic complete response (pCR) (n=51). BPE = background parenchymal enhancement, IER = initial enhancement ratio, NME = nonmass enhancement.
| Pathologic complete response (pCR) | |||
|---|---|---|---|
| Yes | No | P-value | |
| Concentric shrinkage | 0.000 | ||
| Yes | 28/37 (75.7%) | 9/37 (24.3%) | |
| No | 1/14 (7.1%) | 13/14 (92.9%) | |
| Imaging response** | 0.073 | ||
| Yes | 28/45 (62.2%) | 17/45 (37.8%) | |
| No | 1/6 (16.7%) | 5/6 (83.3%) | |
| Lesion type | 0.726 | ||
| Mass | 24/43 (55.8%) | 19/43 (44.2%) | |
| NME | 3/8 (37.5%) | 5/8 (62.5%) | |
| Lesion shape (masses only+) | 0.403 | ||
| Oval/round | 7/11 (63.6%) | 4/11 (36.4%) | |
| Irregular | 17/32 (53.1%) | 15/32 (46.9%) | |
| BPE | 0.780 | ||
| Minimal to mild | 16/29 (55.2%) | (13/29) 44.8% | |
| Moderate to marked | 13/22 (59.1%) | 9/22 (40.9%) | |
| Positive axillary nodes | 0.066 | ||
| Yes | 18/37 (48.6%) | 19/37 (51.4%) | |
| No | 11/14 (78.6%) | 3/14 (21.4%) | |
| Size (average, range) | 4.45 cm (1.3–11.0 cm) | 3.93 (1.2–8.3 cm) | 0.315 |
| ki-67% (average, range) | 54.7% (20–95%) | 40.94% (0–90%) | 0.121 |
| IER | |||
| Reader 1 | 271.83% (145–375%) | 279.12% (133–405%) | 0.665 |
| Reader 2 | 284.22% (102–416%) | 271.0% (113–379%) | 0.766 |
defined by RECIST 2.0 criteria
Insufficient numbers for NME subgroup analysis
Acknowledgments
Grant Support: NIH R01CA219964 (S.G.K.)
Abbreviations
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- IER
initial enhancement percentage over baseline at first post-contrast imaging
- ICC
intra-class correlation coefficient
- NAC
neoadjuvant chemotherapy
- NME
nonmass enhancement
- pCR
pathologic complete response
- PR
progesterone receptor
- R1
reader 1
- R2
reader 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 2009;27(34):5693–5699. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Morales LA, Segui MA, Andreu X, et al. Analysis of the pathologic response to primary chemotherapy in patients with locally advanced breast cancer grouped according to estrogen receptor, progesterone receptor, and HER2 status. Clin Breast Cancer 2007;7(7):559–564. [DOI] [PubMed] [Google Scholar]
- 3.Wiechmann L, Sampson M, Stempel M, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 2009;16(10):2705–2710. [DOI] [PubMed] [Google Scholar]
- 4.de Azambuja E, Cardoso F, de Castro G Jr., et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96(10):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuerer HM, Rauch GM, Krishnamurthy S, et al. A Clinical Feasibility Trial for Identification of Exceptional Responders in Whom Breast Cancer Surgery Can Be Eliminated Following Neoadjuvant Systemic Therapy. Ann Surg 2018;267(5):946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology 2012;263(3):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol 2005;184(3):868–877. [DOI] [PubMed] [Google Scholar]
- 8.McGuire KP, Toro-Burguete J, Dang H, et al. MRI staging after neoadjuvant chemotherapy for breast cancer: does tumor biology affect accuracy? Ann Surg Oncol 2011;18(11):3149–3154. [DOI] [PubMed] [Google Scholar]
- 9.De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 2013;119(10):1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh J, Selmi M, Charles-Edwards G, et al. Changes in primary breast cancer heterogeneity may augment midtreatment MR imaging assessment of response to neoadjuvant chemotherapy. Radiology 2014;272(1):100–112. [DOI] [PubMed] [Google Scholar]
- 11.Henderson S, Purdie C, Michie C, et al. Interim heterogeneity changes measured using entropy texture features on T2-weighted MRI at 3.0 T are associated with pathological response to neoadjuvant chemotherapy in primary breast cancer. Eur Radiol 2017;27(11):4602–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santamaria G, Bargallo X, Fernandez PL, Farrus B, Caparros X, Velasco M. Neoadjuvant Systemic Therapy in Breast Cancer: Association of Contrast-enhanced MR Imaging Findings, Diffusion-weighted Imaging Findings, and Tumor Subtype with Tumor Response. Radiology 2017;283(3):663–672. [DOI] [PubMed] [Google Scholar]
- 13.Chamming’s F, Ueno Y, Ferre R, et al. Features from Computerized Texture Analysis of Breast Cancers at Pretreatment MR Imaging Are Associated with Response to Neoadjuvant Chemotherapy. Radiology 2018;286(2):412–420. [DOI] [PubMed] [Google Scholar]
- 14.Fukada I, Araki K, Kobayashi K, et al. Pattern of Tumor Shrinkage during Neoadjuvant Chemotherapy Is Associated with Prognosis in Low-Grade Luminal Early Breast Cancer. Radiology 2018;286(1):49–57. [DOI] [PubMed] [Google Scholar]
- 15.Heacock L, Lewin AA, Gao Y, et al. Feasibility analysis of early temporal kinetics as a surrogate marker for breast tumor type, grade, and aggressiveness. J Magn Reson Imaging 2018;47(6):1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SY, Cho N, Shin SU, et al. Contrast-enhanced MRI after neoadjuvant chemotherapy of breast cancer: lesion-to-background parenchymal signal enhancement ratio for discriminating pathological complete response from minimal residual tumour. Eur Radiol 2018;28(7):2986–2995. [DOI] [PubMed] [Google Scholar]
- 17.Shin SU, Cho N, Lee HB, et al. Neoadjuvant Chemotherapy and Surgery for Breast Cancer: Preoperative MRI Features Associated with Local Recurrence. Radiology 2018;289(1):30–38. [DOI] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22(8):1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SA, Cho N, Ryu EB, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014;270(3):699–707. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Saha A, Zhu Z, Mazurowski MAJItomi. Hierarchical Convolutional Neural Networks for Segmentation of Breast Tumors in MRI with Application to Radiogenomics. 2018. [DOI] [PubMed]
- 21.Lee HJ, Seo AN, Kim EJ, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol 2014;142(6):755–766. [DOI] [PubMed] [Google Scholar]
- 22.Seol H, Lee HJ, Choi Y, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 2012;25(7):938–948. [DOI] [PubMed] [Google Scholar]