Abstract
Few therapeutic strategies exist for the treatment of metastatic tumor cells in the brain because the blood-brain barrier (BBB) limits drug access. Thus the identification of molecular targets and accompanying BBB permeable drugs will significantly benefit brain metastasis patients. Polo-like kinase 1 (Plk1) is an attractive molecular target because it is only expressed in dividing cells and its expression is upregulated in many tumors. Analysis of a publicly available database of human breast cancer metastases revealed Plk1 mRNA expression was significantly increased in brain metastases compared to systemic metastases (P = 0.0018). The selective Plk1 inhibitor, GSK461364A, showed substantial uptake in normal rodent brain. Using a breast cancer brain metastatic xenograft model (231-BR), we tested the efficacy of GSK461364A to prevent brain metastatic colonization. When treatment was started 3 days post-injection, GSK461364A at 50 mg/kg inhibited the development of large brain metastases 62% (P = 0.0001) and prolonged survival by 17%. GSK461364A sensitized tumor cells to radiation induced cell death in vitro. Previously, it was reported that mutations in p53 might render tumor cells more sensitive to Plk1 inhibition; however, p53 mutations are uncommon in breast cancer. In a cohort of 41 primary breast tumors and matched brain metastases, p53 immunostaining was increased in 61% of metastases; 44% of which were associated with primary tumors with low p53. The data suggest that p53 overexpression occurs frequently in brain metastases and may facilitate sensitivity to Plk1 inhibition. These data indicate Plk1 may be a new druggable target for the prevention of breast cancer brain metastases.
Keywords: Brain metastasis, Breast cancer, Plk1, p53, Targeted therapy
Introduction
The majority of morbidity and mortality from breast cancer is associated with metastatic disease. Brain metastases are frequent in metastatic patients with triple-negative (estrogen and progesterone receptor negative, wild type Her-2) or Her-2 amplified metastatic tumors. The 1-year survival estimate after diagnosis of a central nervous system (CNS) or brain metastasis is only 20% [1].
Brain permeable molecularly targeted agents are needed to treat, or even prevent brain metastatic disease [2]. The blood–brain barrier (BBB) is constructed to keep undesirable agents from entering the brain. The endothelial cells that form the vasculature of the brain are connected by continuous tight junctions and surrounded by a basement membrane, pericytes, and the feet of astrocytes to restrict the movement of molecules into the brain parenchyma. In addition, a myriad of efflux transporters, such as P-glycoprotein and Breast Cancer Resistance Protein, present at the BBB actively pump molecules out of the brain. Using two model systems of brain metastases of breast cancer, we reported that, while most experimental brain metastases have some increased permeability to tracers or traditional chemotherapeutic drugs (i.e., doxorubicin, paclitaxel), the magnitude of increased permeability is low and insufficient for chemotherapy induced cytotoxicity in ∼90% of lesions [3]. These data argue that new brain-permeable drugs will be needed for treatment and prevention of brain metastases.
A hallmark of brain metastases is that their proliferative state is higher than that of the surrounding normal brain. Ki67 staining of 16 resected brain metastases of breast cancer revealed a proliferative rate of 47% [4]. Antimitotics are successful chemotherapy drugs used to treat many types of solid tumors (rev. in [5]). Taxanes are antimitotic agents that target the microtubule function of the mitotic spindle apparatus, and have been used widely in breast cancer therapy. However, taxanes which work by stabilizing microtubules and also vinca alkyloids by depolymerizing them, affect both dividing and non-dividing cells [6]. Further, the taxanes suffer from poor brain permeability and efficacy [3, 7–9]. The next generation of antimitotics are targeted agents that inhibit the activity of a specific protein involved in proliferation [5], rather than general effects on microtubules or nucleotide pools. These molecularly targeted antimitotics are in early phase clinical trials. One exciting target of these drugs is polo-like kinase 1 (Plk1).
Plk1 is one of four polo-like kinase family members. It is a serine-threonine kinase containing a kinase domain near its N-terminus and a polo-box domain critical for protein:protein interactions near its C-terminus. Plk1 is critical for cell division as it controls entry into and exit from mitosis along with centrosome maturation, assembly of the bipolar spindle, sister chromatid splitting, activation of the anaphase-promoting complex and initiation of cytokinesis [6, 10, 11]. Key targets of Plk1 in mitosis include cyclin B1, Cdc25C, Nlp and Tctp [6, 10]. Plk1 pathways also function at cell cycle checkpoints to promote chromosomal stability and Plk1 interacts with Mdm2 and BRCA2 [12, 13]. Plk1 is a valid molecular target for cancer [6, 10, 14–18] and was shown to be overexpressed in a variety of primary tumors, including breast [19]. While Plk1 overexpression was linked to tumor grade, proliferative activity and Her-2 expression, it was not an independent predictor of patient survival [20]. Interestingly, Plk1 expression was necessary but not sufficient for invasion of immortal breast cells in vitro [21]. Furthermore, in melanoma patients, an elevated level of Plk1 expression indicted risk of metastasis [22].
To our knowledge, the role of Plk1 in the metastatic colonization of the brain or any other secondary organ has not been reported. We tested the ability of the Plk1 selective inhibitor, GSK461364A, to prevent the outgrowth of breast cancer cells in the brain using an experimental metastasis xenograft model.
GSK461364A is an imidazotriazine, competitive ATP kinase inhibitor highly specific for Plk1 (Ki = <0.5 nM compared to 860 and 1000 nM for Plk2 and Plk3, respectively) [23]. In a phase I study in 40 patients, limiting toxicities of neutropenia, thrombocytopenia and bone marrow suppression were observed. Increased phospho-Histone H3 (pHH3) in circulating tumor cells was noted as evidence of Plk1 inhibition. Six patients experienced prolonged stable disease lasting at least 3 months [6].
A notable interacting protein of Plk1 is p53. Depletion of Plk1 stabilized p53 and the cytotoxic effects of Plk1 depletion were elevated in p53 null cells [10, 24, 25]. These effects may be mediated by the kinase domain of Plk1 binding to the DNA binding domain of p53, inhibiting both its transactivation and apoptosis induction functions; other reports suggest a direct kinase activity or that Plk1 phos-phorlyates Topors, a topoisomerase binding protein, which then enhances the ubiquitination of p53 [10]. GSK461364A has been shown to have increased sensitivity in TP53 mutant cells [25]. Following drug exposure and washout, an outgrowth assay segregated cell lines in vitro that were sensitive to GSK461364A from those that were resistant. Cell lines deemed sensitive in the outgrowth assay were found to have a high incidence of mutated TP53 or be TP53 null [25]. While primary breast cancers do not have a high frequency of TP53 mutations [26], two small cohort studies identified LOH at the TP53 loci in 40–45% of the breast cancer brain metastases [27, 28], suggesting the intriguing possibility of site-specific Plk1 pathway sensitization.
Herein we report that the p53 immunostaining of matched human primary tumors and brain metastases of breast cancer showed an elevation of p53 expression in a proportion of brain metastases. Using the 231-BR experimental brain metastasis model system, derived from six rounds of in vivo brain selection of triple negative MDA-MB-231 cells [29], GSK461364A significantly prevented the outgrowth of 231-BR cells by 62% (P = 0.0001). Additionally, we show that GSK461364A is a radiosensitizer able to enhance the efficacy of radiation induced cell death in vitro. This is important as radiation therapy, either stereotactic or whole brain, is a mainstay treatment for brain metastatic disease.
Materials and methods
Cell culture and in vitro experiments
A brain metastatic derivative of the breast cancer cell line, MDA-MB-231, was isolated and described previously [29]. These cells, designated 231-BR, were maintained in high glucose DMEM medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) at 37°C in 5% CO2. For cell viability assays 231-BR cells were maintained in 10% FBS and treated with increasing concentration of GSK461364A as indicated. MTT (Sigma, St. Louis, MO) was as previously described [30]. Three separate experiments were performed, with n = 4 for each data point. For cell cycle analysis, cells were harvested after treatment with GSK461364A for 24 h. Samples were prepared by standard procedures and analyzed on a Becton–Dickinson FACSCalibur using ModFit v3.0 software. For western blot analysis, 231-BR cells were treated with GSK461364A at the concentrations indicated for the times given prior to lysis. Immunoblot analysis was preformed per standard procedures. Primary antibodies from Cell Signaling Technologies against the following proteins were used: PLK-1, phosphorylated-Histone H3, cleaved caspase 9, caspase 9, cleaved caspase 8, caspase 8, cleaved caspase 3, caspase 3. Additionally a-tubulin (Oncogene) was used as a protein loading control.
Animal experiments
All experiments were conducted under an approved Animal Use Agreement with the NCI. For efficacy studies 5–7 weekold female nu/nu NRC mice (Charles River Laboratories) were inoculated with 175,000 231-BR cells in the left ventricle of the heart. Three days after tumor cell inoculation mice were randomized to GSK461364A treatment (25 and 50 mg/kg, respectively) and vehicle (2% Cremophor EL, 2% N,N,-dimethylacetamide in acidified (pH5) water) groups. GSK461364A was administered via intraperitoneal (IP) injection once every other day for 27 days. Mice were euthanized, brains excised and bisected along the sagittal plane. Ten serial sections (10 microns thick) every 300 microns through the right hemisphere were analyzed at 50× magnification using an ocular grid with squares of 0.8 mm2. Every micro or large (>300 microns along the longest axis) metastasis in each section was tabulated.
For survival studies, 231-BR cells were administered as described above. Day 3 post injection, one group of mice began treatment with 50 mg/kg and one group began vehicle treatment. Four days later a third group began treatment 7 days post injection and the final group began treatment 13 days post injection. Mice were euthanized when they showed signs of distress —>20% weight loss or paralysis. Brains from all mice were sectioned and H&E stained to confirm the presence of brain metastases.
Whole brain autoradiography
A single dose of 12 mg/kg [14C]GSK461364A was administered to rats by iv injection. Tissue uptake was assessed from whole body sections at various time points O. after drug treatment. Tissue radioactivity was quantified using a Fujifilm FLA7000 phosphoimager with the MCID Analysis program and 14C autoradiography standards (Amersham Biosciences).
Immunohistochemistry and image analysis
Tissue microarrays containing 41 primary breast tumors and patient matched brain metastases from the same patients were prepared, with each sample represented as triplicate cores of 0.6 mm diameter. The tissue microarrays were stained with anti-human p53 antibody (Dako) and the stained slides were scanned with the ScanScope CS (A-perio, Vista, CA) using bright-field imaging at 200× magnification. The three cores representative of each case were outlined at 100× magnification. Digital images of the selected areas were analyzed using an Immunohistochemistry Nuclear Image Analysis Algorithm optimized for breast tissue (Aperio). The performance of the IHC Nuclear Imaging had been validated by certified pathologists according to standard pathology laboratory practices. Briefly, brown (3,3-diamino-benzidine) and blue (hematoxylin) colors were separated spectrally. Cells with blue and brown nuclei were scored as positive and cells with blue and not brown nuclei as negative; the percentage of p53-positive cells was calculated for each core and the average value of the three cores was used as the final score for each case. The average scores obtained on analysis of the primary breast tumor and of the brain metastasis from the same patient were compared. A manual count of the nuclei in the representative areas was also obtained for independent confirmation of the validity of digital scoring. Foci composed predominantly of stroma, blood vessels, adipose tissue, benign glandular epithelium and areas of necrosis were excluded from analysis using Aperio negative pen tools.
DNA sequencing
Exons 2–11 of TP53 were sequenced from eight micron sections of frozen tissue embedded in OCT from resected human breast cancer brain metastases. A board-certified pathologist verified that a sufficient tumor fraction was present in H&E stained sections. OCT was removed by washing samples in 500 μl of TE buffer and centrifuging the tissue for 10 m. DNA was then extracted using the QIAamp DNA Micro Kit (Qiagen). Purified DNA (20 ng) was amplified with M13-tagged primers (Supplemental Table 1) against exons 2–11 in TP53 [31] under the following PCR protocol: 96°C 5 m; then 40 cycles of 94°C 30 s, 60°C 45 s, 72°C 45 s; and a final 72°C 10 m. Amplicons were evaluated for yield by 2% agarose gel electrophoresis and then cleaned using Exo-Sap It (USB, Cleveland, Ohio) according the manufacturer’s protocol. Amplicons were sequenced using a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) using the following cycle sequencing protocol: 96°C for 1 m; then 25 cycles of 96°C 10 s, 50°C 5 s; 60°C 4 m. Sequence products were purified with the Qiagen DyeEx 96 Kit (Qiagen). Labeled samples were sequenced on the 3730 DNA Analyzer (ABI) and GeneBank reference sequence NM_000546 for TP53 was used in the DNA sequence analysis (Mutation Surveyor software, SoftGenetics, State College, PA) using default settings. Synonymous single nucleotide polymorphisms did not require repeat sequencing, while nonsynonymous base pair changes were repeat sequenced for confirmation.
Plk1 expression in brain and systemic metastases
The expression of Plk1 was compared in a panel of 15 brain, 10 bone and 4 lung metastases from breast cancer, for which microarray data was publicly available in the Gene Expression Omnibus (GEO) database under accession number GSE14017 [32]. Gene expression differences of RMA-normalized [33], ln-transformed expression values were analyzed in dChip (http://www.dCHIP.org). Plk1 expression was compared between brain metastases and systemic (bone and lung) metastases using a t-test with unequal variances. Fold-changes, which are reported in retransformed, original units, and P-values are based on expression differences of Affymetrix U133plus2 probe 202240_at, corresponding to the human PLk1 gene (NM_005030).
Statistical analysis
Mixed model analysis of variance (ANOVA) was performed on continuous data (cell viability and in vivo brain metastasis data). Data were transformed as appropriate to meet ANOVA assumptions. Pair-wise P-values were adjusted using Dunnett’s method. Actuarial analysis was performed on the survival data using the Kaplan–Meier method. Data were compared using the Log-rank test. Survival analysis P-values were not adjusted for multiple comparisons.
Results
Plk1 is a molecular target for breast cancer brain metastasis
Previously, we reported a high proliferative rate for breast cancer cells growing in the brain. Ki67 labeling of 16 surgically resected human breast cancer brain metastases showed a mean proliferative index of 47% [4]. A comparable proliferative index was found in an experimental model of breast cancer brain metastasis using a subline of the human "triple negative" MDA-MB-231 cell line (231-BR). In the model system, 53% of the tumor cells in vivo were Ki67 positive [4], suggesting that the model recapitulates the highly proliferative phenotype of human brain metastases.
Molecular pathways regulating tumor cell proliferation may constitute excellent targets for therapy in the brain, as neurons, which are principal targets for deleterious neurocognitive side effects, are post-mitotic. New agents for anti-proliferative therapy are molecularly directed toward a number of protein kinases involved in mitosis, one of which is Plk1 [5, 6, 10, 15]. Using a publicly available database (GEO accession# GSE14017, [32]) of a gene expression analysis of human breast cancer brain metastasis (n = 15) compared to systemic metastases from breast cancer patients (n = 10 for bone and n = 4 for lung) Plk1 mRNA expression was significantly increased in the brain metastasis cohort (P = 0.0018, fold-change = 3.00) compared to the systemic metastases. There was no difference in Plk1 mRNA expression between the lung and bone metastases (P = 0.94). This suggests overexpression of Plk1 may be particularly important for the growth of tumor cells in the brain compared to systemic metastases.
GSK461364A is a potent inhibitor of breast cancer cell growth and crosses the blood brain barrier
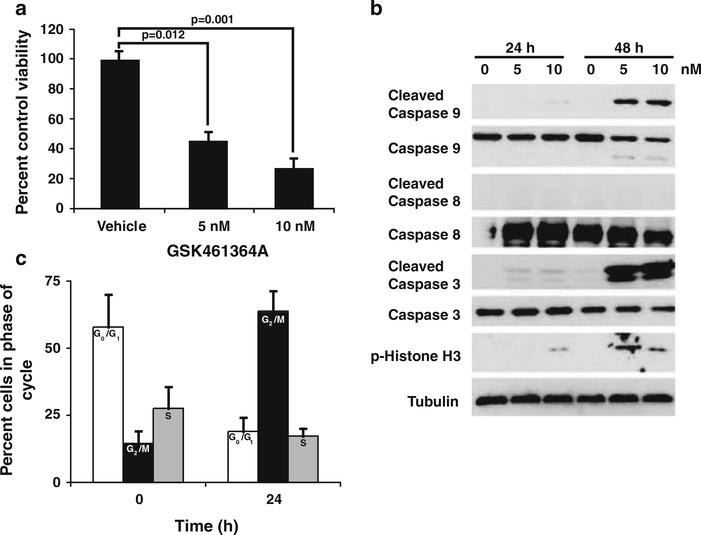
GSK461364A is a Plk1-specific inhibitor in early clinical testing [6, 34]. To determine if this compound was inhibitory to 231-BR cells, MTT-based viability assays were performed over a concentration range of 1–100 nM. GSK461364A inhibited the viability of 231-BR cells with an IC50 of approximately 5 nM after 48 h of continuous drug exposure (Fig. 1a). Apoptotic markers were apparent on Western blot analysis within this same timeframe. Cleaved caspase 9 and cleaved caspase 3 were observed (with a lack of cleaved caspase 8) in 231-BR cells treated with 5 or 10 nM GSK461346 (Fig. 1b), indicating that apoptosis proceeded mainly via the intrinsic cellular pathway under the conditions tested, as would be expected by an anti-mitotic drug.
Fig. 1.
GSK461364A inhibits cell viability by causing cell cycle arrest and inducing apoptosis. a 231-BR cells were treated with 5 and 10 nM GSK641364A, respectively, for 48 h and viability assessed by MTT assay. Mean ± the standard error for data combined from three independent experiments shown as percent of vehicle control viability. A mixed model one-way analysis of variance (ANOVA) was performed on log10(y) data (P = 0.002) and results of the post-hoc Dunnett’s multiple comparison shown. b Western blot analysis of 231-BR cells treated with GSK641364A. Cells were treated for the times indicated with either 5 or 10 nM GSK641364A and cell lystates subjected to SDS-PAGE for standard western blotting. All antibodies used with the exception of Tubulin were from Cell Signaling Technologies. Tubulin (Calbiochem) shown as a protein loading control. c Cell cycle analysis was determined by FACS analysis after 231-BR cells were treated with 5 nM GSK461364A for 24 h. Data shown as percentage of cells in each phase of the cell cycle at the given time point. The mean ± standard deviation of data combined from two experiments is presented. White bars G0/G1 phase, Black bars G2/M phase, Grey bars S phase
A hallmark of PLK1 pathway inhibition is cell cycle arrest in the M phase, leading to mitotic cell death [35]. At time zero, prior to treatment, 14.5% of the 231-BR cell population was in G2M as determined by FACS analysis. After 24 h of exposure to GSK461346 the percentage of cells in G2M rose to 63.8% with concomitant decrease of cells in G1 and S phase (Fig. 1c). Western blot analysis of phosphorylated Histone H3 was conducted to confirm an M phase cell cycle block. Treatment of 231-BR cells with 5 and 10 nM GSK461364A resulted in an increase in pHH3 levels (Fig. 1b). Further, mitotic figures consistent with mitotic cell death were observed after 24 h of treatment with GSK461364A (data not shown). Taken together these data indicate 231-BR cells were blocked in mitosis (M phase) upon drug treatment and preceding cell death.
One of the principal reasons why conventional therapeutics have been ineffective against brain metastases is their poor penetration into the brain [36]. The extent to which clinically apparent brain metastases have a patent blood–brain barrier is debated, and virtually no information exists for occult micrometastases, which would constitute a target for brain metastasis prevention. Drug uptake experiments were therefore conducted in the normal rat brain for [14C]GSK461364A. A single dose of 12.5 mg/kg radiolabeled compound was injected intraperitoneally and animals were sacrificed at various times to quantify drug accumulation by autoradiography (Table 1). Drug uptake in the brain or striatum varied from 3.2–4.3 μg equivalents/g of tissue at 30 min post-injection, a level 25–44% higher than that observed in blood (2.4 μg equivalents/g of tissue). Drug levels decreased in the brain over a 24 h time course, at times faster than blood levels and at other times slower. At 24 h post-injection, GSK461364A was undetectable in the bloodstream but remained at 0.13–0.29 μg equivalents/ g in the brain. The data indicate that GSK461364A is permeable in the normal brain and therefore a plausible candidate for in vivo brain metastasis prevention and/or treatment.
Table 1.
Tissue uptake of a single 12.5 mg/kg dose of [14C]GSK461364A in whole body autoradiography studies
| Tissue | Time (h) |
|||
|---|---|---|---|---|
| 0.5 | 2 | 6 | 24 | |
| Blood (cardiac) | 2.396a | 0.730 | 0.183 | BLQb |
| Brain | 3.192 | 0.363 | 0.088 | 0.126 |
| Brain (striatum) | 4.283 | 0.522 | 0.225 | 0.288 |
| Choroid plexus | 32.560 | 9.734 | 2.790 | 2.342 |
| Meninges | 10.305 | 1.236 | 1.239 | 4.881 |
μg equivalents/g tissue
Below the limit of quantitation (<0.051 μg equivalents/g)
Inhibition of Plk1 prevents experimental brain metastases and improves survival
To determine if GSK461364A could prevent the growth of experimental metastasis of 231-BR cells in the brain, nude mice were injected with tumor cells in the left ventricle of the heart and 3 days post-injection were randomized to GSK461364A or vehicle, administered intraperitoneally every other day. Preliminary dose finding experiments were conducted with 75 and 50 mg/kg. The higher dose was found to be toxic causing acute weight loss (data not shown). Prevention experiments were therefore conducted using 25 and 50 mg/kg dosing starting 3 days post-injection of 231-BR cells and continuing for 27 days. At necropsy the brains were removed and bisected sagittally; 10 H&E stained sections, one every 300 microns through one hemisphere, were counted. An ocular micrometer was used to distinguish micrometastases from large metastases, the latter comparable in one-dimensional size to a magnetic resonance imaging-detectable metastasis in a human brain as reported previously [30]. The combined analyses of two independent experiments testing the efficacy of GSK461364A to prevent growth of metastatic tumor cells in the brain are presented (Table 2). A mean of 6.62 large metastases/section developed in the brains of vehicle treated mice, which declined 62% to 2.53 metastases/section when treated with 50 mg/kg GSK461364A (P = 0.0001). At the lower 25 mg/kg dose, a 43% decline in large metastases to 3.46/section was observed (P = 0.042). Prevention of the outgrowth of large metastases was therefore observed at both doses. For micrometastases, GSK461364A inhibited the number of lesions/ section from 239 to 159 at the 50 mg/kg dose (P = 0.041). At 25 mg/kg a 26% reduction from a mean of 177 compared to the 239 in the vehicle treated mice was observed, which was statistically insignificant.
Table 2.
Effect of GSK461364A on outgrowth of metastatic breast cancer cells in the braina
| Treatment | n | Micrometastases |
Large metastasesb |
||||
|---|---|---|---|---|---|---|---|
| Meanc | 95% confidence interval | Pd | Mean | 95% confidence interval | p | ||
| Vehicle | 22 | 239 | 158–320 | 6.62 | 4.95–8.29 | ||
| 25 mg/kg | 13 | 177 | 94–260 | 0.20 | 3.46 | 1.34–5.59 | 0.042 |
| 50 mg/kg | 18 | 159 | 86–232 | 0.041 | 2.53 | 1.81–3.25 | 0.0001 |
1.75 × 105 231-BR cells were injected into the left ventricle of nu/nu NRC mice on Day 0
Size of metastases determined by 16 mm2 ocular grid. Large metastases >300 microns
Mean number of metastases counted in 10 step sections from one hemisphere of the brain
A mixed model ANOVA was used to determine significance and adjusted post-hoc using Dunnett’s method for the comparison between vehicle and the two doses. All P-values are two-tailed
To determine whether GSK461364A could effectively treat established brain metastases, as compared to preventing their outgrowth, mice that received 231-BR cells in the left cardiac ventricle were randomized to four treatment arms: (1) vehicle, (2) GSK461364A treatment started on day 3 post-injection, (3) GSK461364A treatment started on day 7 post-injection, or (4) GSK461364A treatment started on day 13 post-injection. The latter time is a point where numerous micrometastases and occasional large metastases have formed (data not shown). The endpoint of the experiment was survival; mice were sacrificed when either a 20% weight loss or paralysis was noted in accordance with NIH guidelines. GSK461364A extended survival to the same extent, compared to the vehicle control group, regardless of if treatment was started day 3, 7 or 14 post-injection (Supplemental Fig. 1, experiment 1; P = 0.001 and experiment 2; P = 0.026). No significant difference was observed between the groups that started treatment on the varying days.
The data suggest that inhibition of Plk1 can exert survival benefits in either the prevention or early treatment settings.
GSK461364A sensitizes 231-BR cells to radiation
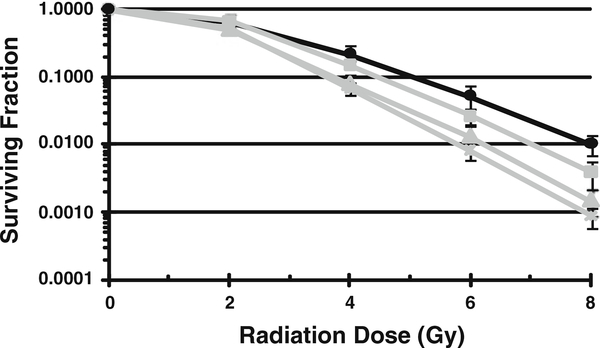
Whole brain radiotherapy (WBR) is a mainstay treatment for micrometastatic disease in the brain and in cases of multiple metastases. Stereotactic radiotherapy delivers a higher dose to a single brain metastatic lesion. Identifying agents that can synergize with radiation to increase its efficacy in tumor but not normal cells is of intense interest to the radiation oncology field. To determine if GSK461364A could sensitize 231-BR cells to radiation induced cell death, clonogenic survival assays were performed after combined exposure. 231-BR cells were seeded as single cells and treated with varying concentrations of GSK461364A for 2 h prior to irradiation. Ten to fourteen days later colonies were counted and survival curves were generated normalized to the survival of cells treated with GSK461364A only in the absence of radiation. A dose dependent effect was observed with a dose enhancement factor of 1.5 for 40 nM GSK461364 at a surviving fraction of 0.1 (Fig. 2). The data in conjunction with the brain permeability of this compound suggest that GSK461364A given prior to WBR could enhance the cytotoxic effect of the radiation on tumor cells in the brain.
Fig. 2.
GSK461364A radiosensitizes 231-BR cells in vitro. Cells were treated with varying concentrations of GSK461364A as indicated 2 h prior to irradiation. Colony forming efficiency was determined 10–14 days later and survival curves were generated after normalizing for survival of cells treated with GSK461364A alone in the absence of radiation. The mean ± the standard error are graphed for a representative experiment of n = 3. Black line vehicle control (radiation alone in the absence of GSK461364A). Grey lines in descending order: square 10 nM GSK461364A, triangle 20 nM GSK461364A, times symbol 40 nM GSK461364A
p53 status is discordant in matched primary breast tumors and brain metastases
Recently, Sur et al. [24], reported that cancer cells with inactivated p53 were more sensitive to inhibition of Plk1 than their wild-type p53 isogenic partners in vitro and in vivo. Additionally, cell lines with mutant TP53 from multiple primary tumor types were shown to be more sensitive to GSK461364A than cell lines with wild-type TP53 [25]. These observations raise the issue of whether Plk1 is a good target in breast cancer, as only 20–35% of primary breast tumors harbor TP53 mutations [26]. However, an equally plausible hypothesis is that primary tumors and metastases may vary in genotype [37, 38] and that selection for an aggressive metastasis such as in the brain may result in a population of tumor cells with more frequent TP53 mutations.
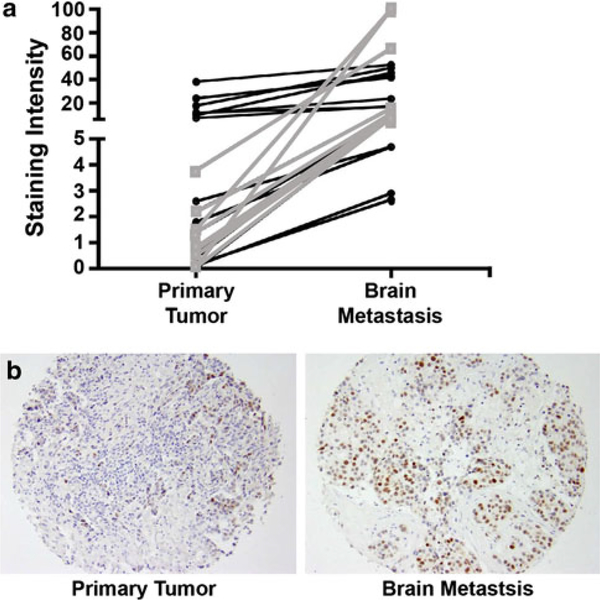
To discriminate between these two possibilities, a tissue microarray cohort of formalin-fixed, paraffin embedded tissue from 41 matched sets of human resected primary tumors and brain metastases was stained for p53 protein by immunohistochemistry. p53 protein stability stands as one measure of p53 functional status due to mutation and consequent inactivation of degradation pathways. Protein staining was quantified by an immunohistochemistry nuclear image analysis algorithm optimized for breast tissue (Aperio). Of the primary tumors, 56% (23 of 41) expressed low staining levels of p53, suggestive of a wild-type pathway. Of these primary tumors, 10 (43%) of the matched brain metastases had higher p53 staining levels, indicating that p53 status likely changed between primary tumor and metastasis. The magnitude of the staining increase is graphed on Fig. 3a and a representative matched set shown on Fig. 3b. Overall, 61% of the brain metastases exhibited high p53 staining, suggesting that breast cancer brain metastases may harbor frequent p53 inactivation. We were unable to sequence TP53 from the archival specimens to determine whether increased expression of p53 was from gene mutation or indirect effects. However, TP53 exons 2–11 were sequenced from 20 frozen resected human brain metastases of breast cancer specimens. Only five of the samples or 25% were found to have TP53 mutations, similar to the percentage commonly observed in primary breast tumors (Supplemental Table 2). The data suggest that direct mutational inactivation of p53 is not common in breast cancer brain metastases.
Fig. 3.
p53 immunoreactivity on a tissue microarray of matched resected primary breast tumors and brain metastases. a Graph represents the magnitude of increase in immunoreactivity between a primary tumor and the matched brain metastatic lesion. Grey (squares) show patient matched samples with the greatest increase from low primary tumor expression to high brain metastasis expression. b Representative images of low staining in a primary tumor sample and high staining in the corresponding brain metastasis sample
Discussion
The FDA has designated brain metastatic disease as an unmet medical need as there are no effective therapies for treatment metastatic disease at this organ specific site. New, effective agents that will cross the BBB and target pathways operative in brain metastatic tumor cells need to be identified and validated. The highly proliferative nature of human brain metastases of breast cancer, coupled with the post-mitotic status of normal neurons in the brain, suggested the hypothesis that molecular pathways regulating proliferation would be of interest. Plk1 is critical to multiple aspects of proliferation. Multiple inhibitors are in preclinical development or early clinical testing (rev. in [6, 10, 15]). Here we present data on the selective Plk1 inhibitor, GSK461364A. Using whole body autoradiography of rodents injected with radioactive drug, GSK461364A was observed in the brain at levels comparable, or higher than that of the bloodstream. Thus, the drug is predicted to be available in brain tissue behind the BBB, unlike virtually all standard chemotherapeutic agents for breast cancer. In vitro, GSK461364A inhibited proliferation of the 231-BR brain tropic cell line, causing cell cycle arrest and mitotic death as predicted. In vivo, a 62% reduction (P = 0.0001) in large metastases was observed when drug was administered 3 days post-injection. At the highest dose tested, GSK461364A (50 mg/kg) significantly inhibited micrometastasis formation, consistent with its brain permeability characteristics. A trend toward increased survival was noted when the drug was administered on several schedules.
These data nominate GSK461364A as a candidate for clinical testing for the prevention and/or treatment of brain metastases of breast cancer. Additionally, this inhibitor may be of interest in lung cancer and melanoma, other cancer types that have high incidence of brain metastasis [2].
Plk1 inhibition is optimal in a TP53 null cellular environment in numerous reports [10, 24, 25]. While TP53 is uncommonly mutated or overexpressed in primary breast carcinomas [26], we hypothesized that alterations may be more frequent in brain metastases. Previously reported LOH data for brain lesions are supportive of this hypothesis: In a cohort of 11 brain metastases, 5 (45%) were found to have LOH at 17p13.1 compared to 23% of unrelated primary tumors analyzed [27]. Likewise, in a cohort of 12 primary tumors with 15 patient matched brain metastases (one patient had two brain metastases and one had three), informative LOH analysis at 17p13.1 was obtained for 10 cases and 40% had LOH in the brain metastasis sample but not the primary tumor sample while another 40% had LOH at both sites [28]. In a cohort of primary breast tumors and patient matched brain metastases we used immunohistochemistry coupled with an automated quantitative imaging analysis to determine p53 protein expression, indicative of inactivated p53 degradation pathways. Overall, 61% of the brain metastasis samples exhibited high p53 immunostaining compared to 44% of the primary tumors. Furthermore, 43% of the primary tumors that showed low p53 immunostaining had matched brain metastasis samples with increased staining. These data indicate that p53 status in brain metastases can be distinct from that of primary tumors, and support the relevance of this site-specific lesion for Plk1 inhibition. Sequencing conducted on independently resected brain metastases for TP53 failed to identify a high frequency of mutations. It remains possible that indirect pathways are operative. Examples include Plk1 phosphorylation of Topors, resulting in inhibition of the sumoylation and increased ubiquitination of p53 [39–41]. Plk1 has also been shown to regulate levels of Mdm2, a p53 ubiquitin ligase [42, 43]. p73 is also phosphorylated by Plk1 and may transcriptionally regulate genes relevant to the p53 pathway [44, 45].
Another topic of concern is the design of brain metastasis trials. Typically patients with one or more lesions who have progressed through whole brain radiotherapy are entered onto trial, with a primary endpoint of shrinkage of the lesions. Based on our day 13 dosing schedule this may be feasible, however, most of our data was conducted in a prevention setting. Brain metastasis prevention trials conducted in the metastatic setting are large and costly. We propose a new "secondary prevention" design, where patients with one or more brain metastases, treated with standard of care but not whole brain radiation therapy, are randomized to placebo or drug. The primary endpoint of interest would not be shrinkage of the existing lesion but the time to the development of a new brain metastasis.
In vitro data suggest that GSK461364A may also enhance radiation therapy, suggesting a Plk1 inhibitor-radiation combination trial. In vivo determination of an interaction between inhibition of Plk1 activity and radiation will be required in the brain. It is interesting to note that, in rectal cancer, Rödel and colleagues [46] showed Plk1 to be a predictor of radiosensitivity. This could be of importance for breast cancer patients as well.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Program of the National Cancer Institute, Department of Defense Breast Cancer Research Program grant #W81XWH-062–0033 and GlaxoSmithKline.
Abbreviations
- BBB
Blood brain barrier
- CNS
Central nervous system
- Plk1
Polo-like kinase 1
- pHH3
Phosphorylated histone H3
- 231-BR
MDA-MB-231 brain metastatic subline
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10585-011-9421-9) contains supplementary material, which is available to authorized users.
Contributor Information
Yongzhen Qian, Laboratory Animal Sciences Program, SAIC-Frederick, NCI, NIH, Frederick, MD, USA.
Emily Hua, Women's Cancers Section, Laboratory of Molecular Pharmacology, NCI, NIH, 37 Convent Drive, Building 37, Room 1126, Bethesda, MD 20892, USA.
Kheem Bisht, Radiation Oncology Branch, NCI, NIH, Bethesda, MD, USA.
Stephan Woditschka, Women's Cancers Section, Laboratory of Molecular Pharmacology, NCI, NIH, 37 Convent Drive, Building 37, Room 1126, Bethesda, MD 20892, USA.
Konstantine W. Skordos, GlaxoSmithKline, Philadelphia, PA, USA
David J. Liewehr, Biostatistics and Data Management Section, NCI, NIH, Bethesda, MD, USA
Seth M. Steinberg, Biostatistics and Data Management Section, NCI, NIH, Bethesda, MD, USA
Edi Brogi, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Muzaffar M. Akram, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
J. Keith Killian, Genetics Branch, NCI, NIH, Bethesda, MD, USA.
Daniel C. Edelman, Genetics Branch, NCI, NIH, Bethesda, MD, USA
Marbin Pineda, Genetics Branch, NCI, NIH, Bethesda, MD, USA.
Stephanie Scurci, Genetics Branch, NCI, NIH, Bethesda, MD, USA.
Yan Y. Degenhardt, GlaxoSmithKline, Philadelphia, PA, USA
Sylvie Laquerre, GlaxoSmithKline, Philadelphia, PA, USA.
Thomas A. Lampkin, GlaxoSmithKline, Philadelphia, PA, USA
Paul S. Meltzer, Genetics Branch, NCI, NIH, Bethesda, MD, USA
Kevin Camphausen, Radiation Oncology Branch, NCI, NIH, Bethesda, MD, USA.
Patricia S. Steeg, Women's Cancers Section, Laboratory of Molecular Pharmacology, NCI, NIH, 37 Convent Drive, Building 37, Room 1126, Bethesda, MD 20892, USA
Diane Palmieri, Women's Cancers Section, Laboratory of Molecular Pharmacology, NCI, NIH, 37 Convent Drive, Building 37, Room 1126, Bethesda, MD 20892, USA.
References
- 1.Weil RJ, Palmieri D, Bronder JL et al. (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167(4): 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steeg PS, Camphausen KA, Smith QR (2011) Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 11(5):352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lockman PR, Mittapalli RK, Taskar KS et al. (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin Cancer Res 16(23):5664–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald DP, Palmieri D, Hua E et al. (2008) Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 25(7): 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt M, Bastians H (2007) Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updates 10(4–5):162–181 [DOI] [PubMed] [Google Scholar]
- 6.Degenhardt Y, Lampkin T (2010) Targeting Polo-like kinase in cancer therapy. Clin Cancer Res 16(2):384–389 [DOI] [PubMed] [Google Scholar]
- 7.Freilich RJ, Seidman AD, DeAngelis LM (1995) Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer 76(2):232–236 [DOI] [PubMed] [Google Scholar]
- 8.Crivellari D, Pagani O, Veronesi A et al. (2001) High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann Oncol 12(3):353–356 [DOI] [PubMed] [Google Scholar]
- 9.Boogerd W, Dalesio O, Bais EM et al. (1992) Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 69(4):972–980 [DOI] [PubMed] [Google Scholar]
- 10.Strebhardt K (2010) Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov 9(8): 643–660 [DOI] [PubMed] [Google Scholar]
- 11.Barr FA, Sillje HH, Nigg EA (2004) Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 5(6): 429–440 [DOI] [PubMed] [Google Scholar]
- 12.Dias SS, Hogan C, Ochocka AM et al. (2009) Polo-like kinase-1 phosphorylates MDM2 at Ser260 and stimulates MDM2-medi-ated p53 turnover. FEBS Lett 583(22):3543–3548 [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Daniels MJ, Venkitaraman AR (2004) Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene 23(4):865–872 [DOI] [PubMed] [Google Scholar]
- 14.Spankuch B, Matthess Y, Knecht R et al. (2004) Cancer inhibition in nude mice after systemic application of U6 promoter-driven short hairpin RNAs against PLK1. J Natl Cancer Inst 96(11): 862–872 [DOI] [PubMed] [Google Scholar]
- 15.Chopra P, Sethi G, Dastidar SG et al. (2010) Polo-like kinase inhibitors: an emerging opportunity for cancer therapeutics. Expert Opin Investig Drugs 19(1):27–43 [DOI] [PubMed] [Google Scholar]
- 16.Spankuch-Schmitt B, Bereiter-Hahn J, Kaufmann M et al. (2002) Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst 94(24):1863–1877 [DOI] [PubMed] [Google Scholar]
- 17.Spankuch-Schmitt B, Wolf G, Solbach C et al. (2002) Downregulation of human polo-like kinase activity by antisense oligonucleotides induces growth inhibition in cancer cells. Oncogene 21(20): 3162–3171 [DOI] [PubMed] [Google Scholar]
- 18.Cogswell JP, Brown CE, Bisi JE et al. (2000) Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25C function. Cell Growth Differ 11(12):615–623 [PubMed] [Google Scholar]
- 19.Wolf G, Hildenbrand R, Schwar C et al. (2000) Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract 196(11): 753–759 [DOI] [PubMed] [Google Scholar]
- 20.Weichert W, Kristiansen G, Winzer KJ et al. (2005) Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch 446(4):442–450 [DOI] [PubMed] [Google Scholar]
- 21.Rizki A, Mott JD, Bissell MJ (2007) Polo-like kinase 1 is involved in invasion through extracellular matrix. Cancer Res 67(23):11106–11110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kneisel L, Strebhardt K, Bernd A et al. (2002) Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol 29(6):354–358 [DOI] [PubMed] [Google Scholar]
- 23.Gilmartin AG, Bleam MR, Richter MC et al. (2009) Distinct concentration-dependent effects of the polo-like kinase 1-specific inhibitor GSK461364A, including differential effect on apoptosis. Cancer Res 69(17):6969–6977 [DOI] [PubMed] [Google Scholar]
- 24.Sur S, Pagliarini R, Bunz F et al. (2009) A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci USA 106(10):3964–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degenhardt Y, Greshock J, Laquerre S et al. (2010) Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function and chromosome instability. Mol Cancer Ther 9(7):2079–2089 [DOI] [PubMed] [Google Scholar]
- 26.Bertheau P, Espie M, Turpin E et al. (2008) TP53 status and response to chemotherapy in breast cancer. Pathobiology 75(2):132–139 [DOI] [PubMed] [Google Scholar]
- 27.Hampl M, Hampl JA, Schwarz P et al. (1998) Accumulation of genetic alterations in brain metastases of sporadic breast carcinomas is associated with reduced survival after metastasis. Invasion Metastasis 18(2):81–95 [DOI] [PubMed] [Google Scholar]
- 28.Hampl M, Hampl JA, Reiss G et al. (1999) Loss of heterozygosity accumulation in primary breast carcinomas and additionally in corresponding distant metastases is associated with poor outcome. Clin Cancer Res 5(6):1417–1425 [PubMed] [Google Scholar]
- 29.Yoneda T, Williams PJ, Hiraga T et al. (2001) A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16(8):1486–1495 [DOI] [PubMed] [Google Scholar]
- 30.Palmieri D, Lockman PR, Thomas FC et al. (2009) Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res 15(19):6148–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjoblom T, Jones S, Wood LD et al. (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314(5797):268–274 [DOI] [PubMed] [Google Scholar]
- 32.Zhang XH, Wang Q, Gerald W et al. (2009) Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16(1):67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irizarry RA, Hobbs B, Collin F et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4(2):249–264 [DOI] [PubMed] [Google Scholar]
- 34.Olmos D, Allred A, Sharma R, et al. (2009) Phase I first-in-human study of the polo-like kinase-1 selective inhibitor, GSK461364, in patients with advanced solid tumors. In: ASCO Annual Meeting American Society of Clinical Oncology, Abstract no 3536 [Google Scholar]
- 35.van Vugt MA, Gardino AK, Linding R et al. (2004) A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol 8(1):e1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeken JF, Loscher W (2007) The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res 13(6):1663–1674 [DOI] [PubMed] [Google Scholar]
- 37.Wu JM, Fackler MJ, Halushka MK et al. (2008) Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res 14(7):1938–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12(8):895–904 [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Li H, Zhou Z et al. (2009) Plk1-mediated phosphorylation of Topors regulates p53 stability. J Biol Chem 284(28): 18588–18592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weger S, Hammer E, Heilbronn R (2005) Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett 579(22): 5007–5012 [DOI] [PubMed] [Google Scholar]
- 41.Rajendra R, Malegaonkar D, Pungaliya P et al. (2004) Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J Biol Chem 279(35):36440–36444 [DOI] [PubMed] [Google Scholar]
- 42.Momand J, Wu HH, Dasgupta G (2000) MDM2—master regulator of the p53 tumor suppressor protein. Gene 242(1–2):15–29 [DOI] [PubMed] [Google Scholar]
- 43.Kreis NN, Sommer K, Sanhaji M et al. (2009) Long-term downregulation of Polo-like kinase 1 increases the cyclin-dependent kinase inhibitor p21(WAF1/CIP1). Cell Cycle 8(3):460–472 [DOI] [PubMed] [Google Scholar]
- 44.Koida N, Ozaki T, Yamamoto H et al. (2008) Inhibitory role of Plk1 in the regulation of p73-dependent apoptosis through physical interaction and phosphorylation. J Biol Chem 283(13):8555–8563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soond SM, Barry SP, Melino G et al. (2008) p73-mediated transcriptional activity is negatively regulated by polo-like kinase 1. Cell Cycle 7(9):1214–1223 [DOI] [PubMed] [Google Scholar]
- 46.Rödel F, Keppner S, Capalbo G et al. (2010) Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol 177(2):918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.