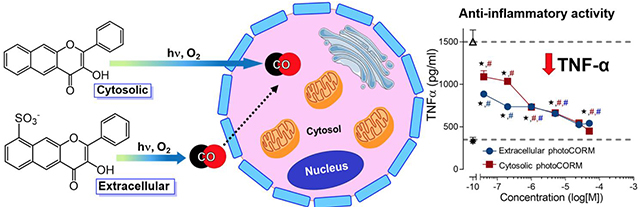
Abstract
Carbon monoxide (CO) is a gasotransmitter produced in humans. An essential unanswered question in the design of carbon monoxide releasing molecules (CORMs) is whether the delivery molecule should be localized extra- or intracellularly to produce desired biological effects. Herein we show that extracellular CO release is less toxic and is sufficient to produce an anti-inflammatory effect similar to that of intracellular CO release at nanomolar concentrations. This information is valuable for the design of CORMs.
Graphical Abstract
INTRODUCTION
Long considered a toxic gas, carbon monoxide (CO) is now recognized as a biologically important signaling molecule.1 Endogenously generated in the breakdown of heme2–4, CO exhibits its biological effects through coordination to low-valent transition metal centers in proteins and enzymes, including to ferrous hemoglobin, which serves as a CO reservoir.1 This stable gas also diffuses extracellularly into surrounding tissues with subsequent exhalation by the lungs.5 Inhalation of controlled amounts of exogenous CO gas has purported health benefits including anti-inflammatory, antioxidant, anti-hypertensive, and vasodilation effects.6 To avoid the dangers associated with the administration of CO gas, inorganic and metal-free CO-releasing molecules (CORMs) have been developed that spontaneously release CO under physiological conditions.7–12 The CORMs that have been used most extensively, thus far, in studies of the biological effects of CO are the transition metal-containing [RuCl2(CO)3]2 (CORM-2)13, [Ru(CO)3Cl(glycinate)] (CORM-3)14, Mn(CO)4{S2CNMe(CH2CO2H)} (CORM-401)15, and the boronocarbonate derivative Na2(H3BCO2).16 Notably, these CORMs, as well as spontaneous metal-free CORMs12, do not offer spatiotemporal control of CO delivery. Using these molecules, it is unclear where and when CO release occurs, as the location of the CORM prior to CO release is unknown. As CO can permeate cellular membranes, the impact of the site of CO release, specifically extra- vs. intracellular delivery, has not been rigorously evaluated to date.
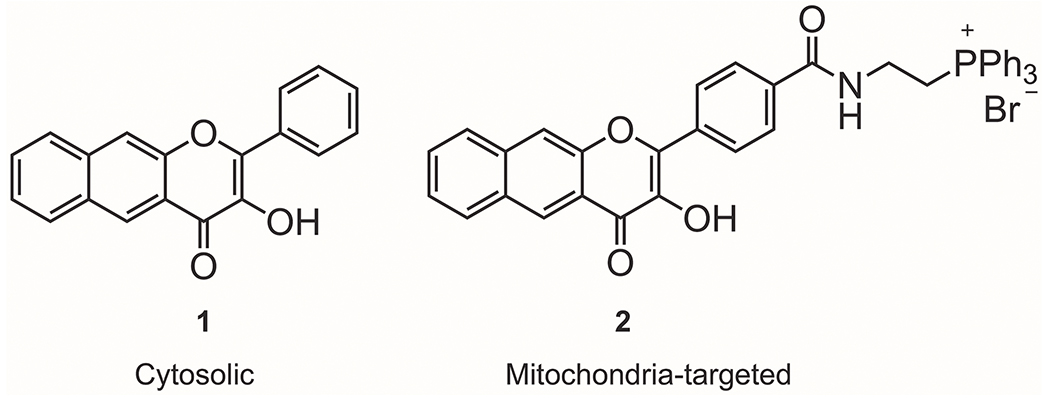
Metal carbonyl17–23 and metal-free24–32 photoCORMs are light-driven CO-releasing molecules that offer high spatiotemporal control of CO delivery in biological systems. Luminescent photoCORMs that exhibit a signal change upon CO release offer the possibility of tracking the location of CO delivery in cellular studies.33 A recent study performed using luminescent, metal-free photoCORMs suggests that the concentration necessary to produce biological effects may be impacted by the site of release.34 Specifically, at a concentration of 10 μM, the intracellular photoCORMs 1 and 2 (Figure 1) induce a decrease in mitochondrial basal respiration, ATP production, maximal respiration, and the reserve capacity of adenocarcinoma human alveolar basal epithelial (A549) cells. Achieving these effects typically requires higher concentrations (>50 μM) of transition metal-based CORMs, which likely release CO extracellularly.35–38
Figure 1.
Flavonol-based non-metal photoCORMs for intracellular CO delivery.
In the study reported herein, we use a pair of structurally similar, trackable organic photoCORMs to test an important fundamental question with regard to the delivery of CO. Specifically, how does extra- versus intracellular CO release impact the biological effects of this stable, diffusible gasotransmitter?
RESULTS AND DISCUSSION
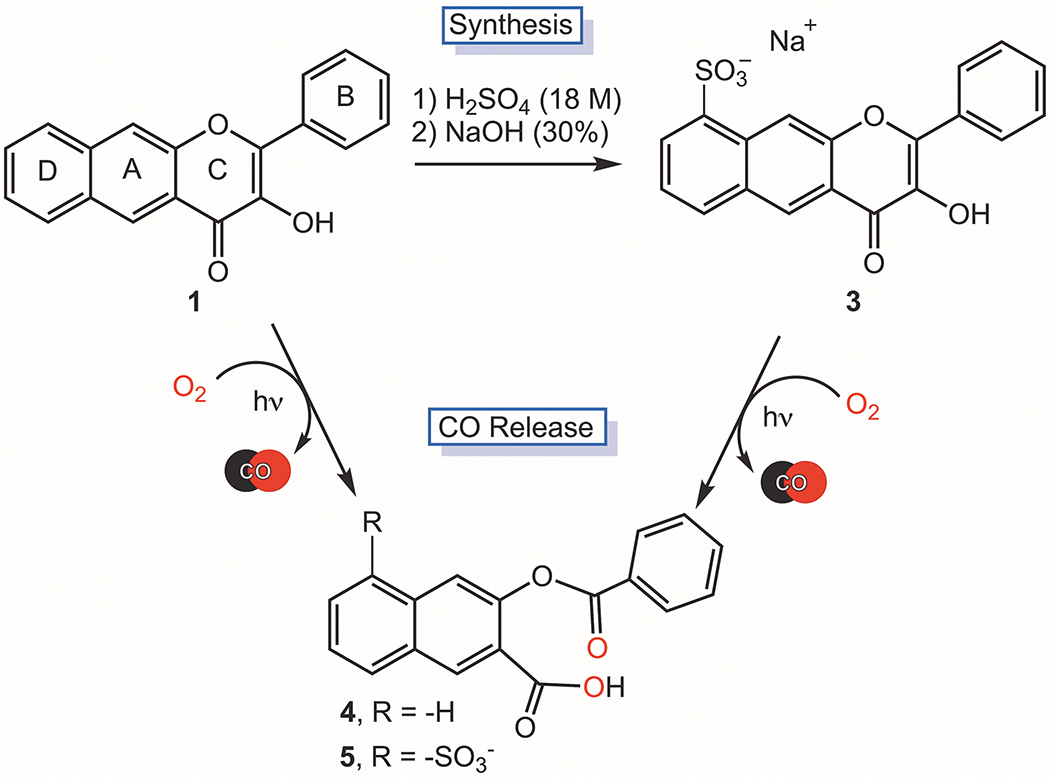
Sulfonation can limit the cellular uptake of small molecules due to decreased lipophilicity and increased water solubility of the scaffold,39,40 with some sulfonated molecules localizing at the external polar surface of the cell membrane.41 Hypoth-esizing that sulfonation of 1 (Figure 1) could provide a water soluble, extracellular analogue of photoCORM 1, we pursued the preparation of such a molecule. Stirring of 1 in 18 M H2SO4 at room temperature for 18 h results in the formation of 3, which is sulfonated on the D ring (Scheme 1). Elemental analysis and HPLC data indicating purity, along with NMR, FTIR, UV-Vis and mass spectrometry data (Figures S1–S6) are consistent with the proposed structure for 3. The assignment of the site of sulfonation was determined based on 2D COSY, HSQC and HMBC NMR data (Figure S1C–E). We note that a minor species (<5%) is always present in samples of 3 by 1H NMR. As only one species is evident by HRMS and HPLC, the 1H NMR features are similar to that of 3, we propose that this minor species is a regioisomer of 3 also sulfonated on the D ring.
Scheme 1.
Preparation of 3 and visible light-induced CO release reactivity of 1 and 3.
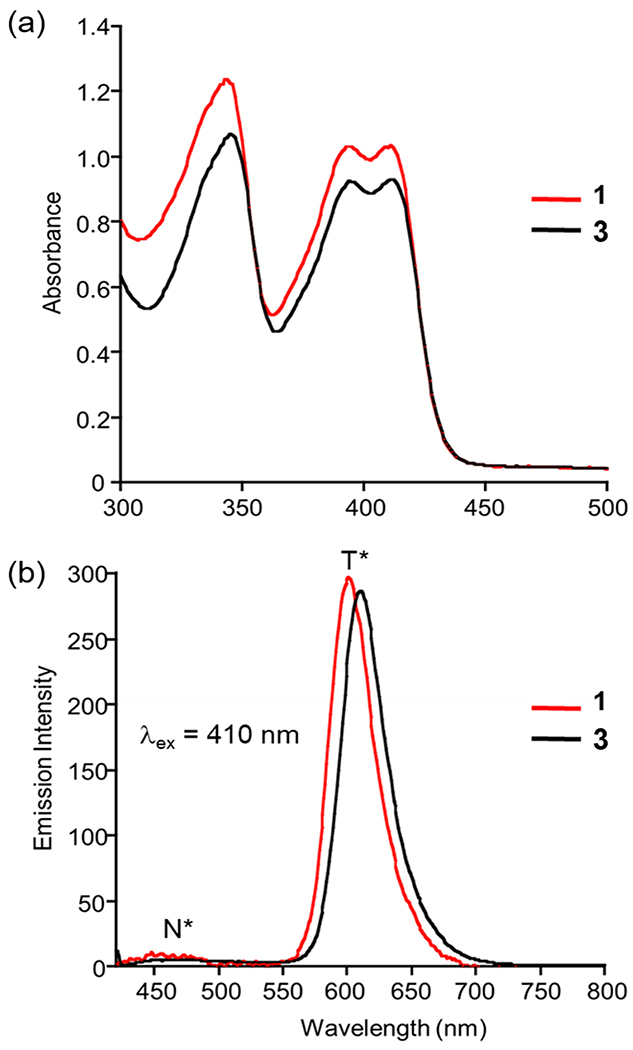
Compound 3 exhibits good solubility in DMSO, water, PBS buffer, and FBS-supplemented cell culture medium at concentrations suitable for spectrophotometric and biological experiments (<50 mM). The compound is stable in the above-mentioned solvents for at least one month if protected from light. The lowest-energy absorption band of 3 in DMSO is centered at ~410 nm (Figure 2(a)). Excitation at this wavelength produces two emission bands centered at ~480 nm and ~610 nm (Figure 2(b)), respectively, corresponding to the excited-state normal (N*) and tautomeric (T*) forms of the neutral flavonol.42 The congruence of the spectral features of 1 and 3 (Figure 2) indicates that sulfonation of the D ring did not affect the photophysical properties of the chromophoric and fluorophoric core. The fluorescence quantum yield and lifetime for 3 (ΦPL = 47.0; 4.8 ns (N2-deaerated DMSO)) are also similar to those of 1 (ΦPL = 34.5; 7.4 ns (N2-deaerated CH3CN)).26
Figure 2.
Absorption (a) and emission (b) spectra of a 100 μM solution of 1 and 3 in DMSO.
Similar to 1, the absorption features of 3 are modulated by the solvent, with an increase in absorbance noted in the 450-500 nm range in aqueous environments (Figure S4). This observation suggests that partial deprotonation of the 3-OH moiety of 3 may occur in the presence of water. Excitation at 410 nm in PBS buffer or water produces a weak emission centered at 560 nm (Figure S6). Notably, the emission is shifted to ~600 nm and is significantly enhanced in intensity in DMEM/F12K media containing 10% fetal bovine serum (Figure S6). This result is likely due to interactions of 3 with serum proteins. The binding constant (Ka) for 3 to bovine serum albumin is 4.1 x 105 (Figure S7), which is >100-fold higher than that found for 1 (3.2 x 103).42 Both 1 and 3 interact in a 1:1 ratio with the protein. Compound 1 releases CO when bound to BSA.42 Excitation of 3 in DMEM/F12K media containing 10% fetal bovine serum (Figure S8) at 467 nm produces a similarly intense emissive feature albeit with the maximum at ~560 nm.
Exposure of an aerobic DMSO solution of 3 to visible light (419 nm) results in loss of the absorption (Figure S9) and emission features of the compound and quantitative CO release (1.0(1) eq). The quantum yield for this CO release reaction is 0.003(3), which is similar to that of 1 (0.006(3)) under identical experimental conditions.30 The organic product resulting from CO release from 3 (5, Scheme 1) is a non-emissive depside which was characterized by 1H and 13C NMR, 2D COSY, HSQC and HMBC (Figure S10A–E), FTIR (Figure S11) and high-resolution mass spectrometry (Figure S12). Overall, these results indicate that 1 and 3 exhibit similar CO release reactivity.
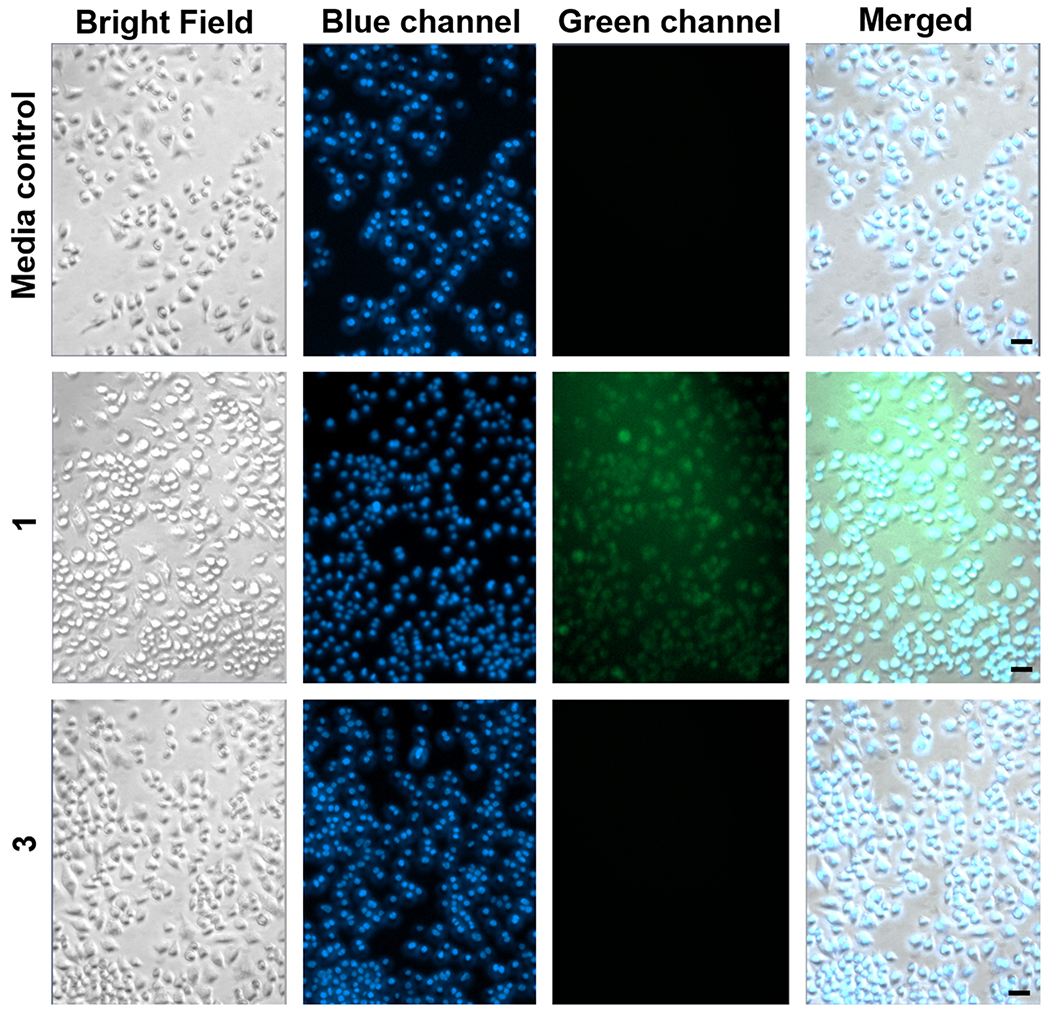
Fluorescence emission studies were performed to evaluate the cellular uptake of 1 versus 3 in RAW 264.7 cells. At 20x magnification, only compound 1 was observed to accumulate intracellularly as evidenced by its green emission (Figure 3). The extracellular compound 3 was not detected when cell washes were performed prior to fluorescence imaging. Using fluorescence microscopy, we compared the washes from the RAW 264.7 cells exposed to 3 to a standard solution containing the initial concentration of 3 (50 μM) introduced to the cells. As shown in Figure S13, the majority of 3 was recovered indicating its limited cell permeability.
Figure 3.
Fluorescence microscopy images of RAW 264.7 cells incubated for 4 h with 1 or 3 (50 μM) followed by washing of the cells prior to imaging. Row 1: Media control for all experiments. Row 2: Cells exposed to 1. Row 3: Cells exposed to 3. The observed green fluorescence in Row 2 is from cytosolic 1. The lack of green emission in Row 3 implies that 3 was not taken up by cells. The cells were co-stained with Hoechst 33342 nuclear dye (blue) to assess cell integrity. Size of bar = 20 μm.
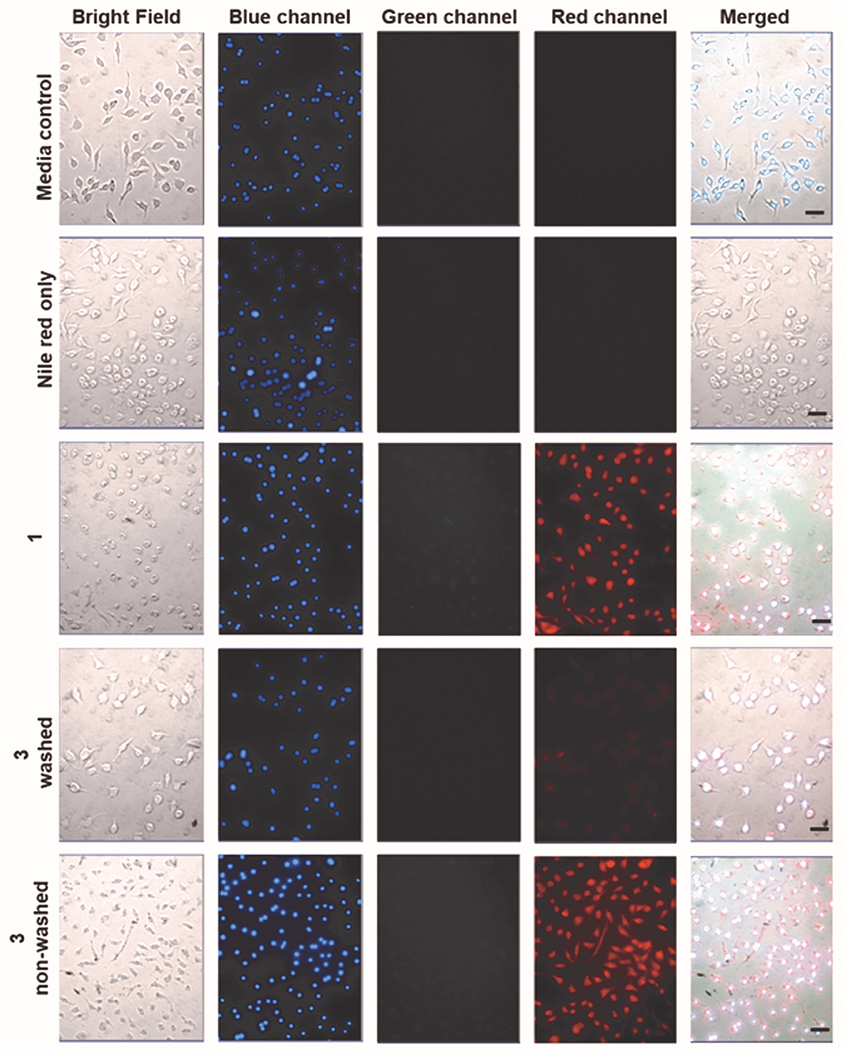
Visible light-induced CO release from both 1 and 3 was examined under conditions wherein cell washes were and were not performed in the presence of a Nile red-based CO sensor (1-Ac, Figure 4).43 Cells exposed to 1 (50 μM), washed and then illuminated with visible light, show bright red emission from the CO sensor (Figure 4, row 3) indicating CO release and detection. We note that the lack of detectable signal in the green channel was expected after 1 h of illumination of 1 as its fluorescence is quenched upon CO release.30 Notably, cells exposed to 3 (50 μM) that were washed prior to light-triggered CO release showed only faint red emission from the sensor (Figure 4, row 4). This is consistent with the removal of the majority of 3 in the cell washes. Cells incubated with 3 (50 μM) and not washed prior to visible light-triggered CO release show bright red emission from the intracellular turn-on CO sensor (Figure 4, row 5). These combined results provide evidence that 1 is a cytosolic compound that releases CO intracellularly, whereas 3 is primarily an extracellular CO donor.
Figure 4.
Fluorescence detection of CO release from 1 and 3 (50 μM) using a Nile red-based CO sensor (1-Ac) in RAW 264.7 cells. Row 1: Media control for all the experiments. Row 2: 1-Ac control after illumination for 1 h. Row 3: Compound 1 incubated for 4 h, followed by cell washes with media, introduction of 1-Ac and illumination for 1 h. Row 4: Compound 3 incubated for 4 h, followed by cell washes with media, introduction of 1-Ac and illumination for 1 h. Row 5: Compound 3 incubated for 4 h, followed by introduction of 1-Ac and illumination for 1 h (cells were not washed prior to addition of the sensor). All cells were co-stained with Hoechst 33342 nuclear dye (blue channel) to assess cell integrity and illuminated with 460 nm LED array (66,3351 lx). Green channel: Detection of fluorescence emission by 1 or 3. Red channel: Detection of CO sensor. Size of bar = 40 μm.
Using MTT assays, both 126 and 3 were found to be nontoxic in RAW 264.7 murine macrophage cells up to 100 μM. However, whereas illumination of 1 to produce CO release results in some cytotoxicity (IC50 22.39 ± 2.98 μM)26, illumination of 3 showed no cytotoxicity up to 100 μM (Figure S14). These results provide evidence that intracellular CO release is more cytotoxic than extracellular release. It should be noted that the depside CO release byproducts (4 and 5) of both compounds are nontoxic in RAW 264.7 cells up to 100 μM (Figure S14).26
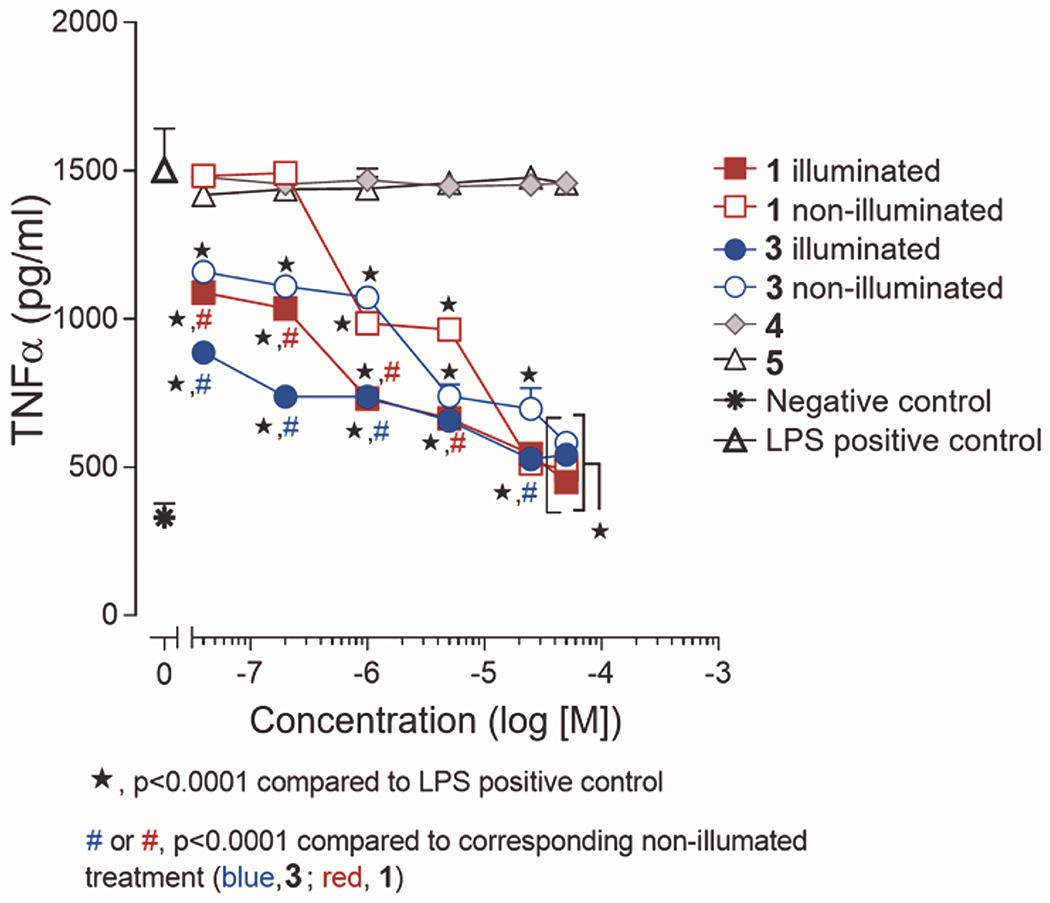
We next comparatively evaluated the effect of CO release from 1 and 3 in the pre-conditioning of LPS-challenged RAW 264.7 murine macrophage cells to suppress TNF-α expression. Two plates of cells were examined for each compound (1 or 3 (concentration up to 50 μM)) and its CO release by-product, with one plate being left in the dark and the other being illuminated for 1 h to induce CO release using a 460 nm LED array. The non-illuminated and illuminated plates were then incubated for additional 6 h at which point they were treated with LPS (1 μg/mL final concentration) for 1 h. The concentration of expressed TNF-α in the supernatant of each well was evaluated using a commercial ELISA kit. As shown in Figure 5, CO release from both 1 and 3 suppresses the TNF-α level to a similar extent at the lowest concentrations examined (40 and 80 nM) when compared to the non-illuminated samples. In other words, the extra- and intra-cellular CO release compounds produce similar CO-induced anti-inflammatory effects. However, the total extent of TNF-α suppression depends on the delivery scaffold, with 3 showing an enhanced suppression over 1 up to a concentration of 1 μM under CO release conditions. The data from the non-illuminated samples provides evidence that the sulfonated framework of 3 is providing an independent contribution to the suppression of TNF-α expression starting at nanomolar concentrations. This anti-inflammatory contribution of 3 independent of CO release is not entirely unexpected as naturally-occurring flavonols such as quercetin have been reported to suppress TNF-α expression in LPS-induced RAW 264.7 macrophages.44
Figure 5.
Anti-inflammatory effects of 1 and 3-5 in RAW 264.7 cells in the presence of light (CO release in situ) or under dark conditions. The results are presented in means ± SEM from three independent experiments. Data were analyzed by two-way ANOVA followed by Sidak’s multiple comparison posthoc tests to compare the effects of all treatments to the LPS positive control or to compare the effects of treatment with compounds 1 and 3 under illuminated and non-illuminated conditions.
CONCLUSION
In summary, we have developed novel trackable organic photoCORMs that have enabled the first comparative studies of the biological effects of CO produced from extra- versus intracellular delivery. Notably, extracellular CO delivery is less toxic but produces similar CO-induced anti-inflammatory effects to intracellular delivery. These results provide important insight into the design of next generation CORMs for achieving various biological effects. The studies presented herein also provide evidence that CO delivery via a donor molecule can be augmented by additional biological activity of the vehicle framework. Future work in our laboratory is directed at further examining the use of flavonol frameworks as multifunctional CO-releasing molecules.
EXPERIMENTAL SECTION
3-Hydroxy-2-phenyl-benzo[g]chromen-4-one (3).
2-hydroxy-2-phenyl-benzo[g]chromen-4-one30 (0.10 g, 0.35 mM, 1 eq.) was suspended in 3 mL of H2SO4 (18 M). This mixture was stirred for 18 hours at room temperature. The solution was then cooled to 0 °C and ddH2O (3 mL) was added slowly, followed by the addition of aqueous NaOH (30%) until the pH = 3.0. A yellow-brown solid was observed to form. This solid was removed by filtration and dried under vacuum (0.12 g, 95%). 1H NMR (DMSO-d6, 500 MHz) δ 9.06 (s, 1H), 8.83 (s, 1H), 8.29 (d, J = 8.8 Hz, 2H), 8.23 (d, J = 8.4 Hz, 1H), 8.08 (d, J = 6.9 Hz, 1H), 7.64-7.58 (m, 3H), 7.56-7.49 (m, 2H); 13C{1H} NMR (DMSO-d6, 125 MHz) δ ppm 174.0, 150.7, 146.3, 143.2, 137.9, 131.5, 131.4, 131.1, 130.2, 130.1, 128.7, 127.9, 126.9, 125.9, 124.6, 120.5, 114.5. (17 signals expected and observed); FTIR (KBr, cm−1) 1637 (νC=O), 1181 (νS=O); UV-vis (DMSO) (ε, M−1cm−1) 344 (10,600), 396 (9,200); (water, nm) (ε, M−1cm−1) 344 (10,400), 396 (8,000); (PBS 10 mM (pH = 7.4), nm) (ε, M−1cm−1) 344 (11,400), 396 (9,200); (DMEM/F12K medium supplemented with 10% FBS, nm) (ε, M−1cm−1) 344 (11,600), 396 (9400); Melting point 132-133 °C; ESI/APCI-MS (relative intensity) calcd. for C19H12O6S [M-H]: 368.0355; found: 368.0380 (100%). Anal. Calcd. for C19H12O6S·1.8H2O: C, 56.94; H, 3.92. Found: C, 57.05; H, 3.73. The presence of H2O in the elemental analysis sample was confirmed using 1H NMR; >95% purity is also indicated by HPLC analysis.
Supplementary Material
ACKNOWLEDGMENT
We thank the NIH (R15GM124596 to L.M.B. and A.D.B.), American Heart Association (18PRE34030099 to T.S.), the USDA National Institute of Food and Agriculture (Hatch Capacity Grant UTA-01178 to A.D.B), the National Science Foundation (CHE-1429195 for Brüker Avance III HD 500 MHz NMR) and the USU Office of Research (PDRF Fellowship to T.S.) for financial support.
ABBREVIATIONS
- A549 cells
adenocarcinoma human alveolar basal epithelial cells
- BSA
bovine serum albumin
- CO
carbon monoxide
- CORM
CO-releasing molecule
- FBS
fetal bovine serum
- LPS
lipopolysaccharide
- RAW 264.7 cells
murine macrophage cells
- photoCORM
light-triggered CORM
- TNF-α
tumor necrosis factor α
Footnotes
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website.
Additional experimental details including (1) characterization data for 3 and 5, (2) experimental data for BSA binding, fluorescence microscopy, MTT assays and TNF-α quantification (PDF); Molecular Smiles strings (CSV).
The authors declare no competing financial interests.
REFERENCES
- (1).Motterlini R; Foresti R Biological Signaling by Carbon Monoxide and Carbon Monoxide-releasing Molecules. Am. J. Physiol. Cell. Physiol 2017, 312, C302–C313. [DOI] [PubMed] [Google Scholar]
- (2).Tenhunen R; Marver HS; Schmid R The Enzymatic Conversion of Heme to Bilirubin by Microsomal Heme Oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Tenhunen R; Marver HS; Schmid R Microsomal Heme Oxygenase. Characterization of the Enzyme. J. Biol. Chem 1969, 244, 6388–6394. [PubMed] [Google Scholar]
- (4).Otterbein LE; Choi AM Heme Oxygenase: Colors of Defense against Cellular Stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1029–L1037. [DOI] [PubMed] [Google Scholar]
- (5).Clanton T; Hogan M; Gladden L Regulation of Cellular Gas Exchange, Oxygen Sensing, and Metabolic Control. Compr. Physiol 2013, 3, 1135–1190. [DOI] [PubMed] [Google Scholar]
- (6).Motterlini R; Otterbein LE The Therapeutic Potential of Carbon Monoxide. Nat. Rev. Drug. Discov 2010, 9, 728–743. [DOI] [PubMed] [Google Scholar]
- (7).Motterlini R; Mann BE; Foresti R Therapeutic Applications of Carbon Monoxide-releasing Molecules. Expert Opin. Invest. Drugs 2005, 14, 1305–1318. [DOI] [PubMed] [Google Scholar]
- (8).Mann BE CO-releasing Molecules: A Personal View. Organometallics 2012, 31, 5728–5735. [Google Scholar]
- (9).Ramão CC; Blättler WA; Seixas JD; Bernardes GJ Developing Drug Molecules for Therapy with Carbon Monoxide. Chem. Soc. Rev 2012, 41, 3571–3583. [DOI] [PubMed] [Google Scholar]
- (10).Heinemann SH; Hoshi T; Westerhausen M; Schiller A Carbon Monoxide – Physiology, Detection and Controlled Release. Chem. Commun 2014, 50, 3644–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Schatzschneider U Novel Lead Structures and Activation Mechasnims for CO-releasing Molecules (CORMs). Br. J. Pharmacol 2015, 172, 2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ji X; Wang B Strategies toward Organic Carbon Monoxide Prodrugs. Acc. Chem. Res 2018, 51, 1377–1385. [DOI] [PubMed] [Google Scholar]
- (13).Motterlini R; Clark JE; Foresti R; Sarathchandra P; Mann BE; Green CJ Carbon Monoxide-releasing Molecules: Characterization of Biochemical and Vascular Activities. Circ. Res 2002, 90, E17–24. [DOI] [PubMed] [Google Scholar]
- (14).Foresti R; Hammad J; Clark JE; Johnson TR; Mann BE; Friebe A; Green CJ; Motterlini R Vasoactive Properties of CORM-3, A Novel Water-soluble Carbon Monoxide Releasing Molecule. Br. J. Pharmacol 2004, 142, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Crook SH; Mann BE; Meijer AJHM; Adams H; Sawle P; Scapens D; Motterlini R [Mn(CO)4{SCNMe(CH2CO2H}], A New Water-soluble CO Releasing Molecule. Dalton Trans. 2011, 40, 4230–4235. [DOI] [PubMed] [Google Scholar]
- (16).Motterinli R; Sawle P; Hammad J; Bains S; Alberto R; Foresti R; Green CJ CORM-A1: A New Pharmacologically Active Carbon-monoxide Releasing Molecule. FASEB 2005, 19, 284–286. [DOI] [PubMed] [Google Scholar]
- (17).Ford PC Metal Complex Strategies for Photo-uncaging the Small Molecule Bioregulators Nitric Oxide and Carbon Monoxide. Coord. Chem. Rev 2018, 376, 548–564. [Google Scholar]
- (18).Mede R; Hoffman P; Neumann C; Görls H; Schmitt M; Popp J; Neugebauer U; Westerhausen M Acetoxymethyl Concept for Intracellular Administration of Carbon Monoxide with Mn(CO)3-based PhotoCORMs. Chem. Eur. J 2018, 24, 3321–3329. [DOI] [PubMed] [Google Scholar]
- (19).Kottelat E; Zobi F Visible-light Activated PhotoCORMs. Inorganics 2017, 5, 24. [Google Scholar]
- (20).Wright MA; Wright JA PhotoCORMs: CO Release Moves into the Visible. Dalton Trans. 2016, 45, 6801–6811. [DOI] [PubMed] [Google Scholar]
- (21).Chakraborty I; Carrington SJ; Mascharak PK Design Strategies to Improve the Sensitivity of Photoactive Metal Carbonyl Complexes (PhotoCORMs) to Visible Light and their Potential as CO-donors to Biological Targets. Acc. Chem. Res 2014, 47, 2603–2611. [DOI] [PubMed] [Google Scholar]
- (22).Gonzales MA; Mascharak PK Photoactive Metal Carbonyl Complexes as Potential Agents for Targeted CO Delivery. J. Inorg. Biochem 2014, 133, 127–135. [DOI] [PubMed] [Google Scholar]
- (23).Schatzschneider U PhotoCORMs: Light-triggered Release of Carbon Monoxide from the Coordination Sphere of Transition Metal Complexes for Biological Applications. Inorg. Chim. Acta 2011, 374, 19–23. [Google Scholar]
- (24).Feng W; Feng S; Feng G CO Release with Ratiometric Fluorescence Changes: A Promising Visible-light-triggered CO Releasing Molecule. Chem. Commun 2019, 55, 8987–8990. [DOI] [PubMed] [Google Scholar]
- (25).Li Y; Shu Y; Liang M; Xie X; Jiao X; Wang X; Tang B A Two-photon H2O2-Activated CO Photoreleaser. Angew. Chem. Int. Ed 2018, 57, 12415–12419. [DOI] [PubMed] [Google Scholar]
- (26).Popova M; Soboleva T; Ayad S; Benninghoff AD; Berreau LM Visible-light-activated Carbon-monoxide-releasing Molecule: Prodrug and Albumin–assisted Delivery Enables Anti-cancer and Potent Anti-inflammatory Effects. J. Am. Chem. Soc 2018, 140, 9721–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Slanina T; Šebej P Visible-light-activated PhotoCORMs: Rational Design of CO-releasing Organic Molecules Absorbing in the Tissue-transparent Window. Photochem. Photobiol. Sci 2018, 17, 692–710. [DOI] [PubMed] [Google Scholar]
- (28).Abeyrathna N; Washington K; Bashur C; Liao Y Non-metallic Carbon Monoxide Releasing Molecules. Org. Biomol. Chem 2017, 15, 8692–8699. [DOI] [PubMed] [Google Scholar]
- (29).Palao E; Slanina T; Muchová L; Šolomek T; Vitek L; Klán P Tranisition metal-free CO-releasing BODIPY Derivatives Activatable by Visible to NIR light as Promising Bioactive Molecules. J. Am. Chem. Soc 2016, 138, 126–133. [DOI] [PubMed] [Google Scholar]
- (30).Anderson SN; Richards JM; Esquer HJ, Benninghoff AD; Arif AM; Berreau LM A Structurally-tunable 3-Hydroxyflavone Motif for Visible Light-induced Carbon Monoxide-releasing Molecules. ChemistryOpen 2015, 4, 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Antony LAP; Slanina T; Šebej P; Šolomek T; Klán P Fluorscein Analogue Xanthene-9-carboxylic Acid: A Transition-metal-free CO Releasing Molecule Activated by Green Light. Org. Lett 2013, 15, 4552–4555. [DOI] [PubMed] [Google Scholar]
- (32).Peng P; Wang C; Shi Z; Johns VK; Ma L; Oyer J; Copik A; Igarashi R; Liao Y Visible-light Activatable Organic CO-releasing Molecules (PhotoCORMs) that Simultaneously Generate Fluorophores. Org. Biomol. Chem 2013, 11, 6671–6674. [DOI] [PubMed] [Google Scholar]
- (33).Soboleva T; Berreau LM Tracking CO Release in Cells via the Luminescence of Donor Molecules and/or their Byproducts. Isr. J. Chem 2019, 359, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Soboleva T; Esquer HJ; Anderson SN; Berreau LM; Benninghoff AD Mitochondrial-localized Versus Cytosolic Intracellular CO-releasing Organic PhotoCORMs: Evaluation of CO Effects using Bioenergetics. ACS Chem. Biol. 2018, 13, 2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kaczara P; Motterlini R; Rosen GM; Augustynek B; Bednarczyk P; Szewczyk A; Foresti R; Chlopicki S Carbon Monoxide Release by CORM-401 Uncouples Mitochondrial Respiration and Inhibits Glycolysis in Endothelial Cells: A Role for Mi-toBKCa Channels. Biochim. Biophys. Acta 2015, 1847, 1297–1309. [DOI] [PubMed] [Google Scholar]
- (36).Wilson JL; Bouillaud F; Almedia AS; Vieira HL; Ouidja MO; Dubois-Randé JL; Foresti R; Motterlini R Carbon Monoxide Reverses the Metabolic Adaptation of Microglia Cells to an Inflammatory Stimulus. Free Radic. Biol. Med. 2017, 104, 311–323. [DOI] [PubMed] [Google Scholar]
- (37).Reiter CEN; Alayash AI Effects of Carbon Monoxide (CO) Delivery by a CO Donor or Hemoglobin on Vascular Hypoxia Inducible Factor 1α and Mitochondrial Respiration. FEBS Open Bio. 2012, 2, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Iacono LL; Boczkowski J; Zini R; Salouage I; Berdeaux A; Motterlini R; Morin D A Carbon Monoxide-releasing Molecule (CORM-3) Uncouples Mitochondrial Respiration and Modulates the Production of Reactive Oxygen Species. Free Radic. Biol. Med. 2011, 50, 1556–1564. [DOI] [PubMed] [Google Scholar]
- (39).Chan W-S; Marshall JF; Svensen R; Bedwell J; Hart IR Effect of Sulfonation on the Cell and Tissue Distribution of the Photosensitizer Aluminum Phthalocyanine. Cancer Res. 1990, 50, 4533–4538. [PubMed] [Google Scholar]
- (40).Fu Y-J; Yao H-W; Zhu X-Y; Guo X-F; Wang H A Cell Surface Specific Two-photon Fluorescent Probe for Monitoring Intracellular Transmission of Hydrogen Sulfide. Anal. Chim. Acta 2017, 994, 1–9. [DOI] [PubMed] [Google Scholar]
- (41).Cardone A; Lopez F; Affortunato F; Busco G; Hofer AM; Mallamaci R; Martinelli C; Colella M; Farinola GM An Aryleneethynylene Fluorophore for Cell Membrane Staining. Biochim. Biophys. Acta 2012, 1818, 2808–2817. [DOI] [PubMed] [Google Scholar]
- (42).Popova M; Soboleva T; Arif AM; Berreau LM Properties of a Flavonol-based PhotoCORM in Aqueous Buffered Solutions: Influence of Metal ions, Surfactants and Proteins on Visible Light-induced CO Release. RSC Adv. 2017, 7, 21997–22007. [Google Scholar]
- (43).Liu K; Kong X; Ma Y; Lin W Rational Design of a Robust Fluorescent Probe for the Detection of Endogenous Carbon Monoxide in Living Zebrafish Embryos and Mouse Tissue. Angew. Chem. Int. Ed 2017, 56, 13489–13492. [DOI] [PubMed] [Google Scholar]
- (44).Cho YH; Kim NH; Khan I; Yu JM; Jung HG; Kim HH; Jang JY; Kim HJ; Kim DI; Kwak JH; Kang SC; An BJ Anti-inflammatory Potential of Quercetin-3-O-β-D-(„2“-galloyl)-glucopyranoside and Quercetin Isolated from Diospyros kaki calyx via Suppression of MAP Signaling Molecules in LPS-induced RAW 264.7 Macrophages. J. Food. Sci 2016, 81, C2447–C2456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.