Abstract
BACKGROUND
Observational studies suggest that beta-blockers may reduce the risk of exacerbations and death in patients with moderate or severe chronic obstructive pulmonary disease (COPD), but these findings have not been confirmed in randomized trials.
METHODS
In this prospective, randomized trial, we assigned patients between the ages of 40 and 85 years who had COPD to receive either a beta-blocker (extended-release metoprolol) or placebo. All the patients had a clinical history of COPD, along with moderate airflow limitation and an increased risk of exacerbations, as evidenced by a history of exacerbations during the previous year or the prescribed use of supplemental oxygen. We excluded patients who were already taking a beta-blocker or who had an established indication for the use of such drugs. The primary end point was the time until the first exacerbation of COPD during the treatment period, which ranged from 336 to 350 days, depending on the adjusted dose of metoprolol.
RESULTS
A total of 532 patients underwent randomization. The mean (±SD) age of the patients was 65.0±7.8 years; the mean forced expiratory volume in 1 second (FEV1) was 41.1±16.3% of the predicted value. The trial was stopped early because of futility with respect to the primary end point and safety concerns. There was no significant between-group difference in the median time until the first exacerbation, which was 202 days in the metoprolol group and 222 days in the placebo group (hazard ratio for metoprolol vs. placebo, 1.05; 95% confidence interval [CI], 0.84 to 1.32; P=0.66). Metoprolol was associated with a higher risk of exacerbation leading to hospitalization (hazard ratio, 1.91; 95% CI, 1.29 to 2.83). The frequency of side effects that were possibly related to metoprolol was similar in the two groups, as was the overall rate of nonrespiratory serious adverse events. During the treatment period, there were 11 deaths in the metoprolol group and 5 in the placebo group.
CONCLUSIONS
Among patients with moderate or severe COPD who did not have an established indication for beta-blocker use, the time until the first COPD exacerbation was similar in the metoprolol group and the placebo group. Hospitalization for exacerbation was more common among the patients treated with metoprolol. (Funded by the Department of Defense; BLOCK COPD ClinicalTrials.gov number, NCT02587351.)
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide. Most of COPD-related morbidity, mortality, and health care costs are driven by exacerbations, particularly those leading to hospitalization.1,2 Since many patients have such exacerbations despite maintenance therapy, new approaches to treatment are needed.2
An exacerbation of COPD may be triggered or made more severe by underlying cardiovascular disease.3 Patients with COPD have up to five times the risk of cardiovascular disease as age-matched controls,4 and cardiovascular disease has been shown to be a risk factor for COPD exacerbations,5 hospitalization for exacerbations,6 in-hospital death,7,8 and reduced survival.9,10
It is well established that beta-blockers reduce mortality in patients after myocardial infarction11 and in those with heart failure.12 Patients with COPD are often not treated with this class of medications, even when they have an evidence-based indication for the use of such drugs, because of concern about possible adverse effects on lung function.13,14 This practice pattern persists despite multiple observational studies suggesting that beta-blockers benefit patients with COPD and coexisting cardiovascular disease, with outcomes similar to those observed in patients without COPD.13,15,16 Several nonrandomized observational studies involving patients with COPD have also suggested that beta-blockers reduce the risk of exacerbations and death, regardless of the presence of cardiac disease.17–20 However, these observational data are subject to biases, which has precluded determinations regarding cause and effect.21
In the BLOCK COPD (Beta-Blockers for the Prevention of Acute Exacerbations of Chronic Obstructive Pulmonary Disease) trial, we investigated the effect of the beta-blocker metoprolol, as compared with placebo, on the risk of COPD exacerbations among patients who were at high risk for such events.22 We hypothesized that the use of metoprolol would lower the risk of exacerbations in these patients without having an adverse effect on lung function, results on a 6-minute walk test, dyspnea, or quality of life.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted this placebo-controlled, double-blind, prospective, randomized trial at 26 centers in the United States. The trial protocol, which was approved by the data and safety monitoring committee and the institutional review board at each trial center, is available with the full text of this article at NEJM.org. The Department of Defense funded the trial but had no role in its design, in the accrual or analysis of the data, or in the preparation of the manuscript. No commercial entity was involved in the trial. Written informed consent was obtained from all the patients.
INCLUSION AND EXCLUSION CRITERIA
We enrolled patients between the ages of 40 and 85 years who had received a clinical diagnosis of COPD and who had at least moderate airflow limitation, as defined by the Global Initiative for Obstructive Lung Disease (GOLD),2 as follows: a forced expiratory volume in 1 second (FEV1) of less than 80% of the predicted value after bronchodilation and a ratio of the FEV1 to the forced vital capacity (FVC) of less than 0.70. We recruited patients who were at increased risk for exacerbations as indicated by at least one of the following factors: the receipt of a course of systemic glucocorticoids or antibiotic agents for respiratory problems during the previous year, a visit to an emergency department or hospitalization for a COPD exacerbation during the previous year, or the receipt of a prescription for supplemental oxygen for use at home for the treatment of COPD. The inclusion criteria were a resting heart rate between 65 and 120 beats per minute and a resting systolic blood pressure of more than 100 mm Hg. We excluded patients who had a proven indication for the use of a beta-blocker, including a history of myocardial infarction or revascularization within the previous 36 months or heart failure with a known left ventricular ejection fraction of less than 40%.23,24
RANDOMIZATION AND INTERVENTION
Randomization was performed by a computer algorithm by means of an interactive website linked to the data coordinating center. The starting dose was one 50-mg tablet of metoprolol or matching placebo taken orally daily. Metoprolol was purchased for use in the trial; matching placebo was manufactured at the Current Good Manufacturing Practices Facility at the Temple University School of Pharmacy. For 42 days after randomization, patients underwent a dose-adjustment period on the basis of their heart rate, systolic blood pressure, changes in FEV1, and assessment of possible beta-blocker side effects. This dose adjustment resulted in a final daily dose of 25 mg, 50 mg, or 100 mg. Patients were followed until completion of the day 336 visit, after which they were weaned off either metoprolol or placebo, and were monitored for symptoms of beta-blocker withdrawal until the day 378 visit.
PRIMARY AND SECONDARY END POINTS
The primary end point was the median time until the first COPD exacerbation of any severity during the treatment period, which was defined as the period from randomization to day 336 for the patients receiving a final dose of 25 mg of metoprolol or placebo or until day 350 for those receiving a dose of 50 mg or 100 mg. This difference in treatment period according to dose was due to the additional time necessary to wean patients from the 50-mg and 100-mg dose levels.
An exacerbation of COPD was defined as an increase in or a new onset of two or more of the following symptoms: cough, sputum production, wheezing, dyspnea, or chest tightness that led to treatment with antibiotics or systemic glucocorticoids for at least 3 days.25,26 The severity of the exacerbation was graded according to the following scale: mild (involving only home management, with or without contact with a health care provider), moderate (leading to a visit to an emergency department), severe (leading to hospitalization), and very severe (leading to intubation and mechanical ventilation). Key secondary end points included the rate of COPD exacerbations, all-cause mortality, all-cause hospitalization, results of spirometry, distance on the 6-minute walk test, dyspnea assessments, and measures of quality of life.
TRIAL VISITS
During in-clinic visits and telephone calls, the patients were queried regarding the efficacy and safety of the trial treatment, including providing details regarding any possible beta-blocker side effects. Spirometry and 6-minute walk tests were performed according to American Thoracic Society–European Respiratory Society guidelines.27,28 Data regarding spirometry that was performed after bronchodilation are presented as a percentage of predicted reference values.29 We evaluated the patients’ disease-specific quality of life using scores on the St. George’s Respiratory Questionnaire30 and the COPD Assessment Test31 and assessed the level of dyspnea using the modified Medical Research Council (mMRC) scale32 and the San Diego Shortness of Breath Questionnaire33 (SOBQ). In addition, we measured the 6-minute walk distance at baseline, at the day 112 visit, and at the day 336 visit. (Scores on the St. George’s Respiratory Questionnaire range from 0 to 100, with lower scores indicating better functioning and with a minimal clinically important difference [MCID] of 4 points.30 Scores on the COPD Assessment Test range from 0 to 40, with lower scores indicating better functioning and with a MCID of 2 points.31 Scores for dyspnea on the mMRC scale range from 0 to 4, with higher scores indicating more severe breathlessness.32 Scores on the San Diego Shortness of Breath Questionnaire range from 0 to 120, with higher scores indicating more severe breathlessness and with an MCID of 5 points.33)
MONITORING PLAN, INTERIM ANALYSIS, AND EARLY TERMINATION
The data and safety monitoring committee met approximately every 6 months to review recruitment, follow-up rates, safety, and efficacy results. Reviews of outcome data involved multiple statistical testing procedures performed on a set of accumulating data, with the use of a sequential monitoring plan based on the alpha spending approach.34
After the first interim analysis on November 30, 2018, the committee recommended that the trial be continued but planned to reconvene before the second interim analysis to review serious adverse events. On March 21, 2019, the committee recommended that the trial be stopped on the basis of the conditional power analyses and concern about safety. (Details regarding the power analyses are provided in the Supplementary Appendix, available at NEJM.org.) Patients who had not yet completed the day 336 visit were contacted early to undergo final assessments and begin weaning from metoprolol or placebo, according to the protocol.
STATISTICAL ANALYSIS
We based the sample size and considerations for statistical power on the primary end point of the time until the first exacerbation of COPD. On the basis of data from previous clinical trials of a similar design,25,26 we estimated that 65% of the patients in the placebo group would have an exacerbation during the 1-year trial and that metoprolol would reduce this risk to 55%. Sample-size calculations that included a two-sided alpha level of 0.05 and a trial power of 90% indicated we would need to enroll 1028 patients on the assumption of a loss to follow-up of approximately 12%.
The primary analysis was based on Kaplan–Meier survival curves that described the probability of remaining exacerbation-free in each of the two groups. We used the log-rank test to compare the two curves. As secondary analyses, we used both unadjusted and adjusted Cox proportional-hazards models to assess the association between the trial-group assignment and the time until the first COPD exacerbation. Adjusted models included the covariates of race, sex, baseline age, FEV1 as a percentage of the predicted value, smoking status, heart rate greater than the median value, number of hospitalizations for COPD during the previous year, number of exacerbations treated with glucocorticoids or antibiotics during the previous year, use of supplemental oxygen, scores on the COPD Assessment Test and the mMRC scale, and trial center.
We used Kaplan–Meier methods and Cox models to perform similar analyses of overall survival and used negative binomial regression models to analyze exacerbation rates. We used Student’s t-tests to compare annualized rates of hospitalization and nonfatal serious adverse events and used mixed-effects models with patient-specific random intercepts to compare between-group differences in changes in continuous measures of secondary end points. All the analyses are based on the intention-to-treat principle.
RESULTS
PATIENTS
From May 2016 through March 2019, a total of 532 patients underwent randomization (268 to the metoprolol group and 264 to the placebo group). The most common reasons for exclusion were not meeting the spirometric criteria for COPD or a resting heart rate that was out of the mandated range. Details regarding screening, randomization, and follow-up are provided in Figure 1.
Figure 1. Screening, Randomization, and Follow-up.
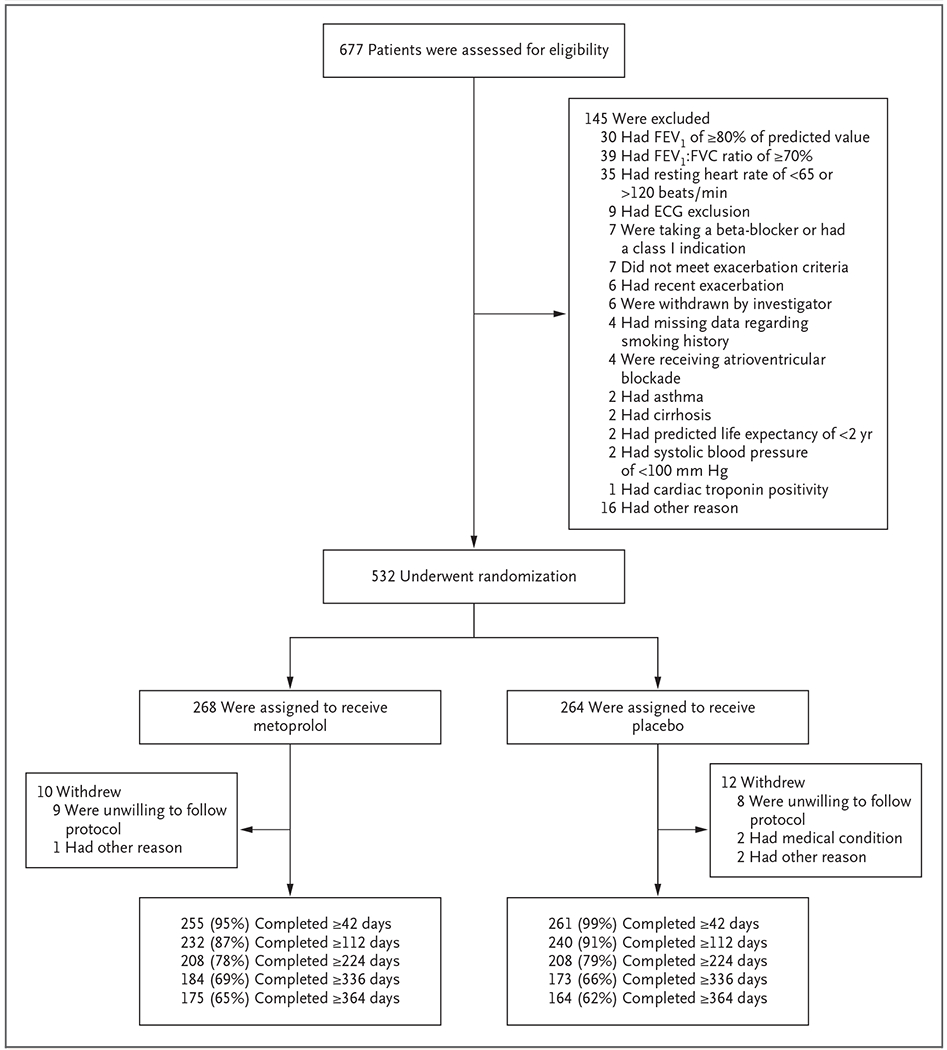
Among the 145 patients who were excluded from the trial, several had more than one reason for exclusion. Patients were excluded from the trial if they had a class I indication for receipt of a beta-blocker (a history of myocardial infarction or revascularization within the previous 36 months or heart failure with a known left ventricular ejection fraction of less than 40%), according to the guidelines of the American College of Cardiology and the American Heart Association. ECG denotes electrocardiography, FEV1 forced expiratory volume in 1 second, and FVC forced vital capacity.
The demographic and clinical characteristics of the patients at baseline are provided in Table 1, with a full list provided in Table S1 in the Supplementary Appendix. The mean (±SD) age of the patients was 65.0±7.8 years, the mean FEV1 was 41.1±16.3% of the predicted value, and the mean smoking exposure was 50.1±29.1 pack-years.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Metoprolol (N = 268) | Placebo (N = 264) |
|---|---|---|
| Age — yr | 65.2±7.6 | 64.8±7.9 |
| Race — no. (%)† | ||
| White | 178 (66.4) | 194 (73.5) |
| Black | 83 (31.0) | 60 (22.7) |
| Other | 7 (2.6) | 10 (3.8) |
| Female sex — no. (%) | 124 (46.3) | 123 (46.6) |
| FEV1 after bronchodilation — % of predicted value | 41.3±16.3 | 40.8±16.2 |
| FEV1:FVC ratio — % | 44.2±11.7 | 45.2±21.6 |
| Smoking history | ||
| No. of pack-yr | 50.7±28.7 | 49.5±29.6 |
| Current smoker — no. (%) | 95 (35.4) | 71 (26.9) |
| COPD medication — no. (%) | ||
| Inhaled glucocorticoid, LABA, and LAMA | 154 (57.5) | 160 (60.6) |
| Inhaled glucocorticoid and LABA | 45 (16.8) | 51 (19.3) |
| LAMA only | 20 (7.5) | 17 (6.4) |
| LABA and LAMA | 11 (4.1) | 13 (4.9) |
| Inhaled glucocorticoid and LAMA | 8 (3.0) | 6 (2.3) |
| Inhaled glucocorticoid only | 5 (1.9) | 2 (0.8) |
| Other | 25 (9.3) | 15 (5.7) |
| Heart rate — beats/min | 85.5±10.8 | 83.6±11.7 |
| Blood pressure — mm Hg | ||
| Systolic | 128.4±16.5 | 130.6±15.9 |
| Diastolic | 77.2±9.2 | 76.8±9.1 |
| No. of courses of systemic glucocorticoids or antibiotic use in previous 12 mo | 1.9±1.5 | 1.9±1.7 |
| No. of hospitalizations in previous 12 mo | 0.7±1.0 | 0.5±1.2 |
| Score on COPD Assessment Test‡ | 20.1±7.3 | 21.3±7.3 |
| Score of >1 on modified Medical Research Council scale — no. (%)§ | 164 (61.2) | 169 (64.0) |
| Enrollment criteria — no. (%) | ||
| Systemic glucocorticoid or antibiotic use in previous 12 mo | 246 (91.8) | 228 (86.4) |
| COPD exacerbation leading to emergency department visit or hospitalization in previous 12 mo | 168 (62.7) | 133 (50.4) |
| Prescription or use of supplemental oxygen in previous 12 mo | 106 (39.6) | 106 (40.2) |
Plus-minus values are means ±SD. Percentages may not total 100 because of rounding. COPD denotes chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, LABA long-acting beta agonist, and LAMA long-acting muscarinic antagonist.
Race was reported by the patients.
Scores on the COPD Assessment Test range from 0 to 40, with lower scores indicating better functioning and with a minimal clinically important difference of 2 points.
Scores for dyspnea on the modified Medical Research Council scale range from 0 to 4, with higher scores indicating more severe breathlessness.
COPD EXACERBATIONS
There was no significant between-group difference in the median time until the first exacerbation, which was 202 days (95% confidence interval [CI], 162 to 282) in the metoprolol group and 222 days (95% CI, 189 to 295) in the placebo group (Fig. 2A). The unadjusted hazard ratio for the comparison between metoprolol and placebo was 1.05 (95% CI, 0.84 to 1.32; P = 0.66), which was similar after adjustment (hazard ratio, 1.12; 95% CI, 0.88 to 1.42). For the time until the first exacerbation of moderate severity or greater, the unadjusted hazard ratio was 1.47 (95% CI, 1.06 to 2.04) and the adjusted hazard ratio was 1.46 (95% CI, 1.03 to 2.06) (Fig. S1A). For severe or very severe exacerbations, the unadjusted and adjusted hazard ratios were 1.91 (95% CI, 1.29 to 2.83) and 2.08 (95% CI, 1.37 to 3.14), respectively (Fig. 2B). The result of the subgroup analysis of the risk of exacerbation is provided in Figure S2.
Figure 2. Exacerbations of Chronic Obstructive Pulmonary Disease (COPD).
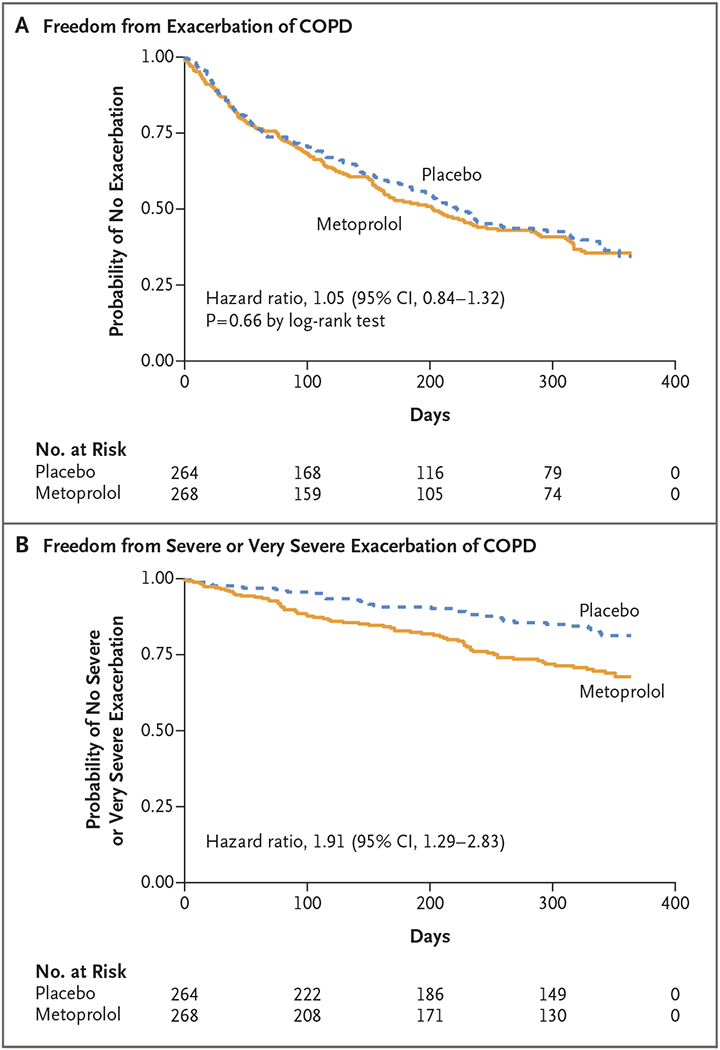
Panel A shows the Kaplan-Meier estimate of freedom from exacerbation of COPD in the two trial groups. The median time until the first exacerbation was 202 days in the metoprolol group and 222 days in the placebo group. Panel B shows the probability of freedom from either a severe exacerbation (leading to hospitalization) or a very severe exacerbation (leading to hospitalization with intubation and mechanical ventilation). Severe or very severe exacerbations occurred in 26.1% of the patients in the metoprolol group and in 14.8% of those in the placebo group.
We found no evidence of a between-group difference in the overall rates of exacerbation, with a rate per person-year of 1.40 (95% CI, 1.21 to 1.61) in the metoprolol group and 1.33 (95% CI, 1.15 to 1.54) in the placebo group (rate ratio, 1.05; 95% CI, 0.85 to 1.28). There was evidence that the metoprolol group had a higher rate of more severe exacerbation than the placebo group, with a rate ratio of 1.51 (95% CI, 1.00 to 2.29) for severe exacerbation and 3.71 (95% CI, 1.10 to 16.98) for very severe exacerbation (Table 2 and Fig. S3).
Table 2.
Rate of Exacerbation of COPD, According to Severity.
| Severity of Exacerbation | Metoprolol (N = 268) | Placebo (N = 264) | Rate Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Events | Rate (95% CI) | Events | Rate (95% CI) | ||
| no. | no. of events/person-yr | no. | no. of events/person-yr | ||
| Any severity | 289 | 1.40 (1.21–1.61) | 272 | 1.33 (1.15–1.54) | 1.05 (0.85–1.28) |
| Mild | 163 | 0.78 (0.65–0.94) | 178 | 0.88 (0.74–1.05) | 0.89 (0.69–1.15) |
| Moderate | 34 | 0.17 (0.11–0.25) | 36 | 0.18 (0.12–0.26) | 0.94 (0.53–1.65) |
| Severe | 81 | 0.40 (0.30–0.52) | 55 | 0.26 (0.19–0.36) | 1.51 (1.00–2.29) |
| Very severe | 11 | 0.05 (0.03–0.10) | 3 | 0.01 (0.00–0.05) | 3.71 (1.10–16.98) |
| Moderate or greater | 126 | 0.62 (0.50–0.77) | 94 | 0.45 (0.35–0.58) | 1.36 (0.98–1.91) |
| Severe or very severe | 92 | 0.45 (0.35–0.58) | 58 | 0.28 (0.21–0.38) | 1.63 (1.10–2.42) |
MORTALITY
During the treatment period, there were 11 deaths in the metoprolol group and 5 in the placebo group, with unadjusted and adjusted hazard ratios for death of 2.18 (95% CI, 0.76 to 6.29) and 2.13 (95% CI, 0.69 to 6.42), respectively (Fig. S1B). The majority of deaths in the metoprolol group were attributed to COPD (7, vs. 1 in the placebo group) (Table 3). After the treatment period, there were 3 additional deaths in the metoprolol group (at 10 to 277 days after the last dose) and 4 additional deaths in the placebo group (at 10 to 26 days after the last dose).
Table 3.
Nonfatal and Fatal Serious Adverse Events.*
| Event | Metoprolol (N = 268) | Placebo (N = 264) | P Value† |
|---|---|---|---|
| Nonfatal adverse events — no. of events per person-yr‡ | |||
| All events | 0.650 | 0.430 | 0.07 |
| Cardiovascular event | |||
| Myocardial infarction | 0.009 | 0.004 | 0.51 |
| Heart failure | 0.008 | 0.014 | 0.57 |
| Stroke | 0.004 | 0.008 | 0.65 |
| Arrhythmias | 0.012 | 0.008 | 0.71 |
| Hypotension | 0 | 0.004 | 0.31 |
| Other cardiovascular event | 0.004 | 0.004 | 0.99 |
| Respiratory event | |||
| COPD exacerbation§ | 0.430 | 0.190 | 0.02 |
| Pneumonia | 0.084 | 0.057 | 0.34 |
| Other respiratory event | 0.020 | 0.004 | 0.16 |
| Fatal events — no. of patients (%)¶ | |||
| All events | 11 (4.1) | 5 (1.9) | 0.14 |
| COPD | 7 (2.6) | 1 (0.4) | — |
| Sudden cardiac death | 0 | 1 (0.4) | — |
| Lung cancer | 1 (0.4) | 0 | — |
| Sepsis | 1 (0.4) | 1 (0.4) | — |
| Unknown | 1 (0.4) | 2 (0.8) | — |
| Other | 1 (0.4) | 0 | — |
Listed are adverse events that were reported as serious by the investigator.
For nonfatal adverse events, P values were calculated by Student’s t-test. For fatal adverse events, the P value for the overall between-group comparison was calculated by the log-rank test; P=0.17 by Fisher’s exact test for the overall comparison among the causes of death.
Nonfatal events are reported as rates per person-year because the patients could have had more than one event. A complete list of nonfatal serious adverse events is provided in Table S2.
COPD exacerbations that are listed here may not meet the protocol-defined criteria for the primary end point.
After the treatment period, three additional deaths occurred in the metoprolol group (two from COPD and one from pneumonia) and four in the placebo group (one from COPD, one from lung cancer, and two from unknown causes).
HOSPITALIZATION AND NONFATAL SERIOUS ADVERSE EVENTS
The rate of hospitalization for any cause was 0.66 per person-year (95% CI, 0.47 to 0.86) in the metoprolol group and 0.42 per person-year (95% CI, 0.30 to 0.55) in the placebo group. The rate of overall nonfatal serious adverse events was 0.65 per person-year in the metoprolol group and 0.43 per person-year in the placebo group. Nonfatal, serious COPD exacerbations occurred at a rate of 0.43 per person-year and 0.19 per person-year, respectively (Table 3 and Table S2).
OTHER PRESPECIFIED MEASURES
There were no significant between-group differences in several prespecified measurements, including the change from baseline in the FEV1, in the 6-minute walk distance, and in the score on the St. George’s Respiratory Questionnaire (Figs. S4, S5, and S6). The patients in the metoprolol group had a greater increase (indicating worse control) from baseline in the score on the COPD Assessment Test than those in the placebo group, with a difference of 1.13 points (95% CI, 0.06 to 2.20) at day 112 and a difference of 1.47 points (95% CI, 0.32 to 2.62) at day 336 (Fig. S7). The metoprolol group also had a greater increase in SOBQ scores from baseline, indicating a worsening in shortness of breath. The between-group difference in the change from baseline was 3.47 points (95% CI, 0.42 to 6.52) at day 112 and 4.80 points (95% CI, 1.52 to 8.07) at day 336 (Fig. S8).
ADVERSE EVENTS AND DISCONTINUATIONS
We observed no evidence of between-group differences in the frequency of patient-reported adverse events that were potentially related to metoprolol (Table S3). Patients in the metoprolol group had a lower mean heart rate than those in the placebo group (difference, 6 to 10 beats per minute) (Fig. S9). Smaller and less consistent effects were seen for systolic and diastolic blood pressure. The discontinuation of metoprolol or placebo occurred more frequently in the metoprolol group than in the placebo group (11.2% vs. 6.1%). The most common reason for discontinuation was an increase in respiratory symptoms (Table S4).
DISCUSSION
In this prospective, multicenter, randomized trial, we did not find evidence of a difference in the risk of COPD exacerbation between the metoprolol group and the placebo group, although the use of metoprolol was associated with a higher risk of exacerbation leading to hospitalization. These results differ from previously reported findings from observational studies suggesting that beta-blockers reduce the risks of exacerbation and death from any cause in patients with COPD.17–19 A meta-analysis of 9 studies showed that patients taking beta-blockers had a lower risk of COPD-related death than those not taking beta-blockers (relative risk, 0.69; 95% CI, 0.62 to 0.78).18 Another meta-analysis of 15 studies also showed a lower risk of death from any cause (relative risk, 0.72; 95% CI, 0.63 to 0.83) or from COPD exacerbation (relative risk, 0.63; 95% CI, 0.57 to 0.71).19 These observational studies have methodologic limitations inherent to their design, including the possibility of residual confounding and immortal time bias, which may have had an effect on the findings.21
A primary concern about the use of beta-blockers in patients with COPD is that the drugs may cause a worsening in lung function. We did not observe this effect, and none was reported in a meta-analysis on the subject.35 We also found no evidence of between-group differences in the 6-minute walk distance or in patients’ reports of possible beta-blocker side effects. However, metoprolol was associated with worsening of dyspnea and of the overall burden of COPD symptoms, as measured by the shortness-of-breath questionnaire and the COPD Assessment Test (although not on the St. George’s Respiratory Questionnaire). In addition, more discontinuations occurred in the metoprolol group than in the placebo group, which suggests the presence of adverse respiratory effects not captured by spirometry.
Our trial has several limitations. First, although the investigators and patients were unaware of trial-group assignments, it was not possible to fully blind the effects of beta blockade, which resulted in reductions in heart rate and blood pressure. Second, our trial population had moderate or severe COPD with a high prevalence of supplemental oxygen use and previous hospitalization for COPD. Thus, we do not know whether our results would apply to patients with mild airflow obstruction or a lower exacerbation risk. Third, in part because the trial was stopped early, we had limited power to detect differences in the risk of severe exacerbation between subgroups and could not identify specific factors that predisposed patients to adverse outcomes when treated with metoprolol. Fourth, we do not know whether these results would be similar for other cardioselective beta-blockers or for non-cardioselective agents, although concern regarding adverse respiratory effects is greater with the latter.36 Finally, we did not enroll patients who had a proven indication for the use of a beta-blocker or who were already taking the drugs, so our results do not inform the risk of COPD exacerbations with metoprolol in such patients.
The risk of exacerbations of COPD was similar in the metoprolol group and the placebo group among patients with moderate or severe COPD who were at increased risk for exacerbations and had no proven indication for beta-blockers. Although observational studies have suggested that the benefits of beta-blockers in patients with recent myocardial infarction and heart failure extend to those with COPD,15,19 this hypothesis has not been prospectively confirmed, and randomized trials to determine the overall risk–benefit ratio in such patients may be needed.
Supplementary Material
Acknowledgments
Supported by a grant (W81XWH-15-1-0705) from the Department of Defense.
Footnotes
REFERENCES
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD executive summary. Am J Respir Crit Care Med 2017;195:557–82. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res 2013;162:237–51. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015;3:631–9. [DOI] [PubMed] [Google Scholar]
- 5.Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res 2017;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest 2007;131:20–8. [DOI] [PubMed] [Google Scholar]
- 7.Ai-Ping C, Lee KH, Lim TK. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective study. Chest 2005; 128:518–24. [DOI] [PubMed] [Google Scholar]
- 8.Dransfield MT, Rowe SM, Johnson JE, Bailey WC, Gerald LB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2008;63:301–5. [DOI] [PubMed] [Google Scholar]
- 9.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155–61. [DOI] [PubMed] [Google Scholar]
- 10.Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376–84. [DOI] [PubMed] [Google Scholar]
- 11.Hjalmarson A, Elmfeldt D, Herlitz J, et al. Effect on mortality of metoprolol in acute myocardial infarction: a double-blind randomised trial. Lancet 1981;2:823–7. [DOI] [PubMed] [Google Scholar]
- 12.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). JAMA 2000;283:1295–302. [DOI] [PubMed] [Google Scholar]
- 13.Quint JK, Herrett E, Bhaskaran K, et al. Effect of β blockers on mortality after myocardial infarction in adults with COPD: population based cohort study of UK electronic healthcare records. BMJ 2013;347:f6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egred M, Shaw S, Mohammad B, Waitt P, Rodrigues E. Under-use of beta-blockers in patients with ischaemic heart disease and concomitant chronic obstructive pulmonary disease. QJM 2005;98:493–7. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 1998;339:489–97. [DOI] [PubMed] [Google Scholar]
- 16.Su TH, Chang SH, Kuo CF, Liu PH, Chan YL. β-Blockers after acute myocardial infarction in patients with chronic obstructive pulmonary disease: a nationwide population-based observational study. PLoS One 2019;14(3):e0213187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax 2016; 71:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etminan M, Jafari S, Carleton B, FitzGerald JM. Beta-blocker use and COPD mortality: a systematic review and meta-analysis. BMC Pulm Med 2012;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Q, Sun Y, Ding N, Lu L, Chen Y. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One 2014;9(11):e113048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol 2006;47:2554–60. [DOI] [PubMed] [Google Scholar]
- 21.Suissa S, Ernst P. Beta-blockers in COPD: a methodological review of the observational studies. COPD 2018;15:520–5. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βBLOCK COPD): a randomised controlled study protocol. BMJ Open 2016;6(6):e012292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128(16):e240–e327. [DOI] [PubMed] [Google Scholar]
- 24.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60(24):e44–e164. [DOI] [PubMed] [Google Scholar]
- 25.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365: 689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Criner GJ, Connett JE, Aaron SD, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med 2014;370:2201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153–61. [DOI] [PubMed] [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 30.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest 2007;132:456–63. [DOI] [PubMed] [Google Scholar]
- 31.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med 2014;2: 195–203. [DOI] [PubMed] [Google Scholar]
- 32.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–6. [DOI] [PubMed] [Google Scholar]
- 33.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire: University of California, San Diego. Chest 1998;113:619–24. [DOI] [PubMed] [Google Scholar]
- 34.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994;13:1341–56. [DOI] [PubMed] [Google Scholar]
- 35.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005;4:CD003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest 2005;127:818–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


