Abstract
Angiotensin-(1–7) [Ang-(1–7)] exerts cerebroprotective effects in ischemic stroke, and this action is associated with a blunting of intracerebral inflammatory processes and microglial activation. Since intracerebral inflammation and microglial activation play key roles in the mechanism of injury and brain damage in both ischemic and hemorrhagic stroke, we have investigated the potential beneficial actions of Ang-(1–7) in stroke-prone Spontaneously Hypertensive Rats (spSHR), an established animal model of hypertension-induced hemorrhagic stroke. Ang-(1–7) was administered by continuous infusion via the intracerebroventricular (ICV) route for six weeks into spSHR fed a high sodium (4%) diet, starting at 49 days of age. This treatment resulted in a significant increase in survival of the spSHR. Median survival was 108 days in control artificial cerebrospinal fluid-infused spSHR and 154 days in Ang-(1–7) treated spSHR. This effect that was partially reversed by ICV infusion of the Mas receptor blocker A-779. This Ang-(1–7) treatment also decreased the number of hemorrhages in the striatum, improved neurological status (reduced lethargy), decreased the number of microglia in the striatum and tended to increase neuron survival at the same site. Importantly, infusions of Ang-(1–7) had no effect on kidney pathology, heart pathology, body weight, serum corticosterone levels, or blood pressure. This study is the first to demonstrate the cerebroprotective actions of Ang-(1–7), including increased survival time, in spSHR. As such, these data reveal a potential therapeutic target for hemorrhagic stroke.
Keywords: Angiotensin-(1–7), stroke prone SHR, cerebrovascular disease
Subject Area: Vascular
Introduction
Stroke pathophysiology is driven by multiple complex systems with hypertension being the leading risk factor for both ischemic and hemorrhagic stroke (Iadecola & Davisson, 2008). Efforts to develop novel therapies for the prevention and treatment of stroke have been hampered by the multifactorial nature of this disease, and so it remains a leading cause of death and serious long-term disability worldwide (Smith, 2011). The renin-angiotensin system (RAS) has been implicated in stroke and many other cardiovascular diseases, chiefly the deleterious effects of an over active angiotensin converting enzyme/angiotensin II/angiotensin type 1 receptor (ACE/AngII/AT1R) axis. Blocking this axis has been a target for the development of potential therapies for stroke. For example, numerous studies have shown that blocking the actions of Ang II at AT1R via ACE inhibitors or AT1R blockers (ARBs) decreases cortical/subcortical infarct size and the ensuing neurological deficits in animal models of stroke (Groth et al. 2003; Mecca et al. 2009). Furthermore, several human clinical trials have suggested that blocking this axis reduces cardiovascular risk and improves stroke prevention (Dahlöf et al. 2002; Papademetriou et al. 2004; Thone-Reineke et al. 2006).
Another axis of the RAS, the angiotensin converting enzyme 2/angiotensin-(1–7)/Mas (ACE2/Ang-(1–7)/Mas) axis, has been the focus of recent attention due to its therapeutic potential in cardiovascular disease and stroke (Santos et al. 2008). Activating this protective arm of the RAS appears to have potential for treating hypertension related pathology, pulmonary hypertension, myocardial infarction, and heart failure (Santos et al. 2008). Previously we demonstrated that intracerebroventricular (ICV) treatment with Ang-(1–7) is cerebroprotective in an endothelin-1 (ET-1) induced middle cerebral artery occlusion (MCAO) model of ischemic stroke, where it decreased the infarct size, improved rats’ performance on neurological exams, and did not alter cerebral blood flow (Mecca et al. 2011). Furthermore, we and others have demonstrated that the neuroprotective actions of Ang-(1–7) in ischemic stroke are associated with anti-inflammatory properties of this peptide (Jiang et al. 2012; Regenhardt et al. 2013).
Here, we investigated the therapeutic potential of Ang-(1–7) in stroke-prone Spontaneously Hypertensive Rats (spSHR) fed a high salt diet, an established animal model of hemorrhagic stroke. This model more closely resembles the human condition, where chronic elevations in blood pressure and cardiovascular disease lead to both ischemic and hemorrhagic strokes. Although most spSHR are believed to die from hemorrhagic stroke (Smeda, 1989), the two types of stroke are inter-related as hypertension is a risk factor for either type and up to 30% of patients with ischemic stroke undergo hemorrhagic transformation (Lyden & Zivin, 1993). There is also overlap in the pathophysiology of ischemic and hemorrhagic stroke with regard to the role of excessive inflammation and vascular damage. Considering our previous data that show anti-inflammatory properties of Ang-(1–7), we also tested whether the cerebroprotective effect of this peptide in spSHR is associated with changes in microglial infiltration and expression of pro-inflammatory cytokines.
Methods
Animals and Ethical Approval
Male spSHR were purchased from Charles River Farms (Wilmington, MA, USA). All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee. In addition, the principles governing the care and treatment of animals, as stated in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (eighth edition, 2011) and adopted by the American Physiological Society, were followed at all times during this study. Rats had ad libitum access to water and were housed in a well-ventilated, specific pathogen-free, temperature-controlled environment (24 ± 1°C; 12 h–12 h light–dark cycle). Rats were obtained at 35 days of age (weaning), and the spSHR were immediately fed a 4.0% high sodium diet (Research Diets, New Brunswick, NJ).
Anesthesia, Analgesia and Euthanasia
For surgical procedures, anesthesia was induced using 100% O2/4% isoflurane, and was maintained throughout the surgeries by the administration of 100% O2/2% isoflurane. During the surgeries/procedures, the level of anesthesia was monitored by checking the eye blink reflex and a reaction to paw pinch, and was adjusted if necessary. Buprenorphine (0.05 mg/kg, s.c., Hospira Inc., Lake Forest, IL, USA) was administered to rats immediately following the survival surgeries. Animals were euthanized by placing them under deep anesthesia with 100% O2/5% isoflurane, followed by either decapitation or transcardial perfusion with 0.9% saline containing 10% formalin.
ICV infusions
ICV infusion of artificial cerebrospinal fluid (aCSF; 0.15 μl/h), Ang-(1–7) [1.0 μg in 0.15 μl aCSF/h] or (D-Ala7)-Ang-(1–7) [A-779; 2.5 μg in 0.15 μl aCSF/h] was achieved via a stainless steel cannula (kit 1, ALZET, Cupertino, CA) implanted into the left lateral cerebroventricle and coupled via vinyl tubing to a 6 week osmotic pump (model 2006, ALZET, Cupertino, CA). Surgical implantations were made when spSHR were 49 days of age and were performed exactly as detailed previously (Mecca et al. 2011). Osmotic pumps were positioned subcutaneously between the scapulae. Infusion started at the time of ICV cannula placement and lasted for 6 weeks, until rats were 91 days old. At 49 days of age, the average weight was 153 g, meaning 157 ug/kg/d Ang-(1–7) was infused. At 91 days of age, the average weight was 248 g, meaning 97 ug/kg/d Ang-(1–7) was infused.
Experimental Protocols
Experiment 1.
The primary goals of this experiment were to assess the effects of ICV administration of Ang-(1–7) on the survival of spSHR and on the numbers and severity of cerebral cortical/subcortical hemorrhages. spSHR were fed a diet containing 4% sodium since 5 weeks of age. From 49 to 91 days of age, rats were infused ICV for 6 weeks with either Ang-(1–7), aCSF, or Ang-(1–7) plus A-779 contained in the same osmotic pump. During these infusions, rats were examined twice daily and time of death was noted. Blood pressure was monitored every 2 to 5 days via indirect tail-cuff plethysmography, and behavioral testing via the Sunflower seed-eating task was performed every 3 days from 56 to 101 days of age. Following death cerebral hemorrhage number and severity was assessed as described below.
Experiment 2.
Experiment 2 was to designed to determine the effects of ICV administration of Ang-(1–7) on the behavioral outcomes of spSHR. spSHR were fed a diet containing 4% sodium since 5 weeks of age. From 49 to 91 days of age rats were infused ICV for 6 weeks with either Ang-(1–7) or aCSF as above. Rats underwent testing for immobility (lethargy) and spontaneous locomotor activity between days 105–108 of age. Rats were all euthanized later on day 108, when the first death occurred. This experiment allowed examination of all animals at the same time point. Plasma corticosterone, serum creatinine and blood urea nitrogen (BUN) were measured on the day of euthansia.
Experiment 3.
The primary goals of this experiment were to determine if the beneficial action of Ang-(1–7) was associated with an anti-inflammatory effect (reduction in microglia numbers) and an increase in neuron survival in the striatum, an area often associated with hemorrhages in spSHR. spSHR were fed high sodium diet and infused ICV with Ang-(1–7) or aCSF as above. All rats were euthanized at 90 days of age, one day before Ang-(1–7) or aCSF treatment was complete. In addition to evaluating the numbers of microglia and neurons in the striatum, we also investigated kidney and heart pathology in these rats to rule out any potential peripheral tissue effects that may account for the beneficial actions of centrally administered Ang-(1–7) on survival.
Experiment 4.
This protocol here was identical to Experiment 3, with all rats being euthanized at 90 days of age. Brains were removed and (minus the olfactory bulbs and cerebellum) were used for qRT-PCR analyses of interleukin-1β (IL-1β) and monocyte chemotactic protein-1 (MCP-1) as described below.
Hemorrhage Number and Severity Assessment
To compare the numbers and severity of hemorrhages at the time of death, brains were removed from spSHR immediately after they were found dead. Neither hemorrhage size nor hemorrhage number were appreciably changed when measured at different time points up to 12 h after death. The external cortex, as well as serial 2 mm coronal sections were photographed. For each animal, the number of brain hemorrhages was counted. In addition, the severity of each hemorrhage was graded on a scale of 1 to 4, where 1 is minor and 4 most severe (Smeda, 1989; Smeda, 1992). Then the sum of the severity scores was calculated to give a global assessment of severity called the “severity sum.” This was done separately for both the external cortex and subcortex (using coronal sections).
Sunflower Seed Task
A sunflower seed-eating task was performed to provide an index of fine motor function and to assess the level of neurological deficits in each rat. This neurological assessment has been well validated as described previously (Gonzalez & Kolb, 2003; Mecca et al. 2011), and was performed from age 56 to 102 days old to monitor sensorimotor function. Animals were allowed to eat until all seeds were consumed, and the number of shell pieces was counted.
Video Tracking and Locomotor Activity
Video tracking of locomotor activity was used to monitor immobility and rotation frequency. Rats were habituated to a circular open field (diameter 55 cm, height 42 cm) that was black in color. For acclimation, rats were placed in the field for 10 min/day for three consecutive days before measurements were taken. Locomotor activity was monitored for 20 min on the fourth day (age 108 days old) and analyzed using Noldus Ethovision Software (Wageningen, The Netherlands) (Bruijnzeel et al. 2011). Immobility and rotation frequency were calculated. Immobility is the time that the running average mobility is below an immobility threshold. It is assessed by calculating the changed area of the rat, indicating movement. Rotation Frequency is the number of turns (either clockwise or counterclockwise) of 360 degrees. As rats are present in a circular field, this data is a measure of activity.
Indirect Blood Pressure measurements
Blood pressure was recorded via an indirect tail-cuff plethysmography method exactly as described previously, including an acclimation period before measurements were recorded (Mecca et al. 2011). Data were collected every 2–5 days from age 70 d to age 129 d, when the sample sizes started to get small due to fewer animals surviving.
Stereology and Immunostaining
For stereology experiments, animals were perfused transcardially with normal saline followed by 10% formalin. Brains were removed and post fixed in 10% formalin for 24 h, cryoprotected in 30% sucrose in 0.1M phosphate buffer, then frozen in dry ice and stored at −80°C. Systematic uniform random sampling was achieved by sectioning brains coronally on a calibrated freezing stage microtome (30 μm) through the full rostro-caudal extent of the striatum, beginning just caudal to the olfactory bulbs and ending at the rostral hippocampus. A registered 1-in-15 series of sections was obtained for each animal and separate series were randomly assigned for processing to detect total numbers of striatal microglia (Iba-1 positive cells) or neurons (NeuN positive cells). Sections were collected into cold 0.1M TBS and stored at 4°C until used for immunostaining procedures with rabbit anti-Iba-1 and mouse anti-NeuN antibodies as detailed previously (Regenhardt et al. 2013).
Stereological quantification of Iba-1 and NeuN immunopositive cells in the striatum was performed using the optical fractionator as detailed previously (Sterio, 1984; West et al. 1991), in which the product of the cells counted in a known, uniformly random sample of the region of interest is multiplied by the reciprocal of the sampling fraction.
In addition to the Iba-1 and NeuN immunostaining described above, co-localization of Mas with NeuN or Ox-42 immunoreactivities (microglial specific marker) was achieved as detailed previously (Regenhardt et al. 2013).
2.10 Corticosterone, Creatinine and BUN
Blood was collected just prior to euthanasia. Serum was used for corticosterone analysis using an enzyme immunoassay kit (K014-H1, Arbor Assays, Ann Arbor, MI), and for creatinine and BUN analyses, performed by the University of Florida Veterinary Pathology Laboratory.
Kidney and Heart Pathology
Rats were perfused transcardially with normal saline followed by 10% formalin. Kidneys and hearts were removed and stored in 10% formalin until sectioned. Kidney sections were stained using hematoxylin and eosin and Periodic acid-Schiff, and heart sections were stained with H&E and Masson’s trichrome, by the histology core at the University of Florida. Kidney and heart pathologies were assessed using scoring systems as detailed previously (Jokinen et al. 2011; Reinhard et al. 1991). Assessments were made by an individual who was blinded to the treatment.
qRT-PCR
IL1β and MCP-1 mRNAs were analyzed using whole brain homogenate (minus the cerebellum and olfactory bulbs) by qRT-PCR exactly as detailed previously (Mecca et al. 2011; Regenhardt et al. 2013), and were normalized against GAPDH expression. The primer assays were ordered from Applied Biosystems with reference numbers Rn00580555_m1 for MCP-1 and Rn00580432_m1 for IL1β.
Chemicals
Ang-(1–7) and A779 were purchased from Bachem Bioscience (Torrance, CA, USA). Mouse anti-NeuN was from Millipore (Bedford, MA). Rabbit anti-Iba-1 (01919741) was from Wako (Richmond, VA). Rabbit anti-Ang-(1–7) Mas receptor antibody was from Alomone Labs (Jerusalem, Israel). Mouse anti-OX-42 antibody was from BD Biosciences (San Jose, CA). Alexafluor donkey anti-rabbit 594 and anti- mouse 488 were from Molecular Probes [Invitrogen] (Carlsbad, CA, USA). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA, USA).
2.14 Data Analyses
Data are expressed as means ± SEM. Statistical significance was evaluated, as specified in the figure legends, with the use of a one-way ANOVA, two-way row matched ANOVA, Newman-Keuls test, Dunn’s test, t-test, or log rank as appropriate. Differences were considered significant at p<0.05.
Results
Central administration of Ang-(1–7) improves survival of spSHR.
Previously, we demonstrated that Ang-(1–7), administered ICV, had cerebroprotective effects against ischemic stroke that are associated with an anti-inflammatory action of this peptide (Mecca et al. 2011; Regenhardt et al. 2013). Since inflammation is a mechanism of cerebral damage common to both ischemic and hemorrhagic strokes, we evaluated the therapeutic effects of Ang-(1–7) in hypertension induced stroke using spSHR. When spSHR received six weeks of central Ang-(1–7) treatment, from 49 to 91 days of age (Experiment 1), the median survival increased from 108 days in control aCSF treated spSHR to 154 days in Ang-(1–7) treated spSHR (Figure 1). Furthermore, this effect was partially attenuated when the Mas antagonist A779 was co-administered with Ang-(1–7) (mean survival 130 days), indicating that the effect on survival involves Mas.
Figure 1. Central Ang-(1–7) administration improves the survival of spSHR.
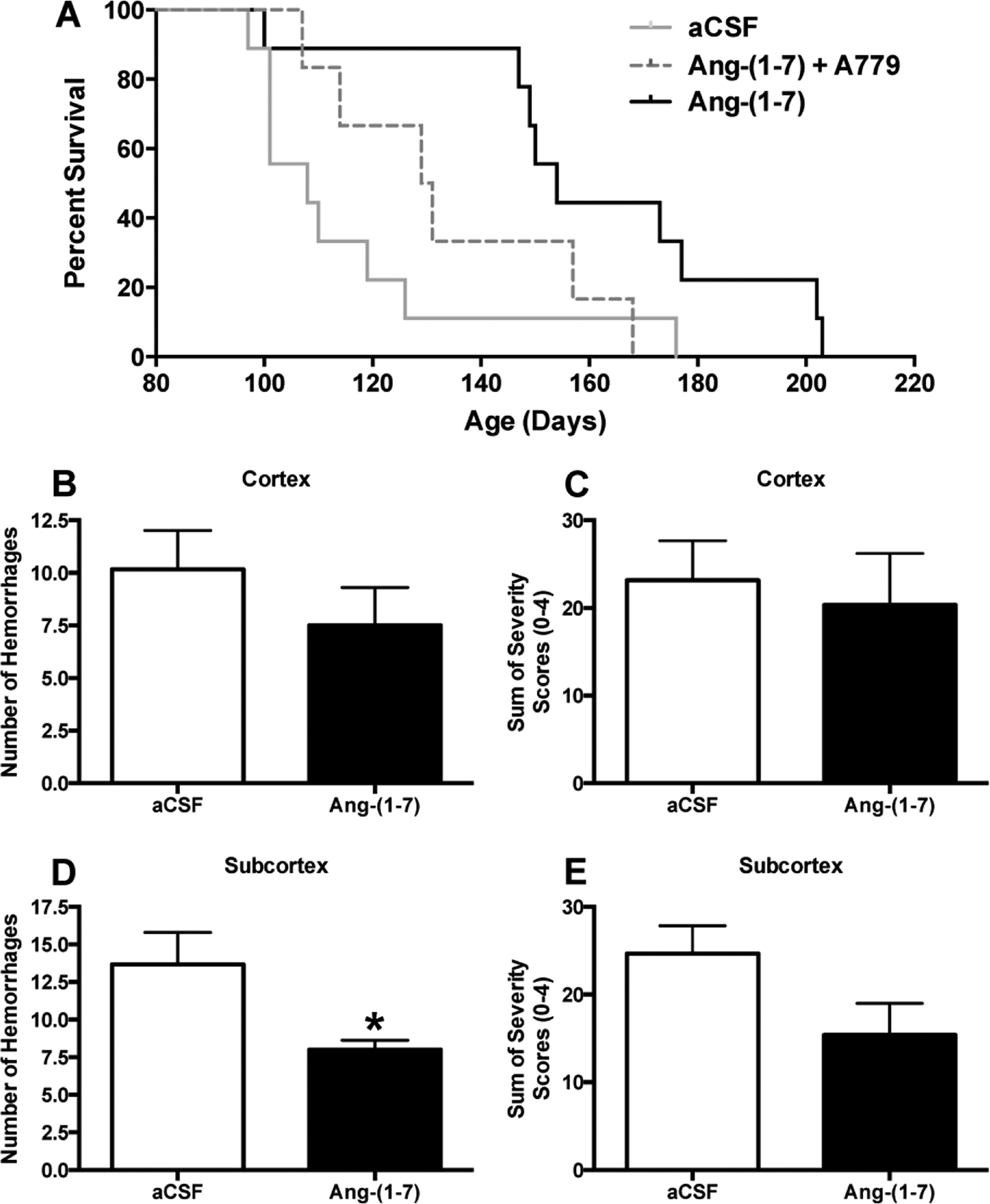
Immediately following weaning at 35 days of age, spSHR were fed a 4% sodium diet. At 49 days of age, rats were infused ICV with either aCSF (0.15 μL/h), Ang-(1–7) [1.0 μg in 0.15 μl aCSF/h] or Ang-(1–7) + A779 (2.5 μg in 0.15 μl aCSF/h) for the next 6 weeks. Animals were monitored and maintained on the 4% sodium diet until death. Following death, brains were removed and the numbers and severity of hemorrhages in the cortex and subcortex/striatum were analyzed as described in the Methods. Panel (A) contains survival curves for spSHR following the above treatments. Vertical black dashed line indicates when the osmotic pumps stopped (91 days of age). N=9 rats for the aCSF and Ang-(1–7) groups; N=6 for the Ang-(1–7) + A779 group. *, P=0.0092, aCSF vs. Ang-(1–7) treatment; P=0.29, aCSF vs. Ang-(1–7) + A-779 treatment; P=0.07, Ang-(1–7) vs. Ang-(1–7) + A-779 treatment. Panels (B) and (D) show the numbers, and panels (C) and (E) the severity of hemorrhages in the cerebral cortex and subcortex/striatum, respectively. Bar graphs are means + SEM. *P<0.05, compared to spSHR infused with aCSF.
Central administration of Ang-(1–7) decreases the number of hemorrhages in the subcortex.
Observational studies of spSHR have shown that they exhibit several cortical and subcortical hemorrhages when they die and these hemorrhages are considered the primary cause of death in most cases (Smeda, 1989; Smeda, 1992). For Experiment 1, rats exhibited signs of cerebrovascular events, including lethargy and seizures, before death. Both cortical and subcortical hemorrhages were counted in brains removed from spSHR immediately after death. Ang-(1–7) did not significantly alter the number or severity of hemorrhages in the cortex (Figure 1B, C) in these brains. However, Ang-(1–7) did elicit a significant decrease in the number of hemorrhages in the subcortex (Figure 1D) and tended to decrease the severity of hemorrhages in these regions (Figure 1E).
Central administration of Ang-(1–7) improves the neurological status of spSHR.
The most apparent signs of neurological disease in spSHR are general sensorimotor dysfunction and lethargy (Smeda, 1989). In Experiment 1, the sunflower seed eating task was performed repeatedly to track general sensorimotor function from 56 to 102 days of age in the absence or presence of Ang-(1–7) treatment. A significant improvement in the efficiency to shell seeds, as indicated by fewer shell pieces, was observed in the Ang-(1–7) treated spSHR compared to aCSF treated rats, with several time points showing very large differences (Figure 2A). In Experiment 2 spontaneous movement was analyzed at 108 days of age following central Ang-(1–7) or aCSF treatment, using video tracking software. Figure 2B demonstrates that Ang-(1–7) decreased the mean immobility of spSHR, suggesting that the rats treated with Ang-(1–7) exhibited less lethargic behavior. In addition, Ang-(1–7) significantly increased the rotation frequency within a circular arena, indicating that spontaneous movement is increased (Figure 2C).
Figure 2. Central Ang-(1–7) treatment improves the neurological status of spSHR.
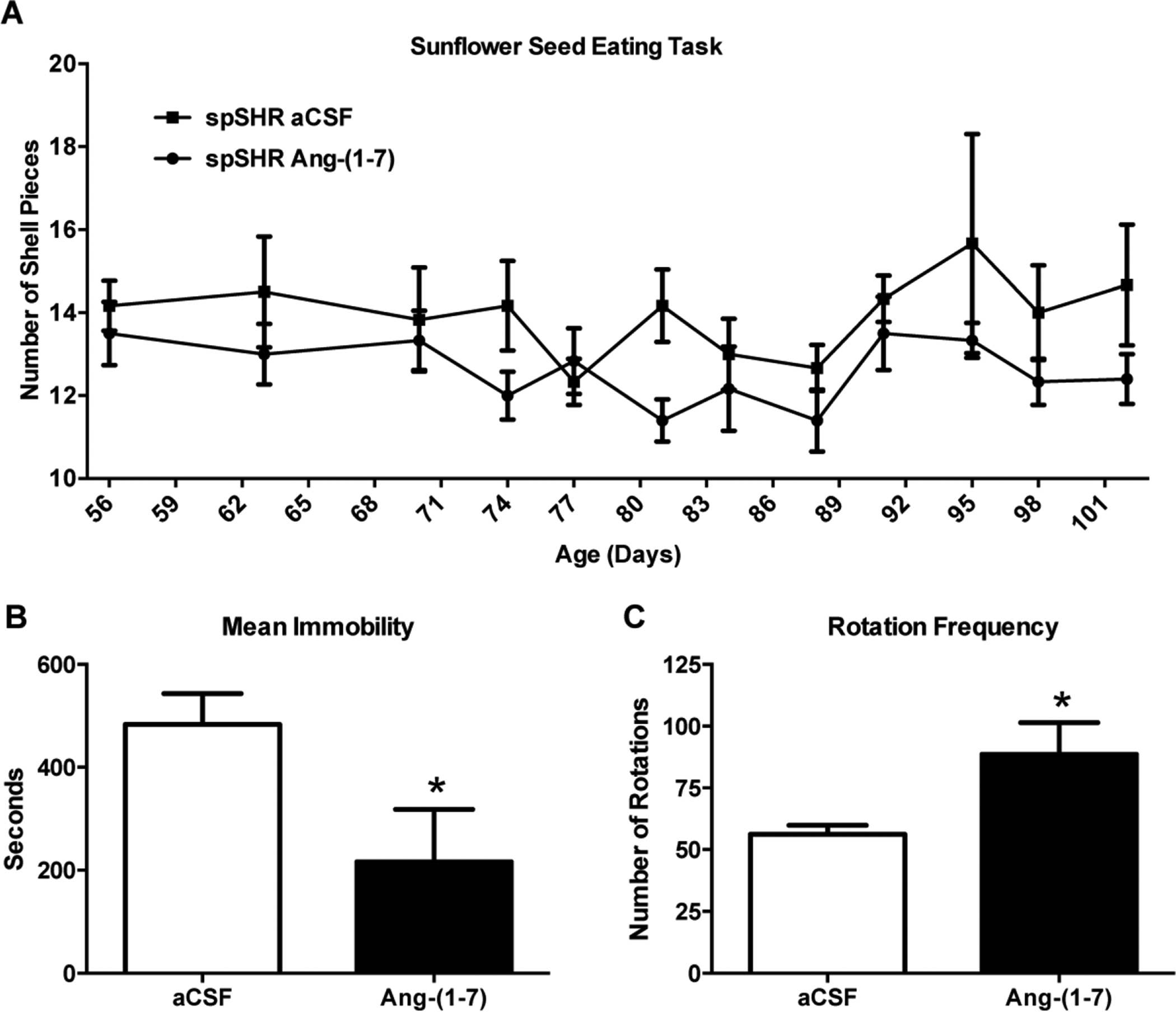
Panel (A), Sunflower seed eating task: Rats were fed a 4% sodium diet and infused ICV with either aCSF (0.15 μL/h) or Ang-(1–7) [1.0 μg in 0.15 μl aCSF/h] as described in the legend to Figure 1, and underwent behavioral testing via the Sunflower seed-eating task every 3 days from 56 to 102 days of age. Vertical black dashed line indicates when the osmotic pumps stopped (91 days of age). Shown here is the number of shell pieces plotted against age. The timeline shows that the number of remaining shell pieces after eating 5 seeds is reduced by Ang-(1–7) treatment, as indicated by 2 way row matched ANOVA when comparing the lines. Data are means ± SEM. N=6 rats per group. Panels (B) and (C), Immobility and Rotation Frequency: Rats were subjected to the same treatments as in panel (A) and underwent testing for spontaneous locomotor activity between days 105–108 of age as described in the Methods. Bar graphs are means + SEM showing Immobility (B) and Rotation Frequency (C). N=9 rats for the aCSF treatment and 7 for Ang-(1–7). *P<0.05 compared to aCSF-treated spSHR.
Central Ang-(1–7) infusion does not alter mean arterial blood pressure of spSHR.
The rats in Experiment 1 were subjected to measurement of MAP both during and after ICV Ang-(1–7) infusion had ceased. Figure 3 reveal that there were no significant differences in MAP between the spSHR infused with Ang-(1–7) and those infused with aCSF throughout the recording period. This suggests that the effects of Ang-(1–7) to increase lifespan and improve behavioral outcomes are independent of changes in MAP.
Figure 3. Central Ang-(1–7) infusion does not alter mean arterial blood pressure of spSHR.
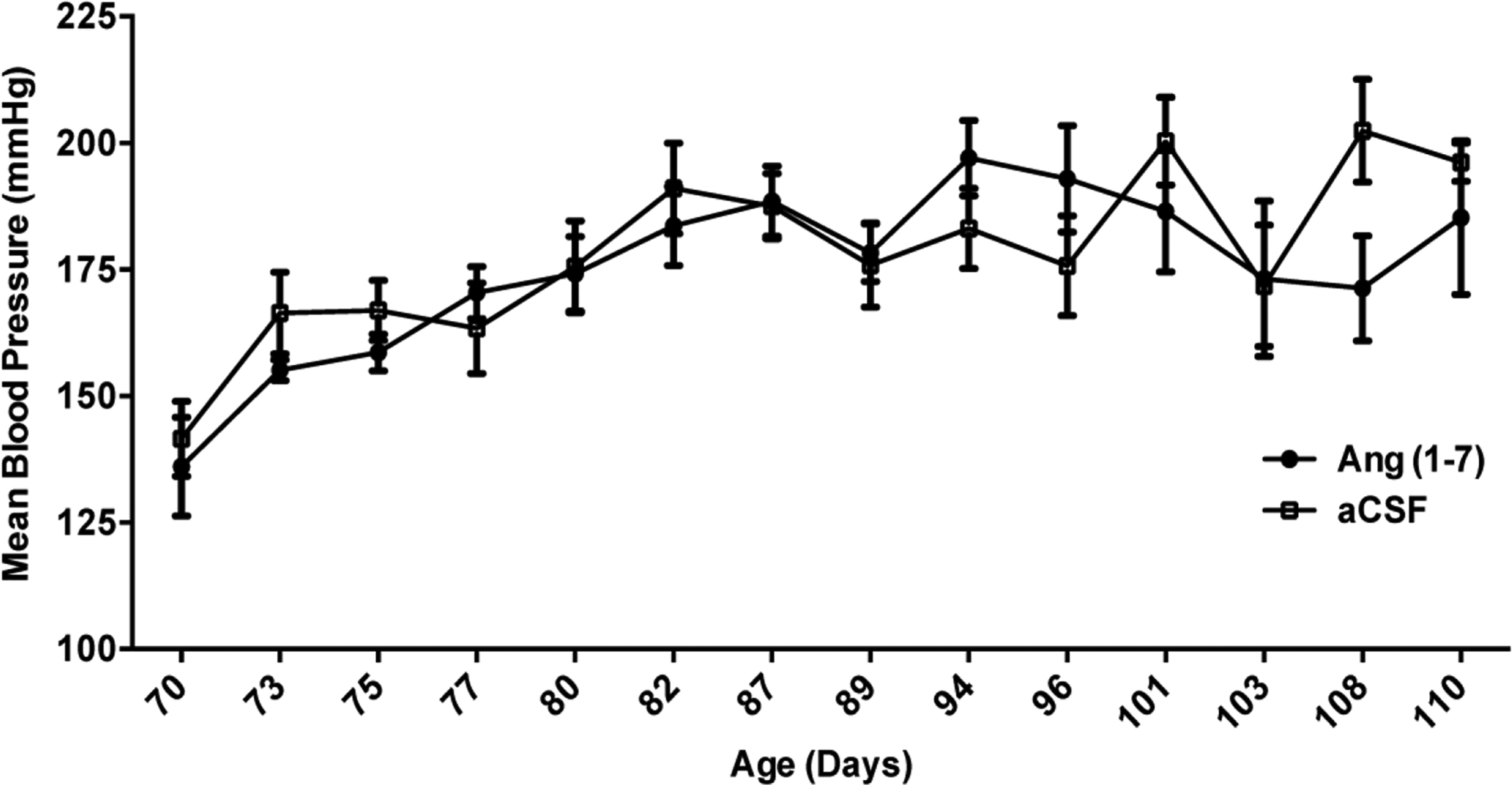
Rats were fed a 4% sodium diet, infused ICV with either aCSF (0.15 μL/h) or Ang-(1–7) [1.0 μg in 0.15 μl aCSF/h] as described in the legend to Figure 1, and underwent measurements of MAP via tail cuff plethysmography every 2 to 5 days between days 70 and 110 of age. Vertical black dashed line indicates when the osmotic pumps stopped (91 days of age). Data are means ± SEM. N=8 rats per group.
Central administration of Ang-(1–7) decreases the number of microglia in the striatum.
In previous studies we demonstrated an inhibitory effect of Ang-(1–7) on macrophage/ microglia activation in an ischemic model of stroke, suggesting that the cebroprotective actions of this peptide include an anti-inflammatory action (Regenhardt et al. 2013). Thus, in Experiment 3 we tested whether the beneficial actions of centrally infused Ang-(1–7) in spSHR were associated with altered numbers of microglia in the striatum as described in Experiment 3. We focused on the striatum because it was the brain region where Ang-(1–7) significantly decreased the number of hemorrhages. Using stereology to count the number of Iba-1 immunoreactive cells, Ang-(1–7) was shown to decrease the number of macrophages/microglia in the striatum compared with the aCSF-infused rats (Figure 4A, B, E). In addition to microglia, we also quantified the numbers of neurons in the striatum to determine if their survival was affected by Ang-(1–7) treatment. The data demonstrate that even though the mean number of NeuN immunoreactive cells in the striatum from Ang-(1–7) treated rats was increased by ~1.1 million, this effect was not statistically significant (Figure 4C,D and F). Co-immunostaining for the Ang-(1–7) receptor Mas and Iba-1 demonstrated that Mas was localized on microglia and neurons within spSHR cortex and striatum (data not shown), similar to its localization in SD rat brain (Regenhardt et al. 2013). In Experiment 4, the potential anti-inflammatory action of centrally-administered Ang-(1–7) was further investigated via qRT-PCR analyses of pro-inflammatory chemokines and cytokines. These analyses showed non-significant trends for MCP-1 and IL-1β mRNAs to be decreased in the whole brain (minus cerebellum and olfactory bulbs) of Ang-(1–7) infused spSHR (Figure 4G,H).
Figure 4. Anti-inflammatory action of centrally administered Ang-(1–7) in the striatum of spSHR.
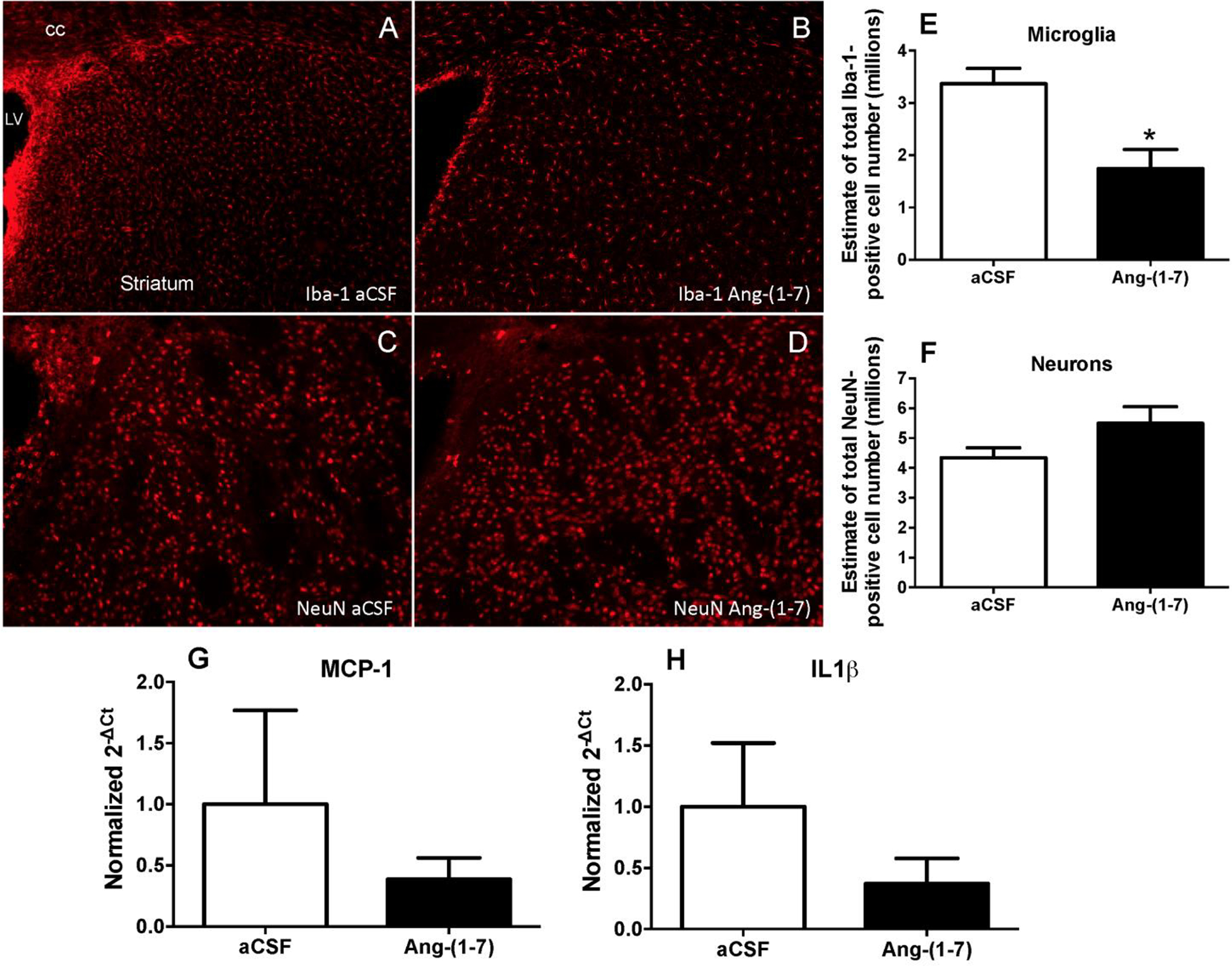
(A-F) Rats were fed a 4% sodium diet, infused ICV with either aCSF (0.15 μL/h) or Ang-(1–7) [1.0 μg in 0.15 μl aCSF/h]. (A-F): At 90 days of age rats were deeply anesthetized, perfused transcardially with saline followed by 10% formalin and brains removed for immunostaining as described in the Methods. Panels A and B are representative fluorescence micrographs showing Iba-1 immunoreactivity in the striatum of aCSF and Ang-(1–7) infused rats, respectively. Panels C and D are representative fluorescence micrographs showing NeuN immunoreactivity in the striatum of aCSF and Ang-(1–7) infused rats, respectively. LV= lateral ventricle; cc = Corpus callosum. Stereological analyses of the striatum showing the estimated numbers of Iba-1 positive cells (microglia) and NeuN positive cells (neurons) are shown in panels E and F respectively. The bar graphs are means + SEM. N=5–6 rats/group. *P<0.05 compared to aCSF treated spSHR. (G, H): At 90 days of age, rats were euthanized, brains removed and processed for RT-PCR analyses of MCP-1 (G) and IL-1β (H) respectively. The bar graphs are means + SEM. N=5 rats/group.
Central administration of Ang-(1–7) did not alter indices of adrenal, kidney or heart pathological changes in spSHR.
We examined, as described in Experiments 2 and 3, whether the beneficial actions of ICV infused Ang-(1–7) were associated with improvements in the peripheral organ pathological changes that occur in spSHR with the progression of hypertension. There was no significant effect of Ang-(1–7) infusion on serum corticosterone, creatinine, and BUN levels (data not shown). In addition, Ang-(1–7) infusion did not alter body weight [289.0 ± 4.6 g (n=9) and 291.7 ± 5.7 g (n=7) in aCSF and Ang-(1–7) infused rats, respectively]. In-depth analysis of kidney and heart pathologies via assessment of tuft atrophy, Bowman’s capsule thickening, periglomerular fibrosis, membranoproliferative changes, tubular interstitial fibrosis and cell infiltration, tubular degeneration/regeneration, heart degeneration, and heart fibrosis and inflammation revealed that for each metric there was no beneficial effects of ICV infusion of Ang-(1–7) versus the spSHR that were infused with aCSF (Figure 5).
Figure 5. Co-localization of Mas with NeuN- and OX-42-immunopositive cells in the striatum.
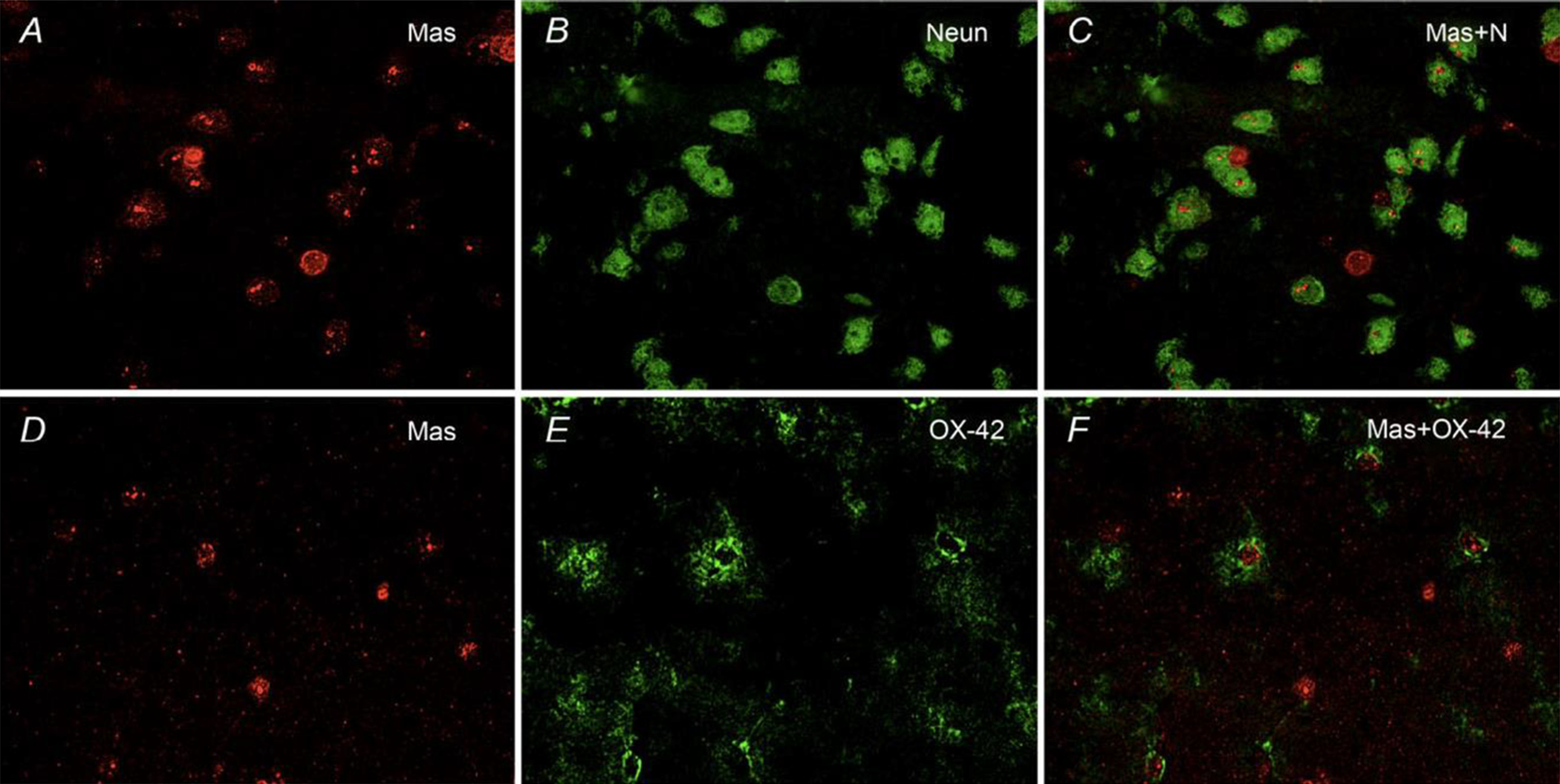
The spSHRs were fed a 4% sodium diet and infused ICV with aCSF (0.15 μl h−1). At 90 days of age, rats were deeply anaesthetized, perfused transcardially with saline followed by 10% formalin and brains removed for immunostaining as described in the Methods. The representative fluorescence micrographs taken from the striatum at ×40 magnification are as follows: Mas (A); NeuN (B); co-localization of Mas and NeuN immunostaining (Mas+N; C); Mas (D); OX-42 (E); and Co-localization of Mas and OX-42 immunostaining (Mas+OX-42; F).
Discussion
This study demonstrates, for the first time, the cerebroprotective effects of central Ang-(1–7) in spSHR. The strongest evidence for this is the ability of Ang-(1–7) to increase survival in this model of severe hypertension and stroke. This effect appears to involve the receptor Mas, since the survival time in spSHR showed a strong trend (P=0.07) to be reduced when Ang-(1–7) mediated Mas activation was inhibited with A779. The inability of A-779 to completely reverse the effects of Ang-(1–7) on survival may indicate that the dose of this antagonist used was too low. The dose of A-779 used here was based on our previous studies which demonstrated complete blockade of the effects of Ang-(1–7) on cerebral damage elicited by ischemic stroke (Mecca et al. 2011). Thus, while the current data suggest a role for Mas in the protective effects of Ang-(1–7) in spSHR, further studies that involve using higher doses of A-779 may elucidate whether the increase in survival elicited by Ang-(1–7) also involves a Mas-independent mechanism.
The observation that Ang-(1–7) also decreased the number of hemorrhages in the striatum/subcortex in spSHR provides additional evidence for its cerebroprotective efficacy. Ang-(1–7) trended to decrease the number of hemorrhages in the cortex, but this effect did not reach statistical significance. One possibility is that Ang-(1–7) could better diffuse to the striatum relative to the cortex from its infusion site in the ventricular system. Furthermore, our data showing decreased numbers of microglia in the striatum suggests that the mechanism of protection may be more anti-inflammatory in nature rather than a result of preventing the initial hemorrhages.
The neurological data provide strong additional support that Ang-(1–7) exerts a cerebroprotective action against hypertension induced stroke. One of the most common neurological signs in spSHR as the pathology progresses is lethargy (Smeda, 1989). Central Ang-(1–7) treatment reduces immobility and increases rotation frequency in a circular open field designed to measure spontaneous activity. In addition, spSHR treated with Ang-(1–7) exhibit improved performance on the sunflower seed eating task which translates to an improvement in global sensorimotor function. We believe these functional improvements add greatly to the evidence supporting a cerebroprotective effect of Ang-(1–7).
Previously, we performed an immunohistochemical co-localization study to determine which brain cell types Sprague Dawley rats contain Mas (Regenhardt et al. 2013). The present study confirmed a similar distribution in the spSHR brain, with Mas immunoreactivity present on neurons, microglia, and endothelial cells. The presence of Mas on microglia suggested that the cerebroprotective effects of Ang-(1–7) in spSHR may involve an anti-inflammatory action. As previously stated, inflammation has a strong role in the mechanism of injury in both hemorrhagic and ischemic forms of stroke. The inflammatory response begins soon after hemorrhage and peaks several days later in humans and animal models (Enzmann et al. 1981; Gong et al. 2000). The infiltration of neutrophils occurs within 2 days, and the activation of microglia may continue for up to one month (Gong et al. 2004). Evidence supports a role of microglia in hemorrhagic stroke pathology since inhibiting microglial activation reduces cerebral damage (Wang & Tsirka, 2005a; Wang & Tsirka, 2005b).
Stereological techniques were used to observe changes in numbers of microglia and neurons in the striatum at 90 days of age, just prior to completion of Ang-(1–7) ICV infusions. We focused on the striatum since this was the region where Ang-(1–7) had the greatest effect to reduce the number of visible hemorrhages at the time of death. Ang-(1–7) decreased the number of microglia and showed a trend to increase the numbers of neurons, perhaps reflecting improved neuron survival. While we recognize that changes in microglial number do not necessarily reflect the changes in microglial activation that occur during stroke, it has been demonstrated that the total numbers of microglia (resting and activated) are elevated in the brains of spSHR versus WKY control rats (Marks et al. 2001). Thus, our data showing that Ang-(1–7) decreases the number of microglia in the striatum of spSHR suggests an anti-inflammatory action of this peptide. Ongoing work in our laboratory is assessing the role of Ang-(1–7) in microglial activation.
In our previous reports using the ET-1 induced MCAO model of ischemic stroke, Ang-(1–7) blunted pathological increases in iNOS, pro-inflammatory cytokines, and CD11b. Although there was a trend for attenuation of IL1β and MCP-1, we did not observe any statistically significant treatment effect on pro-inflammatory cytokines. It is possible that the pathological changes were not excessive enough to elicit measurable changes in gene expression at this time point. Alternatively, it is possible that changes in gene expression in spSHR brain in the current study might have been apparent if we had focused on a specific region such as the striatum, rather than the entire brain where alterations in mRNA levels may be diluted out (Mecca et al. 2011). However, we believe this is the only way to perform a gene expression study in the spSHR model because the hemorrhages occur spontaneously and unpredictably throughout the brain. Although these data support the idea that the anti-inflammatory effects of Ang-(1–7) contribute to the increased survival and improved neurological status, we cannot rule out the possibility that effects on blood flow also contribute to these beneficial actions of Ang-(1–7). In addition to our data, others have also described anti-inflammatory properties of Ang-(1–7) in brain that may account for this peptide’s cerebroprotective effects (Jiang 2012).
Another consideration was whether or not centrally administered Ang-(1–7) could have any peripheral or systemic effects. We investigated the possibility that an effect of this nature might be the mechanism for increased survival. The heart and kidney pathology as well as well as serum BUN, creatinine, and corticosterone data reported here indicate that it is unlikely that centrally administered Ang-(1–7) is having a significant effect outside of the brain.
One potential limitation of this study, in terms of translation to the bedside, is the invasive nature of intracerebroventricular infusion. We purposefully designed this study to measure central effects and limit peripheral effects, and we believe ICV infusion is the most direct approach. However, we are currently investigating novel treatment modalities that will make the activation of the ACE2/Ang-(1–7)/Mas axis more feasible in patients. Some strategies include intraperitoneal injection of a putative ACE2 activator, the use of orally active cyclic Ang-(1–7), and the peripheral intravenous injection of endothelial progenitor cell that over express a secretable form of Ang-(1–7) which home to areas of endothelial damage.
In summary, this study provides the first evidence that activating the ACE2/Ang-(1–7)/Mas axis may have therapeutic potential in hypertension-induced stroke. Ang-(1–7) administered ICV is cerebroprotective in spSHR and increases their survival. This bolsters our previous studies indicating Ang-(1–7)’s cerebroprotective effect in a model of ischemic stroke (Mecca et al. 2011). The stereology data reported here also corroborate our previous finding that Ang-(1–7) has anti-inflammatory effects during stroke, however ongoing studies are further investigating microglial activation, phenotype, and migration. The findings of this study are robust as survival, physical endpoints, and neurological/behavioral endpoints are all improved by Ang-(1–7). In addition to the current findings, further studies will need to be performed to better understand the molecular mechanisms of cerebroprotection mediated by Ang-(1–7).
Figure 6. Central Ang-(1–7) infusion does not reduce the pathological changes in kidney and heart structure that occur in spSHR.
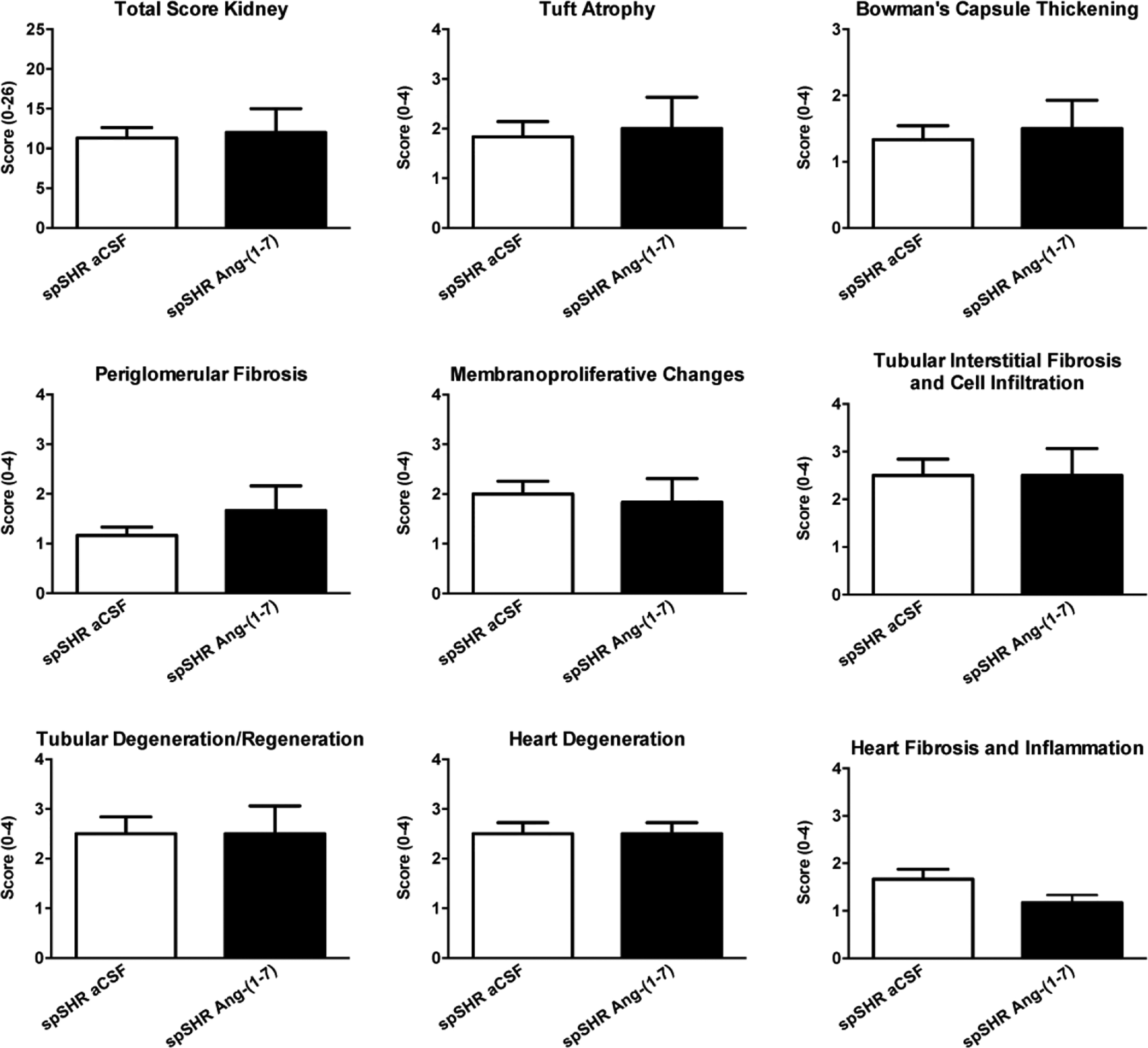
Following euthanization of the 90- day-old rats used for analysis of microglial and neuronal numbers (Figure 4E,F), kidneys and hearts were removed and used to assess pathological changes. The bar graphs are means + SEM (N=5 rats/group) of scores obtained from the indicated kidney and heart pathological changes.
Funding
This work was supported by grants from the American Heart Association Greater Southeast Affiliate (09GRNT2060421), the University of Florida Clinical and Translational Science Institute, the National Heart Lung and Blood Institute (T32 HL083810) and the National Institute for Neurological Disorders and Stroke (F30 NS060335).
Footnotes
Conflict of Interest
None declared
References
- Bruijnzeel AW, Rodrick G, Singh RP, Derendorf H & Bauzo RM (2011). Repeated pre-exposure to tobacco smoke potentiates subsequent locomotor responses to nicotine and tobacco smoke but not amphetamine in adult rats. Pharmacol Biochem Behav 100, 109–118. [DOI] [PubMed] [Google Scholar]
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group (2002) Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359, 995–1003. [DOI] [PubMed] [Google Scholar]
- Enzmann DR, Britt RH, Lyons BE, Buxton JL & Wilson DA (1981). Natural history of experimental intracerebral hemorrhage: sonography, computed tomography and neuropathology. AJNR Am J Neuroradiol 2, 517–526. [PMC free article] [PubMed] [Google Scholar]
- Gong C, Hoff JT & Keep RF (2000). Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res 871, 57–65. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT & Xi G (2004). Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke 35, 2571–2575. [DOI] [PubMed] [Google Scholar]
- Gonzales CL & Kolb B (2003). A comparison of different models of stroke on behavior and brain morphology. Eur J Neurosci 18, 1950–1962. [DOI] [PubMed] [Google Scholar]
- Groth W, Blume A, Gohlke P, Unger T & Culman J (2003). Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J.Hypertens 21, 2175–2182. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL (2008). Hypertension and cerebrovascular dysfunction. Cell Metab 7,476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Gao L, Guo J, Lu J, Wang Y, Zhang Y (2012). Suppressing inflammation by inhibiting the NF-κB pathway contributes to the neuroprotective effect of angiotensin-(1–7) in rats with permanent cerebral ischaemia. Br J Pharmacol 167,1520–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen MP, Lieuallen WG, Boyle MC, Johnson CL, Malarkey DE & Nyska A (2011). Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicol Pathol 39, 850–860. [DOI] [PubMed] [Google Scholar]
- Lyden PD & Zivin JA (1993). Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc Brain Metab Rev 5, 1–16. [PubMed] [Google Scholar]
- Marks L, Carswell HV, Peters EE, Graham DI, Patterson J, Dominiczak AF, Macrae IM (2001). Characterization of the microglial response to cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension 38,116–22. [DOI] [PubMed] [Google Scholar]
- Mecca AP, O’Connor TE, Katovich MJ & Sumners C (2009). Candesartan pretreatment is cerebroprotective in a rat model of endothelin-1-induced middle cerebral artery occlusion. Exp Physiol 94, 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca AP, Regenhardt RW, O’Connor TE, Joseph JP, Raizada MK, Katovich MJ & Sumners C (2011). Cerebroprotection by angiotensin-(1–7) in endothelin-1-induced ischaemic stroke. Exp Physiol 96, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papademetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A (2004). Study on Cognition and Prognosis in the Elderly study group. Stroke prevention with the angiotensin II type 1-receptor blocker candesartan in elderly patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol 44,1175–80. [DOI] [PubMed] [Google Scholar]
- Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C (2013). Anti-inflammatory effects of angiotensin-(1–7) in ischemic stroke. Neuropharmacology 71, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard MK, Hottendorf GH & Powell ED (1991). Differences in the sensitivity of Fischer and Sprague-Dawley rats to aminoglycoside nephrotoxicity. Toxicol Pathol 19, 66–71. [DOI] [PubMed] [Google Scholar]
- Santos RA, Ferreira AJ, Simoes E, & Silva AC (2008). Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol 93, 519–527. [DOI] [PubMed] [Google Scholar]
- Smeda JS (1992). Cerebral vascular changes associated with hemorrhagic stroke in hypertension. Can J Physiol Pharmacol 70, 552–564. [DOI] [PubMed] [Google Scholar]
- Smeda JS (1989). Hemorrhagic stroke development in spontaneously hypertensive rats fed a North American, Japanese-style diet. Stroke 20, 1212–1218. [DOI] [PubMed] [Google Scholar]
- Smith SC Jr (2011). Reducing the global burden of ischemic heart disease and stroke: a challenge for the cardiovascular community and the United Nations. Circulation 124, 278–279. [DOI] [PubMed] [Google Scholar]
- Sterio DC (1984). The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134, 127–136. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C, Steckelings UM & Unger T (2006). Angiotensin receptor blockers and cerebral protection in stroke. J Hypertens Suppl 24, S115–21. [DOI] [PubMed] [Google Scholar]
- Wang J & Tsirka SE (2005). Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 128, 1622–1633. [DOI] [PubMed] [Google Scholar]
- Wang J & Tsirka SE, (2005). Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke 36, 613–618. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L & Gundersen HJ (1991). Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231, 482–497. [DOI] [PubMed] [Google Scholar]


