Abstract
Background:
Perfluoroalkyl substances (PFAS) are a group of widely used persistent chemicals with suspected immunotoxic effects.
Objectives:
The present study aimed to examine the association between infant PFAS exposure and antibody responses to measles vaccination as well as morbidity in a low-income country.
Methods:
In a randomized controlled trial, children from Guinea-Bissau, West Africa, were followed from inclusion (4–7 months of age) through 2 years of age. Half the children received two measles vaccinations (at inclusion and at 9 months of age), and the other half received only one (at 9 months of age). In a subset of 237 children, six PFAS were quantified in serum at inclusion, and measles antibody concentrations were assessed at inclusion and at approximately 9 months and 2 years of age. At inclusion and at the 9-month visit, mothers were interviewed about infant morbidity.
Results:
All but one child had detectable serum concentrations of all six PFAS, although levels were lower than seen elsewhere. A doubling in perfluorooctane sulfonic acid (PFOS) and perfluorodecanoic acid (PFDA) were associated with 21% (95% CI: 2, 37%) and 25% (95% CI: 1, 43%), respectively, lower measles antibody concentrations at the 9-month visit among the children who had received a measles vaccine at inclusion. Elevated serum PFAS concentrations were also associated with reduced prevaccination measles antibody concentrations and increased morbidity.
Discussion:
The present study documents that PFAS exposure has reached West Africa and that infants show PFAS-associated increases in morbidity and decreases in measles-specific antibody concentrations before and after vaccination. These findings support the evidence on PFAS immunotoxicity at comparatively low serum concentrations. https://doi.org/10.1289/EHP6517
Introduction
Perfluoroalkyl substances (PFAS) are a group of persistent chemicals produced since the 1940s and applied in industrial and commercial products such as repellents for outdoor clothing, furniture textiles, food packaging materials, cookware, and firefighting foams (ATSDR 2018; Sunderland et al. 2019). Humans are exposed to PFAS through contaminated food and water and through inhalation and ingestion of dust (ATSDR 2018; Domingo and Nadal 2017; Sunderland et al. 2019). Furthermore, PFAS are transferred across the placenta and into breast milk (Manzano-Salgado et al. 2015; Mogensen et al. 2015; Pan et al. 2017; Verner et al. 2016), thereby causing peak exposures in infancy.
Due to their widespread use and resistance to breakdown, PFAS are now globally distributed in the environment (Wang et al. 2017), and the presence of PFAS in humans and associations with adverse health effects have been documented in numerous studies from Asia, Europe, and North America (ATSDR 2018; Jian et al. 2018; Rappazzo et al. 2017). PFAS have been detected in the serum of pregnant South African women (Hanssen et al. 2010) and mothers in Tanzania (Müller et al. 2019), but little is known about serum PFAS concentrations in African children.
Developmental exposure to PFAS has previously been associated with immunotoxicity in experimental models (DeWitt et al. 2019) and with increased morbidity risk (Dalsager et al. 2016; Goudarzi et al. 2017; Granum et al. 2013; Impinen et al. 2018, 2019; Kvalem et al. 2020) and decreased antibody concentrations after routine immunizations (Grandjean et al. 2012, 2017; Granum et al. 2013; Stein et al. 2016) in children from Nordic countries, Japan, and the United States. Based on estimated exposures, serum PFAS concentrations in the first months of life are more important predictors of subsequent reduced antibody concentrations than PFAS concentrations measured later in childhood (Grandjean et al. 2017), but studies of immunotoxicity relying on PFAS concentrations measured in infancy are lacking.
Although the mortality rate in West Africa has declined by more than 50% since 1990, it is still at 9% with infectious diseases being among the leading causes of death (UNICEF 2019). Given the public health importance of successful measles vaccination and the high incidence of infectious disease, the aim of the present study was to examine the association between PFAS exposure in infancy and immune response to measles vaccination as well as morbidity among children in Guinea-Bissau. We hypothesized that higher PFAS concentrations would be associated with increased morbidity and decreased antibody concentrations after vaccination. Furthermore, we examined the association between PFAS concentrations and prevaccination antibody concentrations.
Methods
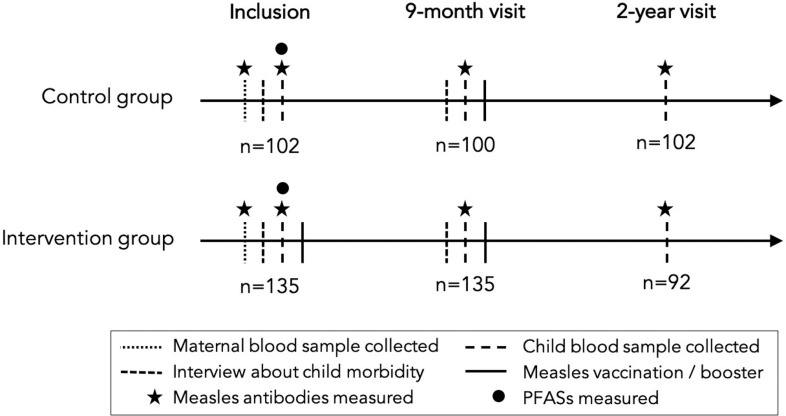
This study is based on a subset of data from a randomized controlled trial (RCT) of early measles vaccination conducted in Guinea-Bissau from 2012 through 2015 (Fisker et al. 2018). The RCT compared two doses of measles vaccine (Edmonston-Zagreb strain) at 4–7 and at 9 months of age (intervention) vs. the usual single measles vaccination at 9 months (control). Children living in villages in three rural regions within a 2-h drive from the capital, Bissau, were enrolled at 4–7 months of age. At 9 months of age, the child was invited back for examination and measles vaccination. The main outcome of the original trial was mortality until 3 years of age. Furthermore, at inclusion and at the 9-month visit, mothers were interviewed about child fever, diarrhea, coughing, and vomiting on the day of the visit, in addition to duration of breastfeeding and introduction of solids. Information about maternal education and parity was obtained prior to enrollment through routine surveillance data (Thysen et al. 2019). A subgroup study among 422 infants assessed measles antibodies using finger prick blood samples obtained at inclusion, at the 9-month visit, and at 2 years of age. In the subgroup study, maternal blood samples were also obtained at enrollment. We included in the present study those who had measles antibodies measured at least once after receiving a measles vaccination () and had sufficient serum from finger prick blood samples at inclusion to measure PFAS, thus resulting in 237 included infants: 135 from the intervention group and 102 from the control group (Figure 1). Measles IgG antibody titers were measured at Rijksinstituut voor Volksgezondheid en Milieu, Bilthoven, Netherlands, using a multiplex immunoassay, as described by Smits et al. (2012).
Figure 1.
Overview of visits. Note: PFAS, perfluoroalkyl substances.
Assessment of PFAS Exposure
PFAS analyses were conducted at the University of Southern Denmark. The six types of PFAS usually found in measurable concentrations in serum samples, that is, perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), and perfluoroundecanoic acid (PFUnDA) were quantified using online solid-phase extraction followed by liquid chromatography and triple quadrupole mass spectrometry, as described by Haug et al. (2009). The analysis was performed on serum from finger prick blood samples. National Institute of Standards and Technology (NIST) Standard Reference Material 1957 and NIST1958 samples as well as in-house–made samples were included in the sample series for quality control. The between-batch imprecision was , and the limit of detection (LOD) was . Values below the LOD were replaced by LOD/2. The accuracy of the PFAS methods are continuously secured by regular participation in the German Quality Assessment program organized by the German Society of Occupational Medicine.
One extreme PFNA value () was observed, and serum was not available for duplicate analysis. We did not have an explanation for this outlier and in order to avoid excessive influence from this single measurement, it was excluded from the statistical analyses.
Statistics
Associations between maternal/child characteristics and maternal/child antibody concentrations were tested using the Wilcoxon rank-sum test (binary variables) and the Kruskal-Wallis test (). The primary hypothesis of PFAS affecting the antibody response to measles vaccination was examined by testing the associations between serum PFAS concentrations at inclusion and antibody concentrations at the 9-month visit (intervention group only) and the 2-y visit (both control and intervention group; groups were analyzed separately) using linear regression. Furthermore, we tested the association between PFAS and baseline levels of measles antibodies at inclusion (both control and intervention group) and at the 9-month visit (control group only) also using linear regression. Associations between PFAS and infant morbidity at inclusion and at the 9-month visit were examined using logistic regression models. Only 14 children vomited on the day of inclusion, and only 9 children vomited on the day of the 9-month visit. Vomiting on its own was, therefore, not used as an outcome in the logistic regression models. However, fever, diarrhea, coughing, and vomiting were combined into a new variable indicating whether the child had had any of the four symptoms on the day of the visit.
Distributions of child antibody and PFAS concentrations were skewed to the right and were therefore transformed when included in the regression models in order not to violate model assumptions and to avoid high values being overly influential. Estimates from the linear and logistic regression models were back-transformed to express percentage difference in antibody concentrations and odds ratio (OR) of morbidity, respectively, with a doubling in serum PFAS concentrations.
Potential confounding variables were identified using directed acyclic graphs (DAGs) for the associations between PFAS and measles antibody concentrations (Figure 2A) and between PFAS and child morbidity (Figure 2B) based on a priori knowledge. Maternal PFAS exposure have been shown to increase the risk of preterm birth (Meng et al. 2018), and the placenta/maternal serum PFAS ratio might increase across gestation, thus perhaps suggesting higher child PFAS exposure at increasing fetal age (Mamsen et al. 2019). In addition, infants born preterm could have an immature immune system with reduced ability to fight pathogens and bacteria (Melville and Moss 2013), and preterm birth has been associated with a reduction in transfer of maternal antibodies (Okoko et al. 2001). Preterm birth could thus constitute a potential confounder. Information about gestational length was, however, not available, and weight (continuous to nearest ) and age in days at inclusion (continuous) were, therefore, used as a proxy for preterm birth and included in the adjusted analyses.
Figure 2.
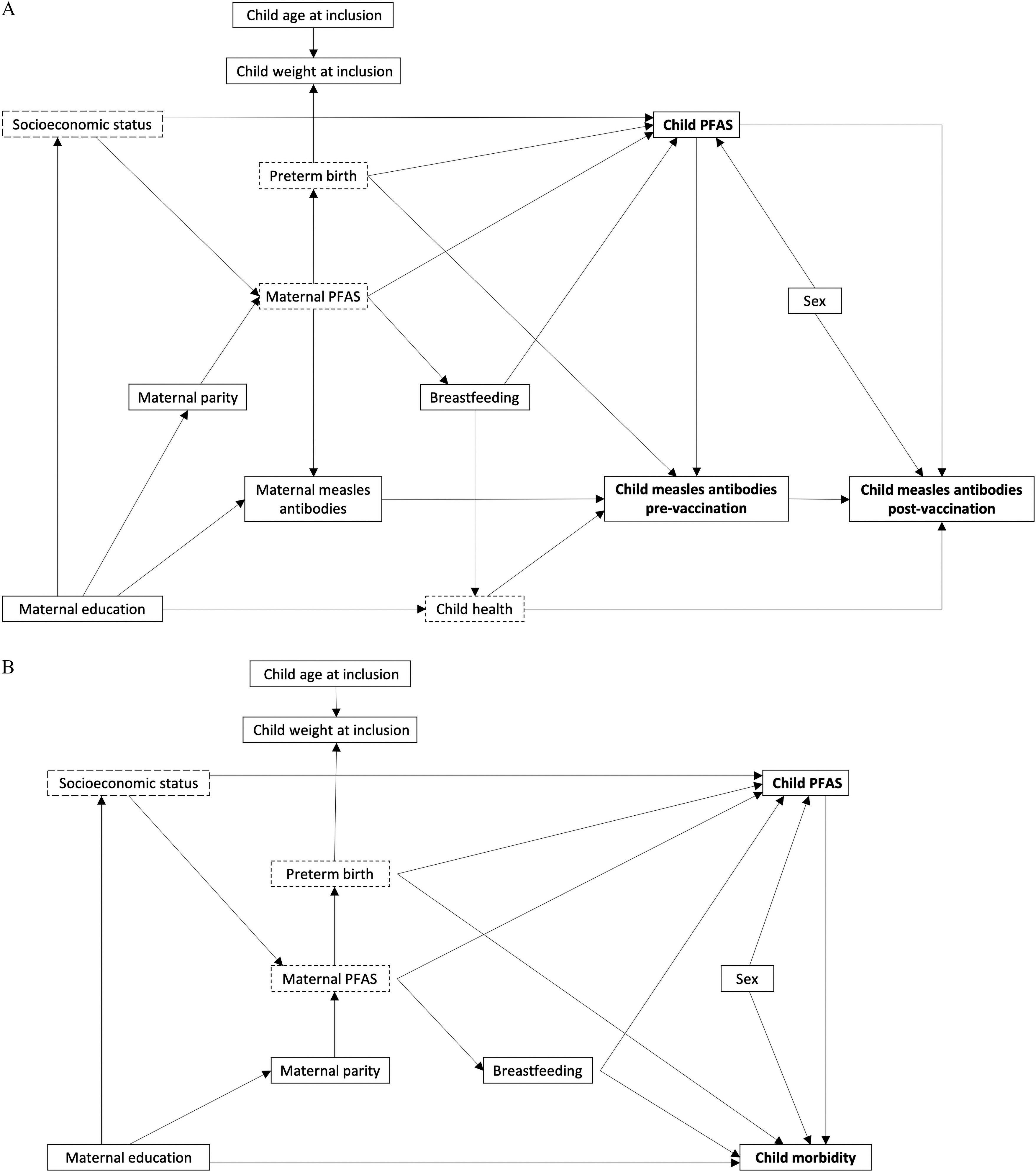
Directed acyclic graphs for the hypothesized associations between child PFAS concentrations and (A) measles antibody concentrations or (B) child morbidity. Arrows indicate a priori assumptions of associations. Dotted squares indicate unobserved variables. Note: PFAS, perfluoroalkyl substances.
Maternal education might affect child morbidity and child health and thus indirectly the antibody concentrations. Furthermore, with little knowledge about the routes of PFAS exposure in Guinea-Bissau, we cannot exclude the possibility of socioeconomic status, as reflected by maternal education, being associated with PFAS exposure. Information about maternal education was, however, missing for 10% of the participants, and in order not to reduce the sample size when including this variable in the adjusted regression models, education was divided into three categories (any, none, and unknown).
Maternal PFAS concentrations have been associated with reduced duration of breastfeeding among U.S. and Faroese mothers (Romano et al. 2016; Timmermann et al. 2017), and breastfeeding acts as an important pathway for PFAS exposure in young children (Haug et al. 2011; Mogensen et al. 2015). In addition, breastfeeding is expected to have beneficial effects on the immune system development (Plaza-Díaz et al. 2018) and reduce child morbidity (Victora et al. 2016). Breastfeeding could, therefore, potentially act as a confounder in this study. In Guinea-Bissau, virtually all children are breastfed during the first months of life, and at inclusion, 43% of the children in our study had not yet had porridge or other solid food introduced, whereas this was the case for only 4% of the children at the 9-month visit. However, 99% of the children were still being breastfed at the 9-month visit. Consequently, analyses examining outcomes at inclusion were adjusted for whether or not the child was still being breastfed without complementary solids, whereas analyses examining outcomes at the 9-month visit were adjusted for duration of breastfeeding without solids until 9 months (measured in days), and analyses examining outcomes at the 2-y visit were likewise adjusted for duration of breastfeeding without solids (days).
Infant antibody concentrations prior to vaccination depend on the maternal antibody concentrations (Niewiesk 2014), and if PFAS are immunotoxic, the mother’s exposure could affect her own serum antibody concentrations. Furthermore, PFAS are transferred across the placenta (Eryasa et al. 2019; Needham et al. 2011), and the infant’s serum PFAS concentrations are thus affected by the maternal PFAS concentrations. To avoid confounding from this backdoor path, the analyses of measles antibody concentrations were adjusted for the maternal antibody concentrations.
Analyses of the antibody concentration after vaccination were first performed without adjustment for the child’s antibody concentration prior to vaccination in order to explore overall effects on postvaccination concentrations. We then carried out an analysis with adjustment for the antibody concentration prior to the vaccination to elucidate whether PFAS were associated with a reduced ability to produce new antibodies upon vaccination. When adjusting for prevaccination antibody concentrations, maternal measles antibody concentrations and preterm birth did not constitute potential confounders (Figure 2A), and maternal measles antibody concentrations and the proxy variables weight and age at inclusion were, therefore, not included in these analyses.
Pregnancies with male fetuses might have a higher placenta/maternal serum PFAS ratio (Mamsen et al. 2019), which could result in higher PFAS concentrations among boys compared with girls. In addition, boys are more vulnerable to morbidity in infancy (Zhao et al. 2017) and have been shown to have lower antibody concentrations after measles vaccination with the Edmonston-Zagreb strain (Martins et al. 2013). Child sex was thus included as a potential confounder in the adjusted analyses of morbidity and postvaccination antibody concentrations.
Postvaccination antibody concentrations depend on time since vaccination, as the antibody concentration decrease over time. In the present study, the interval between vaccination at inclusion (intervention group) and blood sampling at the 9-month visit was 2.0–9.2 months (mean: 4.1) and the interval between the 9-month vaccination/booster and blood sampling at the 2-y visit was 7.2–23.6 months (mean: 15.4). Thus, to account for the variance in time interval and improve model efficiency, the analyses of antibody concentrations after vaccination were adjusted for time (days) since vaccination, in addition to the potential confounders identified in the DAGs.
To sum up, four different sets of covariates were used for the adjusted analyses; one set for analyses of prevaccination antibody concentrations, one set for analyses of postvaccination antibody concentrations without adjustment for prevaccination antibody concentrations, one set for postvaccination antibody concentrations with adjustment for prevaccination antibody concentrations, and one for set for morbidity analyses. The covariates included in each model are listed in Tables 3 and 4.
Table 3.
Percentage difference in measles antibody concentrations at inclusion and at the 9-month and 2-y visits with a doubling of serum PFAS concentrations at inclusion.
| PFAS | Measles antibody concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion | 9-month visit/control | 9-month visit/intervention | 2-y visit/control | 2-y visit/intervention | ||||||
| (no measles vaccination) | (no measles vaccination) | (1 measles vaccination) | (1 measles vaccination) | (2 measles vaccinations) | ||||||
| Percentage difference (95% CI) | Percentage difference (95% CI) | Percentage difference (95% CI) | Percentage difference (95% CI) | Percentage difference (95% CI) | ||||||
| PFHxS | ||||||||||
| Crude | 236 | (, 15) | 100 | (, 29) | 134 | 6 (, 38) | 102 | (, 19) | 92 | 10 (, 48) |
| Adjusted | 236 | (, 18)a | 100 | (, 43)a | 134 | (, 25)b | 102 | (, 9)b | 92 | 12 (, 51)b |
| Adjustedc | — | — | 133 | 8 (, 41) | 100 | (, 5) | 91 | 0 (, 29) | ||
| Sensitivityd | 230 | (, )a | 97 | (, 24)a | 127 | (, 9)c | 95 | (, )c | 88 | (, 12)c |
| PFOS | ||||||||||
| Crude | 236 | (, 5) | 100 | (, ) | 134 | (, ) | 102 | 2 (, 27) | 92 | (, 11) |
| Adjusted | 236 | (, 4)a | 100 | (, )a | 134 | (, )b | 102 | (, 24)b | 92 | (, 12)b |
| Adjustedc | — | 100 | — | 133 | (, ) | 100 | (, 18) | 91 | (, 17) | |
| Sensitivityd | 229 | (, )a | 96 | (, )a | 129 | (, )c | 98 | (, )c | 88 | (, 6)c |
| PFOA | ||||||||||
| Crude | 236 | (, 8) | 100 | (, 17) | 134 | 10 (, 41) | 102 | (, 25) | 92 | 9 (, 37) |
| Adjusted | 236 | (, 7)a | 100 | (, 22)a | 134 | 7 (, 35)b | 102 | (, 18)b | 92 | 12 (, 40)b |
| Adjustedc | — | 100 | — | 133 | 13 (, 47) | 100 | (, 12) | 91 | 5 (, 28) | |
| Sensitivityd | 229 | (, )a | 97 | (, 6)a | 129 | (, 15)c | 97 | (, )c | 87 | (, 9)c |
| PFNA | ||||||||||
| Crude | 235 | (, 7) | 100 | (, 13) | 133 | (, 15) | 102 | (, 17) | 92 | 1 (, 21) |
| Adjusted | 235 | (, 4)a | 100 | (, 12)a | 133 | (, 18)b | 102 | (, 16)b | 92 | 0 (, 21)b |
| Adjustedc | — | — | 132 | (, 17) | 100 | (, 15) | 91 | 4 (, 21)c | ||
| Sensitivityd | 225 | (, )a | 99 | (, 10)a | 127 | (, )c | 96 | (, )c | 88 | (, 14)c |
| PFDA | ||||||||||
| Crude | 236 | (, 10) | 100 | (, 8) | 134 | (, 0) | 102 | 4 (, 32) | 92 | (, 32) |
| Adjusted | 236 | (, 6)a | 100 | (, 7)a | 134 | (, )b | 102 | 4 (, 33)b | 92 | (, 29)b |
| Adjustedc | — | — | 133 | (, ) | 100 | (, 24) | 91 | 6.5 (, 36) | ||
| Sensitivityd | 227 | (, )a | 97 | (, )a | 129 | (, )c | 99 | (, 13)c | 87 | (, 14)c |
| PFUnDA | ||||||||||
| Crude | 236 | (, 11) | 100 | (, 27) | 134 | (, 10) | 102 | 13 (, 44) | 92 | (, 35) |
| Adjusted | 236 | (, 10)a | 100 | (, 24)a | 134 | (, 2)b | 102 | 11 (, 43)b | 92 | (, 23)b |
| Adjustedc | — | — | 133 | (, 2) | 100 | 5 (, 36) | 91 | (, 30) | ||
| Sensitivityd | 233 | (, 1)a | 99 | (, 24)a | 130 | (, )c | 97 | (, 15)c | 88 | (, 10)c |
Note: —, not applicable; CI, confidence interval; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
Adjusted for weight (to nearest ) and age (days) at inclusion, maternal education (none/any/unknown), breastfeeding without solids (inclusion: yes/no, 9-month visit: duration in days), maternal measles antibody concentration (IU/mL).
Adjusted for weight (to nearest ) and age (days) at inclusion, maternal education (none/any/unknown), breastfeeding without solids (duration in days), maternal measles antibody concentration (IU/mL), sex, and time from vaccination to blood sampling (days).
Adjusted for measles antibody concentration at previous visit (IU/mL, log-transformed), maternal education (none/any/unknown), duration of breastfeeding without solids (duration in days), sex, and time from vaccination to blood sampling (days).
The most influential observations were removed from the analyses.
Table 4.
Odds ratio (OR) for the presence of fever, coughing, diarrhea, and any morbidity at inclusion and at the 9-month visit with a doubling of serum PFAS concentrations at inclusion.
| Morbidity and PFAS | Inclusion | 9-month visit | ||
|---|---|---|---|---|
| n/N | OR (95% CI) | n/N | OR (95% CI) | |
| Fever | ||||
| PFHxS | ||||
| Crude | 47/236 | 1.29 (0.78, 2.14) | 19/236 | 1.02 (0.47, 2.18) |
| Adjusteda | 47/236 | 1.31 (0.78, 2.21) | 19/236 | 0.95 (0.43, 2.08) |
| PFOS | ||||
| Crude | 47/236 | 1.30 (0.84, 2.00) | 19/236 | 1.16 (0.62, 2.17) |
| Adjusteda | 47/236 | 1.40 (0.90, 2.20) | 19/236 | 1.19 (0.62, 2.31) |
| PFOA | ||||
| Crude | 47/236 | 0.96 (0.59, 1.56) | 19/236 | 1.56 (0.77, 3.17) |
| Adjusteda | 47/236 | 0.93 (0.56, 1.56) | 19/236 | 1.62 (0.74, 3.54) |
| PFNA | ||||
| Crude | 47/235 | 1.28 (0.89, 1.84) | 19/235 | 1.32 (0.77, 2.26) |
| Adjusteda | 47/235 | 1.29 (0.89, 1.86) | 19/235 | 1.20 (0.70, 2.07) |
| PFDA | ||||
| Crude | 47/236 | 1.54 (0.94, 2.52) | 19/236 | 1.24 (0.61, 2.53) |
| Adjusteda | 47/236 |
1.49 (0.89, 2.49) |
19/236 | 1.16 (0.56, 2.44) |
| PFUnDA | ||||
| Crude | 47/236 | 1.27 (0.81, 1.99) | 19/236 | 0.85 (0.41, 1.78) |
| Adjusteda | 47/236 | 1.29 (0.81, 2.07) | 19/236 | 0.71 (0.33, 1.54) |
| Coughing | ||||
| PFHxS | ||||
| Crude | 71/236 | 1.33 (0.85, 2.10) | 32/237 | 1.96 (1.13, 3.41) |
| Adjusteda | 71/236 | 1.28 (0.81, 2.05) | 32/237 | 2.15 (1.17, 3.97) |
| PFOS | ||||
| Crude | 71/236 | 1.04 (0.72, 1.50) | 32/237 | 1.41 (0.85, 2.33) |
| Adjusteda | 71/236 | 1.00 (0.69, 1.47) | 32/237 | 1.54 (0.92, 2.59) |
| PFOA | ||||
| Crude | 71/236 | 1.07 (0.70, 1.64) | 32/237 | 1.63 (0.92, 2.89) |
| Adjusteda | 71/236 | 1.06 (0.69, 1.64) | 32/237 | 1.87 (1.02, 3.45) |
| PFNA | ||||
| Crude | 71/235 | 1.05 (0.77, 1.42) | 32/236 | 1.37 (0.89, 2.10) |
| Adjusteda | 71/235 | 1.06 (0.78, 1.45) | 32/236 | 1.34 (0.87, 2.07) |
| PFDA | ||||
| Crude | 71/236 | 0.79 (0.51, 1.24) | 32/237 | 1.16 (0.65, 2.07) |
| Adjusteda | 71/236 | 0.77 (0.49, 1.22) | 32/237 | 1.17 (0.64, 2.14) |
| PFUnDA | ||||
| Crude | 71/236 | 0.94 (0.62, 1.42) | 32/237 | 1.01 (0.59, 1.75) |
| Adjusteda | 71/236 | 0.93 (0.60, 1.44) | 32/237 | 1.00 (0.57, 1.75) |
| Diarrhea | ||||
| PFHxS | ||||
| Crude | 27/236 | 1.16 (0.62, 2.19) | 22/237 | 1.54 (0.83, 2.84) |
| Adjusteda | 27/236 | 1.25 (0.65, 2.39) | 22/237 | 1.58 (0.81, 3.09) |
| PFOS | ||||
| Crude | 27/236 | 1.03 (0.60, 1.75) | 22/237 | 1.01 (0.56, 1.82) |
| Adjusteda | 27/236 | 1.14 (0.66, 1.96) | 22/237 | 1.20 (0.62, 2.31) |
| PFOA | ||||
| Crude | 27/236 | 1.07 (0.58, 1.98) | 22/237 | 1.31 (0.67, 2.53) |
| Adjusteda | 27/236 | 1.09 (0.56, 2.09) | 22/237 | 1.54 (0.72, 3.29) |
| PFNA | ||||
| Crude | 27/235 | 0.97 (0.62, 1.51) | 22/236 | 1.26 (0.77, 2.08) |
| Adjusteda | 27/235 | 0.97 (0.63, 1.49) | 22/236 | 1.22 (0.73, 2.03) |
| PFDA | ||||
| Crude | 27/236 | 1.13 (0.61, 2.10) | 22/237 | 1.22 (0.62, 2.38) |
| Adjusteda | 27/236 | 1.03 (0.54, 1.97) | 22/237 | 1.17 (0.55, 2.51) |
| PFUnDA | ||||
| Crude | 27/236 | 0.99 (0.54, 1.79) | 22/237 | 1.01 (0.53, 1.92) |
| Adjusteda | 27/236 | 0.99 (0.53, 1.84) | 22/237 | 0.91 (0.42, 1.94) |
| Any morbidityb | ||||
| PFHxS | ||||
| Crude | 99/235 | 1.36 (0.87, 2.11) | 52/236 | 1.76 (1.08, 2.88) |
| Adjusteda | 99/235 | 1.32 (0.84, 2.07) | 52/236 | 1.82 (1.06, 3.11) |
| PFOS | ||||
| Crude | 99/235 | 1.14 (0.81, 1.62) | 52/236 | 1.25 (0.83, 1.89) |
| Adjusteda | 99/235 | 1.13 (0.80, 1.62) | 52/236 | 1.36 (0.88, 2.10) |
| PFOA | ||||
| Crude | 99/235 | 1.05 (0.71, 1.55) | 52/236 | 1.81 (1.11, 2.93) |
| Adjusteda | 99/235 | 1.03 (0.68, 1.54) | 52/236 | 2.02 (1.20, 3.41) |
| PFNA | ||||
| Crude | 99/234 | 1.03 (0.77, 1.37) | 52/235 | 1.26 (0.89, 1.79) |
| Adjusteda | 99/234 | 1.03 (0.77, 1.38) | 52/235 | 1.23 (0.86, 1.75) |
| PFDA | ||||
| Crude | 99/235 | 1.00 (0.67, 1.51) | 52/236 | 1.24 (0.77, 2.00) |
| Adjusteda | 99/235 | 0.98 (0.65, 1.49) | 52/236 | 1.23 (0.75, 2.03) |
| PFUnDA | ||||
| Crude | 99/235 | 1.01 (0.69, 1.49) | 52/236 | 0.99 (0.62, 1.56) |
| Adjusteda | 99/235 | 0.99 (0.67, 1.47) | 52/236 | 0.92 (0.57, 1.48) |
Note: CI, confidence interval; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
Adjusted for weight (to nearest ) and age (days) at inclusion, sex, maternal education (none/any/unknown), and breastfeeding without solids (inclusion: yes/no, 9-month visit: duration in days).
Fever, diarrhea, coughing, or vomiting.
In vitro data have shown that leukocytes obtained from adult female donors were more sensitive to the toxic effects of PFAS than were leukocytes from adult male donors (Corsini et al. 2012), and the development of the immune system may be dependent on sex before puberty (Klein and Flanagan 2016). Thus, we examined potential immunotoxic effects of PFAS exposure separately for boys and girls. Sex-differential effects of PFAS on measles antibody concentrations and child morbidity were tested by including interaction terms in the regression models.
At inclusion, one child had a measles antibody concentration of (more than five times the 90th percentile for this group). An elevated antibody concentration is likely due to prior measles infection or unregistered measles vaccination, and the single observation of was, therefore, excluded from the analyses. Slightly outlying values were found for other children as well, and to ensure that single points did not overly influence the regression line, sensitivity analyses were performed excluding highly influential observations from the linear regression models [] (Belsley et al. 1980). Up to 10 observations were removed with an average of 4 observations per analysis (3% of observations).
The assumptions underlying the linear regression models about homoscedasticity and normal distribution of the residuals were inspected visually using plots of residuals against fitted values and quantile-normal plots of the standardized residuals, respectively. Goodness-of-fit for the logistic regression models were tested using the Hosmer-Lemeshow test. Log-linearity of PFAS was tested by including log(PFAS) squared along with log(PFAS) in the regression model.
All analyses were performed using Stata/IC (version 16.1; StataCorp). A 5% level of significance were used when testing associations and interactions.
Ethics
The original trial was approved by the ethical review committee in Guinea-Bissau (Comité Nacional de Ética na Saúde) and received subsequent approval in Denmark [Danish Central Ethical Committee (consultative approval)]. The trial was registered with ClinicalTrials.gov, Identifier: NCT01644721. The present study relied on anonymized data and linked samples and, therefore, did not require further approvals.
Results
At inclusion, the children were between 4.2 and 7.1 months of age (mean: 5.6), at the 9-month visit between 8.9 and 18.2 months (mean: 9.9), and at the 2-y visit between 21.9 and 32.7 months (mean: 25.3). The intervals between PFAS measurement at inclusion and the 9-month and 2-y visit were 2.0–12.1 months (mean: 4.3) and 16.4–25.6 months (mean: 19.5), respectively. In the control group, the intervals between PFAS measurement at inclusion and vaccination at the 9-month visit was 2.1–12.1 months (mean: 4.5).
One child had a serum PFUnDA concentration below the LOD. All other children had detectable serum concentrations of all six PFAS at inclusion. Median infant serum PFAS concentrations ranged between (PFHxS) and (PFOS). At inclusion, 20%, 11%, 30%, and 6% of the children reportedly had current fever, diarrhea, coughing, and vomiting, respectively, and at the 9-month visit, the corresponding frequencies were 8%, 9%, 14%, and 3%. At inclusion, 42% reported any current morbidity and at the 9-month visit, 22% reported any current morbidity (Table 1).
Table 1.
Distribution of PFAS concentrations, measles antibody concentrations, and child morbidity.
| Categories | Median (25th, 75th percentile) or (%) | |
|---|---|---|
| PFAS (ng/mL serum) | ||
| PFHxS | 237 | 0.10 (0.09, 0.14) |
| PFOS | 237 | 0.77 (0.53, 1.02) |
| PFOA | 237 | 0.68 (0.53, 0.92) |
| PFNA | 236 | 0.21 (0.13, 0.31) |
| PFDA | 237 | 0.19 (0.15, 0.25) |
| PFUnDA | 237 | 0.12 (0.10, 0.16) |
| Measles antibody concentrations (mIU/mL) | ||
| Maternal sample at inclusion | 237 | 882 (412, 1,668) |
| Child at inclusion | 236 | 54 (29, 124) |
| Child at 9-month visit | ||
| Control group | 100 | 10 (6, 21) |
| Intervention group | 134 | 432 (264, 749) |
| Child at 2-y visit | ||
| Control group | 102 | 772 (441, 1,083) |
| Intervention group | 92 | 577 (364, 1,102) |
| Reported child morbidity | ||
| Fever | ||
| At inclusion | 236 | 47 (20) |
| At 9-month visit | 236 | 19 (8) |
| Diarrhea | ||
| At inclusion | 236 | 27 (11) |
| At 9-month visit | 237 | 22 (9) |
| Coughing | ||
| At inclusion | 236 | 71 (30) |
| At 9-month visit | 237 | 32 (14) |
| Vomiting | ||
| At inclusion | 235 | 14 (6) |
| At 9-month visit | 237 | 8 (3) |
| Any morbidity | ||
| At inclusion | 235 | 99 (42) |
| At 9-month visit | 236 | 52 (22) |
Note: PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
The protective serum concentration of measles antibodies is not known for certain but is often set to (Chen et al. 1990; Cohen et al. 2007; Ratnam et al. 1995). At inclusion, 5% (12/237) of mothers and 74% (176/237) of the children had measles antibody concentrations below . At the 9-month visit, 99% (99/100) of children in the control group (with no previous vaccination) and 10% (14/134) of children in the intervention group (vaccinated at inclusion) were below , and at the 2-y visit, 2% (2/102) of children in the control group (with one previous vaccination) and 1% (1/92) of children in the intervention group (with two previous vaccinations) were below .
Girls tended to have higher measles antibody concentrations after vaccination compared with boys, but no difference was seen prior to vaccination (Table 2). Neither maternal education, child weight at inclusion, nor breastfeeding without solids at inclusion were significantly associated with child measles antibody concentrations in crude analyses (Table 2).
Table 2.
Child measles antibody concentrations at inclusion and at the 9-month and the 2-y visit by maternal and child characteristics.
| Maternal and child characteristics | (%) | Measles antibody concentrations [mIU/mL median (25th, 75th percentile)] | ||||
|---|---|---|---|---|---|---|
| Inclusion | 9-month visit/control | 9-month/intervention | 2-y visit/control | 2-y visit/intervention | ||
| (No measles vaccination) | (No measles vaccination) | (1 measles vaccination) | (1 measles vaccination) | (2 measles vaccinations) | ||
| Maternal parity | 235 (100) | |||||
| 0 | 34 (14) | 32 (15, 93) | 5 (3, 17) | 535 (467, 978) | 1,000 (797, 1,603) | 1,059 (505, 1,146) |
| 1–2 | 80 (34) | 71 (31, 108) | 11 (8, 19) | 354 (166, 682) | 630 (401, 962) | 494 (301, 1,106) |
| 3–4 | 61 (26) | 55 (30, 153) | 8 (6, 18) | 471 (202, 758) | 750 (292, 1,074) | 684 (415, 1,146) |
| 5–11 | 60 (26) | 59 (32, 127) | 10 (6, 25) | 421 (295, 725) | 839 (699, 1,093) | 570 (345, 830) |
| -Valuea | 0.06 | 0.18 | 0.18 | 0.06 | 0.21 | |
| Maternal education | 237 (100) | |||||
| None | 102 (43) | 67 (34, 149) | 12 (7, 22) | 471 (295, 794) | 735 (427, 1,058) | 605 (373, 1,134) |
| 1–11 y | 111 (47) | 49 (25, 96) | 8 (5, 17) | 396 (217, 687) | 895 (471, 1,325) | 570 (378, 1,100) |
| Missing | 24 (10) | 68 (28, 103) | 10 (6, 18) | 739 (188, 1,267) | 590 (426, 893) | 469 (330, 1,146) |
| -Valuea | 0.07 | 0.11 | 0.38 | 0.39 | 0.96 | |
| Child sex | 237 (100) | |||||
| Boy | 123 (52) | 58 (27, 136) | 10 (6, 21) | 376 (219, 749) | 641 (398, 988) | 570 (345, 891) |
| Girl | 114 (48) | 53 (29, 115) | 9 (5, 21) | 487 (317, 758) | 871 (550, 1,401) | 830 (390, 1,146) |
| -Valueb | 0.75 | 0.63 | 0.27 | 0.03 | 0.13 | |
| Child weight at inclusion | 237 (100) | |||||
| 131 (55) | 53 (26, 137) | 8 (5, 21) | 532 (305, 765) | 763 (427, 1,074) | 562 (345, 1,018) | |
| 106 (45) | 58 (31, 100) | 11 (7, 21) | 375 (198, 745) | 782 (442, 1,103) | 601 (391, 1,146) | |
| -Valueb | 0.73 | 0.16 | 0.13 | 0.96 | 0.67 | |
| Breastfeeding without solids at inclusion | 237 (100) | |||||
| Yes | 102 (43) | 54 (30, 135) | 10 (6, 21) | 373 (219, 1,199) | 913 (506, 1,389) | 601 (347, 1,100) |
| No | 135 (57) | 55 (27, 111) | 9 (6, 21) | 467 (293, 699) | 702 (426, 1,004) | 570 (378, 1,103) |
| -Valueb | 0.71 | 0.80 | 0.90 | 0.07 | 0.98 | |
Associations tested using the Kruskal-Wallis test.
Associations tested using the Wilcoxon rank-sum test.
Among the children who had received a measles vaccination at inclusion (intervention group), a doubling in PFOS and PFDA was associated with 21% [95% confidence interval (CI): 2, 37%] and 25% (95% CI: 1, 43%) lower measles antibody concentrations at the 9-month visit (adjusted analyses), and when removing the most influential points , the same trend was seen for all six PFAS, although not significant for PFHxS and PFOA (Table 3). When excluding the most influential points, the trend persisted through the 2-y visit, although weakened and not statistically significant. Among the children who received their first measles vaccination at the 9-month visit (control group), elevated PFAS concentrations at inclusion was likewise associated with reduced measles antibody concentrations at the 2-y visit, but the associations were significant (for PFHxS, PFOS, PFOA, and PFNA) only after removal of the most influential points (Table 3).
Elevated concentrations of all six PFAS were associated with lower measles antibody concentrations at inclusion, but the associations were not statistically significant unless the most influential points were removed in the sensitivity analyses (Table 3). Among the children who did not receive a vaccination at inclusion (control group), the associations persisted at the 9-month visit, but the association was significant only for PFOS, where a doubling in serum PFOS was associated with a 27% (95% CI: 4, 44%) lower measles antibody concentration. Again, the associations were strengthened by removal of the most influential points most pronounced for PFOS [40% (95% CI: 19, 56%)] and PFDA [23% (95% CI: 8, 53%)] (Table 3).
When examining the associations between PFAS and morbidity at inclusion and at the 9-month visit, most (35 of 48) analyses showed increased odds of morbidity at higher serum PFAS concentrations at inclusion, although only a few of the associations were statistically significant (Table 4). The effects were generally more pronounced at the 9-month visit, and the strongest results were seen for PFHxS and PFOA in relation to coughing and any morbidity. At the 9-month visit, ORs for coughing in association with a doubling of PFHxS and PFOA were 2.15 (95% CI: 1.17, 3.97) and 1.87 (95% CI: 1.02, 3.45), respectively, whereas ORs for any morbidity were 1.82 (95% CI: 1.06, 3.11) and 2.02 (95% CI: 1.20, 3.41), respectively (Table 4).
Most of the associations between PFAS and diarrhea and morbidity at inclusion were positive () for boys and negative () for girls (see Table S1), but this trend did not persist across all outcomes, and among the 78 interaction analyses, only 4 were statistically significant (see Tables S1–S2). PFDA was associated with higher odds of diarrhea at inclusion in boys [OR 1.66 (95% CI: 0.76, 3.59)] and with lower odds in girls [OR 0.35 (95% CI: 0.10, 1.20)] (). Similarly, PFOS and PFUnDA were associated with higher odds of any morbidity at inclusion in boys and with lower odds of any morbidity in girls ( and 0.03, respectively) (see Table S1). In contrast, among those vaccinated at the 9-month visit only (control group), measles antibody concentrations at the 2-y visit were 22% higher in association with a doubling of PFOS in boys (95% CI: , 66%) but were 28% lower in girls (95% CI: , ) () (Table S2).
For most of the linear regression models, plots of residuals against fitted values and quantile-normal plots of standardized residuals suggested that assumptions regarding homoscedasticity and normal distribution of the residuals were met (data not shown). Visual evidence of slight heteroscedasticity was diminished for PFAS and measles antibodies at inclusion, and in the intervention group at the 9-month visit, when highly influential observations , 1.3–4.3% and 2.3–4.5% of observations, respectively) were removed in the sensitivity analyses. In general, log(PFAS) squared was not significantly associated with measles antibodies when added to the models, suggesting that assumptions regarding log-linear associations were met in most cases (data not shown). Exceptions were models of PFOA and PFNA with measles antibodies at inclusion (p-values for higher-order terms of 0.049 and 0.024, respectively). The deviation was no longer significant for PFOA after 7 influential observations were removed () but remained significant for PFNA (10 influential observations removed, ) with a negative slope for PFNA concentrations (see Figure S1). In the logistic regression models, the Hosmer-Lemeshow test revealed a poor model fit when examining the associations between PFHxS and coughing at the 9-month visit () and between PFOS and any morbidity at the 9-month visit (). However, the model fit was acceptable in most of the analyses, and we therefore chose not to change the DAG a posteriori.
Discussion
The present study extends the documentation on the distribution of PFAS exposure to West African infants and reports evidence of immunotoxicity even at low PFAS exposures. Data on infant PFAS exposure is limited, but a recent study measuring PFOS and PFOA in stored dried blood spots from newborns in Upstate New York found median concentrations of 1.74 and , respectively (Ghassabian et al. 2018), and it should be noted that concentrations in whole blood are approximately half of those measured in serum (Poothong et al. 2017). In Faroese children, at 18 months of age, median PFHxS, PFOS, PFOA, PFNA, and PFDA concentrations in serum (Grandjean et al. 2017) were between 1.5 (PFDA) and 24 (PFOS) times higher than concentrations in the present study. In the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES), median serum concentrations (in nanograms per milliliter) of PFHxS (0.74), PFOS (3.41), PFOA (1.80), and PFNA (0.62) in children 3–5 years of age were also higher than those found in the present study, whereas the median PFDA serum concentration was lower () (Ye et al. 2018). Thus, the children in this study generally had lower serum PFAS concentrations than children in other parts of the world, but notably, we found detectable levels of five of the investigated substances in all serum samples analyzed, and PFUnDA was detected in all but one of the samples.
The sources of PFAS exposure in Guinea-Bissau are unknown. The population is among the poorest in the world, and exposure from consumer products such as new furniture and outdoor clothing is, therefore, expected to be minimal. However, the diet in rural Guinea-Bissau includes fish caught in small lakes and rivers, and in some of the villages also marine fish, which could constitute a PFAS source along with potentially contaminated drinking water (Jian et al. 2017). In Faroese children, PFUnDA has been shown to be a marker of marine food exposure (Dassuncao et al. 2018), and interestingly, in the present study, we found PFUnDA in concentrations slightly higher than PFHxS, thus indicating possible exposure from sea food. To our knowledge, there is no relevant industry in the area that could leak PFAS to the environment.
PFAS and Postvaccination Antibodies
Despite the relatively low serum PFAS concentrations among infants in the present study, we found that elevated concentrations of PFOS and PFDA in the intervention group were significantly associated with lower measles antibody concentrations at the 9-month visit after vaccination at inclusion (4–7 months of age). After removal of the most influential points, the same trend was seen for all six PFAS. A similar trend was seen at the 2-y visit among the children who were first vaccinated at the 9-month visit (control group). These results correspond to previous findings in Faroese 5- and 7-y-olds from two cohorts vaccinated against tetanus and diphtheria (Grandjean et al. 2012, 2017) and to the findings of decreased rubella antibodies after vaccination in Norwegian 3-y-olds prenatally exposed to PFAS (Granum et al. 2013). The Norwegian study also showed a trend toward reduced measles antibody concentrations with elevated concentrations of PFHxS, PFOS, PFOA, and PFNA, but the associations were not significant, possibly due to the small sample size () (Granum et al. 2013). Recent studies have analyzed data on adolescents in the cross-sectional 1999–2000 and 2003–2004 NHANES. Although one found no association between serum PFAS concentrations and rubella antibodies among 1,012 12- to 18-y-olds (Pilkerton et al. 2018), the other found PFAS to be associated with reduced concentrations of mumps and rubella, although not measles, antibodies among 1,191 12- to 19-y-olds (Stein et al. 2016). However, the vaccination status of the U.S. adolescents was unknown (Stein et al. 2016).
Mechanisms for potential immunotoxic effects of PFAS are uncertain, but in experimental studies, PFAS have been reported to suppress T-cell-dependent antibody responses in rodents (DeWitt et al. 2019; Dong et al. 2009; Keil et al. 2008). Furthermore, an in vitro study of human immune cells showed that PFAS can affect nuclear factor (NF) activation, with PFDA and PFOS being more active than PFOA (Corsini et al. 2012), which is consistent with our findings.
PFAS and Prevaccination Antibodies
Findings from the present study also suggested that higher serum PFAS concentrations were associated with lower measles antibody concentrations before vaccination. Prior to vaccination (or infection), infants are dependent on measles antibodies transferred from the mother across the placenta mainly during third trimester (Leuridan and Van Damme 2007). During the present study there was no measles epidemics and we do not expect the infants to have been exposed to measles infection. Lower infant measles antibody concentrations prior to vaccination is thus a sign of either reduced maternal measles antibody concentrations, reduced placental transfer, or increased metabolization and, thus, increased waning of the antibodies. Associations between PFAS and prevaccination titers persisted after adjustment for the mother’s measles antibody concentrations, suggesting that PFAS may disrupt the transfer of maternal antibodies or increase the rate at which they decline after birth. In the absence of prior studies on this issue, interpretation of our findings is tentative at present.
Infants with low prevaccination antibodies tend to have a more robust humoral response to vaccination (Niewiesk 2014). In the present study, those with higher serum PFAS concentrations had lower prevaccination antibodies. However, instead of higher postvaccination antibodies, PFAS were associated with lower postvaccination antibody responses. This suggests that PFAS exposure may reduce the robust humoral response to vaccination that is typically observed in infants with low prevaccination antibodies.
PFAS and Infant Morbidity
We saw a consistent trend toward increased odds of morbidity with higher serum PFAS concentrations. Although previous studies on child morbidity did not have access to serum PFAS concentrations in infancy, our findings are in line with findings from Norwegian, Danish, and Japanese studies of early life PFAS exposure and morbidity episodes during the first years of life (Dalsager et al. 2016; Goudarzi et al. 2017; Granum et al. 2013; Impinen et al. 2018, 2019). The first Norwegian study showed that higher prenatal exposure to PFOA and PFNA was associated with more episodes of the common cold in the first 3 y of life, and higher prenatal exposure to PFOA and PFHxS was associated with more episodes of gastroenteritis (Granum et al. 2013). The study from Denmark showed an increased risk of fever in 1- to 4-y-olds at higher maternal pregnancy concentrations of PFOS and PFOA (Dalsager et al. 2016). The study from Japan showed increased odds of total infectious disease in the first 4 y of life at higher prenatal exposure to PFOS (Goudarzi et al. 2017). The second Norwegian study showed more lower respiratory tract infections in the first 10 y of life with increasing cord serum concentrations of PFAS, including PFOS, PFOA, and PFNA (Impinen et al. 2018), whereas a the third Norwegian study showed associations between prenatal exposure to PFAS, including PFOS, PFOA, and PFHxS, and bronchitis/pneumonia in the first 3 y of life (Impinen et al. 2019). However, results were not consistent across all outcomes (Impinen et al. 2019). In older children, a recent Norwegian study found an increased risk of lower respiratory tract infections between 10 and 16 years of age with higher serum PFAS concentrations, including PFOS, PFOA and PFNA, measured at 10 years of age (Kvalem et al. 2020). However, at 16 years of age, a trend was seen toward a reduced risk of common colds in the past 12 months with higher serum PFAS concentrations at 10 years of age (Kvalem et al. 2020). Furthermore, a Danish and a Japanese study found no associations between maternal serum concentrations of PFOS and PFOA and hospitalizations due to infection in early childhood (Fei et al. 2010) and otitis media during the first 18 months of life (Okada et al. 2012), respectively. However, the validity of the PFAS measurements in the Danish cohort have been questioned (Bach et al. 2015).
Sex-Specific Associations
Significant differences between boys and girls were found in only 4 of 78 regression analyses, thus our findings do not support differences in the association between PFAS and serological vaccine responses or morbidity by sex. Given the limited statistical power of the present study, a minor sex-related difference in PFAS susceptibility is, of course, possible.
Sensitivity Analyses
For the linear regression models, sensitivity analyses were performed excluding influential observations , to ensure that the associations were not driven by outlying values. When removing the influential points, the negative associations between PFAS and measles antibodies were strengthened, thus supporting the notion that the associations were not merely chance findings.
Strengths and Weaknesses
The present study was performed on a subset of data collected for another purpose, and a main limitation of this opportunistic approach was that information was not available about all potential confounding variables. Preterm birth could constitute a potential confounding path between child PFAS exposure and prevaccination antibodies, but we did not have information about gestational length. Instead, we adjusted for weight and age at inclusion, which resulted in only a minor impact on the findings.
Information about morbidity was based on information from the mother without clinical measures, and we did not have information about the causes of fever, diarrhea, coughing, and vomiting. Furthermore, past morbidity was not taken into account. Nonetheless, the results from the morbidity analyses substantiate the hypothesis of PFAS affecting the immune system.
Precision and accuracy of the methods used to assess PFAS and measles antibodies were high, thus reducing the risk of information bias. At inclusion, the serum PFAS concentrations and morbidity outcomes were assessed simultaneously, whereas at the 9-month visits, the time interval between exposure and outcome assessment varied by 2–12 months. However, due to the long half-life of the PFAS, the differences will probably not have influenced the findings of the study to any substantial degree. Similarly, variations in the interval between PFAS assessment and vaccination in the control group (2–12 months) should matter little. Measurements of the mothers’ antibody concentrations were performed on average 5.6 months after childbirth, which could introduce some imprecision and, thus, residual confounding in the analyses adjusted for maternal antibody concentrations.
Information about PFAS exposure sources in Africa is sparse, and the causal pathways hypothesized in the DAG may, therefore, be insufficient. However, by adjusting for the most important predictors for the outcomes, we believe that the risk of additional confounding is minimal. Furthermore, the living conditions, nutritional intake, and health status of these children is very different from other cohorts, where similar associations between PFAS exposures and vaccine antibodies have been found. It is, therefore, unlikely that any overlooked confounding factors would be the same across the different settings in this and previous studies.
Only children who had measles antibodies measured at least once after receiving a measles vaccination were included in the study. More children were thus included from the intervention group than from the control group. However, because separate analyses were performed for control and intervention children after vaccination, this selection should not have affected the results.
In this study, we examined six types of PFAS and five different outcomes at two or three time points. Thus, a few significant findings are to be expected merely by chance, and we therefore focused on general trends in the data. Overall, our findings are in agreement with the hypothesis of adverse immune system effects of early life exposure to PFAS, even at comparatively low exposure levels.
Most recently, the European Food Safety Authority (EFSA) published a draft scientific opinion on PFAS, suggesting a lowered tolerable weekly intake of PFHxS, PFOS, PFOA, and PFDA based on epidemiological evidence that PFAS have immunotoxic effects (CONTAM Panel et al. 2020). The EFSA also emphasized the need for more longitudinal epidemiological studies using different populations, examining infections and more varied types of vaccines (CONTAM Panel et al. 2020). The present study adds to the strength of the evidence suggesting that PFAS are immunotoxic to infants even at lower serum concentrations than previously examined.
Conclusions
In this study of West African children with low PFAS exposures, a doubling of serum PFOS and PFDA concentrations in children vaccinated at 4–7 months of age was associated with 21% (95% CI: , ) and 25% (95% CI: , ) lower measles antibody concentrations (respectively) at approximately 9 months of age. Furthermore, we saw a trend toward reduced measles antibody concentrations at 4–7 months of age (before vaccination) and higher odds of morbidity with higher PFAS concentrations.
Supplementary Material
Acknowledgments
This study was supported by the Danish Health Foundation (Helsefonden) (17-B-0255). In addition, the original trial was supported by the European Union FP7 support for Optimising the Impact and Cost-Effectiveness of Child Health Intervention Programmes of Vaccines and Micronutrients in Low-Income Countries (OPTIMUNISE; Health-F3-2011-261375). The Research Center for Vitamins and Vaccines is supported by the Danish National Research Foundation (grant DNRF108). K.J.J. is supported by a grant from Novo Nordisk Foundation (grant NNF14OC0012169). P.G. is supported by the National Institutes of Health/National Institute of Environmental Health Sciences (P42ES027706).
References
- ATSDR (Agency for Toxic Substances and Disease Registry). 2018. Toxicological Profile for Perfluoroalkyls. Draft for Public Comment. Atlanta, GA: ATSDR; https://www.atsdr.cdc.gov/ToxProfiles/tp200-p.pdf [accessed 24 July 2020]. [Google Scholar]
- Bach CC, Henriksen TB, Bossi R, Bech BH, Fuglsang J, Olsen J, et al. . 2015. Perfluoroalkyl acid concentrations in blood samples subjected to transportation and processing delay. PLoS One 10(9):e0137768, PMID: 26356420, 10.1371/journal.pone.0137768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley DA, Kuh E, Welsch RE. 1980. Detecting influential observations and outliers. In: Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Belsley DA, Kuh E, Welsch RE, eds. Hoboken, NJ: John Wiley & Sons, 6–84. [Google Scholar]
- Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, et al. . 1990. Measles antibody: reevaluation of protective titers. J Infect Dis 162(5):1036–1042, PMID: 2230231, 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- Cohen BJ, Audet S, Andrews N, Beeler J, WHO Working Group on Measles Plaque Reduction Neutralization Test. 2007. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 26(1):59–66, PMID: 18063236, 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- CONTAM Panel (Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman J, del Mazo J, et al. . 2020. Draft scientific opinion on the Risk for Human Health Related to the Presence of Perfluoroalkyl Substances in Food. http://www.efsa.europa.eu/en/consultations/call/public-consultation-draft-scientific-opinion-risks-human-health [accessed 19 March 2020].
- Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, et al. . 2012. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol Appl Pharmacol 258(2):248–255, PMID: 22119708, 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Host A, et al. . 2016. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4 years among 359 children in the Odense Child Cohort. Environ Int 96:58–64, PMID: 27608427, 10.1016/j.envint.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM. 2018. Shifting global exposures to poly- and perfluoroalkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood-consuming population. Environ Sci Technol 52(6):3738–3747, PMID: 29516726, 10.1021/acs.est.7b06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Blossom SJ, Schaider LA. 2019. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol 29(2):148–156, PMID: 30482935, 10.1038/s41370-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. 2017. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem 65(3):533–543, PMID: 28052194, 10.1021/acs.jafc.6b04683. [DOI] [PubMed] [Google Scholar]
- Dong G-H, Zhang Y-H, Zheng L, Liu W, Jin Y-H, He Q-C. 2009. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch Toxicol 83(9):805–815, PMID: 19343326, 10.1007/s00204-009-0424-0. [DOI] [PubMed] [Google Scholar]
- Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, et al. . 2019. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int 130:104874, PMID: 31200157, 10.1016/j.envint.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. 2010. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ Res 110(8):773–777, PMID: 20800832, 10.1016/j.envres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Fisker AB, Nebie E, Schoeps A, Martins C, Rodrigues A, Zakane A, et al. . 2018. A two-center randomized trial of an additional early dose of measles vaccine: effects on mortality and measles antibody levels. Clin Infect Dis 66(10):1573–1580, PMID: 29177407, 10.1093/cid/cix1033. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bell EM, Ma W-L, Sundaram R, Kannan K, Buck Louis GM, et al. . 2018. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut 243(pt B):1629–1636, PMID: 30296759, 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi H, Miyashita C, Okada E, Kashino I, Chen C-J, Ito S, et al. . 2017. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4 years of age. Environ Int 104:132–138, PMID: 28392064, 10.1016/j.envint.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, et al. . 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307(4):391–397, PMID: 22274686, 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A, et al. . 2017. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol 14(1):188–195, PMID: 28805477, 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, et al. . 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 10(4):373–379, PMID: 23350954, 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- Hanssen L, Röllin H, Odland JØ, Moe MK, Sandanger TM. 2010. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. J Environ Monit 12(6):1355–1361, PMID: 20424796, 10.1039/b924420d. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. 2011. Characterisation of human exposure pathways to perfluorinated compounds—comparing exposure estimates with biomarkers of exposure. Environ Int 37(4):687–693, PMID: 21334069, 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. 2009. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A 1216(3):385–393, PMID: 19026423, 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Impinen A, Longnecker MP, Nygaard UC, London SJ, Ferguson KK, Haug LS, et al. . 2019. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environ Int 124:462–472, PMID: 30684804, 10.1016/j.envint.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impinen A, Nygaard UC, Lødrup Carlsen KC, Mowinckel P, Carlsen KH, Haug LS, et al. . 2018. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ Res 160:518–523, PMID: 29106950, 10.1016/j.envres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Jian J-M, Chen D, Han F-J, Guo Y, Zeng L, Lu X, et al. . 2018. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ 636:1058–1069, PMID: 29913568, 10.1016/j.scitotenv.2018.04.380. [DOI] [PubMed] [Google Scholar]
- Jian J-M, Guo Y, Zeng L, Liang-Ying L, Lu X, Wang F, et al. . 2017. Global distribution of perfluorochemicals (PFCs) in potential human exposure source—a review. Environ Int 108:51–62, PMID: 28800414, 10.1016/j.envint.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Keil DE, Mehlmann T, Butterworth L, Peden-Adams MM. 2008. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci 103(1):77–85, PMID: 18252804, 10.1093/toxsci/kfn015. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16(10):626–638, PMID: 27546235, 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Kvalem HE, Nygaard UC, Lødrup Carlsen KC, Carlsen KH, Haug LS, Granum B. 2020. Perfluoroalkyl substances, airways infections, allergy and asthma related health outcomes—implications of gender, exposure period and study design. Environ Int 134:105259, PMID: 31733527, 10.1016/j.envint.2019.105259. [DOI] [PubMed] [Google Scholar]
- Leuridan E, Van Damme P. 2007. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 25(34):6296–6304, PMID: 17629601, 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Björvang RD, Mucs D, Vinnars M-T, Papadogiannakis N, Lindh CH, et al. . 2019. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 124:482–492, PMID: 30684806, 10.1016/j.envint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa M-J, Ballester F, Basterrechea M, Grimalt JO, et al. . 2015. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res 142:471–478, PMID: 26257032, 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Martins C, Garly M-L, Bale C, Rodrigues A, Benn CS, Whittle H, et al. . 2013. Measles antibody levels after vaccination with Edmonston-Zagreb and Schwarz measles vaccine at 9 months or at 9 and 18 months of age: a serological study within a randomised trial of different measles vaccines. Vaccine 31(48):5766–5771, PMID: 23994379, 10.1016/j.vaccine.2013.08.044. [DOI] [PubMed] [Google Scholar]
- Melville JM, Moss TJM. 2013. The immune consequences of preterm birth. Front Neurosci 7:79, PMID: 23734091, 10.3389/fnins.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. 2018. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health 15(9):1832, PMID: 30149566, 10.3390/ijerph15091832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol 49(17):10466–10473, PMID: 26291735, 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MHB, Polder A, Brynildsrud OB, Grønnestad R, Karimi M, Lie E, et al. . 2019. Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ Res 170:433–442, PMID: 30634139, 10.1016/j.envres.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG Jr, et al. . 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol 45(3):1121–1126, PMID: 21166449, 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiesk S. 2014. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 5:446, PMID: 25278941, 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, et al. . 2012. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res 112:118–125, PMID: 22030285, 10.1016/j.envres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Okoko BJ, Wesuperuma LH, Ota MO, Banya WA, Pinder M, Gomez FS, et al. . 2001. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr 19(2):59–65, PMID: 11503348, 10.3329/jhpn.v19i2.77. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhu Y, Zheng T, Cui Q, Buka SL, Zhang B, et al. . 2017. Novel chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: placental transfer and relationship with serum albumin and glomerular filtration rate. Environ Sci Technol 51(1):634–644, PMID: 27931097, 10.1021/acs.est.6b04590. [DOI] [PubMed] [Google Scholar]
- Pilkerton CS, Hobbs GR, Lilly C, Knox SS. 2018. Rubella immunity and serum perfluoroalkyl substances: sex and analytic strategy. PLoS One 13(9):e0203330, PMID: 30248109, 10.1371/journal.pone.0203330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Díaz J, Fontana L, Gil A. 2018. Human milk oligosaccharides and immune system development. Nutrients 10(8):1038, PMID: 30096792, 10.3390/nu10081038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poothong S, Thomsen C, Padilla-Sanchez JA, Papadopoulou E, Haug LS. 2017. Distribution of novel and well-known poly- and perfluoroalkyl substances (PFASs) in human serum, plasma, and whole blood. Environ Sci Technol 51(22):13388–13396, PMID: 29056041, 10.1021/acs.est.7b03299. [DOI] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E, Hines EP. 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health 14(7):691, PMID: 28654008, 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam S, Gadag V, West R, Burris J, Oates E, Stead F, et al. . 1995. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol 33(4):811–815, PMID: 7790442, 10.1128/JCM.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, et al. . 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246, PMID: 27179585, 10.1016/j.envres.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits GP, van Gageldonk PG, Schouls LM, van der Klis FRM, Berbers GAM. 2012. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin Vaccine Immunol 19(3):396–400, PMID: 22237896, 10.1128/CVI.05537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS. 2016. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatr Res 79(2):348–357, PMID: 26492286, 10.1038/pr.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: 30470793, 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thysen SM, Rodrigues A, Aaby P, Fisker AB. 2019. Out-of-sequence DTP and measles vaccinations and child mortality in Guinea-Bissau: a reanalysis. BMJ Open 9(9):e024893, PMID: 31492774, 10.1136/bmjopen-2018-024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann CA, Budtz-Jørgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, et al. . 2017. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol 68:164–170, PMID: 27421579, 10.1016/j.reprotox.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (United Nations International Children’s Fund). 2019. Children in Africa: Key Statistics on Child Survival and Population. New York, NY: UNICEF; https://data.unicef.org/resources/children-in-africa-child-survival-brochure/ [accessed 24 July 2020]. [Google Scholar]
- Verner MA, Ngueta G, Jensen ET, Fromme H, Völkel W, Nygaard UC, et al. . 2016. A simple pharmacokinetic model of prenatal and postnatal exposure to perfluoroalkyl substances (PFASs). Environ Sci Technol 50(2):978–986, PMID: 26691063, 10.1021/acs.est.5b04399. [DOI] [PubMed] [Google Scholar]
- Victora CG, Bahl R, Barros AJ, França GVA, Horton S, Krasevec J, et al. . 2016. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387(10017):475–490, PMID: 26869575, 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518, PMID: 28224793, 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong L-Y, Jia T, Kalathil A, Latremouille J, et al. . 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health 221(1):9–16, PMID: 28993126, 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Zou L, Lei X, Zhang Y. 2017. Gender differences in infant mortality and neonatal morbidity in mixed-gender twins. Sci Rep 7(1):8736, PMID: 28821800, 10.1038/s41598-017-08951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.