Abstract
Purpose
A comparison of stage at cancer diagnosis and cancer treatment rates between people with HIV (PWH) and the general US population is needed to identify any disparities by HIV status.
Methods
We compared 236 PWH in clinical care diagnosed with cancer from 1997 to 2014 to a sample from NCI’s Surveillance, Epidemiology and End Results (SEER) Program, presumed to be HIV negative. We performed G-computation using random forest methods to estimate stage and treatment percent differences (PD) by HIV. We conducted sensitivity analyses among non-AIDS-defining cancers (NADC), by sex and by CD4 ≤ 200 or > 200 cells/mm3.
Results
PWH were less likely to be diagnosed at localized stage (PD = − 16%; 95% CI − 21, − 11) and more likely to be diagnosed at regional stage (PD = 14%; 95% CI 8, 19) than those in SEER. Cancer treatment rates were 13% lower among PWH as compared to SEER (95% CI − 18, − 8). The difference in percent receiving cancer treatment was more pronounced for those with lower CD4 at cancer diagnosis (PD −15%; 95% CI − 27, − 6). Lower treatment rates were observed among NADC, males, and women with CD4 ≤ 200.
Conclusion
Cancer care for PWH could be improved by diagnosis at earlier stages and increasing rates of cancer treatment.
Keywords: HIV infection, Cancer, Cancer stage, Cancer treatment, CD4
Introduction
Cancer is a leading cause of morbidity and mortality among people with HIV (PWH) [1–3]. There is some evidence, although inconsistent, that PWH are diagnosed at more advanced stages of cancer [4, 5] and have lower rates of cancer treatment [6, 7]. Several factors may affect cancer treatment and outcomes in PWH compared to the general population. In particular, immunosuppression from HIV could result in the development of more aggressive cancers and may reduce tolerability of treatment or physician willingness to provide cancer treatment to PWH [4, 6]. In addition, PWH also experience higher rates of other risk factors, such as smoking and viral co-infections, that may affect cancer stage at presentation, treatment, and mortality [8–11]. Barriers in access to and engagement in care also play an important role in cancer outcomes among PWH [10, 11]. In this study, we compare stage at cancer diagnosis and initial cancer treatment rates between PWH engaged in clinical HIV care and individuals in the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER). SEER is representative of cancer cases among the general US population [12] and the vast majority are HIV negative [13–15]. We hypothesized that PWH would be diagnosed with a more advanced stage cancer [4] and would receive cancer treatment at lower rates [6, 7] than the general US population.
Methods
Data sources
We identified 382 incident, first cancer cases among PWH enrolled in the Johns Hopkins HIV Clinical Cohort (JHHCC) between 1 January 1997 and 30 September 2014 using a protocol developed by the Centers for AIDS Research Network of Integrated Clinical Systems [16]. Of the 382 total cases, 296 (77%) were linked to stage and treatment data in Maryland Cancer Registry (MCR).1 We excluded all Kaposi’s sarcoma cases, non-specific cancer types (e.g., skin cancers), and cancers with limited staging information in SEER (e.g., oral/pharynx), resulting in 236 cancers among PWH. A total of 1,756,229 incident, first cancers that matched the cancer types found in the JHHCC and had non-missing stage, were identified among persons aged 21–80 reported to SEER registries between 2000 and 2014.
Statistical analysis
Stage at diagnosis was categorized into localized, regional, distant, or unstaged based on the SEER Summary Stage 2000 classification [17]. Cancer treatment was defined as any chemotherapy, any radiation, and/or any surgery for the first course of treatment. We used G-computation to account for the differences in the covariate distribution by HIV status [18, 19], which is comparable to direct standardization where the standard population is PWH diagnosed with an incident, first cancer who are enrolled in the JHHCC [18, 20]. We fitted random forest models [21] for the outcome of interest separately for those with HIV (JHHCC) and for those without HIV (SEER) [19]. We included the following covariates in the random forest models for stage at diagnosis: cancer type, age, sex, race, and year of diagnosis. The cancer treatment random forest models included cancer type, age, sex, race, year, and stage at diagnosis. Random forest is an ensemble machine learning algorithm formed by averaging tree-based learners (e.g., recursive partitioning). Random forest creates an ensemble learner by taking a random sample with replacement of the data and restricting the classification variables to a random subset of the total covariates as a way to introduce sufficient variation in each tree, allowing for the model to learn from each additional tree that is added to the forest until the point of diminishing return [21]. We then predicted the potential outcomes with and without HIV infection. The estimated effect of HIV infection is the average difference in the predicted potential outcomes. The results are presented as percent differences (PD). We repeated the analyses for each outcome among several subgroups, including those diagnosed with a nonAIDS-defining cancer (NADC) (N = 183 in JHHCC), males (N = 163 in JHHCC), and females (N = 73 in JHHCC). Analyses restricted to males or females excluded sex from the covariates. Due to large computational time, we estimated the random forest models using a 25% random sample of the SEER data (439,057 cases). We obtained all 95% confidence intervals (CI) using the bootstrap variance of the effect estimates based on 200 iterations. The bootstrap included a random sampling procedure and estimated the random forest models in each iteration. To address potential variation in the results by cancer type, we present forest plots of the estimated differences by HIV status in stage at diagnosis and percent receiving initial cancer treatment in the supplemental materials (Supplemental Figs. 1–4). All analyses were performed in R [22], including the randomForest [23] package.
Results
Among the 236 PWH in the JHHCC, 163 (69%) were male and 184 (78%) were non-Hispanic black. Their median age was 50 years (IQR 44–56). Common cancer types in the JHHCC include non-Hodgkin’s lymphoma (NHL) (N = 53, 23%), lung cancer (N = 42, 18%), liver cancer (N = 23, 10%), Hodgkin lymphoma (N = 18, 8%), prostate cancer (N = 18, 8%), breast cancer (N = 16, 7%), and anal cancer (N = 15, 6%). Refer to the supplemental materials for a table with a full list of the cancer types in the JHHCC and the 25% SEER sample, as well as information on the unadjusted distributions of stage at diagnosis.
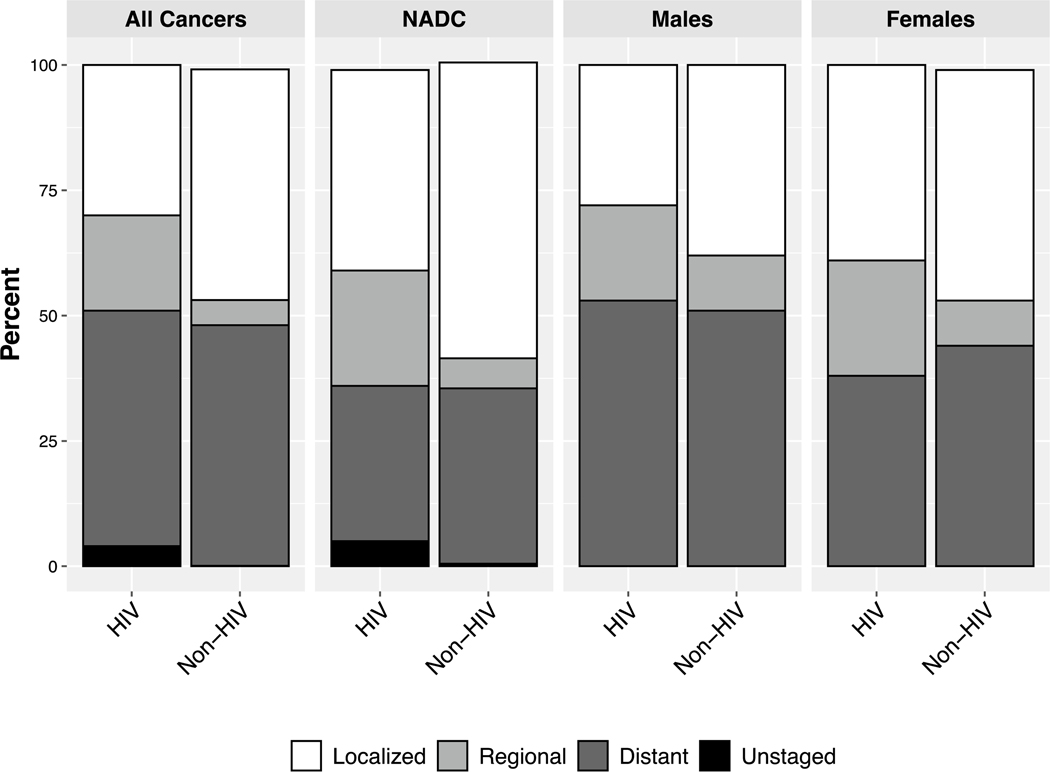
The probability of being diagnosed at each stage shown in percentages are provided for all cancers, all NADC, males, and females by HIV status and are presented in Fig. 1. These results can be interpreted as what the stage distribution is for PWH and what would be the stage distribution had those individuals not had HIV, adjusting for differences in cancer type, age, sex, race, and year of diagnosis. Cancer cases were less likely to be diagnosed as localized and more likely to be diagnosed as regional, if persons had HIV. Similar rates of diagnosis at distant stages were observed by HIV status. Among all cancers, 30% of individuals with HIV were diagnosed at a localized stage as compared to 46% of individuals without HIV, resulting in a 16% lower probability among PWH (95% CI − 22, − 10). A total of 19% of PWH were diagnosed at a regional stage as compared to 5% of those without HIV, yielding a 14% difference (95% CI 8, 19). Rates of diagnosis at a distant stage were similar with 47% of PWH as compared to 48% of those without HIV, yielding a − 2% percent difference (PD) (95% CI − 6, 5). Those with HIV were more likely to be unstaged (PD = 3%; 95% CI 1, 6). The results are similar when the data are restricted to NADC and males. Among females, PWH were more likely to be diagnosed at a regional stage (PD = 13%; 95% CI 1, 26) but had similar rates at localized and distant stages. Unstaged cancers were excluded from the male and female models due to small sample size. In the sensitivity analyses, the results were fairly consistent across cancer types; however, lung cancer appeared to be more localized among those with HIV (Supplemental Fig. 1).
Fig. 1.
Distribution of stage at cancer diagnosis stratifed by HIV status for all cancers, non-AIDS-defning cancers (NADC), males, and females
a All models adjusted for age, sex, race, calendar year, and cancer type.
b Models among males and females do not include sex in the adjustment and excluded unstaged outcome due to small sample size.
Probabilities of initial cancer treatment, presented as percentages in Table 1, are similarly lower among PWH than what we would have expected had they not had HIV. High rates of cancer treatment are expected based on data from SEER; however, among all cancers, 82% of PWH receive any initial cancer treatment as compared to 94% of those without HIV (PD = − 13%; 95% CI − 18, − 8), accounting for differences in cancer type, age, sex, race, year of diagnosis, and stage at diagnosis. There is a larger difference in initial cancer treatment for all cancers among PWH with CD4 ≤ 200 as compared to had they been HIV negative with a PD of − 15% (95% CI − 27, − 6); however, PWH with CD4 > 200 were also less likely to receive cancer treatment (PD = − 9; 95% CI − 14, − 2). Similar results are seen when restricting the analysis to NADC and males. Differences in rates of treatment were only observed for women with HIV and CD4 ≤ 200. This difference is pronounced, although there is significant variability in the estimate (PD = − 20%; 95% CI − 42, − 4). When stratified by cancer type, differences in treatment probability appeared consistent across types (Supplemental Fig. 4).
Table 1.
Percent receiving any cancer treatment among those with an incident, first cancer between 1997 and 2014 stratified by HIV status and the difference in percent receiving any cancer treatment (HIV–non-HIV)
| Population | CD4 strata | HIV percent (95% CI) | Non-HIV percent (95% CI) | Difference (95% CI) |
|---|---|---|---|---|
| All cancers | All CD4 | 82 (76, 87) | 94 (92, 97) | − 13 (− 18, − 8) |
| CD4 ≤ 200 | 80 (67, 89) | 94 (90, 99) | − 15 (− 27, − 6) | |
| CD4 > 200 | 85 (79, 91) | 94 (91, 97) | − 9 (− 14, − 2) | |
| NADC | All CD4 | 81 (75, 87) | 93 (90, 96) | − 12 (− 18, − 5) |
| CD4 ≤ 200 | 77 (61, 89) | 91 (85, 98) | − 14 (− 29, − 4) | |
| CD4 > 200 | 83 (78, 91) | 93 (90, 96) | − 10 (− 16, − 2) | |
| Males | All CD4 | 79 (71, 85) | 93 (90, 95) | − 14 (− 21, − 8) |
| CD4 ≤ 200 | 81 (68, 92) | 93 (86, 99) | − 12 (− 23, − 1) | |
| CD4 > 200 | 79 (71, 88) | 92 (88, 96) | − 13 (− 20, − 3) | |
| Females | All CD4 | 88 (80, 96) | 95 (91, 98) | − 7 (− 15, 3) |
| CD4 ≤ 200 | 75 (52, 93) | 96 (89, 100) | − 20 (− 42, − 4) | |
| CD4 > 200 | 94 (89, 100) | 94 (88, 100) | 0 (− 8, 10) | |
NADC non-AIDS-defining cancer
Discussion
We compared cancer stage at diagnosis and percent receiving any initial cancer treatment between PWH enrolled in HIV care (in the JHHCC) and the outcomes that we would expect had those individuals not had HIV, using the SEER data. Prior studies that have found PWH present at a later stage than those without HIV [4, 5, 24]. In this study, we found PWH were less likely to be diagnosed at a local stage and more likely to be diagnosed at a regional stage than had they not had HIV infection. The proportion of individuals diagnosed at a distant stage were similar by HIV status. To address this difference in stage at diagnosis by HIV status, more evidence on the benefits and harms of enhanced monitoring or early detection of cancer among PWH are needed (Table 1).
The percent of individuals receiving any initial cancer treatment was lower among people with HIV. This result echoes prior studies that have found lower cancer treatment rates for PWH [6, 7]. In contrast, a few prior studies have found no disparities in cancer treatment rates by HIV among those enrolled in managed care organizations [25, 26]. Our analysis was restricted o persons with HIV in continuity care, albeit with varying rates of engagement in care over time; however, we were limited our ability to study the role that access to care may play in disparities observed elsewhere. Yet we still found evidence of lower treatment, suggesting that poor retention in care or cancer treatment tolerability issues may impact cancer treatment uptake in this population. More evidence on the tolerability and efficacy of cancer treatment among PWH, particular among women and those with low CD4 cell counts, is needed [16].
Our sample of cancer cases among PWH came from a single institution, limiting the generalizability of our findings and precluding analyses of particular cancer types. It is important to note that our population is not representative of all PWH with cancer in the US, as it comprises individuals linked to clinical care. We are also comparing cancer outcomes with a sample of the general US population, who may or may not have had similar access to healthcare. We also had relatively limited information on cancer treatment. Our analyses focused on receipt of any initial cancer treatment and could not delineate whether the treatments were appropriate based on the guidelines and stage at diagnosis. Based on prior knowledge about lower treatment rates and issues with tolerability [6, 7], it is possible that PWH receiving initial treatment may not have received guideline appropriate treatment and then our results would be biased toward the null.
Another limitation of our analysis was the small sample size, which required an aggregation of the results across all cancer types. It is possible that the results are heavily influenced by the most common cancers found in the JHHCC, and this approach does not allow for an examination of heterogeneity by cancer type. We attempted to address these limitations with sensitivity analyses. First, we examined only those with NADCs, given that AIDS-defining cancers, particularly NHL, have known differences in subtype distributions by HIV status [27]. However, the NADC results were fairly consistent with the results among all cancers. Secondly, though underpowered, we presented results of the main analyses stratified by cancer type for the most common cancers to partially address this concern. A replication of this analysis among individual cancer types with sufficient sample size would allow further assessment of any heterogeneity in the effect of HIV on stage and treatment by cancer type.
Our study had several strengths, including the linkage between a cancer registry and HIV clinical cohort to directly compare cancer outcomes by HIV status among a collection of cancer registries that use the same data collection procedures. We were also able to perform sub-analyses by CD4 level to assess the role of immunosuppression in receipt of cancer treatment among PWH. Our use of random forest methods coupled with G-computation provided flexibility in determining the association of the covariates with outcomes of interest and an efficient estimator to offset the smaller sample size of those with HIV [18, 19, 21, 23]. Finally, individuals with cancer in the JHHCC are a largely non-Hispanic Black (78%), low income population with a high prevalence of hepatitis C co-infection (59%), and/or a history of injection drug use (39%), making it a unique population of PWH in which to explore cancer outcomes.
Conclusion
HIV infection was associated with a lower probability of being diagnosed with cancer at a local stage and a higher probability of being diagnosed with cancer a regional stage. Information on predictors of earlier detection of cancers among PWH would improve prognosis for these individuals. We did observed a lower probability of receiving initial cancer treatment by HIV status after accounting for demographic and cancer stage information. The role of HIV-related immune suppression in the receipt and tolerability of cancer treatment should be explored further.
Supplementary Material
Acknowledgments
We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Diseases Control and Prevention for the funds that support the collection and availability of the cancer registry data.
Funding This work was supported by the National Institutes of Health (Grant Numbers U01-DA036935, P30-AI094189), National Institute of Allergy and Infectious Diseases (Grant No. U01 AI069918) and National Cancer Institute (Grant No. T32 CA009314).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10552-020-01289-x) contains supplementary material, which is available to authorized users.
Cancer incidence data were provided by the Maryland Cancer Registry, Center for Cancer Prevention and Control, Maryland Department of Health, 201 W. Preston Street, Room 400, Baltimore, MD 21201, USA, https://phpa.health.maryland.gov/cancer/Pages/mcr_home.aspx, 410-767-4055.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH et al. (2011) Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 103(9):753–762. 10.1093/jnci/djr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg MJ, Lau B, Achenbach CJ et al. (2015) Cumulative incidence of cancer among HIV-infected individuals in North America. Ann Intern Med 163(7):507–518. 10.7326/M14-2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel P, Armon C, Chmiel JS et al. (2014) Factors associated with cancer incidence and with all-cause mortality after cancer diagnosis among human immunodeficiency virus-infected persons during the combination antiretroviral therapy era. Open Forum Infect Dis. 10.1093/ofid/ofu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Copeland G, Goodman MT et al. (2015) Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer 121(12):2063–2071. 10.1002/cncr.29324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coghill AE, Han X, Suneja G, Lin CC, Jemal A, Shiels MS (2019) Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer 125(16):2868–2876. 10.1002/cncr.32158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suneja G, Shiels MS, Angulo R et al. (2014) Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol 32(22):2344–2350. 10.1200/JCO.2013.54.8644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA (2013) Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS Lond Engl 27(3):459–468. 10.1097/QAD.0b013e32835ad56e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helleberg M, May MT, Ingle SM et al. (2015) Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 29(2):221–229. 10.1097/QAD.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimiaga MJ, Reisner SL, Grasso C et al. (2013) Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems Cohort. Am J Public Health 103(8):1457–1467. 10.2105/AJPH.2012.301162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy ME, Wilton L, Phillips G et al. (2014) Understanding structural barriers to accessing HIV testing and prevention services among black men who have sex with men (BMSM) in the United States. AIDS Behav 18(5):972–996. 10.1007/s10461-014-0719-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, Heffelfinger JD (2011) Clinical and behavioral characteristics of adults receiving medical care for HIV infection—Medical Monitoring Project, United States, 2007. Morb Mortal Wkly Rep 60(11):1–20 [PubMed] [Google Scholar]
- 12.About the SEER Program - SEER. https://seer.cancer.gov/about/overview.html. Accessed 3 Jan 2018
- 13.Shiels MS, Koritzinsky EH, Clarke CA, Suneja G, Morton LM, Engels EA (2014) Prevalence of HIV Infection among U.S. Hodgkin lymphoma cases. Cancer Epidemiol Biomark Prev 23(2):274–281. 10.1158/1055-9965.EPI-13-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL (2011) Use of surveillance, epidemiology, and end results-medicare data to conduct case–control studies of cancer among the US elderly. Am J Epidemiol 174(7):860–870. 10.1093/aje/kwr146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlader N, Shiels MS, Mariotto AB, Engels EA (2016) Contributions of HIV to non-hodgkin lymphoma mortality trends in the United States. Cancer Epidemiol Prev Biomark 25(9):1289–1296. 10.1158/1055-9965.EPI-16-0273 [DOI] [PubMed] [Google Scholar]
- 16.Achenbach CJ, Cole SR, Kitahata MM et al. (2011) Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS 25(5):691–700. 10.1097/QAD.0b013e3283437f77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz AG et al. (2001) SEER summary staging manual-2000 codes and coding instructions. National Cancer Institute, Bethesda [Google Scholar]
- 18.Snowden JM, Rose S, Mortimer KM (2011) Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol 173(7):731–738. 10.1093/aje/kwq472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC (2012) Using ensemble-based methods for directly estimating causal effects: an investigation of tree-based G-computation. Multivar Behav Res 47(1):115–135. 10.1080/00273171.2012.640600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vansteelandt S, Keiding N (2011) Invited commentary: G-computation–lost in translation? Am J Epidemiol 173(7):739–742. 10.1093/aje/kwq474 [DOI] [PubMed] [Google Scholar]
- 21.Breiman L (2001) Random forests. Mach Learn 45(1):5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 22.R Core Team (2017) R: A language and environment for statistical computing R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/ [Google Scholar]
- 23.Liaw A, Wiener M (2015) RandomForest: Breiman and Cutler’s random forests for classification and regression. https://CRAN.R-project.org/package=randomForest. Accessed 13 Mar 2018
- 24.Wasserberg N, Nunoo-Mensah JW, Gonzalez-Ruiz C, Beart RW, Kaiser AM (2007) Colorectal cancer in HIV-infected patients: a case control study. Int J Colorectal Dis 22(10):1217–1221. 10.1007/s00384-007-0285-z [DOI] [PubMed] [Google Scholar]
- 25.Marcus JL, Chao C, Leyden WA et al. (2015) Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Prev Biomark 24(8):1167–1173. 10.1158/1055-9965.EPI-14-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigel K, Crothers K, Dubrow R et al. (2013) Prognosis in HIV-infected patients with non-small cell lung cancer. Br J Cancer 109(7):1974–1980. 10.1038/bjc.2013.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA (2014) Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS Lond Engl 28(15):2313–2318. 10.1097/QAD.0000000000000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.