Summary
Background:
The real-world, long-term benefits of sustained virologic response (SVR) on the risk of variceal bleeding remain unclear.
Aim:
We aimed to assess the association between DAA-induced SVR and post-treatment variceal bleeding.
Methods:
We identified patients who initiated DAA-only antiviral treatments in the Veterans Affairs healthcare system from 2013–2015. We followed patients until 01/01/2019 for the development of gastroesophageal variceal bleeding defined by diagnostic codes. We used multivariable Cox proportional hazards regression to assess the association between SVR and development of variceal bleeding, adjusting for potential confounders.
Results:
Among 33,582 DAA-treated patients, 549 (1.6%) developed variceal bleeding after treatment (mean follow-up 3.1 years). Compared to no-SVR, SVR was associated with a significantly lower incidence of variceal bleeding among all patients (0.46 vs. 1.26 per 100-patient years, adjusted hazard ratio [AHR] 0.66, 95% CI 0.52–0.83), among patients with pre-treatment cirrhosis (1.55 vs 2.96 per 100 patient-years, AHR 0.73, 95% CI 0.57–0.93) and among patients without pre-treatment cirrhosis (0.07 vs. 0.29 per 100 patient-years, AHR 0.33, 95% CI 0.17–0.65). The risk of variceal bleeding after treatment was lower in those who achieved SVR versus no SVR among patients who had non-bleeding varices (3.5 vs 4.9 per 100 patient-years) or bleeding varices (12.9 vs 16.4 per 100 patient-years) diagnosed before treatment, but these differences were not statistically significant in adjusted analyses.
Conclusion:
DAA-induced SVR is independently associated with lower risk of variceal bleeding during long-term follow-up in patients with and without pre-treatment cirrhosis. These findings demonstrate an important real-world benefit of DAA treatment.
Keywords: cirrhosis, haemorrhage, portal hypertension, liver disease
Introduction
Gastrooesophageal varices are a common complication of cirrhosis that can lead to substantial morbidity and mortality1–3. Hepatitis C virus (HCV) infection is a leading cause of cirrhosis in the US4. With the advent of direct-acting antiviral agents (DAA), HCV cure rates have increased dramatically and the majority of patients with HCV are now expected to achieve sustained virologic response (SVR)5. Furthermore, SVR can now be achieved in the majority of patients with established cirrhosis, including patients with advanced or decompensated cirrhosis, such as those with a history of ascites, encephalopathy, varices and variceal bleeding.
Since all available randomized, placebo-controlled trials of antiviral treatment have very short follow-up, some have argued that the long-term clinical benefits of antiviral treatment and sustained virologic response (SVR) have not yet been demonstrated6. As such, some insurance providers and states require that significant fibrosis be present before covering HCV antiviral treatment7. It is therefore imperative to evaluate the long-term benefits of DAA-induced SVR in adequately powered and designed observational studies. DAAs have now led to eradication of HCV in unprecedented numbers of patients with cirrhosis and decompensated cirrhosis and we now have long-term follow up data, enabling us to address these questions8.
We hypothesized that HCV eradication may lead to a reduced risk of variceal bleeding both in patients with and those without pre-treatment cirrhosis during long-term follow-up. In patients without pre-treatment cirrhosis, HCV eradication may reduce the risk of progressing to cirrhosis thereby reducing the risk of developing varices and variceal bleeding. HCV eradication in patients with cirrhosis may stop fibrosis progression or even cause fibrosis regression, leading to improved portal hypertension and reduced risk of variceal bleeding9. Finally, HCV eradication might reduce the risk of variceal bleeding even in the highest risk patients, i.e. those who with known varices or variceal bleeding prior to SVR.
In this study, we used Veterans Affairs Healthcare System (VAHS) data to examine associations between DAA-induced HCV eradication and the risk of variceal bleeding, in clinically relevant subgroups such as the ones outlined above. Furthermore, we aimed to investigate other characteristics that are associated with variceal bleeding in patients who received antiviral treatment and achieved SVR.
Methods
Data Source
The VAHS is the largest integrated healthcare provider of HCV antiviral treatment in the United States8. Nationwide, the VA uses a single comprehensive electronic healthcare information network which integrates all care applications into a single, common database. We obtained data on all patients who initiated antiviral therapy for chronic HCV in the VA system using the VA Corporate Data Warehouse (CDW), a national, continually updated repository of healthcare data including all patient pharmacy prescriptions, demographics, inpatient and outpatient visits, problem lists, procedures, vital signs, diagnostic tests, and laboratory tests10. The study was approved by the Institutional Review Board of the Veterans Affairs Puget Sound Healthcare System.
Study Population
Using VA pharmacy prescription data, we identified all DAA-only HCV antiviral regimens initiated in the VA from 2013 to 2015 (Figure 1). We defined sustained virologic response (SVR) as a serum HCV RNA viral load test below the lower limit of detection performed at least 12 weeks after the end of antiviral treatment11. We excluded patients with missing SVR data or prior liver transplant. Patients were excluded if variceal bleed, death or last follow-up visit occurred within the first 90 days after the start of DAA treatment. For patients who received multiple DAA regimens, we analyzed only the results of the first one and censored them at the point of a subsequent regimen that resulted in SVR, if one existed. The most common DAA regimen was sofosbuvir/ledipasvir (58.1%) followed by Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir (19.1%), Sofosbuvir (±daclatasvir) (13.1%), and Sofosbuvir+Simeprevir (9.6%).
Figure 1. Flow diagram of HCV patients included in our analysis cohort.
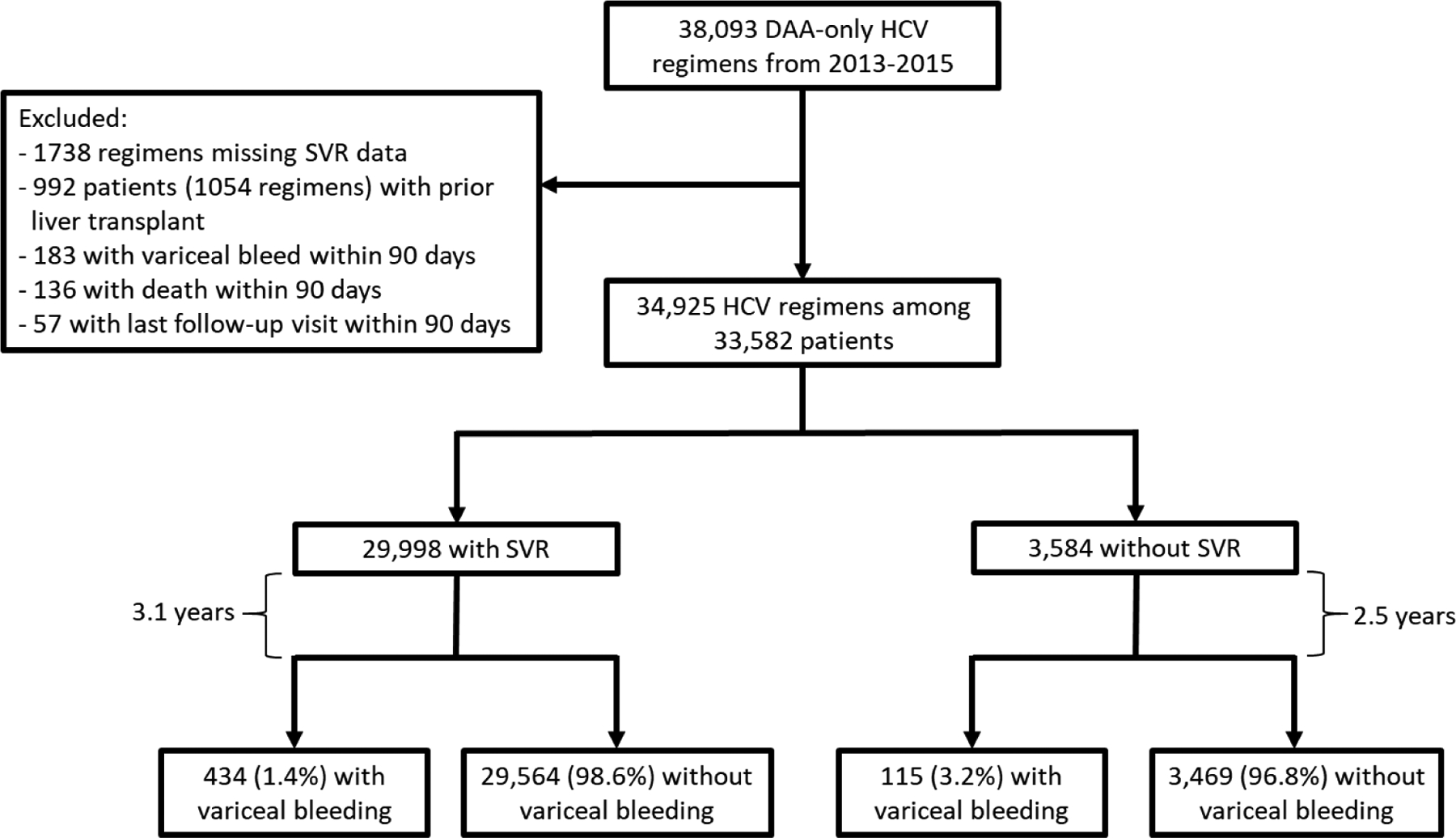
Our cohort included all DAA-treated patients within the national VA from 2013–2015, excluding those with missing data, prior liver transplant, or variceal bleed, death or liver transplant within 90 days of DAA treatment. Of our resulting cohort of 33,582 patients, 29,998 (89.3%) had SVR and 3,584 (10.6%) had no SVR. Patients were followed for a mean of 3.1 years to assess for the development of variceal bleeding.
Gastrooesophageal Varices
Gastrooesophageal varices without bleeding were defined by the presence of appropriate diagnostic codes (ICD-9 codes 456.1, 456.21 or ICD-10 codes I85.00, I86.4 or I85.10) recorded at least twice. These diagnostic codes have been validated within the VA and have a positive predictive value of approximately 90% for identifying oesophageal varices compared to chart review12,13. Gastrooesophageal varices with bleeding were defined by the presence of appropriate diagnostic codes (ICD-9 codes 456.0, 456.20 or ICD-10 codes I85.10, I85.01, I86.41, or I85.11) recorded at least once. A single recording was required for bleeding varices (rather than two or more) because many patients may only have a single bleeding episode.
Nonselective beta blockers (NSBBs), frequently used to prevent variceal bleeding, were not included in the diagnostic criteria for gastrooesophageal varices because they have many other indications (hypertension, angina prophylaxis, essential tremor, migraine prophylaxis, post-traumatic stress disorder) and therefore were not considered specific enough. However, we did evaluate NSBB as a potential confounder of the association between SVR and variceal bleeding.
Baseline Patient Characteristics and Potential Confounders
We collected baseline data including age, sex, race/ethnicity, diabetes, body mass index (BMI), HCV genotype, HCV viral load, and receipt of prior antiviral treatment. We extracted all clinical factors and laboratory tests [including components of the model for end-stage liver disease (MELD) score14] prior to treatment and recorded the value of each test closest to the treatment starting date within the preceding 6 months. We defined hepatitis B virus (HBV) coinfection by positive HBV surface antigen or viral load. We also determined the presence of cirrhosis, or complications of cirrhosis (ascites, spontaneous bacterial peritonitis, encephalopathy, gastrooesophageal varices and hepatorenal syndrome), type 2 diabetes mellitus, alcohol use disorders, substance use disorders, based on appropriate ICD-9 or ICD-10 codes recorded at least twice prior to treatment initiation in any inpatient or outpatient encounter. These ICD-based definitions of cirrhosis and other comorbidities have been widely used and validated in studies using VA medical records15–20. We assessed treatment with NSBBs (i.e. nadolol, propranolol or carvedilol) at the time of DAA initiation, since NSBBs may reduce the risk of variceal bleeding or may be associated with the presence of large gastrooesophageal varices or prior variceal bleeding requiring prophylaxis21–23.
In addition to determining the history of alcohol use disorders by ICD-9/10 codes, we used the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) score to estimate the severity of alcohol use at baseline. AUDIT-C is a validated screening tool for assessing alcohol misuse and scores range from 0–12, with higher scores reflecting higher amounts of alcohol consumption24,25. Since 2004, AUDIT-C has been used to screen all veterans for unhealthy alcohol use annually in the outpatient setting26. Baseline alcohol use was defined by the AUDIT-C score reported within 1 year before initiation of antiviral treatment and categorized into abstinent (score of 0), low-level drinking (score of 1–3 in men, 1–2 in women), and unhealthy drinking (score of 4–12 in men, 3–12 in women)27,28.
Statistical Analysis
We used Cox proportional hazards regression to compare patients who achieved SVR to those who did not achieve SVR with respect to the risk of developing gastrooesophageal variceal bleeding after antiviral treatment (or, more accurately 90 days after the initiation of antiviral treatment). Our comparison group was DAA-treated patients who did not achieve SVR, rather than patients who were never treated with DAAs, to avoid the risk of health initiator bias (selective provision of treatments to healthy and health-conscious patients and avoidance of treatment of frail individuals29,30) and immortal time bias (treated patients experience “immortal time” prior to treatment during which outcomes cannot occur). Any episodes of variceal bleeding that occurred within 90 days of initiation of treatment were excluded because they occurred during the DAA treatment and might have caused failure of treatment, thus resulting in a spurious association between failure of treatment and variceal haemorrhage. Analyses were adjusted for the following potential confounders that may be associated with both SVR and the risk of progressive liver disease and variceal bleeding: cirrhosis, prior history of varices, variceal bleeding, ascites, bacterial peritonitis or encephalopathy, age, sex, race/ethnicity, body mass index, HBV co-infection, type 2 diabetes mellitus, hepatocellular carcinoma, alcohol use disorders, substance use disorder, baseline alcohol use, NSBB use, platelet count, serum bilirubin, serum creatinine, serum albumin, INR, and hemoglobin levels. Continuous variables were categorized and modeled as dummy categorical variables.
Follow-up for development of variceal bleeding extended until 01/01/2019 so that even the patients treated in late 2015 (i.e. the most recent in our cohort) had adequate follow-up. Patients without incident variceal bleeding were censored at the time of death or last follow-up in the VA. We presented subgroup analyses according to prior history of varices or variceal bleeding, cirrhosis, MELD score, FIB-4 score, and alcohol use disorders (suggesting comorbid alcohol-related liver disease).
Survival analyses were stratified by the Veterans Affairs facility at which the antiviral treatment was administered. We analyzed only the first antiviral regimen administered between 2013–2015. Patients who did not achieve SVR with this regimen and were subsequently treated again at any point up to 01/01/2019 and achieved SVR, were censored at the time of initiation of the regimen that led to SVR.
Results
Characteristics of study population
Patients with (n=29,998) and without SVR (n=3,584) were of similar age (61.1 vs. 60.6 years), sex (96.6% vs. 98.1% male), and race/ethnicity (52.4% vs. 52.7% white/non-Hispanic) (Table 1). Patients without SVR were more likely to have cirrhosis (41.1% vs. 26.4%), non-bleeding varices (11.6% vs. 5.0%), and bleeding varices (2.9% vs. 1.4%).
Table 1.
Baseline characteristics of HCV-infected patients who received antiviral treatment with DAAs from 2013–2015, according to whether they achieved SVR
| No SVR (n=3,584) | SVR (n=29,998) | P-value | |
|---|---|---|---|
| Age, yrs (mean [SD]) | 60.6 [6.6] | 61.1 [6.5] | < 0.001 |
| BMI, (mean [SD]) | 28.7 [5.8] | 28.1 [5.4] | < 0.001 |
| Male (%) | 98.1 | 96.6 | < 0.001 |
| Race/Ethnicity (%) | < 0.001 | ||
| White, non-Hispanic | 52.7 | 52.4 | |
| Black, non-Hispanic | 30.6 | 33.1 | |
| Hispanic | 7.4 | 5.2 | |
| Other | 2 | 1.7 | |
| Declined to answer/missing | 7.3 | 7.8 | |
| Non-Genotype 1 (%) | 28.2 | 14.7 | < 0.001 |
| HBV co-infection(%) | 0.8 | 1.3 | 0.01 |
| Cirrhosis (%) | 41.1 | 26.4 | < 0.001 |
| Varices (%) | < 0.001 | ||
| No varices | 85.5 | 93.6 | |
| Varices, but no bleeding | 11.6 | 5.0 | |
| Varices with bleeding | 2.9 | 1.4 | |
| Ascites (%) | 0.6 | 0.3 | < 0.01 |
| Encephalopathy (%) | 13 | 6.3 | < 0.001 |
| Hepatocellular Carcinoma (%) | 6.7 | 2 | < 0.001 |
| Diabetes (%) | 32.9 | 29 | < 0.001 |
| Non-selective beta blocker (%) | 10.6 | 7.9 | < 0.001 |
| AUDIT-C scores* (%) | 0.09 | ||
| Abstinent | 67.2 | 66.2 | |
| Low-level use | 22.4 | 24 | |
| Unhealthy use | 10.4 | 9.8 | |
| Alcohol Use Disorder (%) | 50.3 | 44 | < 0.001 |
| MELD score ≥ 9 | 25.2 | 19.4 | < 0.001 |
| Charlson Comorbidity Index (%) | < 0.001 | ||
| 0 | 24.9 | 17.4 | |
| 1 | 26.6 | 33.7 | |
| 2 | 15 | 18.6 | |
| > 2 | 33.5 | 30.3 | |
| Laboratory Results (mean [SD]) | |||
| Alpha Fetoprotein, ng/mL | 7.3 [4.7] | 6.2 [4.3] | < 0.001 |
| Hemoglobin, g/dL | 14.3 [1.7] | 14.5 [1.6] | < 0.001 |
| Platelet Count, k/μL | 155.8 [74.1] | 178.6 [70.6] | < 0.001 |
| Creatinine, mg/dL | 1.0 [0.5] | 1.0 [0.5] | < 0.01 |
| Bilirubin, g/dL | 0.8 [0.6] | 0.7 [0.5] | < 0.001 |
| Albumin g/dL | 3.7 [0.6] | 3.9 [0.5] | < 0.001 |
| INR | 1.2 [0.9] | 1.2 [0.9] | 0.42 |
| FIB-4 | 5.3 [13.6] | 3.9 [16.5] | < 0.001 |
| MELD | 8.6 [3.3] | 8.3 [3.4] | < 0.001 |
DAA = direct acting antivirals, HBV = hepatitis B virus, IFN = interferon, INR = international normalized ratio, MELD = model for end-stage liver disease, SVR = sustained virologic response
Abstinent from alcohol: AUDIT-C score 0. Low-level alcohol use: AUDIT-C 1–3 in men, 1–2 in women. Unhealthy alcohol use: AUDIT-C 4–12 in men, 3–12 in women
There were 9,399 patients with cirrhosis, including 7,927 (84.3%) who achieved SVR and 1,472 (15.7%) who did not achieve SVR (Table 2). Baseline demographic characteristics and MELD scores were similar among cirrhotic patients who did and did not achieve SVR. Patients without SVR had a higher proportion of complications of cirrhosis, including varices without bleeding (27.5% vs. 18.2%), varices with bleeding (7.1% vs. 5.1%), encephalopathy (28.2% vs 18.3%), and hepatocellular carcinoma (13.5% vs. 6.1%).
Table 2.
Baseline characteristics of HCV-infected patients with cirrhosis who received antiviral treatment with DAAs from 2013–2015, according to whether they achieved SVR or not
| No SVR (n=1,472) | SVR (n=7,927) | P-value | |
|---|---|---|---|
| Age, yrs (mean [SD]) | 61.3 [5.4] | 62.0 [5.3] | < 0.001 |
| BMI, (mean [SD]) | 29.4 [5.9] | 28.7 [5.5] | < 0.001 |
| Male (%) | 98.7 | 97 | < 0.001 |
| Race/Ethnicity (%) | < 0.001 | ||
| White, non-Hispanic | 55.9 | 53.8 | |
| Black, non-Hispanic | 24.5 | 29.2 | |
| Hispanic | 9.7 | 7.5 | |
| Other | 2.1 | 1.8 | |
| Declined to answer/missing | 7.8 | 7.7 | |
| Non-Genotype 1 (%) | 27.7 | 12.9 | < 0.001 |
| HBV co-infection(%) | 0.7 | 1.8 | < 0.01 |
| Varices (%) | < 0.001 | ||
| No varices | 65.4 | 76.6 | |
| Varices, but no bleeding | 27.5 | 18.2 | |
| Varices with bleeding | 7.1 | 5.1 | |
| Ascites (%) | 1.4 | 1 | 0.2 |
| Encephalopathy (%) | 28.2 | 18.3 | < 0.001 |
| Hepatocellular Carcinoma (%) | 13.5 | 6.1 | < 0.001 |
| Diabetes (%) | 38.2 | 37.9 | 0.79 |
| Non-selective beta blocker (%) | 19.2 | 16.6 | 0.02 |
| AUDIT-C scores* (%) | 0.44 | ||
| Abstinent | 75.3 | 74.5 | |
| Low-level use | 17.1 | 18.3 | |
| Unhealthy use | 7.6 | 7.1 | |
| Alcohol Use Disorder (%) | 52.9 | 48 | < 0.001 |
| MELD score ≥ 9 | 39.5 | 31.5 | < 0.001 |
| Charlson Comorbidity Index (%) | < 0.001 | ||
| 0 | 25.5 | 14.1 | |
| 1 | 16.8 | 24.2 | |
| 2 | 10.7 | 16.1 | |
| > 2 | 46.9 | 45.6 | |
| Laboratory Results (mean [SD]) | |||
| Alpha Fetoprotein, ng/mL | 8.5 [4.7] | 7.7 [4.6] | < 0.001 |
| Hemoglobin, g/dL | 13.9 [1.7] | 14.0 [1.7] | < 0.01 |
| Platelet Count, k/μL | 109.7 [56.7] | 130.7 [63.9] | < 0.001 |
| Creatinine, mg/dL | 0.9 [0.5] | 1.0 [0.5] | < 0.01 |
| Bilirubin, g/dL | 1.1 [0.8] | 0.9 [0.7] | < 0.001 |
| Albumin g/dL | 3.4 [0.6] | 3.6 [0.5] | < 0.001 |
| INR | 1.3 [1.0] | 1.3 [1.2] | 0.7 |
| FIB-4 | 8.6 [20.5] | 7.3 [29.9] | 0.11 |
| MELD | 9.6 [3.6] | 9.2 [3.8] | < 0.001 |
DAA = direct acting antivirals, HBV = hepatitis B virus, IFN = interferon, INR =international normalized ratio, MELD = model for end-stage liver disease, SVR = sustained virologic response
Abstinent from alcohol: AUDIT-C score 0. Low-level alcohol use: AUDIT-C 1–3 in men, 1–2 in women. Unhealthy alcohol use: AUDIT-C 4–12 in men, 3–12 in women
Association between SVR and variceal bleeding
All patients
During a mean follow-up time of 3.1 years, 549 patients developed variceal bleeding (incidence 0.53 per 100 patient-years) (Table 3). Among patients with SVR (n=29,998), 434 developed variceal bleeding (incidence 0.46 per 100 patient-years) compared to 115 of 3,584 patients without SVR (1.26 per 100 patient-years) and this difference was statistically significantly different after adjustment for baseline characteristics (adjusted hazards ratio [AHR] 0.66, 95% CI 0.52–0.83). SVR was also associated with a reduced risk of variceal bleeding in many clinically relevant sub-groups that we evaluated, such as patients with cirrhosis (AHR 0.73, 95% CI 0.57–0.93), without cirrhosis (AHR 0.33, 95% CI 0.17–0.65), MELD <9 (AHR 0.41, 95% CI 0.28–0.61), alcohol use disorder (AHR 0.65, 95% CI 0.48–0.88), and no alcohol use disorder (AHR 0.67, 95% CI 0.45–0.99).
Table 3.
Association between DAA-induced SVR and the risk of developing variceal bleeding.
| Number of patients (%) | Mean Follow-up (Years) | Number who developed variceal bleeding (%) | Variceal bleeding incidence per 100 patient-years | Crude hazard ratio (95% CI) | Adjusted* hazard ratio (95% CI) | ||
|---|---|---|---|---|---|---|---|
| All patients | No SVR | 3,584(10.7) | 2.5 | 115(3.2) | 1.26 | 1 | 1 |
| SVR | 29,998(89.3) | 3.1 | 434(1.4) | 0.46 | 0.38(0.31–0.47) | 0.66(0.52–0.83) | |
| No prior varices | No SVR | 3,064(9.8) | 2.6 | 45(1.5) | 0.56 | 1 | 1 |
| SVR | 28,070(90.2) | 3.2 | 152(0.5) | 0.17 | 0.31(0.22–0.43) | 0.52(0.35–0.76) | |
| Prior Varices without Bleeding | No SVR | 416(21.7) | 2.1 | 42(10.1) | 4.87 | 1 | 1 |
| SVR | 1,502(78.3) | 3.0 | 155(10.3) | 3.48 | 0.69(0.48–0.99) | 0.77(0.52–1.15) | |
| Prior variceal bleeding | No SVR | 104(19.6) | 1.6 | 28(26.9) | 16.38 | 1 | 1 |
| SVR | 426(80.4) | 2.3 | 127(29.8) | 12.86 | 0.76(0.47–1.23) | 0.60(0.33–1.09) | |
| Cirrhosis | No SVR | 1,472(15.7) | 2.2 | 98(6.7) | 2.96 | 1 | 1 |
| SVR | 7,927(84.3) | 3.1 | 382(4.8) | 1.55 | 0.55(0.44–0.69) | 0.73(0.57–0.93) | |
| No Cirrhosis | No SVR | 2,112(8.7) | 2.7 | 17(0.8) | 0.29 | 1 | 1 |
| SVR | 22,071(91.3) | 3.1 | 52(0.2) | 0.07 | 0.26(0.15–0.45) | 0.33(0.17–0.65) | |
| MELD < 9 | No SVR | 2,106(9.8) | 2.7 | 43(2.0) | 0.76 | 1 | 1 |
| SVR | 19,487(90.2) | 3.2 | 136(0.7) | 0.22 | 0.28(0.20–0.40) | 0.41(0.28–0.61) | |
| MELD ≥ 9 | No SVR | 1,204(13.2) | 2.3 | 67(5.6) | 2.45 | 1 | 1 |
| SVR | 7,889(86.8) | 3.0 | 277(3.5) | 1.15 | 0.50(0.38–0.66) | 0.77(0.57–1.04) | |
| Alcohol use disorder | No SVR | 1,802(12.0) | 2.5 | 70(3.9) | 1.53 | 1 | 1 |
| SVR | 13,187(88.0) | 3.1 | 236(1.8) | 0.57 | 0.38(0.29–0.50) | 0.65(0.48–0.88) | |
| No alcohol use disorder | No SVR | 1,782(9.6) | 2.5 | 45(2.5) | 0.99 | 1 | 1 |
| SVR | 16,811(90.4) | 3.2 | 198(1.2) | 0.37 | 0.38(0.28–0.53) | 0.67(0.45–0.99) |
Adjusted for cirrhosis, prior history of varices, variceal bleeding, ascites, bacterial peritonitis or encephalopathy, age, sex, race/ethnicity, body mass index, HBV co-infection, type 2 diabetes mellitus, hepatocellular carcinoma, alcohol use disorders, AUDIT-C score, non-selective beta blocker, substance use disorder, Charlson Comorbidity Index, platelet count, serum bilirubin, serum creatinine, serum albumin, INR and blood hemoglobin levels. The laboratory tests were categorized into quartiles and modeled as dummy categorical variables; MELD = model for end-stage liver disease, SVR = sustained virologic response
Patients with cirrhosis
Among 9,399 patients with cirrhosis, 480 (5.1%) developed variceal bleeding during 3.07 years of mean follow-up (incidence 1.66 per 100 patient years). The incidence of variceal bleeding was lower among those with SVR (1.55 per 100 patient-years) compared to those without SVR (2.96 per 100 patient-years) and this difference remained statistically significantly after multivariable adjustment in Cox proportional hazards models (AHR 0.73, 95% CI 0.57–0.93) (Table 3/Figure 2).
Figure 2. Cumulative incidence curves among patients with SVR versus no SVR by presence of cirrhosis, varices and variceal bleeding.
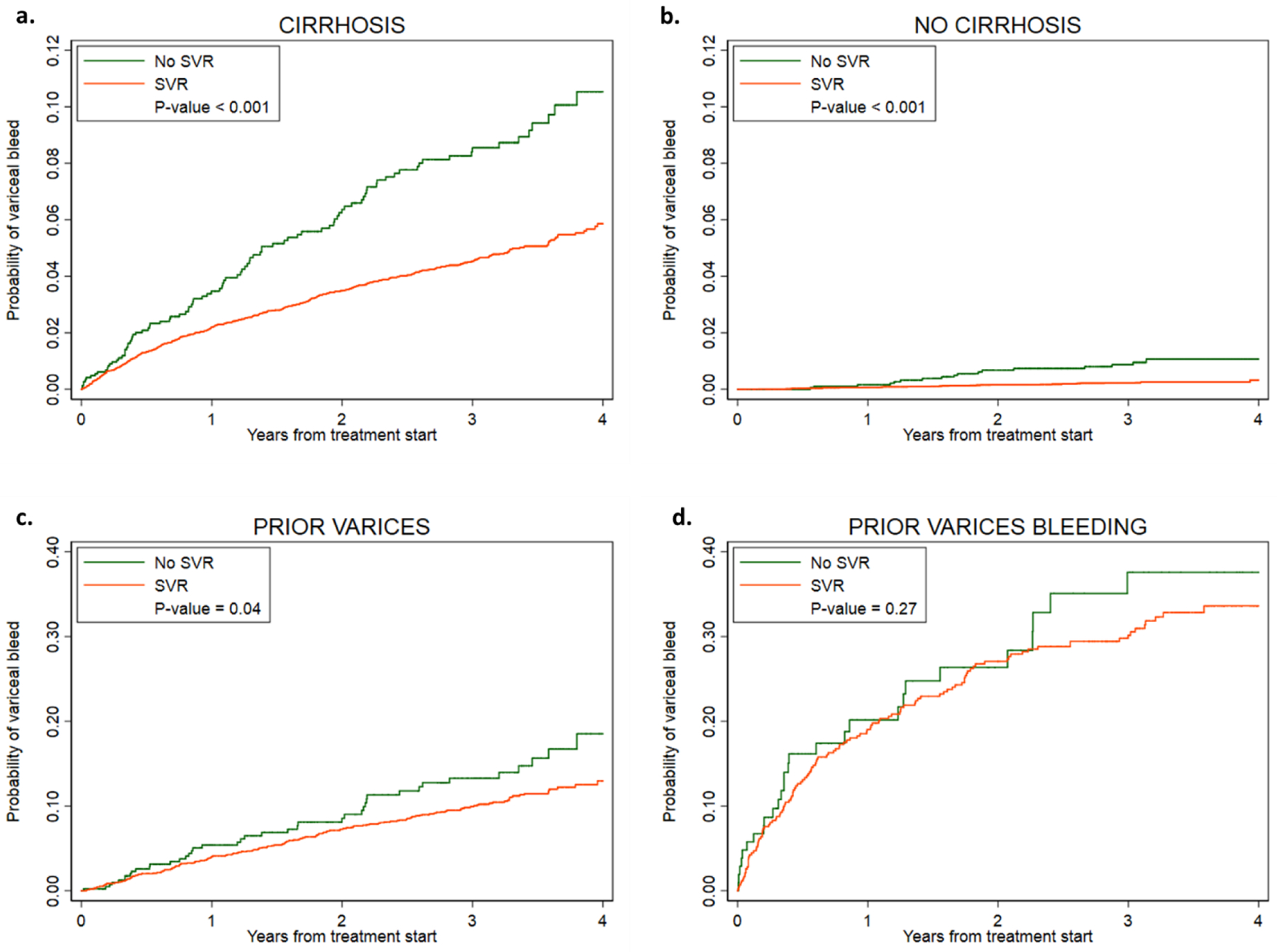
The cumulative incidence of variceal haemorrhage among patients with and without sustained virologic response (SVR) after direct acting antiviral (DAA) treatment for hepatitis C virus (HCV) in (a) patients with cirrhosis, (b) patients without prior cirrhosis, (c) patients with prior varices, and (d) patients with prior variceal bleed.
Patients with a prior history of varices or variceal bleeding
The absolute incidence of variceal bleeding after antiviral treatment was much greater in patients who had a prior history of variceal bleeding (13.38 per 100 patient-years) or varices without bleeding (3.71 per 100 patient-years) than in patients who had no prior history of varices (0.20 per 100 patient-years). Patients with SVR had a lower incidence of variceal bleeding than patients without SVR among those without prior varices (0.17 vs 0.56 per 100 patient-years), with prior non-bleeding varices (3.48 vs 4.87 per 100 patient-years), and with prior bleeding varices (12.86 vs 16.38 per 100 patient-years) (Table 3/Figure 2). In the Cox proportional hazards models, the difference in variceal bleeding rate by SVR was statistically significant among patients without prior varices (AHR 0.52, 95% CI 0.35–0.76) but not among those with non-bleeding varices (AHR 0.77, 95% CI 0.52–1.15) or bleeding varices (AHR 0.60, 95% CI 0.33–1.09)
Characteristics associated with variceal bleeding
In the multivariable Cox proportional hazards model among patients with cirrhosis (Table 4), characteristics associated with risk of variceal bleeding included prior varices without bleeding (AHR 3.09, 95% CI 2.39–4.00), prior varices with bleeding (AHR 9.39, 95% CI 7.08–12.46), non-selective beta-blocker use (AHR 1.37, 95% CI 1.08–1.72), ascites (AHR 1.71, 95% CI 1.02–2.87), spontaneous bacterial peritonitis (SBP) (AHR 2.15, 95% CI 1.31–3.52), PLT >100–150 (AHR 4.42, 95% CI 1.08–18.07) and PLT ≤100 (AHR 6.68, 95% CI 1.64–27.17) vs PLT >250, and hemoglobin ≤13.6 vs >15.6 (AHR 2.12, 95% CI 1.43–3.15). Increasing MELD scores were associated with increased risk of variceal bleed, although this did not meet statistical significance for MELD 16–19 (AHR 1.31, 95% CI 0.69–2.51) or MELD >19 (AHR 2.00, 95% CI 0.90–4.43) potentially due to fewer patients with advanced cirrhosis receiving DAAs. Variables associated with a decreased risk of variceal bleeding included SVR (AHR 0.68, 95% CI 0.53–0.86) and black/non-Hispanic race/ethnicity (AHR 0.74, 95% CI 0.56–0.99).
Table 4.
Characteristics associated with variceal bleeding in patients with cirrhosis following DAA-based antiviral treatment
| Variceal bleeding incidence per 100 patient-years | Adjusted* hazard ratio (95% CI) | |
|---|---|---|
| No SVR | 2.96 | 1 |
| SVR | 1.55 | 0.68(0.53–0.86) |
| No Prior Varices | 0.64 | 1 |
| Prior Varices without bleeding | 3.66 | 3.09(2.39–4.00) |
| Prior varices with bleeding | 13.92 | 9.39(7.08–12.46) |
| Non-selective beta blocker use | ||
| No | 1.21 | 1 |
| Yes | 4.47 | 1.37(1.08–1.72) |
| Ascites | ||
| No | 1.67 | 1 |
| Yes | 7.67 | 1.71(1.02–2.87) |
| Bacterial peritonitis | ||
| No | 1.65 | 1 |
| Yes | 13.14 | 2.15(1.31–3.52) |
| MELD score | ||
| ≤6 | 0.55 | 1 |
| 7–11 | 1.60 | 1.34(0.85–2.12) |
| 12–15 | 4.03 | 1.73(1.06–2.85) |
| 16–19 | 3.19 | 1.31(0.69–2.51) |
| >19 | 2.44 | 2.00(0.90–4.43) |
| Platelet count | ||
| >250 | 0.17 | 1 |
| >200–250 | 0.30 | 1.51(0.30–7.53) |
| >150–200 | 0.54 | 2.86(0.68–12.06) |
| >100–150 | 1.27 | 4.42(1.08–18.07) |
| ≤ 100 | 3.30 | 6.68(1.64–27.17) |
| Hemoglobin, g/dL | ||
| >15.6 | 0.69 | 1 |
| >14.6–15.6 | 1.05 | 1.31(0.84–2.04) |
| >13.6–14.6 | 1.36 | 1.29(0.84–1.96) |
| ≤13.6 | 2.91 | 2.12(1.43–3.15) |
| Race/Ethnicity (%) | ||
| White, non-Hispanic | 2.07 | 1 |
| Black, non-Hispanic | 1.01 | 0.74(0.56–0.99) |
| Hispanic | 2.22 | 0.95(0.67–1.36) |
| Other | 1.77 | 0.79(0.39–1.57) |
| Declined to answer/missing | 1.46 | 0.86(0.58–1.28) |
| BMI | ||
| <25 | 1.58 | 1 |
| 25-< 30 | 1.97 | 1.28(0.99–1.66) |
| 30-< 35 | 1.52 | 1.08(0.81–1.45) |
| ≥35 | 1.53 | 1.00(0.70–1.41) |
Adjusted by Cox proportional hazards regression for all the characteristics shown in the Table
Discussion
Variceal bleeding is a life-threatening complication of cirrhosis. In this large cohort of 33,582 US Veterans who underwent DAA treatment and were followed for a mean of 3.1 years after treatment, we extend our knowledge of the associations between SVR and variceal bleeding in several ways. First, we have shown that DAA-induced SVR was clearly associated with a reduced risk of variceal bleeding both among patients with established cirrhosis and among those without cirrhosis prior to antiviral treatment. Second, our results suggest that even among patients with a prior history of varices or variceal bleeding, which are the highest risk groups, DAA-induced SVR was associated with a lower risk of variceal bleeding (although this difference did not reach statistical significance in our multivariable models). Finally, we found that although in cirrhotic patients SVR reduces the risk of variceal bleeding, a substantial residual risk remains and several important predictors of variceal bleeding are evident. Taken together, our results point to a beneficial effect of DAA-induced SVR with regard to the potentially deadly complication of variceal bleeding and add to the growing literature demonstrating the clinical benefits of HCV eradication with DAAs31–33.
Our results demonstrate that DAAs reduced the risk of variceal haemorrhage among patients without cirrhosis or pre-treatment varices, underscoring the importance of “early” HCV treatment. Given the nature of this study, although we do not know the histological stage of fibrosis of the cohort, natural history studies demonstrate that, among all-comers with untreated HCV, cirrhosis will develop in approximately 15% over 5 years34 and, among patients with compensated cirrhosis, varices will occur in 7% annually35. Further, our data suggest the likelihood of variceal haemorrhage can be substantially reduced with early treatment of HCV – in our study, successful treatment of HCV prior to the development of cirrhosis nearly eliminated the risk of future variceal bleeding (incidence 0.07 per 100-person years). These findings provide further support for calls to expand of HCV screening36 and for treatment of all patients with HCV regardless of fibrosis stage37.
We have also shown that SVR is associated with reduction in the risk of variceal bleeding among patients with cirrhosis, whether varices are present or not. This may be due to fibrosis reversion after successful DAA therapy, resulting in reduced portal pressures. Biologic plausibility for this is provided by studies showing that HCV treatment is associated with fibrosis reversion38–41, improvement in portal hypertension42–45, and decreased risk of oesophageal varices in compensated cirrhosis46. Not surprisingly, in our multivariable model low platelet count, an indirect measure of portal hypertension, and other sequelae of portal hypertension (e.g. ascites, SBP) were associated with an increased risk of variceal bleeding.
Despite SVR, certain patients developed variceal bleeding, including 4.8% of patients with pre-treatment cirrhosis. These data suggest that while some patients may have an improvement in portal hypertension (we speculate as a result of reduced fibrosis) with a concomitant reduction in the risk of variceal bleeding, this does not occur in all patients. This finding has several implications. First, the data suggest that there are likely highly specific biologic responses to SVR – leading to a reduction in portal pressure in some, but not all, patients. Further study to better understand these biologic responses, and the predictors of them, will be essential. Our multivariable model identified some known predictors of variceal bleeding including history of varices, other decompensation events, and low platelet count. NSBB use was also associated with an increased risk of variceal bleeding, as expected since these medications are preferentially used in patients with high-risk varices (e.g. large, red wale signs). Lastly, black race was associated with a lower risk of variceal bleeding, which has previously been reported and deserves more study47. Second, it suggests that it is important to continue endoscopic surveillance for varices and prophylaxis for variceal bleeding if indicated following SVR. This was particularly true among patients with pre-treatment varices. Conversely, it may be that cirrhosis patients without varices who achieve SVR may be able to safely receive screening endoscopies less frequently than every 2–3 years, as is currently recommended by the American Association for the Study of Liver Diseases (AASLD)48. Future prospective and cost-effectiveness studies could help answer these questions.
This study is strengthened by its large, geographically distributed cohort of HCV patients with prolonged follow-up after treatment with DAAs. However, this study has some potential limitations. First, all patients were derived from a single, national healthcare system with fairly uniform antiviral treatment practices and guidelines across its facilities. Second, data were derived from the national VA healthcare system where male sex predominates. Third, since this is by necessity an observational study, we cannot exclude the possibility that residual confounding may have contributed to the associations we observed between SVR and variceal haemorrhage. However, the associations persisted after careful adjustment for >20 baseline characteristics known or suspected to be associated with SVR and variceal bleeding.
In conclusion, these findings demonstrate that successful treatment of HCV with DAAs is associated with a reduced risk of subsequent variceal bleeding. This provides further evidence supporting the real-world benefits of DAAs in patients with and without cirrhosis and emphasizes the importance of early identification and treatment of HCV-infected patients.
Funding:
The study was funded by a NIH/NCI grant R01CA196692 and VA CSR&D grant I01CX001156 to GNI. This research was supported in part by NIH grant T32 DK007634 (AMM).
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- AHR
adjusted hazards ratio
- AUDIT-C
Alcohol Use Disorders Identification Test-Consumption
- BMI
body mass index
- CDW
Corporate Data Warehouse
- DAA
direct acting antiviral
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ICD
International Classification of Diseases
- INR
international normalized ratio
- MELD
model for end-stage liver disease
- NSBBs
nonselective beta blockers
- SBP
spontaneous bacterial peritonitis
- SVR
sustained virologic response
- VAHS
Veterans Affairs Healthcare System
Footnotes
Publisher's Disclaimer: Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the US Government.
Declaration of Personal Interests: None
References
- 1.Reverter E, Tandon P, Augustin S, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146(2):412–419 e413. [DOI] [PubMed] [Google Scholar]
- 2.Amitrano L, Guardascione MA, Manguso F, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107(12):1872–1878. [DOI] [PubMed] [Google Scholar]
- 3.Fortune BE, Garcia-Tsao G, Ciarleglio M, et al. Child-Turcotte-Pugh Class is Best at Stratifying Risk in Variceal Haemorrhage: Analysis of a US Multicenter Prospective Study. J Clin Gastroenterol. 2017;51(5):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 5.Chhatwal J, Wang X, Ayer T, et al. Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016;64(5):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobsen J, Nielsen E, Feinberg J, et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Viral Hepatitis Roundtable. Hepatitis C: The State of Medicaid Access. Harvard Law School;2017. [Google Scholar]
- 8.Moon AM, Green PK, Berry K, Ioannou GN. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Alimentary pharmacology & therapeutics. 2017;45(9):1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockey DC. Fibrosis reversal after hepatitis C virus elimination. Curr Opin Gastroenterol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veterans Affairs Corporate Data Warehouse. Available at: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm Last accessed on 12/19/16.
- 11.Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61(1):41–45. [DOI] [PubMed] [Google Scholar]
- 12.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clin Gastroenterol Hepatol. 2018;16(10):1677–1678. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan PM, Kramer JR, El-Serag HB, et al. The quality of care provided to patients with varices in the department of Veterans Affairs. Am J Gastroenterol. 2014;109(7):934–940. [DOI] [PubMed] [Google Scholar]
- 14.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. New England Journal of Medicine. 2008;359(10):1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beste LA, Ioannou GN, Larson MS, Chapko M, Dominitz JA. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clin Gastroenterol Hepatol. 2010;8(11):972–978. [DOI] [PubMed] [Google Scholar]
- 16.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37–47. [DOI] [PubMed] [Google Scholar]
- 17.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85–93. [DOI] [PubMed] [Google Scholar]
- 18.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–1188 e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27 Suppl 2:B10–21. [DOI] [PubMed] [Google Scholar]
- 20.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–1482 e1475; quiz e1417–1478. [DOI] [PubMed] [Google Scholar]
- 21.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19(4):475–505. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50(3):825–833. [DOI] [PubMed] [Google Scholar]
- 23.Shah HA, Azam Z, Rauf J, et al. Carvedilol vs. oesophageal variceal band ligation in the primary prophylaxis of variceal haemorrhage: a multicentre randomized controlled trial. J Hepatol. 2014;60(4):757–764. [DOI] [PubMed] [Google Scholar]
- 24.Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108(1–2):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 26.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 27.Williams EC, Rubinsky AD, Lapham GT, et al. Prevalence of clinically recognized alcohol and other substance use disorders among VA outpatients with unhealthy alcohol use identified by routine alcohol screening. Drug Alcohol Depend. 2014;135:95–103. [DOI] [PubMed] [Google Scholar]
- 28.Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48(6 Suppl):S114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturmer T, Jonsson Funk M, Poole C, Brookhart MA. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology. 2011;22(3):298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153(4):996–1005 e1001. [DOI] [PubMed] [Google Scholar]
- 33.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8(3):280–288, 288.e281. [DOI] [PubMed] [Google Scholar]
- 34.Butt AA, Yan P, Lo Re V 3rd, et al. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med. 2015;175(2):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. [DOI] [PubMed] [Google Scholar]
- 36.Chhatwal J, Sussman NL. Universal Screening for Hepatitis C: An Important Step in Virus Elimination. Clin Gastroenterol Hepatol. 2019;17(5):835–837. [DOI] [PubMed] [Google Scholar]
- 37.Buckley GJ, Strom BL. A National Strategy for the Elimination of Viral Hepatitis Emphasizes Prevention, Screening, and Universal Treatment of Hepatitis C. Ann Intern Med. 2017;166(12):895–896. [DOI] [PubMed] [Google Scholar]
- 38.Goodman ZD, Stoddard AM, Bonkovsky HL, et al. Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology. 2009;50(6):1738–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McHutchison J, Goodman Z, Patel K, et al. Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology. 2010;138(4):1365–1373, 1373 e1361–1362. [DOI] [PubMed] [Google Scholar]
- 40.D’Ambrosio R, Aghemo A, Rumi MG, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56(2):532–543. [DOI] [PubMed] [Google Scholar]
- 41.Poynard T, Moussalli J, Munteanu M, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59(4):675–683. [DOI] [PubMed] [Google Scholar]
- 42.Afdhal N, Everson GT, Calleja JL, et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat. 2017;24(10):823–831. [DOI] [PubMed] [Google Scholar]
- 43.Roberts S, Gordon A, McLean C, et al. Effect of sustained viral response on hepatic venous pressure gradient in hepatitis C-related cirrhosis. Clin Gastroenterol Hepatol. 2007;5(8):932–937. [DOI] [PubMed] [Google Scholar]
- 44.Rincon D, Ripoll C, Lo Iacono O, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101(10):2269–2274. [DOI] [PubMed] [Google Scholar]
- 45.Lens S, Alvarado-Tapias E, Marino Z, et al. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153(5):1273–1283 e1271. [DOI] [PubMed] [Google Scholar]
- 46.Bruno S, Crosignani A, Facciotto C, et al. Sustained virologic response prevents the development of oesophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51(6):2069–2076. [DOI] [PubMed] [Google Scholar]
- 47.Wollenman CS, Chason R, Reisch JS, Rockey DC. Impact of ethnicity in upper gastrointestinal haemorrhage. J Clin Gastroenterol. 2014;48(4):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. [DOI] [PubMed] [Google Scholar]


