Abstract
In recent years, interventional cardiac magnetic resonance imaging (iCMR) has evolved from attractive theory to clinical routine at several centers. Real-time cardiac magnetic resonance imaging (CMR fluoroscopy) adds value by combining soft-tissue visualization, concurrent hemodynamic measurement, and freedom from radiation. Clinical iCMR applications are expanding because of advances in catheter devices and imaging. In the near future, iCMR promises novel procedures otherwise unsafe under standalone X-Ray guidance.
Keywords: Congenital heart disease, Interventional MRI, MR fluoroscopy, Magnetic resonance imaging (MRI)
Background
Incremental advances in cardiovascular imaging have dramatically improved care for children with heart disease. Non-invasive imaging has supplanted invasive cardiac catheterization for most diagnosis and treatment planning. Interventional cardiology has grown in the number, range, and complexity of transcatheter procedures. By contrast, imaging guidance of catheter procedures has evolved less. Operators continue to rely on X-Ray fluoroscopy and radiocontrast angiography. Cardiac magnetic resonance imaging (CMR) provides excellent soft-tissue characterization, simultaneous multi-planar imaging, 3-dimensional anatomic depiction, and large field-of-view thoracic context. CMR offers hemodynamics such as function and blood flow. Myocardial tissue characteristics can be imaged such as inflammation, edema, collagen content, perfusion, and tumor. It is radiation-free. It has been over fifteen years since clinical CMR catheterization was first performed [1]. In the last five years, centers performing diagnostic CMR catheterization have increased [2-4]. In the next five years, we anticipate centers performing clinical interventional CMR catheterization will similarly increase.
Main Body
Why?
iCMR combines advanced imaging, hemodynamics, and potential intervention in a single radiation-free encounter. Conventional X-Ray fluoroscopy depicts soft tissue poorly. X-Ray requires pattern recognition of two-dimensional gray and white images. Contrast angiography is necessary. Adjunctive real-time soft-tissue imaging is often desirable. It is true that experienced operators have become adept at performing X-Ray guided cardiac intervention. It is also true that X-Ray fluoroscopy requires operators to make assumptions and educated guesses that may not reflect true anatomy. CMR offers superior soft-tissue visualization continuously depicted in any plane (Fig. 1). This could improve procedural success and safety. For example, improved myocardial tissue characterization could improve myocardial biopsy yield and electrophysiology (EP) ablation success [5, 6]. Continuous visualization would enable quicker detection of potential complications such as bleeding. In a coarctation stenting animal model, intentional aortic perforation was immediately visible with continuous real-time CMR [7]. Early detection of bleeding prior to hemodynamic instability is more difficult with X-Ray [8]. These advantages could improve conventional procedures and enable future interventions deemed too risky with X-Ray guidance alone. One example is non-surgical large vessel anastomosis, such as transcatheter cavopulmonary shunt (Fig. 1).
Fig. 1.
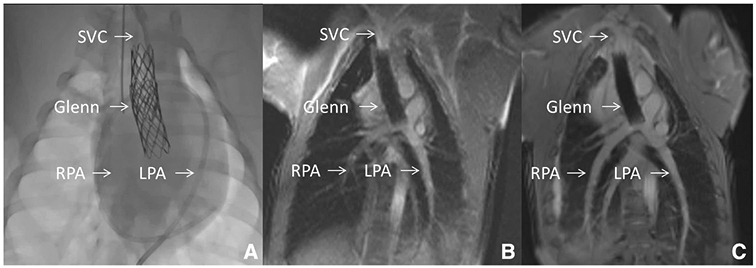
X-Ray Fluoroscopy versus Interventional Cardiac MRI (iCMR). This figure demonstrates a novel percutaneous intervention: transcatheter cavopulmonary anastomosis [58]. a Shows traditional X-Ray fluoroscopy of the endograft based cavopulmonary anastomosis (Glenn) in situ. Superior Vena Cava (SVC), Right and Left Pulmonary Artery (RPA, LPA) expected locations are shown. b Shows real-time CMR fluoroscopy with superior anatomic visualization. SVC, RPA, LPA and entire thoracic context are clearly and continuously displayed. c Shows higher quality diagnostic CMR (steady-state free precession “white” blood imaging) of the Glenn. During iCMR cases, intermittent higher quality non-real-time CMR can be obtained at any time
iCMR offers simultaneous invasive pressure, quantitative blood flow and regurgitant volume, myocardial function as well as detailed tissue characterization including edema, inflammation, and fibrosis assessment. iCMR hemodynamic data quality is better than X-Ray catheterization [9]. It can overcome shortcomings in conventional oximetric flow measurements. Traditionally, we rely on Fick quantification for cardiac output, shunt calculation, and pulmonary vascular resistance. This is flawed. Direct measurement of VO2 is cumbersome and infrequent in clinical catheterization labs. Typically, we assume VO2 based on heart rate. This is inexact. Oxygen consumption (VO2) variations, over time and during different hemodynamic conditions, significantly change calculated flows. Accurate oximetric calculations require simultaneous sampling across multiple cardiac chambers and vessels, but simultaneous measurements are not realistic. In complex anatomy, catheter blood sampling can be challenging and time-consuming. Hemodynamic variation, whether induced by catheter manipulation, pharmacologic provocation, or spontaneous fluctuation, also significantly impacts data veracity. Fick-based oximetric flow calculations are known to be inaccurate during oxygen or nitric oxide inhalation [9], or when there are multiple sources of pulmonary blood flow. Thermodilution cardiac output measures are limited in patients with congenital heart disease (intracardiac shunts, valvar regurgitation, etc.) or low cardiac output. By contrast, diagnostic CMR paired with invasive catheter pressure measurement overcomes these shortcomings, generating more accurate and comprehensive hemodynamic evaluations. The quality of these data is crucial because they are key determinants of surgical, transcatheter, or pharmacologic management.
iCMR is radiation-free. X-Ray exposes children to the risk of cancer from ionizing radiation [10]. Growing children may be particularly sensitive. Children often require multiple X-Ray procedures over time [11]. There is evidence of early chromosomal damage in these children after X-Ray cardiac catheterization [12, 13]. Some of the most vulnerable congenital heart disease patients (e.g. single-ventricle, heart transplant) receive the most cumulative radiation exposure [11]. CMR catheterization may be most compelling in these patients. Operators also risk cancer from radiation exposure [14].
Where?
CMR Room Configuration for CMR Catheterization
There are MRI room requirements to perform CMR-guided catheterization (Table 1). 1.5 Tesla MRI has been used as is typical for most diagnostic CMR. 3.0 T offers increased signal versus 1.5 T, but also increased device heating and inferior workhorse “white blood” cine (balanced steady-state free precession) imaging. Shorter and wider bore systems are available that improve operator access to the patient. Ergonomics remain limited compared with X-Ray systems. CMR of the beating heart in real-time requires dedicated hardware (fast gradients, dedicated phased-array torso coils, etc.) and advanced computational systems that may not accompany standard brain and body purchase options. CMR catheterization is possible using off-the-shelf CMR systems. Dedicated iCMR computer systems are available to simplify workflow including real-time CMR guidance of catheter navigation and post-procedure image review.
Table 1.
Interventional cardiac MRI (iCMR): what you need to start
| Interventional cardiac MRI (iCMR): what you need to start |
| Interventional Cardiac CMR suite (1.5 T CMR & X-Ray Fluoroscopy) |
| Real-time CMR & user interface |
| Hemodynamic recording system |
| Hemodynamic monitoring system |
| Video display |
| CMR surface coils (dedicated cardiac) |
| Noise canceling communication headsets |
| Standard operating procedures → safety/efficiency |
CMR catheterization requires simple additions to the scanner room. Real-time MR images should be displayed to the operator alongside instantaneous hemodynamics, ECG rhythm, and pulse oximetry. This is easily accomplished using inexpensive RF-shielded projectors or more-expensive RF-shielded LCD monitors. The display should be positioned to allow operators to work from the head or the foot of the bed. CMR interferes with ECG signals, but conventional fluid-filled blood pressure transducers work well. At present there is no commercially-available interface to high-fidelity hemodynamic recording systems used in catheterization laboratories. Several programs have adopted PRiME (Physiological Recording in MRI Environment), a set of NIH-developed open-source plans that can be taken to a custom electronics vendor for assembling and testing [15] (see Table 2). PRiME connects MRI-conditional ECG electrodes and high-fidelity pressure signals from MRI catheterization to conventional hemodynamic recording systems. This allows catheter teams to “feel at home” and supports seamless integration into the medical record. A less-attractive alternative is to connect catheter pressure transducers to widely-used low-fidelity portable radiologic hemodynamic monitors. Because CMR scans are loud, catheter staff communication is difficult; hand-signals are not suitable. Although fairly expensive, we strongly recommend that programs interested in MRI catheterization invest in purpose-built MRI staff audio communication systems. One wireless noise-canceling solution (Fig. 2b; Optoacoustics, Yehuda, Israel) allows all staff to speak using “open microphones.” [2, 3] The heating, ventilation, and air conditioning (HVAC) systems in most hospitals afford satisfactory air handling for sterile heart catheterization inside CMR scanner rooms. Sterile draping is straightforward [16].
Table 2.
Interventional cardiac mRI (iCMR): useful online references
| Interventional cardiac MRI (iCMR): useful online references |
|---|
| General/background (presentations for download) https://icmr.nhlbi.nih.gov |
| Representative talks/topics: |
| “Setting up an iCMR suite” |
| “How to begin CMR cath in patients using commercial catheters” CMR-guided electrophysiology |
| Case presentations |
| Taped cases |
| Future directions |
| PRiME (Physiological Recording in MRI Environment) system https://nhlbi-mr.github.io/PRiME/ |
| CMR Right Heart Catheterization (Video) https://www.youtube.com/watch?v=JvdcCEaml9w. |
| CMR Right and Left Heart Catheterization (Video) https://www.youtube.com/watch?v=7JxBNIoJLrQ. |
| CMR right heart catheterization typical slice planes https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5585983/ |
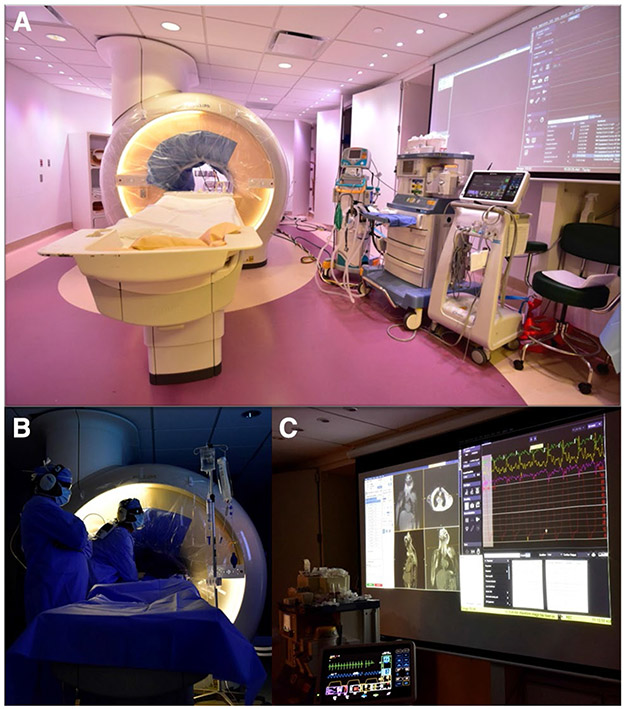
Fig. 2.
Interventional cardiac MRI (iCMR) room, a Demonstrates the iCMR room at Children’s Medical Center of Dallas. The CMR bore has a sterile drape. CMR compatible ventilator, nitric oxide setup, and hemodynamic monitoring system are visible. b The interventional team is performing clinical CMR catheterization. c Shows a large projected image of real-time CMR as catheterization is being performed and live high fidelity hemodynamic recording. Images are courtesy Dr. Surendranath Veeram Reddy and Children’s Medical Center of Dallas team
Interventional Cardiac MRI Suite (X-Ray and MRI in the Same or Adjoining Rooms Within a Single Suite)
It may be helpful to position CMR and X-Ray rooms next to each other (Fig. 3). Such rooms can have adjoining doors so that they can be operated independently and in parallel for standard clinical procedures or together for iCMR cases. It seems helpful for both CMR and X-ray systems to be pro-cured from the same vendor, to simplify patient transfer between systems on detachable tables. Adjoining imaging systems also simplify same-session co-registered (X-Ray Fused with CMR, XFM) procedures, post-catheterization/post-ablation “exit” CMR, and combined procedures during a single anesthesia encounter.
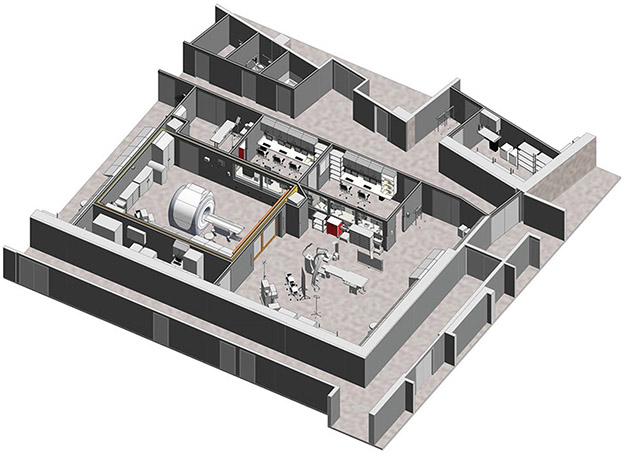
Fig. 3.
Interventional Cardiac MRI (iCMR) suite (co-localized, adjacent CMR and X-Ray cardiac catheterization rooms). A 3-D rendering of future iCMR suite at Rady Children’s Hospital—San Diego show co-localized, adjacent CMR and X-Ray cardiac catheterization rooms separated by sliding radiofrequency shielded doors. Image is courtesy Rady Children’s Hospital—San Diego
Especially during pediatrics procedures, management of “lines” can be complicated because patients may have intravascular devices (lines, sheaths, catheters, etc.), vasoactive medications, endotracheal tubes, and electrodes (for ECG, oximetry, and capnography) connected to their bodies. Patient transfer seems easier when performed in a straight line, through the largest possible door, and with scanners having the same orientation in both rooms (Fig. 3). The straight-line configuration is helpful during rare emergencies needing X-Ray transcatheter or even surgical bailout. Even for such short transfers, all attached equipment, cables and invasive sheaths must be secured, and checklists should assure ferromagnetic items have been removed [17].
Retrofitting Existing MRI Room (Remote X-Ray Cardiac Catheterization Room)
Space, funding, and timing may preclude co-localized suites. Several programs have adopted clinical iCMR without co-localized X-ray systems [18]. The downside of this strategy is that space must be made immediately available for emergency patient management outside the high magnetic field, such as advanced airway and rhythm management. Typical diagnostic CMR rooms also do not have space for the additional staff and equipment used in X-ray catheterization rooms.
Many hospitals have hybrid neurosurgical suites containing mobile MRI systems. These may be underutilized; however, we are not aware of successful clinical CMR catheterization in such retrofitted neurosurgical operative suites.
How?
MR Imaging
Operators need to see MR images in real-time to watch catheter devices as they are moved through the body. Diagnostic CMR collects image information over relatively long periods of time to create high resolution pictures, often assembled by combining information from multiple heartbeats. iCMR requires rapid image acquisition, reconstruction and display all within a fraction of a second to create images that appear in “real-time” to the catheter operator. X-ray operators are accustomed to imaging at 15 frames per second, but many use lower frame rates (3 frames/second) to decrease patient, operator, and staff radiation exposure. Real-time CMR can readily achieve between 8 and 10 frames/second, at the cost of lower spatial resolution compared with diagnostic CMR or X-ray. But because CMR fluoroscopy depicts soft tissues without contrast agents, it is felt to be more information-rich than X-ray, so the lower spatial resolution seems an acceptable compromise. Moreover, CMR fluoroscopy can depict the entire anatomic context, such as critical neighboring structures. The operator can view any desired imaging plane and multiple imaging planes at the same time. Simply put, real-time CMR allows operators to see more anatomy, in more detail, all the time.
Higher quality non-real-time CMR imaging can be obtained before or after introducing catheters as well as intra-procedure. This includes white blood (gradient echo, steady-state free precession), black blood (spin echo), flow (phase contrast), MR angiography, and delayed enhancement as well as newer and research pulse sequences (Fig. 1). Of course, CMR provides quantitative hemodynamics (cardiac output, pulmonary blood flow, myocardial function, regurgitant volumes) and tissue characterization (edema, fibrosis). While retained metallic implants (sternal wires, embolization coils, stents) can limit the image quality of CMR, iCMR procedures can still be performed in patients with these devices [3, 19].
Workflow and Safety Considerations
A common critique of iCMR is that iCMR procedures take more time than X-Ray cardiac catheterization. While it is true that total iCMR procedure time may be longer, more information is collected (invasive pressure + CMR) and key flow data are significantly more accurate [9].
Workflows can be tailored to iCMR. For example, we choose imaging planes to guide navigation before catheter operators enter the room (see Table 2). We also choose which patients do not need adjunctive imaging such as late-gadolinium enhancement. Procedure times all shorten dramatically once teams get experience [3, 18], and CMR right heart catheter times can be similar to X-Ray [3]. In a series of over 100 adult patients, CMR right heart catheterization time averaged 20 min [2]. In a more recent series of 50 adult patients, in-room time was one hour and catheter time was less than five minutes [18]. In 50 consecutive pediatric CMR right heart catheterizations, procedure time was 15 min per hemodynamic condition [3].
Almost all patients can be safely managed in the iCMR setting [17]. Anesthesia providers need easy access to the patient and a clear view of patient comfort and respiratory effort. Lines and tubes should exit the foot of the bore (versus the head) in case of emergency evacuation. This requires longer lines and tubes than X-Ray; the larger “dead space” is surmountable and has not been clinically relevant using this strategy. Teams should run evacuation drills to ensure timely patient movement to perform potential resuscitation. Evacuation can be accomplished in less than one minute [20]. This may not be necessary in the future as MR compatible defibrillators become available [21].
MRI safety concepts may be new to some team members; all staff must undergo formal safety training. iCMR poses unique but surmountable safety challenges. In conventional diagnostic MRI, metallic objects are removed far from the MRI system. In iCMR, metallic objects (for example needles) may be used during adjunctive X-ray procedures. Special safety procedures must be adopted systematically to assure ferromagnetic items are not left on the catheter field, because these can become projectiles when patients are transferred into the MRI system. Most iCMR teams employ approved protocols for patient transfer, such as a “metal time-out” [17].
Catheterization Devices
Visualization
CMR catheter devices are classified based on whether they are visualized “passively” or “actively.” CMR passive visualization refers to devices imaged based on intrinsic material properties [22]. Small amounts of metal can serve as passive markers to create black spots for CMR visualization (Fig. 1b, c). Excessive amounts of metal in the marker can create excessive black spots that distort the images. Passively visualized devices do not require tethering cables.
Active visualization refers to devices imaged using incorporated electronic components (Figs. 5, 6). These devices may be plugged into the scanner. They are presented to the operator as continuously updating color overlay on real-time CMR [23]. Thicker imaging slices are more likely to capture these devices (passive, active) though spatial resolution suffers. Thinner slices may miss out-of-plane devices though spatial resolution is improved. “Active-tracking” devices have embedded radiofrequency receiver coils that are tracked by CMR-tracking pulse sequences. This strategy is used to automatically keep devices in the imaging plane [24].
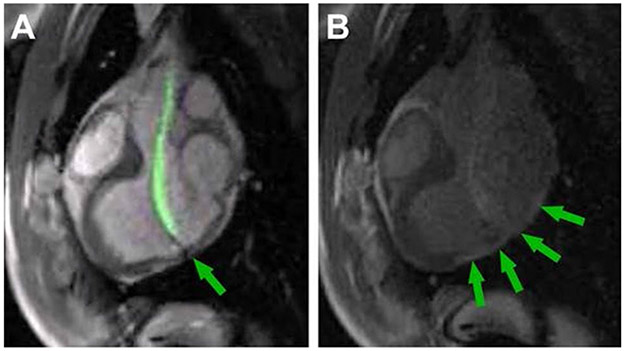
Fig. 5.
CMR-Guided Endomyocardial Biopsy. a Demonstrates real-time MR-guided endomyocardial biopsy within the left ventricle using an “active” visualization device (green). The arrow indicates the jaws of the bioptome. b demonstrates the late-gadolinium enhancement of biopsy targets (arrows) [5]
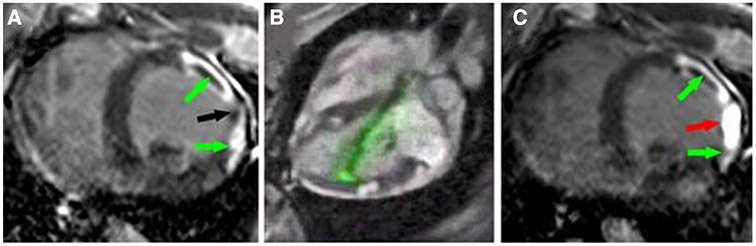
Fig. 6.
CMR-Guided Chemoablation for Ventricular Arrhythmia Therapy. a Late-gadolinium enhancement highlights two areas of infarct (green arrows) with an isthmus of normal myocardium (black arrow). b Shows real-time MR–guided chemoablation with an “active” visualization injection needle in green. c Post-chemoablation the areas of infarct continue to be highlighted with green arrows and the red arrow demonstrates a transmural chemoablation lesion eliminating the gap between infarct zones [55]
Catheters
Most X-Ray catheters incorporate metal braiding for support and to transmit torque. In CMR, this metal creates imaging artifacts and causes unsafe radiofrequency (RF) heating. A few commonly used X-Ray catheters do not have metal braiding. Balloon tipped flow directed catheters are an example and have been used for CMR catheterization [1-4, 18]. The inflatable balloon tip can be seen during MRI because it is filled with CO2 gas or dilute gadolinium contrast agent [25, 26]. The plastic shaft is largely invisible by CMR because it is thin and contains no water. Catheter visualization is difficult when the balloon tip is out-of-plane. More MRI catheter options would improve clinical translation. Recently, Yildirim et al. described a metal braided catheter design with good mechanical integrity and minimal heating [27].
Guidewires
The lack of a visible and safe guidewire has been the biggest barrier to wider iCMR adoption [28]. Unlike with invasive catheters, there have been no X-Ray guidewires available for CMR use. X-Ray guidewires use metal for desirable characteristics including torque response, tracking without prolapse around obstacles, and stiffness to help deliver catheters. Guidewire metals cause imaging artifacts, depending on the material, and more important cause heating because of electrical conductivity. One potential solution is to build non-metallic guidewires. Recently, two such non-metallic polymer iCMR guidewires were approved for clinical use. Both have a fiber core and tip markers for passive visualization (Nano4Imaging, Aachen, Germany; EP Flex, Dettingen an der Erms, Germany) [18, 29, 30]. Without metal, these guidewires lack stiffness and torque responsiveness. iCMR guidewires with familiar X-Ray guidewire mechanical properties are in development [31, 32]. One solution employs insulated nitinol rod segments. These are short enough to avoid RF heating and mechanically coupled to resemble ordinary metallic guidewires. There are iron oxide tip markers for passive visualization [28]. Another promising guidewire design approach uses active electronic visualization [29]. Recently, a low-specific absorption rate (SAR) imaging strategy was reported allowing use of some conventional metallic X-Ray guidewires in 1.5 T CMR scanners. In a small series of patients, commercial X-Ray nitinol guidewires were used safely without significant heating [30].
Electrophysiology
A commercial iCMR electrophysiology mapping and radiofrequency ablation system is available in Europe, and has been used in cavotricuspid isthmus ablation for atrial flutter [6]. More advanced electrophysiology procedures such as cryoablation have been demonstrated in a pre-clinical setting [31, 32]. Circular array catheters are under development for iCMR ablation of atrial fibrillation [33].
Other Catheterization Devices
Commercially-available CMR access needles have been used for CMR-guided pericardiocentesis in the pre-clinical setting [34]. An iCMR trans-septal needle and endomyocardial biopsy forceps are being developed [5, 35, 36].
Future Directions
Individual iCMR device development requires time-consuming iteration including extensive quality systems to allow regulatory approval. Two recent approaches focus on the imaging system rather than development of custom iCMR catheter devices. The aim is to change the conditions that make metallic X-ray catheter devices heat during CMR. Low-specific absorption rate (low-SAR) imaging refers to low radiofrequency excitation energy used to generate MRI pictures. Satisfactory iCMR using metallic guidewires has been performed in patients simply by reducing the amount of radio energy applied to create the pictures [30]. This approach has been generalized in an exciting new iCMR solution to lower the main magnetic field. At lower magnetic fields, the radio energy required to make images declines exponentially, and therefore reduces device heating dramatically [37, 38]. Prototype low-field iCMR systems have already been used to perform MRI catheterization in adults [38]. It is likely that low-field MRI will open some of the vast catalog of X-ray devices to iCMR.
Applications
Diagnostic
The most common clinical iCMR application is CMR-guided diagnostic cardiac catheterization [1]. The majority of cases have been right heart catheterization in children [3] and adults [2] (Table 2 video link). Antegrade left heart catheterization via atrial communication (Table 2 video link) and retrograde left heart catheterization have also been performed. A leading indication for these studies is to determine pulmonary vascular resistance and reactivity in pulmonary hypertensive patients [4, 39]. Other clinical indications include post-heart transplant surveillance, cardiomyopathy, congenital heart disease, and intracardiac shunt. Data are often obtained in multiple hemodynamic conditions including pulmonary vasodilator testing, volume loading, and Fontan fenestration test occlusion [40].
X-Ray Fused with MRI (XFM)
X-Ray fused with MRI (XFM) is a strategy to use CMR soft-tissue imaging in the familiar X-Ray workspace (Fig. 4). CMR-derived regions of interest are displayed as image overlay on live X-Ray fluoroscopy. The anatomic overlay decreases the need for contrast angiography [41, 42]. Radiation exposure is also reduced [41, 42]. This is true for diagnostic cases and some interventions. For example, XFM fluoroscopy times are shorter than matched historical controls in patients undergoing intervention for pulmonary artery stenosis, aortic coarctation, conduit stenosis/insufficiency and ventricular septal defect [43]. Common complex procedures, such as percutaneous pulmonary valve implantation, can be simplified with XFM [43] (Fig. 4). The target window for valve implantation is shown continuously without contrast administration. This is helpful in balloon sizing, valve positioning, and valve deployment. XFM is also helpful in less common clinical scenarios. It helps clearly identify anatomy that is difficult to see with X-Ray angiography; left ventricle to right atrium shunt is an example [44]. XFM may enable novel approaches to traditional interventions, such as antegrade wire crossing for ventricular septal defect device closure [45]. There are limitations. Image overlay can be imperfect with target registration error of a few millimeters. In addition, CMR static roadmaps do not account for respiratory and cardiac motion or anatomic deformation from stiff wires or rigid catheter devices (Fig. 4c). Overall, XFM can make procedures that are cumbersome, lengthy or dangerous with traditional X-Ray fluoroscopy easier, shorter, and safer.
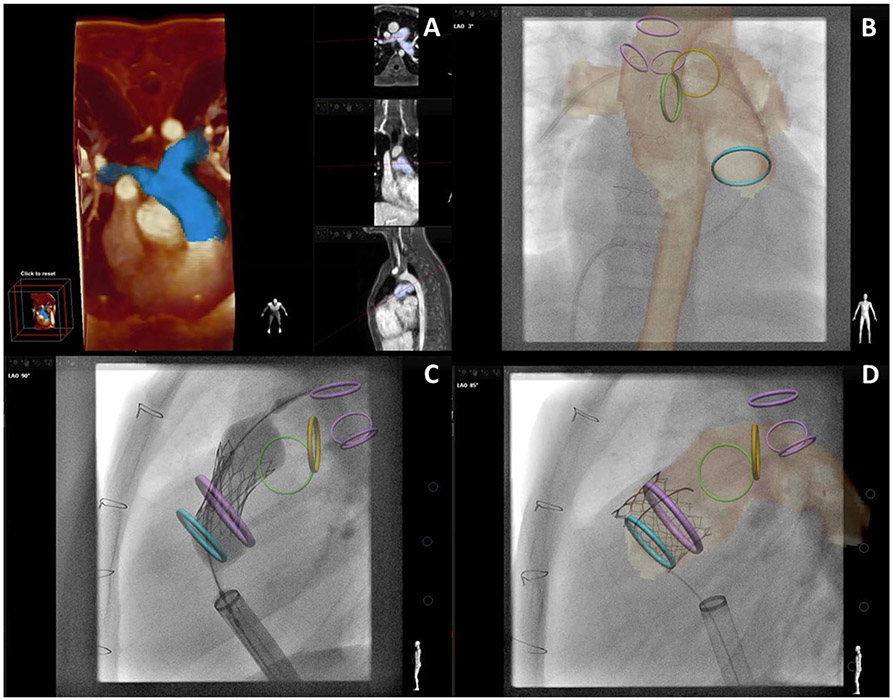
Fig. 4.
X-Ray Fused with MRI (XFM) Guidance of Percutaneous Pulmonary Valve Implantation. a Demonstrates three-dimensional (3D) reconstruction of the right ventricular outflow tract and proximal branch pulmonary arteries (blue). Multiple imaging slices of that region of interest are shown in the vertical panel. b–d demonstrate critical structures depicted by CMR with overlay on live X-Ray fluoroscopy. Three purple rings (most posterior in c, d) show main trachea and right, left bronchus. Blue ring shows proximal right ventricular outflow tract. Green and yellow rings show proximal right and left pulmonary artery, respectively. Large purple circle in c, d highlights intended landing zone. b Shows soft-tissue CMR contour of aorta and pulmonary artery circulation as well as a venous catheter advanced to the right pulmonary artery. c Shows implantation of a percutaneous pulmonary valve. Rigid guidewires displace anatomy making XFM less accurate (see wire in left pulmonary artery above yellow or left pulmonary artery ring). d Shows percutaneous pulmonary valve after implantation and removal of rigid guidewire restoring XFM accuracy. Co-registration was performed with trachea, bronchus, and spine markers. Images are courtesy Dr. Sebastian Goreczny [Polish Mother’s Memorial Hospital, Research Institute (Lodz, Poland) and Children’s Hospital Colorado (Aurora, CO)] [60]
Intervention
Initial iCMR academic work aimed to replicate X-Ray procedures such as simple atrial septal defect device closure [46] or coarctation stent angioplasty [7]. In the former, CMR guidance is not necessary. In the latter, there are advantages such as continuous lesion visualization and precise stent positioning. iCMR also offers a safety advantage; complications may be immediately evident during iCMR analogous to when a surgeon nicks a blood vessel, without waiting for hemodynamic deterioration or angiographic demonstration [7].
Pre-clinical reports continue to suggest that iCMR may improve transcatheter intervention. CMR-guided endomyocardial biopsy is one example. While X-Ray guided endomyocardial biopsy is straightforward, it suffers from sampling error particularly in disease processes that are focal or non-uniform [47]. CMR imaging can depict affected myocardium to guide targeted biopsy. In a pre-clinical series, real-time CMR guidance increased diagnostic biopsy yield when compared with X-Ray guidance (Fig. 5) [5]. iCMR can also simplify complex X-Ray procedures. Real-time CMR was used to guide perventricular access to close ventricular septal defects through the chest wall without surgery [48]. Defects were closed with a modified nitinol occluder and the right ventricular free wall was closed with a commercial device typically used for arterial access closure.
Clinical application of iCMR intervention has been limited by the lack of CMR safe and visible devices. The lack of CMR guidewires has been particularly problematic. For example, non-metallic guidewires that proved mechanically unsatisfactory were employed in CMR-guided balloon pulmonary valvuloplasty [40] in one patient and coarctation of the aorta balloon angioplasty in a few others [49]. With adequate transcatheter tools, iCMR clinical adoption may accelerate.
Electrophysiology
Most interventional electrophysiology procedures are performed using electroanatomic mapping and optional intracardiac echocardiography. These do not depict the global anatomic context or myocardial lesion formation. iCMR may address these shortcomings. iCMR may also be especially useful to guide navigation through complex congenital and postoperative anatomy. In 2012, the Leipzig team performed the first series of CMR-guided diagnostic EP studies in 5 patients [6]. In 2013, cavotricuspid isthmus ablation was accomplished in a human subject [50]. Since then, CMR-guided radiofrequency ablation experience has continued to grow [51].
One of the more promising applications is substrate-based ablation of ventricular tachycardia using iCMR targeting [52]. Cardiac CMR shows thicker ventricular myocardium in great detail including scar and ablation lesions. A pediatric series of exit CMR after ventricular tachycardia ablation demonstrates this potential [53]. Precise catheter positioning on the ventricular myocardium could lead to improved success and safety [54].
CMR-guided chemoablation is attractive to obliterate deep or heterogeneous myocardial targets. In scar induced ventricular tachycardia, the treatment goal is obliteration of any conductive isthmus between scarred regions that contribute to reentrant circuits. Radiofrequency ablation for ventricular tachycardia may not be able to access these deep targets. Moreover, radiofrequency ablation creates lesions that are surrounded by edema associated with reversible conduction block. As a result, lesion recurrence is common despite successful radiofrequency ablation. More important, intra-procedural lesion assessment is challenging using only functional (mapping and pacing) approaches. By contrast, chemoablation can immediately abolish conductive sub-strate, in lesions that can be readily visualized for location and contiguity. In a pre-clinical demonstration, iCMR needle chemoablation obliterated the conductive isthmus and abolished local abnormal voltage activity in areas of heterogeneous scar (Fig. 6) [55].
Novel Procedures
iCMR improves diagnostic cardiac catheterization and can improve conventional cardiac intervention and electrophysiology. Perhaps more exciting, iCMR could guide novel procedures. Visualization of the entire thoracic context offers a new paradigm to access the heart. Real-time CMR can guide transthoracic access and closure through the right or left ventricle [34, 48, 56]. iCMR could guide posterior entry for in-line access to structures such as the mitral valve [57]. iCMR offers surgical type anatomic exposure with a closed chest and a beating heart. This is an opportunity to replicate traditional open heart surgery requiring cardiopulmonary bypass with a minimally-invasive approach. For example, iCMR has been used to implant non-surgical cavopulmonary anastomosis endografts that divert superior cava blood flow directly to both pulmonary arteries in swine. This could replace a standard surgery (Glenn operation) in single-ventricle congenital heart disease patients [58] (Fig. 1). Indeed, experience from iCMR simulation enabled the first human transcatheter cavopulmonary anastomosis, albeit using more technically challenging X-Ray guidance [59].
Conclusions
Previously, interventional cardiac MRI always seemed a few years away. Today, iCMR is a viable option. iCMR offers superior visualization and more accurate hemodynamics. It is radiation-free. As clinical iCMR experience has increased, these procedures have become easier. Ease has led to increased interest with more institutions exploring and building iCMR programs. A wider array of device choices will likely drive additional clinical translation.
Acknowledgments
Funding Funding was funded by National Heart, Lung, and Blood Institute (Grant No: Z01-HL005062).
Abbreviations
- iCMR
Interventional cardiac magnetic resonance imaging
- CMR
Cardiac magnetic resonance imaging
- MRI
Magnetic resonance imaging
- ECG
Electrocardiogram
- EP
Electrophysiology
- RF
Radiofrequency
- SAR
Specific absorption rate
- XFM
X-Ray fused with MRI
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Razavi R, Hill DL, Keevil SF et al. (2003) Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. The Lancet 362:1877–1882. 10.1016/S0140-6736(03)14956-2 [DOI] [PubMed] [Google Scholar]
- 2.Rogers T, Ratnayaka K, Khan JM et al. (2017) CMR fluoroscopy right heart catheterization for cardiac output and pulmonary vascular resistance: results in 102 patients. J Cardiovasc Magn Reson. 10.1186/s12968-017-0366-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratnayaka K, Kanter JP, Faranesh AZ et al. (2017) Radiation-free CMR diagnostic heart catheterization in children. J Cardiovasc Magn Reson. 10.1186/s12968-017-0374-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pushparajah K, Tzifa A, Bell A et al. (2015) Cardiovascular Magnetic Resonance catheterization derived pulmonary vascular resistance and medium-term outcomes in congenital heart disease. J Cardiovasc Magn Reson 17:28 10.1186/s12968-015-0130-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers T, Ratnayaka K, Karmarkar P et al. (2016) Real-time magnetic resonance imaging guidance improves the diagnostic yield of endomyocardial biopsy. JACC Basic Transl Sci 1:376–383. 10.1016/j.jacbts.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer P, Grothoff M, Eitel C et al. (2013) Feasibility of real-time magnetic resonance imaging-guided electrophysiology studies in humans. Europace 15:101–108. 10.1093/europace/eus230 [DOI] [PubMed] [Google Scholar]
- 7.Raval AN, Telep JD, Guttman MA et al. (2005) Real-time magnetic resonance imaging-guided stenting of aortic coarctation with commercially available catheter devices in swine. Circulation 112:699–706. 10.1161/CIRCULATIONAHA.105.542647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esch JJ, Shah PB, Cockrill BA et al. (2013) Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant 32:381–387. 10.1016/j.healun.2013.01.1049 [DOI] [PubMed] [Google Scholar]
- 9.Muthurangu V, Atkinson D, Sermesant M, et al. (2005) Measurement of total pulmonary arterial compliance using invasive pressure monitoring and MR flow quantification during MR-guided cardiac catheterization. Am J Physiol Heart Circ Physiol. 10.1152/ajpheart.00957.2004 [DOI] [PubMed] [Google Scholar]
- 10.Kleinerman RA (2006) Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol 36:121–125. 10.1007/s00247-006-0191-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JN, Hornik CP, Li JS et al. (2014) Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 130:161–167. 10.1161/CIRCULATIONAHA.113.005425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreassi MG, Ait-Ali L, Botto N, et al. (2006) Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J 27:2703–2708. 10.1093/eurheartj/eh1014 [DOI] [PubMed] [Google Scholar]
- 13.Beels L, Bacher K, De Wolf D et al. (2009) γ-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation 120:1903–1909. 10.1161/CIRCULATIONAHA.109.880385 [DOI] [PubMed] [Google Scholar]
- 14.Tamer E-S, Patel AS, Cho JS et al. (2017) Radiation-induced DNA damage in operators performing endovascular aortic repair. Circulation 136:2406–2416. 10.1161/CIRCULATIONAHA.117.029550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakareka JW, Faranesh AZ, Pursley RH et al. (2018) Physiological recording in the MRI environment (PRiME): MRI-compatible hemodynamic recording system. IEEE J Transl Eng Health Med 6:4100112 10.1109/JTEHM.2018.2807813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazal JR, Rogers T, Schenke WH et al. (2016) Interventional-cardiovascular MR: role of the interventional MR technologist. Radiol Technol 87:261–270 [PMC free article] [PubMed] [Google Scholar]
- 17.Deutsch N, Swink J, Matisoff AJ et al. (2019) Anesthetic considerations for magnetic resonance imaging-guided right-heart catheterization in pediatric patients: a single institution experience. Pediatr Anesth 29:8–15. 10.1111/pan.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight DS, Kotecha T, Martinez-Naharro A et al. (2019) Cardiovascular magnetic resonance-guided right heart catheterization in a conventional CMR environment: predictors of procedure success and duration in pulmonary artery hypertension. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 21:57 10.1186/s12968-019-0569-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivieri LJ, Cross RR, O’Brien KE et al. (2015) Optimized protocols for cardiac magnetic resonance imaging in patients with thoracic metallic implants. Pediatr Radiol 45:1455–1464. 10.1007/s00247-015-3366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers T, Lederman RJ (2015) Interventional CMR: clinical applications and future directions. Curr Cardiol Rep 17:31 10.1007/s11886-015-0580-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt EJ, Watkins RD, Zviman MM et al. (2016) A magnetic resonance imaging-conditional external cardiac defibrillator for resuscitation within the magnetic resonance imaging scanner bore. Circ Cardiovasc Imaging. 10.1161/CIRCIMAGING.116.005091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratnayaka K, Faranesh AZ, Guttman MA et al. (2008) Interventional cardiovascular magnetic resonance: still tantalizing. J Cardiovasc Magn Reson 10:62 10.1186/1532-429X-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dick AJ, Guttman MA, Raman VK et al. (2003) Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation 108:2899–2904. 10.1161/01.CIR.0000095790.28368.F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilbert S, Sommer P, Gutberlet M et al. (2016) Real-time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series. Europace 18:572–577. 10.1093/europace/euv249 [DOI] [PubMed] [Google Scholar]
- 25.Ratnayaka K, Faranesh AZ, Hansen MS et al. (2013) Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J 34:380–389. 10.1093/eurheartj/ehs189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velasco Forte MN, Pushparajah K, Schaeffter T et al. (2017) Improved passive catheter tracking with positive contrast for CMR-guided cardiac catheterization using partial saturation (pSAT). J Cardiovasc Magn Reson. 10.1186/s12968-017-0368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildirim KD, Basar B, Campbell-Washburn AE, et al. (2019) A cardiovascular magnetic resonance (CMR) safe metal braided catheter design for interventional CMR at 1.5 T: freedom from radiofrequency induced heating and preserved mechanical performance. J Cardiovasc Magn Reson 21:16 10.1186/s12968-019-0526-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basar B, Rogers T, Ratnayaka K et al. (2015) Segmented nitinol guidewires with stiffness-matched connectors for cardiovascular magnetic resonance catheterization: preserved mechanical performance and freedom from heating. J Cardiovasc Magn Reson. 10.1186/s12968-015-0210-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonmez M, Saikus CE, Bell JA et al. (2012) MRI active guidewire with an embedded temperature probe and providing a distinct tip signal to enhance clinical safety. J Cardiovasc Magn Reson 14:38 10.1186/1532-429X-14-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell-Washburn AE, Rogers T, Stine AM, et al. (2018) Right heart catheterization using metallic guidewires and low SAR cardiovascular magnetic resonance fluoroscopy at 1.5 Tesla: first in human experience. J Cardiovasc Magn Reson. 10.1186/s12968-018-0458-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kholmovski EG, Coulombe N, Silvernagel J et al. (2016) Real-time MRI-guided cardiac cryo-ablation: a feasibility study. J Cardiovasc Electrophysiol 27:602–608. 10.1111/jce.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichter J, Kholmovski EG, Coulombe N et al. (2019) Real-time magnetic resonance imaging-guided cryoablation of the pulmonary veins with acute freeze-zone and chronic lesion assessment. EP Europace 21:154–162. 10.1093/europace/euy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elbes D, Magat J, Govari A et al. (2017) Magnetic resonance imaging-compatible circular mapping catheter: an in vivo feasibility and safety study. Europace 19:458–464. 10.1093/europace/euw006 [DOI] [PubMed] [Google Scholar]
- 34.Halabi M, Faranesh AZ, Schenke WH et al. (2013) Real-time cardiovascular magnetic resonance subxiphoid pericardial access and pericardiocentesis using off-the-shelf devices in swine. J Cardiovasc Magn Reson 15:61 10.1186/1532-429X-15-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arepally A, Karmarkar PV, Weiss C et al. (2005) Magnetic resonance image-guided trans-septal puncture in a swine heart. J Magn Reson Imaging 21:463–467. 10.1002/jmri.20262 [DOI] [PubMed] [Google Scholar]
- 36.Lossnitzer D, Seitz SA, Krautz B et al. (2015) Feasibility of real-time magnetic resonance imaging-guided endomyocardial biopsies: an in-vitro study. World J Cardiol 7:415–422. 10.4330/wjc.v7.i7.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonetti OP, Rizwan A (2017) Low-field cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 10:e005446 10.1161/CIRCIMAGING.117.005446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell-Washburn AE, Ramasawmy R, Restivo MC et al. (2019) Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 293:384–393. 10.1148/radiol.2019190452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandya B, Quail MA, Steeden JA et al. (2014) Real-time magnetic resonance assessment of septal curvature accurately tracks acute hemodynamic changes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging 7:706–713. 10.1161/CIRCIMAGING.113.001156 [DOI] [PubMed] [Google Scholar]
- 40.Aphrodite T, Krombach GA, Nils K et al. (2010) Magnetic resonance-guided cardiac interventions using magnetic resonance-compatible devices. Circ Cardiovasc Interv 3:585–592. 10.1161/CIRCINTERVENTIONS.110.957209 [DOI] [PubMed] [Google Scholar]
- 41.Abu Hazeem AA, Dori Y, Whitehead KK et al. (2014) X-ray magnetic resonance fusion modality may reduce radiation exposure and contrast dose in diagnostic cardiac catheterization of congenital heart disease. Catheter Cardiovasc Interv 84:795–800. 10.1002/ccd.25473 [DOI] [PubMed] [Google Scholar]
- 42.Glöckler M, Halbfaβ J, Koch A et al. (2013) Multimodality 3D-roadmap for cardiovascular interventions in congenital heart disease: a single-center, retrospective analysis of 78 cases. Catheter Cardiovasc Interv 82:436–442. 10.1002/ccd.24646 [DOI] [PubMed] [Google Scholar]
- 43.Grant EK, Kanter JP, Olivieri LJ et al. (2019) X-ray fused with MRI guidance of pre-selected transcatheter congenital heart disease interventions. Catheter Cardiovasc Interv. 10.1002/ccd.28324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant EK, Faranesh AZ, Cross RR et al. (2015) Image fusion guided device closure of left ventricle to right atrium shunt. Circulation 132:1366–1367. 10.1161/CIRCULATIONAHA.115.013724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratnayaka K, Raman VK, Faranesh AZ et al. (2009) Antegrade percutaneous closure of membranous ventricular septal defect using X-ray fused with magnetic resonance imaging. JACC Cardiovasc Interv 2:224–230. 10.1016/j.jcin.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rickers C, Jerosch-Herold M, Hu X et al. (2003) Magnetic resonance image-guided transcatheter closure of atrial septal defects. Circulation 107:132–138. 10.1161/01.cir.0000039343.95540.cf [DOI] [PubMed] [Google Scholar]
- 47.Cunningham KS, Veinot JP, Butany J (2006) An approach to endomyocardial biopsy interpretation. J Clin Pathol 59:121–129. 10.1136/jcp.2005.026443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratnayaka K, Saikus CE, Faranesh AZ et al. (2011) Closed-chest transthoracic magnetic resonance imaging-guided ventricular septal defect closure in swine. JACC Cardiovasc Interv 4:1326–1334. 10.1016/j.jcin.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krueger JJ, Ewert P, Yilmaz S et al. (2006) Magnetic resonance imaging-guided balloon angioplasty of coarctation of the aorta: a pilot study. Circulation 113:1093–1100. 10.1161/CIRCULATIONAHA.105.578112 [DOI] [PubMed] [Google Scholar]
- 50.Christopher P, Matthias G, Thomas G et al. (2013) Cavotricuspid isthmus ablation guided by real-time magnetic resonance imaging. Circ Arrhythm Electrophysiol 6:e7–e10. 10.1161/CIRCEP.112.973719 [DOI] [PubMed] [Google Scholar]
- 51.Paetsch I, Sommer P, Jahnke C et al. (2019) Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Eur Heart J Cardiovasc Imaging 20:147–156. 10.1093/ehjci/jey143 [DOI] [PubMed] [Google Scholar]
- 52.Dickfeld T, Tian J, Ahmad G et al. (2011) MRI-Guided ventricular tachycardia ablation: integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol 4:172–184. 10.1161/CIRCEP.110.958744 [DOI] [PubMed] [Google Scholar]
- 53.Grant EK, Berul CI, Cross RR et al. (2017) Acute cardiac MRI assessment of radiofrequency ablation lesions for pediatric ventricular arrhythmia: feasibility and clinical correlation. J Cardiovasc Electrophysiol 28:517–522. 10.1111/jce.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee RK, Whitaker J, Williams SE et al. (2018) Magnetic resonance imaging guidance for the optimization of ventricular tachycardia ablation. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol 20:1721–1732. 10.1093/europace/euy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers T, Mahapatra S, Kim S et al. (2016) Transcatheter myocardial needle chemoablation during real-time magnetic resonance imaging: a new approach to ablation therapy for rhythm disorders. Circ Arrhythm Electrophysiol. 10.1161/CIRCEP.115.003926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbash IM, Saikus CE, Faranesh AZ et al. (2011) Direct percutaneous left ventricular access and port closure: pre-clinical feasibility. JACC Cardiovasc Interv 4:1318–1325. 10.1016/j.jcin.2011.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers T, Ratnayaka K, Schenke WH et al. (2015) Fully percutaneous transthoracic left atrial entry and closure as a potential access route for transcatheter mitral valve interventions. Circ Cardiovasc Interv. 10.1161/CIRCINTERVENTIONS.114.002538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratnayaka K, Rogers T, Schenke WH et al. (2016) Magnetic resonance imaging-guided transcatheter cavopulmonary shunt. JACC Cardiovasc Interv 9:959–970. 10.1016/j.jcin.2016.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ratnayaka K, Moore JW, Rios R et al. (2017) First-in-human closed-chest transcatheter superior cavopulmonary anastomosis. J Am Coll Cardiol 70:745–752. 10.1016/j.jacc.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goreczny S, Moszura T, Dryzek P et al. (2017) Three-dimensional image fusion guidance of percutaneous pulmonary valve implantation to reduce radiation exposure and contrast dose: a comparison with traditional two-dimensional and three-dimensional rotational angiographic guidance. Neth Heart J Mon J Neth Soc Cardiol Neth Heart Found 25:91–99. 10.1007/s12471-016-0941-4 [DOI] [PMC free article] [PubMed] [Google Scholar]