Abstract
Understanding how the brain coordinates energy status with the motivation to eat is crucial to identify strategies to improve disordered body weight. The ventral tegmental area (VTA), known as the core of the mesolimbic system, is of particular interest in this regard because it controls the motivation to consume palatable, calorie-dense foods and to engage in volitional activity. The VTA is largely composed of dopamine (DA) neurons, but modulating these DA neurons has been alternately linked with promoting and suppressing feeding, suggesting heterogeneity in their function. Subsets of VTA DA neurons have recently been described based on their anatomical distribution, electrophysiological features, connectivity and molecular expression, but to date there are no signatures to categorize how DA neurons control feeding. Assessing the neuropeptide receptors expressed by VTA DA neurons may be useful in this regard, as many neuropeptides mediate anorexic or orexigenic responses. In particular, the lateral hypothalamic area (LHA) releases a wide variety of feeding-modulating neuropeptides to the VTA. Since VTA neurons intercept LHA neuropeptides known to either evoke or suppress feeding, expression of the cognate neuropeptide receptors within the VTA may point to VTA DA neuronal mechanisms to promote or suppress feeding, respectively. Here we review the role of the VTA in energy balance and the LHA neuropeptide signaling systems that act in the VTA, whose receptors might be used to classify how VTA DA neurons contribute to energy balance.
Keywords: feeding, lateral hypothalamic area, orexin/hypocretin, neurotensin, melanin-concentrating hormone
The Importance of Feeding and Energy Balance
Perhaps the most frequent behaviors that animals engage in are eating and drinking. The physiological processes required for life (e.g. thermogenesis, respiration, waste filtration, movement etc.) constantly consume onboard energy and water, so these resources must be replenished. Since energy (from food) and water can only be obtained via ingestion, survival depends on detecting the need for these resources and motivating the behavior to seek and consume them. Multiple neural circuits that contribute to feeding have been characterized, and serve to tune feeding to the environmental, interoceptive and emotional state of the organism1,2. Yet, despite the fundamental necessity of ingestion, the neural mechanisms by which the brain modulates the motivation to seek and consume food are yet to be fully elucidated. Understanding these processes is paramount for understanding basic biology and survival.
Feeding implicitly impacts “energy balance”, a term used to describe the linked relationship of energy intake and energy expenditure that has important ramifications for health. Energy intake refers to the calories obtained from ingested food and fluids, while energy expenditure refers to the energy that is consumed through basal metabolic rate, thermogenesis and physical activity3. Body weight is a “read-out” of energy balance, since it depends on the amount of calories consumed to calories burned. Changes in the extent of feeding or energy expended accordingly change body weight. There are periods when it is advantageous to be “imbalanced”. For example, during development, more calories must be consumed than expended to support growth, including increased body weight. In adulthood, energy balance should be more stable, such that day to day energy intake and output are relatively equal, and weight is maintained3. Even in this state, however, dynamic fluctuations of energy intake occur across the day as energy consumption creates need to replenish energy reserves. Energy deficiency registers as hunger and increases the drive to find and ingest food. Feeding restores satiety, which reduces the drive to eat while enabling for locomotor activity, thermogenesis and other energy dependent behaviors3. These actions are largely orchestrated by neurons in the hindbrain and mediobasal hypothalamus, collectively referred to as the homeostatic system. Thus, cues of energy status inform the body about food “need” and promote appropriate behaviors to resolve any energy imbalance.
Yet, feeding may also occur in the absence of need, and can threaten energy balance. For example, despite being satiated from a meal we might still eat dessert simply because it tastes good. Hence, external cues and/or the anticipated reward from food can potentiate excess caloric intake, and accordingly, weight gain. Overweight and obesity are energy balance disorders that develop due to elevated consumption of palatable and calorie-dense foods. Since overconsumption often occurs in combination with sedentary lifestyle and reduced energy expenditure, this leads to a “perfect storm” for weight gain4. More than one-third of US adults are obese, placing them at increased risk to develop chronic co-morbidities such as cardiovascular diseases and type-2 diabetes that diminish life quality and length5,6. Curbing so called “reward feeding” is therefore a major goal to treat and prevent the development of overweight and obesity. While there are some pharmacotherapies to support acute weight loss, they are minimally effective in the long-term to restrain feeding, and have yet to stem the rising disease incidence4,7,8. As a result, combined diet and exercise remain the most widely prescribed treatment for overweight and obesity. However, dieting increases appetitive drive that can spur overeating such that most individuals regain weight with time9–13. Since altered motivation and drive to eat negatively impacts health it is vital to understand how the brain coordinates feeding behavior, to pinpoint how and why it goes awry in disease states.
The Ventral Tegmental Area Modulates Feeding
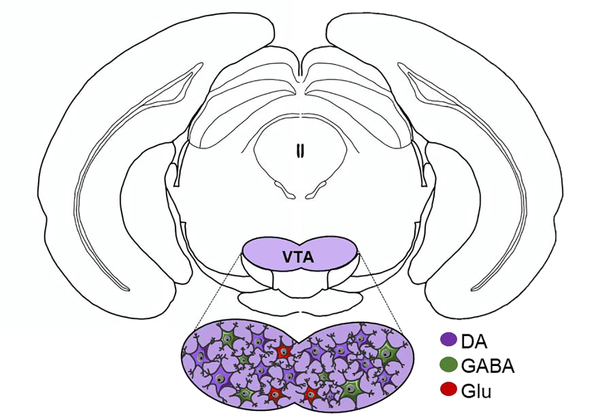
The ventral tegmental area (VTA) is well known to modify intake of pharmacological and natural rewards, including food. While the neural mechanisms of this process remain incompletely understood, the VTA appears to coordinate energy status and external cues so as to influence feeding behavior. The VTA is located near the base of the midbrain and it contains dopamine (DA) neurons (~60%−65%), GABA neurons (~30%−35%) and a small subset of glutamate neurons (~2%−3%)14,15. Given these proportions, it is of little surprise that most research has focused on the role of the DA neurons within the VTA and their roles in feeding behaviors. However, recent work confirms that some subpopulations of VTA GABA neurons16–18 and VTA glutamate neurons19–21 can modify behaviors independently of DA neurons, some of which are relevant to feeding. VTA glutamate neurons are located primarily in the rostral and medial portions of the VTA, and their activation has been shown to drive conditioned place preference, reinforcement of instrumental behavior and aversive conditioning in mice19–21. GABA expressing neurons also have a role in aversion17,22, but, on the other hand, are distributed throughout the VTA16,23,24 and have been shown to suppress the activity and excitability of neighboring DA neurons16.
Owing to their abundance, however, VTA DA neurons are the most studied VTA population, and are involved in an array of motivated behaviors22,25–27, from positive and negative reinforcement, decision making, working memory, stimulus salience and aversion28–33. VTA DA neurons also respond to cues that predict rewards34 and DA is a key substrate in the incentive and reinforcing aspects of food intake35–39, locomotor activity and body weight40. This participation of VTA DA neurons in so many actions suggests a degree of functional heterogeneity, and has prompted speculation on whether there are subsets of DA neurons to mediate specific behaviors – including, perhaps, specific feeding behaviors. In potential support of this idea, experimental activation of VTA DA neurons has yielded a “mixed bag” of feeding responses, from enhancement to suppression41–44. One possible explanation may be the methods used to target and activate the VTA DA neurons, which at least in some cases only activated a portion of all VTA DA neurons41,42. It is possible, therefore, that these experiments manipulated subsets of VTA DA neurons that differ in how they control feeding. Similarly, while not examining feeding specifically, recent work demonstrates that modulating the activity of subsets of VTA DA neurons differentially modifies behavioral reinforcement44. Moreover, manipulation of specific subsets does not always produce the same behavior as manipulation of all VTA DA neurons44. In combination, these findings support examination of methods to classify VTA DA neurons, to identify whether there are subsets with different roles in regulating feeding.
There has been a recent explosion of work directed toward identifying subsets of VTA DA neurons. They have been geographically classified by their distribution across the VTA; DA neurons in the ventral VTA for example, are excited by noxious foot shocks, in contrast to dorsal VTA DA neurons, which are inhibited30,45. VTA DA neurons can also be differentiated according to their electrophysiological firing properties46,47. As of yet, however, there is no consensus on specific electrophysiological or anatomical signatures that predict how VTA DA neurons modify feeding. However, VTA DA neurons can also be described by their anatomical inputs and projections44,48,49, and the latter may provide insight into functional subsets and feeding control. The majority of VTA DA neurons project to the nucleus accumbens (NAc), where DA release has been shown to control the “wanting” of pharmacological and natural rewards and the goal-directed behaviors to obtain them (for review on VTA DA innervating NAc and its influence in motivation see Salamone and Correa, 2012)33. Other VTA DA neurons project to the prefrontal cortex, amygdala, hippocampus and other brain regions that alter top-down control, anxiety and learning, all of which can impact the extent of feeding50. Given this range in projection targets, it seems plausible that the differences in VTA DA neuronal connectivity might track with separate functionality, and possibly differentiable molecular signatures. Recent work supports that there is variable gene expression across VTA DA neurons, and the existence of subpopulations with differing expression of transcription factors, channels, DA related genes and receptors for neurotransmitters, hormones and neuropeptides51–53.
Of these, peptide receptors have garnered considerable interest to parse VTA DA neurons that might influence feeding. Peptide signals can reach the VTA via two routes: via the circulation (as peptide hormones) and through being released from neurons (as neuropeptides). For example, circulating cues of energy status, such as the peptide hormones insulin, leptin and ghrelin act directly upon hypothalamic neurons as well as on VTA neurons54,55. Expression of these hormone receptors on VTA DA neurons can identify subsets of neurons that likely contribute to anorectic or orexigenic responses. Yet, hypothalamic regions have more rapid access to circulating cues than the VTA, due to being adjacent to the “leaky” median eminence that permissively allows circulating factors access to the brain. By contrast, the VTA lies deep within the brain and far from the median eminence. Hence, the VTA is capable of directly responding to circulating peptide cues of energy status, but is not considered to be the “first-responder” to them that can rapidly inform the direction of feeding.
The VTA is also modulated by neurons in other brain areas that release neurotransmitters and neuropeptides to VTA neurons. In order for VTA neurons to intercept these released messages they must express the cognate receptors. For example, VTA neurons express classical neurotransmitter receptors, and receive excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmitter input from various areas in the brain (for a review on specific glutamatergic or GABAergic inputs onto specific neuronal populations in the VTA see Morales and Margolis, 2017)46. GABA and glutamate are important modulators of VTA DA neuronal activity but are also critical in regulating a wide array of physiology and aren’t solely linked with changing feeding. By contrast, some neuropeptides are directly implicated in modulating feeding, may be anorectic or orexigenic in nature, and mediate these actions via their cognate peptide receptors. Indeed, reports have begun to emerge that classify subsets of VTA DA neurons via their expression of neuropeptide receptors, and at least some of these track with specific projection targets and control of behavioral reinforcement44,56. These early studies have only categorized neuropeptide-receptor expressing neurons without assessing the role of the neuropeptide signals on VTA DA subsets nor from where the neuropeptides originated. Defining the neurons that release feeding-modulating neuropeptides to the VTA, as well as which VTA DA neurons respond to them, holds promise to reveal the mechanisms underlying feeding.
The Lateral Hypothalamic Area Engages the VTA to Modify Feeding
The VTA receives neuronal projections from various areas in the brain, but the three main input contributors are the ventral pallidum, dorsal raphe nucleus and hypothalamus57,58. Of all of the subregions of the hypothalamus, the VTA receives its densest inputs from the lateral hypothalamic area (LHA)57, a region well-studied for its role in energy balance and neuropeptide expressing neurons59. The LHA is viewed as a coordinating center, since it receives afferent input concerning internal status (e.g. energy, osmolality, temperature, pH, etc.) and sends projections to brain sites capable of modifying behavior to address any homeostatic deviations (For a review of the LHA control of energy balance, see Kurt, Woodworth and Leinninger, 2017). While the LHA projects widely throughout the brain to orchestrate homeostasis, the LHA→VTA connection is specifically linked with coordinating energy sensing with goal-directed feeding behavior. Indeed, lesion of either the LHA or the VTA cause similar deficits in feeding, while stimulation of either site can incite ingestion60–66. Moreover, LHA neurons directly and indirectly receive cues of energy status, and then accordingly modulate the activity of target neurons (including those in the VTA) to modify feeding67. Thus, the LHA works in concert with the VTA to coordinate energy sensing and ingestion50,57,58.
The nature of LHA communication with the VTA is complex, however, since the LHA exhibits a high degree of cellular heterogeneity. There are multiple molecularly distinct neuronal subpopulations within the LHA that are implicated in the control of diverse physiology, including sleep/arousal, reward, food intake, liquid intake, locomotor activity, stress, and response to inflammation68. LHA neurons can be molecularly classified by their transmitters, and typically contain one classical neurotransmitter (either excitatory glutamate or inhibitory GABA) along with several neuropeptides69–73. Both of these types of signals can be released to target neurons in the VTA, and hence LHA classical transmitters and neuropeptides can impact VTA-mediated behaviors55. This leads to enormous complexity in assessing how any “LHA neuron” that projects to the VTA actually modulates feeding, as it could simultaneously release a mix of signals that mediate different effects. Intriguingly, at least some projection-specified subsets of VTA DA neurons respond differently to the same LHA-derived signals, though the mechanism of how this occurs is yet unclear74. One possibility is that subsets of VTA DA neurons differentially express receptors for classical transmitters or neuropeptides, or express them in different proportions that influences what message is received. Since neurotransmitter and neuropeptide signals differ in important ways, including their mechanism and site of release, it is important to consider these features in regard to how LHA signals modulate VTA neurons.
Classical neurotransmitters are released at the active zone of the axon terminal, then diffuse across the synaptic cleft (a magnitude of nanometers) to bind to ionotropic receptors on the post-synaptic neuron. Classical transmitter binding to these receptors mediates rapid changes in ionic current and activity of the postsynaptic neurons. Signaling is terminated by enzymatic degradation of the classical transmitter or active reuptake, either of which reduce the amount of transmitter in the synaptic cleft available to bind to postsynaptic receptors. LHA classical neurotransmitter input to the VTA can support a variety of behaviors, depending on context. For example, experimental activation of all LHA GABAergic neurons that project to the VTA promotes feeding73,75. However, LHA GABA neurons show heterogeneity in their neuronal responses during food seeking and consumption76, suggesting that not every GABA neuron is functionally the same. Indeed, it is now clear that there are multiple subpopulations of GABA neurons within the LHA that differ in their co-expressed neuropeptide content77. Moreover, subsets of LHA GABA neurons appear to be activated by distinct peripheral stimuli78. Thus, while many LHA neurons that project to the VTA release GABA, it is physiologically unlikely that all LHA GABA neurons are activated at the same time, or that they all exert the same effects within the VTA. The LHA also contains glutamatergic neurons, some of which project to the VTA and can stimulate feeding and arousal68,79–81. However, many LHA glutamatergic neurons project to the lateral habenula, and their stimulation suppresses food intake82. Thus, the effect that an LHA neuron will have on the VTA or feeding behavior cannot be predicted by its classical transmitter content alone. Hence, the expression of GABA or glutamate receptors are not likely to distinguish VTA DA neurons and their control of feeding.
The neuropeptide content of LHA neurons is a more reliable predictor of how they engage the VTA and modulate feeding. Indeed, LHA subpopulations can be differentiated by their neuropeptide expression, which hints that they exert distinct, neuropeptide-determined effects on target neurons expressing the cognate receptors. For this reason, LHA neurons are typically referred to by their neuropeptide content, such as “Orexin/Hypocretin neurons (OX/HCRT)” or “Melanin Concentrating Hormone (MCH)” neurons. Although classical neurotransmitters and neuropeptides can be released by the same neurons, they are fundamentally different signaling molecules. (For a review on neuropeptides and their involvement in modulating the central nervous system, see van del Pol, 2012.) Briefly, neuropeptides are short sequences of amino acids (3–36) that are synthesized in the soma83,84. They are packaged into dense core granules for release, which can occur at any part of the membrane and is not restricted to the active zone (as is the case for neurotransmitters)83. In addition, neuropeptides can diffuse much further than classical transmitters, in the magnitude of microns. Neuropeptides are also thought to have a long extracellular half-life, and so can have prolonged actions on target neurons83. Generally, neuropeptides bind to G protein-coupled receptors (GPCRs) that can be expressed on soma, dendrites, and axon terminals, depending on receptor subtype. Neuropeptide binding to receptors modulates intracellular signaling pathways and phosphorylation of several target proteins that can lead to long-term changes in gene transcription and neuronal function83. They can also impact the likelihood of a neuron to be activated in response to classical transmitters83. Thus, neuropeptides are considered neuromodulators because their release can broadly affect many neurons in the vicinity and invoke lasting changes in function and activity. However, there are numerous neuropeptides, and each can differentially regulate distinct populations of neurons and behaviors. For example, LHA neuropeptides such as MCH, OX/HCRT and galanin promote feeding, whilst the neuropeptide neurotensin (Nts) suppresses feeding59. This range of neuropeptide-regulation indicates that they utilize different mechanisms to modify feeding. Because VTA neurons receive and can respond to these neuropeptides, it is possible that the distribution of neuropeptide receptors on VTA neurons could be used to distinguish VTA populations that differentially modify feeding behavior.
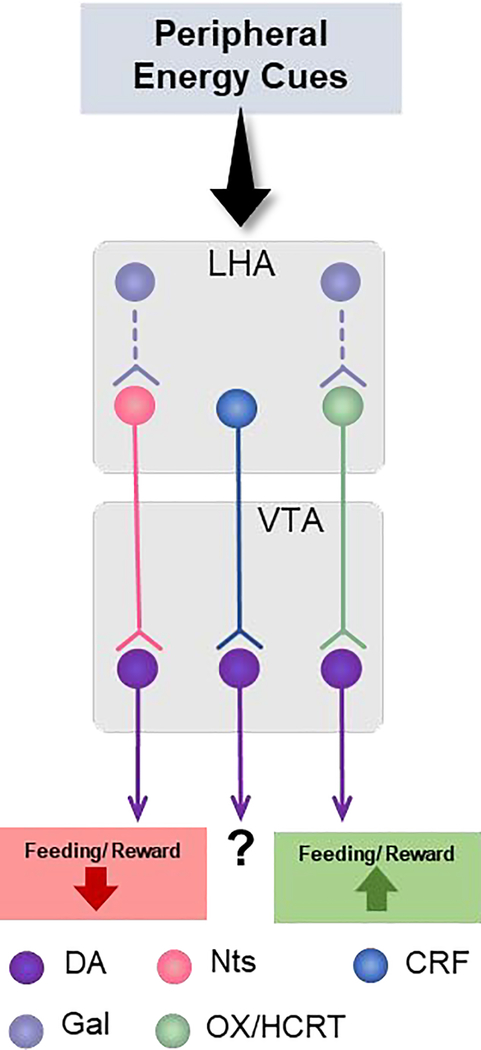
To be clear, neuropeptides are not just limited to the LHA, as they are expressed in many neuronal populations throughout the brain83. Moreover, neuropeptides throughout the brain have been implicated in disorders of excessive consumption, including drug and alcohol abuse and eating disorders84, and at least some of their effects are mediated via the VTA. However, it is notable that many LHA neuropeptide-expressing neurons have been shown to modulate behavior via directly engaging the VTA, including feeding, drinking and physical activity. The varied neuropeptide release from LHA neurons to the VTA indicates a potential mechanism by which target VTA DA neurons might differ: in their expression of the cognate neuropeptide receptors. Some subsets of VTA DA neurons have already been classified by their expression of a neuropeptide receptor, and some of these are receptors for neuropeptide ligands released from the LHA44,56. However, it is unlikely that subpopulations of VTA DA neurons are defined by a single neuropeptide receptor. Indeed, VTA DA neurons almost certainly receive multiple, simultaneous peptidergic inputs from the LHA and/or other areas, via which they coordinate dopaminergic output in target regions and modulation of behaivor55. It is possible that some VTA DA neurons might express a suite of receptors that are indicative of how they modify feeding behavior. Thus, deciphering the neuropeptide signaling systems that mediate hypothalamic-VTA interactions could provide insight into how the brain coordinates energy need and ingestive behavior. Below, and summarized in Figure 2, we review the evidence for LHA neuropeptide regulation of the VTA, given the known importance of the LHA→VTA connection to feeding behavior. Specifically, we address which of the associated neuropeptide receptors might discriminate VTA DA subsets with unique contributions to feeding and body weight.
Figure 2.
Summary of LHA neuropeptidergic input to VTA DA neurons. VTA DA neurons are directly innervated by neuropeptide-containing neurons from the LHA that can decrease or promote feeding and reward behaviors. From the LHA, projecting neurons include neurotensin (Nts), corticortropin releasing factor (CRF) and orexin/hypocretin (OX/HCRT). Although CRF neurons also project to VTA DA neurons, their contribution to feeding/reward behavior is still not clear. Galanin (Gal) does not directly innervate DA neurons but rather projects to OX/HCRT neurons within the LHA that may project to the VTA.
Lateral Hypothalamic Area Neuropeptides that Modulate VTA DA Neurons
Orexin/Hypocretin
LHA neurons expressing the neuropeptides orexin/hypocretin (OX/HCRT) project widely throughout the brain, including to VTA DA neurons85. Interestingly, OX/HCRT is not a stimulator of food intake per se, but a stimulator of arousal, which in turn promotes feeding67,86–88. OX/HCRT promotes intake of natural and drug rewards via actions on VTA neurons70,89–93. Indeed, OX/HCRT neurons are activated during cue-induced feeding, and they in turn activate VTA DA neurons and promote DA release into the NAc and PFC70,89,90. OX/HCRT signaling in the VTA promotes neuronal activity by enhancing excitation and suppressing inhibition of DA neurons70,94,95. This promotes DA release necessary to drive reward seeking90–93. Thus, OX/HCRT acts via modulating VTA DA neurons to promote the ingestion of highly salient substances, including goal-directed responding for palatable foods. These responses are attenuated by pharmacologically antagonizing OX/HCRT receptors, indicating that the OX neuropeptide is a key signal form OX/HCRT neurons that is necessary to promote feeding (Figure 2). There are two identified receptors for OX/HCRT, the G-protein coupled receptors, OX Receptor-1 (OXR-1) and OX receptor-2 (OXR-2). These receptors are expressed throughout the brain, including the VTA, where they are typically coupled to Gq proteins96–98. Intriguingly, OX/HCRT differentially regulates firing of projection-specified populations of VTA DA neurons, and notably activates those projecting to the NAc but not those projecting to the amygdala74. This difference could be due, in part, to differential expression of OX/HCRT receptors on VTA neurons projecting to the NAc vs. the amygdala, though this has yet to be shown definitively. Going forward, it will be instructive to determine if OX/HCRT receptor expression in the VTA can be used to distinguish DA subpopulations and their functions.
Neurotensin
Many neurons expressing the neuropeptide neurotensin (Nts) provide input to the VTA, with the largest populations originating from the medial preoptic area, NAc and the LHA99. Interestingly, at least some LHA Nts-expressing neurons are regulated by leptin; approximately 30% of Nts neurons co-express the long form of the leptin receptor (LepRb)67. Given that leptin itself suppresses feeding, leptin acting on LHA Nts-containing neurons might promote anorexic effects, perhaps via Nts itself. While this has yet to explored explicitly, activating LHA Nts-expressing neurons does increase Nts release into the VTA100,101, and pharmacologic Nts treatment into the VTA has been shown to reduce feeding102,103. Moreover, pharmacologic administration of Nts in the VTA potentiates excitatory synaptic transmission and increases the firing rate of DA neurons, which in turn promotes the release of DA in the NAc104,105. This leads to feeding restriction, increased locomotor activity and ultimately weight loss in obese rodents106–108. In combination, these data confirm that Nts modulates VTA DA neurons and can exert anorectic effects. It is notable that both Nts and OX/HCRT are reported to activate VTA DA neurons, but exert opposing effects on feeding. One possible hypothesis to explain this conundrum is that these neuropeptides might engage separate subsets of VTA DA neurons that differentially modify feeding. If true, expression of receptors for OX/HCRT vs. Nts could be a means of discerning which VTA neurons respond to these neuropeptides, and whether they comprise separate subpopulations. This hypothesis has yet to be directly explored. However, comparing the distributions of OX/HCRT and Nts receptors in the VTA will be vital to assess how each of these neuropeptides can promote activation of VTA DA neurons but yield completely different control of behavior.
The VTA can directly respond to Nts since it contains both of the signaling forms of the neurotensin receptors, neurotensin receptor −1 and −2 (NtsR1 and NtsR2)56,109,110. Intriguingly, Nts action via NtsR2 is not a direct modulator of VTA DA neurons, since NtsR2 is almost exclusively expressed in VTA astrocytes111. By contrast, NtsR1 is the predominant receptor isoform expressed by VTA DA neurons56, and is vital for Nts-mediated DA release into the NAc101. Moreover, only a subset of VTA DA neurons express NtsR1, and these neurons preferentially project to the NAc and not to the PFC56. Thus, NtsR1 represents a molecular marker of primarily mesolimbic, not mesocortical VTA DA neurons, but the specific role of these VTA NtsR1 neurons remains incompletely understood. Loss of NtsR1 increases feeding but also further elevates energy expenditure (primarily through an increase in physical activity), and this energy imbalance promotes leanness111. Furthermore, intact NtsR1 expression is required for LHA Nts-LepRb neurons to restrain feeding, indicating the functional integration of leptin and Nts/NtsR-1 action72. At least some of these feeding restraint effects are mediated via the LHA Nts neurons that project densely to the VTA100,101, and depends on NtsR1112. Taken together, these data indicate that Nts input to the VTA can promote weight loss behaviors, at least in part, due to regulation of NtsR1-expressing DA neurons. It yet remains unclear how the VTA NtsR1 neurons specifically mediate anorectic signals, or if they are sufficient to promote weight loss behaviors, which warrants examination in the future.
Galanin
LHA galanin neurons partially overlap with LepRb-expressing neurons or Nts neurons113–116. Since some LepRb and Nts neurons project to the VTA it was long reasoned that these neurons release the neuropeptide galanin into the VTA as well. Intriguingly, recent evidence suggests that LHA galanin neurons are one of the few LHA populations that do not engage the VTA directly, and instead project locally within the LHA to regulate OX/HCRT neurons that express the galanin receptor. LHA galanin neurons do impact feeding via this local circuit, specifically modifying nutrient preference (sucrose > fat)114. Mice that lack the LepRb from their LHA galanin expressing neurons show an increase in OX/HCRT neuronal activation114, which suggests that LHA galanin-LepRb expressing neurons inhibit OX/HCRT neurons under baseline conditions. Importantly, it remains possible that LHA galanin neurons can indirectly impact activation of VTA DA neurons via modulating OX/HCRT neurons that project to the VTA. On the other hand, activation of LHA Nts neurons, some of which co-express galanin115, can modestly suppress feeding100. Since many LHA Nts neurons project to the VTA99, these data suggest that there must be a population of Nts-only neurons in the LHA that project to the VTA, in addition to an LHA subset that co-expresses Nts and galanin and projects locally. It is therefore possible that the LHA Nts-only neurons that project to the VTA mediate Nts-mediated anorectic actions via the NtsR1 expressed on VTA NtsR1-DA neurons.
Melanin-Concentrating Hormone
Melanin-concentrating Hormone (MCH) is a defining neuropeptide of the LHA, as its expression is restricted to this brain region. While some studies have described MCH fibers and mRNA from MCH receptors in the VTA, others show low to no expression of MCH receptors in the VTA96,117–120. Although the LHA as a whole provides dense input to the VTA, the widely accepted view is that MCH neurons are a notable exception, and do not significantly project to or directly regulate the VTA118. Nevertheless, MCH has well-established connections with other sites that contribute to changing behavior (cerebral cortex, LHA, amygdala, and NAc121–124), where it has been implicated in orexigenic intake of foods, as well as in cocaine- and amphetamine-induced reward and reward learning125,126. Thus, while MCH is an LHA neuropeptide with important roles in modulating feeding, it does not exert these actions via direct engagement of VTA DA neurons.
Corticotropin Releasing Factor
Corticotropin releasing factor (CRF) is widely expressed throughout the brain, including regions of the forebrain, the VTA and within a subset of LHA neurons that are activated by dehydration anorexia127–129. In addition, pharmacologic and endogenous CRF suppress feeding in both lean and obese mice130. CRF signals through the CRF receptor-1 and −2 (CRFR1, CRFR2). Not much is known about CRFR2 signaling, but the CRF/CRFR1 system has been repeatedly associated with stress and addiction131,132. Accordingly, CRFR1 is expressed in DA neurons of the VTA and the adjacent substantia nigra pars compacta (SNc)133. A recent study revealed that VTA CRFR1 expressing DA neurons primarily project to the NAc core, rather than the NAc shell, and their activation is important for associating stimuli and behaviors more so than augmenting the wanting of those stimuli44. While feeding was not specifically studied, it may be that VTA CRFR1 neurons play a role in learned feeding behaviors, and this will merit further attention. Going forward it will be important to investigate if LHA-derived CRF signaling modulates feeding via the VTA CRFR1 neurons, and what the role of the CRF neuropeptide is in this process vs. other signals released from LHA CRF neurons.
Summary and Implications
VTA DA neurons are now recognized to be heterogenous via multiple criteria, and this opens the door to investigating whether subsets of VTA DA neurons may mediate different aspects of feeding behavior. Given the significant diversity in the types of signals that access the VTA, it seems possible that VTA DA neurons may accordingly tune feeding behavior in response to them. Going forward, “mapping” the populations of VTA DA neurons via their neuropeptide receptor expression could prove instructive, as it might identify subsets that contribute to orexigenic or feeding restraint behaviors. Given that LHA neurons project to, and act in concert with VTA DA neurons to modify behavior, assessing VTA DA neurons that express receptors for LHA-released signals might suggest which neurons modulate feeding, and how they do so. However, it is likely that subsets of VTA DA neurons will not express a single neuropeptide receptor subtype, but in fact, a suite of neuropeptide receptors. Such a system could enable VTA DA neurons to flexibly respond to different cues and from different inputs, not all of which may be active at the same time. Thus, it remains possible that there could be subsets of DA neurons that primarily mediate orexigenic responses vs. those that constrain feeding or other rewards and mediate these responses via a collection of expressed neuropeptide receptors. There remains much to learn, however, regarding the mechanisms by which neuropeptides modulate the VTA and feeding. For example, neuropeptides from the LHA (and elsewhere) may change the balance of tonic vs. burst-firing of DA neurons, the latter of which is associated with changing goal-mediated behavior. Thus, in the future it will be important to characterize how specific neuropeptides, or combinations of them, impact the firing pattern of DA neurons. It is also likely that neuropeptides may modulate other VTA neuronal populations (e.g. GABA or glutamate containing neurons) or glial populations, which can in turn indirectly modify the activity of local DA neurons or other external targets. While we have focused on how LHA neuropeptides can directly engage DA neurons that express their cognate receptors, neuropeptide modulation of other VTA cells could have powerful effects on feeding behavior and warrant further study. In any case, going forward it will be important to determine if neuropeptide receptors can be used to molecularly distinguish the projection and functionally specified populations of DA neurons, or other VTA cell types that impact feeding. These studies would not only advance understanding of the basic biology regulating energy balance but could also suggest how to design molecular-based tools to dissect the function of specific VTA cell subsets.
Figure 1.
Neuronal composition of the VTA illustrated on a coronal section of mouse brain. The VTA is primarily composed of DA neurons, followed by GABA and glutamate (Glu)-containing neurons.
Highlights.
Ventral tegmental area (VTA) dopamine neurons modulate feeding behavior
VTA dopamine neurons are heterogeneous
Lateral hypothalamic area neurons release neuropeptides to the VTA to alter feeding
Neuropeptide receptors could indicate VTA dopamine neurons that impact feeding
Acknowledgements
Krystal Santiago-Colon was supported by the NIH-NINDS Bridge to the PhD in Neuroscience (BPNP)- ENDURE Program (R25-NS090989). This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under awards to Patricia Perez-Bonilla (F31-DK121373) and Gina Leinninger (R01-DK103808).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sohn JW, Elmquist JK & Williams KW Neuronal circuits that regulate feeding behavior and metabolism. Trends in Neurosciences vol. 36 504–512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sternson SM & Eiselt A-K Three Pillars for the Neural Control of Appetite. Annu. Rev. Physiol. 79, 401–423 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Hall KD et al. Energy balance and its components: Implications for body weight regulation. American Journal of Clinical Nutrition (2012) doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obesity and Overweight Fact Sheet. World Health Organization www.who.int/mediacentre/factsheets/fs311/en/ (2017).
- 5.Apovian CM Obesity: definition, comorbidities, causes, and burden. The American journal of managed care vol. 22 s176–s185 (2016). [PubMed] [Google Scholar]
- 6.Kitahara CM et al. Association between Class III Obesity (BMI of 40–59 kg/m 2 ) and Mortality: A Pooled Analysis of 20 Prospective Studies. PLoS Med. 11, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadde KM, Martin CK, Berthoud HR & Heymsfield SB Obesity: Pathophysiology and Management. Journal of the American College of Cardiology vol. 71 69–84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessesen DH & Van Gaal LF Progress and challenges in anti-obesity pharmacotherapy. The Lancet Diabetes and Endocrinology vol. 6 237–248 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Polidori D, Sanghvi A, Seeley RJ & Hall KD How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity 24, 2289–2295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grodstein F et al. Three-year follow-up of participants in a commercial weight loss program. Can you keep it off? Arch. Intern. Med. 156, 1302–6 (1996). [PubMed] [Google Scholar]
- 11.Mann T et al. Medicare’s search for effective obesity treatments: diets are not the answer. Am. Psychol. 62, 220–233 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Pietiläinen KH, Saarni SE, Kaprio J & Rissanen A Does dieting make you fat A twin study. Int. J. Obes. 36, 456–464 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Jacquet J, Montani JP & Schutz Y How dieting makes the lean fatter: From a perspective of body composition autoregulation through adipostats and proteinstats awaiting discovery. Obesity Reviews vol. 16 25–35 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Nair-Roberts RG et al. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience (2008) doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungless MA & Grace AA Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends in Neurosciences (2012) doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Zessen R, Phillips JL, Budygin EA & Stuber GD Activation of VTA GABA Neurons Disrupts Reward Consumption. Neuron (2012) doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan KR et al. GABA Neurons of the VTA Drive Conditioned Place Aversion. Neuron doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamatakis AM et al. A Unique Population of Ventral Tegmental Area Neurons Inhibits the Lateral Habenula to Promote Reward. Neuron (2013) doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Root DH, Mejias-Aponte CA, Qi J & Morales M Role of glutamatergic projections from ventral tegmental area to lateral Habenula in aversive conditioning. J. Neurosci. (2014) doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HL, Qi J, Zhang S, Wang HL & Morales M Rewarding effects of optical stimulation of ventral tegmental area glutamatergic neurons. J. Neurosci. (2015) doi: 10.1523/JNEUROSCI.3428-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi J et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. (2016) doi: 10.1038/nn.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen JY, Haesler S, Vong L, Lowell BB & Uchida N Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature (2012) doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson VG & Nestler EJ Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse (2007) doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- 24.Margolis EB, Toy B, Himmels P, Morales M & Fields HL Identification of rat ventral tegmental area GABAergic neurons. PLoS One (2012) doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammel S, Lim BK, Ran C, Huang KW & Betley MJ Input-specific control of reward and aversion in the ventral tegmental area. Cell 491, 212–217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard JM, Castro DC, DiFeliceantonio AG, Robinson MJF & Berridge KC Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews (2013) doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faure A, Reynolds SM, Richard JM & Berridge KC Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J. Neurosci. (2008) doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B & Gabrieli JDE Reward-Motivated Learning: Mesolimbic Activation Precedes Memory Formation. Neuron (2006) doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Berridge KC The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology vol. 191 391–431 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Brischoux F, Chakraborty S, Brierley DI, U. M., Brischoux F, Chakraborty S, Brierley DI & Ungless MA Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U. S. A. 106, 4894–4899 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz W Getting formal with dopamine and reward. Neuron (2002) doi: 10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 32.Bromberg-Martin ES, Matsumoto M & Hikosaka O Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron (2010) doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salamone JD & Correa MM The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron vol. 76 470–485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz W, Dayan P & Montague PR A neural substrate of prediction and reward. Science (80-. ). (1997) doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 35.Salamone JD, Correa M, Mingote S & Weber SM Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. Journal of Pharmacology and Experimental Therapeutics (2003) doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- 36.Wise RA Role of brain dopamine in food reward and reinforcement. Philosophical Transactions of the Royal Society B: Biological Sciences (2006) doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fields HL, Hjelmstad GO, Margolis EB & Nicola SM Ventral Tegmental Area Neurons in Learned Appetitive Behavior and Positive Reinforcement. Annu. Rev. Neurosci. (2007) doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 38.Palmiter RD Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. (2007) doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Narayanan NS, Guarnieri DJ & DiLeone RJ Metabolic hormones, dopamine circuits, and feeding. Frontiers in Neuroendocrinology (2010) doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q-Y & Palmiter RD Dopamine-Deficient Mice Are Severely Hypoactive, Adipsic, and Aphagic. Cell 83, 1197–1209 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Boekhoudt L et al. Enhancing excitability of dopamine neurons promotes motivational behaviour through increased action initiation. Eur. Neuropsychopharmacol. (2018) doi: 10.1016/j.euroneuro.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Boekhoudt L et al. Does activation of midbrain dopamine neurons promote or reduce feeding? Int. J. Obes (2017) doi: 10.1038/ijo.2017.74. [DOI] [PubMed] [Google Scholar]
- 43.Adamantidis AR et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 31, 10829–10835 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heymann G et al. Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement. Neuron (2020) doi: 10.1016/j.neuron.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto M & Hikosaka O Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales M & Margolis EB Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nature Reviews Neuroscience vol. 18 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Margolis EB, Lock H, Hjelmstad GO & Fields HL The ventral tegmental area revisited: Is there an electrophysiological marker for dopaminergic neurons? J. Physiol. (2006) doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lammel S, Ion DI, Roeper J & Malenka RC Projection-Specific Modulation of Dopamine Neuron Synapses by Aversive and Rewarding Stimuli. Neuron 70, 855–862 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lammel S et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beier KT et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell (2015) doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poulin JF et al. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 9, 930–943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderegg A, Poulin JF & Awatramani R Molecular heterogeneity of midbrain dopaminergic neurons - Moving toward single cell resolution. FEBS Letters vol. 589 3714–3726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poulin JF et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu S & Borgland SL Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience vol. 289 19–42 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Godfrey N & Borgland SL Diversity in the lateral hypothalamic input to the ventral tegmental area. Neuropharmacology (2019) doi: 10.1016/j.neuropharm.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Woodworth HL, Perez-Bonilla PA, Beekly BGBG, Lewis TJTJ & Leinninger GMGM Identification of neurotensin receptor expressing cells in the ventral tegmental area across the lifespan. eNeuro 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N Whole-Brain Mapping of Direct Inputs to Midbrain Dopamine Neurons. Neuron (2012) doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Faget L et al. Afferent Inputs to Neurotransmitter-Defined Cell Types in the Ventral Tegmental Area. Cell Rep. (2016) doi: 10.1016/j.celrep.2016.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurt G, Woodworth HL & Leinninger GM Lateral Hypothalamic Control of Energy Balance. Colloq. Ser. Integr. Syst. Physiol. From Mol. to Funct. (2017) doi: 10.4199/c00159ed1v01y201711isp079. [DOI] [Google Scholar]
- 60.Teitelbaum P & Epstein AN The lateral hypothalamic syndrome: Recovery of feeding and drinking after lateral hypothalamic lesions. Psychol. Rev. (1962) doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 61.Hoebel BG & Teitelbaum P Hypothalamic control of feeding and self-stimulation. Science (80-. ) (1962) doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 62.Morgane PJ Distinct ‘feeding’ and ‘hunger motivating’ systems in the lateral hypothalamus of the rat. Science (80-. ). (1961) doi: 10.1126/science.133.3456.887. [DOI] [PubMed] [Google Scholar]
- 63.Ungerstedt U Adipsia and Aphagia after 6-Hydroxydopamine Induced Degeneration of the Nigro-striatal Dopamine System. Acta Physiol. Scand. (1971) doi: 10.1111/j.1365-201X.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 64.Marshall JF & Teitelbaum P A comparison of the eating in response to hypothermic and glucoprivic challenges after nigral 6-hydroxydopamine and lateral hypothalamic electrolytic lesions in rats. Brain Res. (1973) doi: 10.1016/0006-8993(73)90507-6. [DOI] [PubMed] [Google Scholar]
- 65.Szczypka MS et al. Feeding behavior in dopamine-deficient mice. Proc. Natl. Acad. Sci. U. S. A. (1999) doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szczypka MS, Rainey MA & Palmiter RD Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat. Genet. (2000) doi: 10.1038/75484. [DOI] [PubMed] [Google Scholar]
- 67.Brown JA, Woodworth HL & Leinninger GM To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front. Syst. Neurosci. (2015) doi: 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonnavion P, Mickelsen LE, Fujita A, de Lecea L & Jackson AC Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. (2016) doi: 10.1113/JP271946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris GC, Wimmer M & Aston-Jones G A role for lateral hypothalamic orexin neurons in reward seeking. Nature (2005) doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 70.Borgland SL, Taha SA, Sarti F, Fields HL & Bonci A Orexin a in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron (2006) doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 71.Kempadoo KA et al. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J. Neurosci. (2013) doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Opland D et al. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol. Metab. 2, 423–434 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nieh EH et al. Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron 1–13 (2016) doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baimel C, Lau BK, Qiao M & Borgland SL Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep. (2017) doi: 10.1016/j.celrep.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 75.Jennings JH, Rizzi G, Stamatakis AM, Ung RL & Stuber GD The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science (80-. ). doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jennings JH et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mickelsen LE et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown JA et al. Distinct Subsets of Lateral Hypothalamic Neurotensin Neurons are Activated by Leptin or Dehydration. Sci. Rep. 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borgland SL et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci. 29, 11215–11225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muschamp JW et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. U. S. A. (2014) doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giardino WJ & de Lecea L Hypocretin (orexin) neuromodulation of stress and reward pathways. Current Opinion in Neurobiology (2014) doi: 10.1016/j.conb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stamatakis AM et al. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J. Neurosci. (2016) doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van den Pol AN Neuropeptide Transmission in Brain Circuits. Neuron 76, 98–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thiele TE (University of N. C. at C. H. Neuropeptides and Addiction: An Introduction. Int. Rev. Neurobiol. 136, 1–3 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Peyron C et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haynes AC et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides (1999) doi: 10.1016/S0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 87.Yamanaka A, Sakurai T, Katsumoto T, Masashi Yanagisawa & Goto, K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. (1999) doi: 10.1016/S0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- 88.McGregor R, Wu MF, Barber G, Ramanathan L & Siegel JM Highly specific role of hypocretin (Orexin) neurons: Differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J. Neurosci. (2011) doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baimel C & Borgland SL Orexin/hypocretin in the Ventral Tegmental Area is Necessary for Morphine-Induced Synaptic Plasticity of Dopamine Neurons. in Catecholamine Research in the 21st Century: Abstracts and Graphical Abstracts, 10th International Catecholamine Symposium, 2012 (2013). doi: 10.1016/B978-0-12-800044-1.00212-9. [DOI] [Google Scholar]
- 90.Thompson JL & Borgland SL A role for hypocretin/orexin in motivation. Behavioural Brain Research (2011) doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 91.Harris GC & Aston-Jones G Arousal and reward: a dichotomy in orexin function. Trends in Neurosciences (2006) doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 92.Harris GC, Wimmer M, Randall-Thompson JF & Aston-Jones G Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav. Brain Res. (2007) doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cason AM & Aston-Jones G Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl). (2013) doi: 10.1007/s00213-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baimel C & Borgland SL Orexin signaling in the VTA gates morphine-induced synaptic plasticity. J. Neurosci. (2015) doi: 10.1523/JNEUROSCI.4385-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baimel C et al. Themed Section: Opioids: New Pathways to Functional Selectivity Orexin/hypocretin role in reward: implications for opioid and other addictions LINKED ARTICLES. Br. J. Pharmacol. (2015) doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL & Brown RE Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J. Neurosci. 23, 7–11 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahler SV, Moorman DE, Smith RJ, James MH & Aston-Jones G Motivational activation: A unifying hypothesis of orexin/hypocretin function. Nature Neuroscience vol. 17 1298–1303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakurai T et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. - PubMed - NCBI. Cell 92, 573–585 (1998). [DOI] [PubMed] [Google Scholar]
- 99.Woodworth HL, Brown JA, Batchelor HM, Bugescu R & Leinninger GM Determination of neurotensin projections to the ventral tegmental area in mice. Neuropeptides (2018) doi: 10.1016/j.npep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woodworth HL et al. Lateral Hypothalamic Neurotensin Neurons Orchestrate Dual Weight Loss Behaviors via Distinct Mechanisms. Cell Rep. 21, 3116–3128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patterson CM et al. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 156, 1692–1700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cador M, Kelley AE, Le Moal M & Stinus L Ventral tegmental area infusion of substance P, neurotensin and enkephalin: Differential effects on feeding behavior. Neuroscience (1986) doi: 10.1016/0306-4522(86)90061-8. [DOI] [PubMed] [Google Scholar]
- 103.Hawkins MF Aphagia in the rat following microinjection of neurotensin into the ventral tegmental area. Life Sci. (1986) doi: 10.1016/0024-3205(86)90606-5. [DOI] [PubMed] [Google Scholar]
- 104.Kalivas PW, Burgess SK, Nemeroff CB & Prange AJJ Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience 8, 495–505 (1983). [DOI] [PubMed] [Google Scholar]
- 105.Sotty F et al. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: a combined in vivo electrochemical and electrophysiological study. Neuroscience 85, 1173–1182 (1998). [DOI] [PubMed] [Google Scholar]
- 106.Cador M, Kelley AE, Le Moal M & Stinus L Ventral tegmental area infusion of substance P, neurotensin and enkephalin: differential effects on feeding behavior. Neuroscience 18, 659–669 (1986). [DOI] [PubMed] [Google Scholar]
- 107.Feifel D, Goldenberg J, Melendez G & Shilling PD The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology 58, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elliott PJ & Nemeroff CB Repeated neurotensin administration in the ventral tegmental area: effects on baseline and D-amphetamine-induced locomotor activity. Neurosci. Lett. 68, 239–244 (1986). [DOI] [PubMed] [Google Scholar]
- 109.Lepee-Lorgeoux I, Betancur C, Rostene W & Pelaprat D Differential ontogenetic patterns of levocabastine-sensitive neurotensin NT2 receptors and of NT1 receptors in the rat brain revealed by in situ hybridization. Brain Res. Dev. Brain Res. 113, 115–131 (1999). [DOI] [PubMed] [Google Scholar]
- 110.Palacios JM, Pazos A, Dietl MM, Schlumpf M & Lichtensteiger W The ontogeny of brain neurotensin receptors studied by autoradiography. Neuroscience 25, 307–317 (1988). [DOI] [PubMed] [Google Scholar]
- 111.Woodworth HL et al. Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance. Cell Rep. 20, 1881–1892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woodworth HL et al. Lateral Hypothalamic Neurotensin Neurons Orchestrate Dual Weight Loss Behaviors via Distinct Mechanisms. Cell Rep. 21, 3116–3128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mickelsen LE et al. Neurochemical heterogeneity among lateral hypothalamic hypocretin/orexin and melanin-concentrating hormone neurons identified through single-cell gene expression analysis. eNeuro 4, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laque A et al. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol. Metab. (2015) doi: 10.1016/j.molmet.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laque A et al. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. - Endocrinol. Metab. 304, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qualls-Creekmore E et al. Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J. Neurosci. (2017) doi: 10.1523/JNEUROSCI.0155-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saito Y, Cheng M, Leslie FM & Civelli O Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J. Comp. Neurol. (2001) doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 118.Chee MJSS, Pissios P & Maratos-Flier E Neurochemical characterization of neurons expressing melanin-concentrating hormone receptor 1 in the mouse hypothalamus. J. Comp. Neurol. 521, 2208–2234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hervieu GJ et al. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the ratt. Eur. J. Neurosci. 12, 1194–1216 (2000). [DOI] [PubMed] [Google Scholar]
- 120.Liu JJ, Bello NT & Pang ZP Presynaptic regulation of leptin in a defined lateral hypothalamus–ventral tegmental area neurocircuitry depends on energy state. J. Neurosci. 37, 11854–11866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M & Baker BI Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature (1983) doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- 122.Oshima N et al. Action of melanin-concentrating hormone (MCH) on teleost chromatophores. Gen. Comp. Endocrinol. (1986) doi: 10.1016/0016-6480(86)90072-9. [DOI] [PubMed] [Google Scholar]
- 123.Zamir N, Skofitsch G, Bannon MJ & Jacobowitz DM Melanin-concentrating hormone: Unique peptide neuronal system in the rat brain and pituitary gland. Proc. Natl. Acad. Sci. U. S. A. (1986) doi: 10.1073/pnas.83.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mouri T et al. Melanin-concentrating hormone in the human brain. Peptides (1993) doi: 10.1016/0196-9781(93)90158-D. [DOI] [PubMed] [Google Scholar]
- 125.Sherwood A et al. The role of melanin-concentrating hormone in conditioned reward learning. Eur. J. Neurosci. (2012) doi: 10.1111/j.1460-9568.2012.08207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kong D et al. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by ucp2, and regulates peripheral glucose homeostasis. Cell Metab. (2010) doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watts AG, Sanchez-Watts G & Kelly AB Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration- induced anorexia. J. Neurosci. (1999) doi: 10.1523/jneurosci.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alon T et al. Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology (2009) doi: 10.1210/en.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watts AG & Boyle CN The functional architecture of dehydration-anorexia. Physiol. Behav. (2010) doi: 10.1016/j.physbeh.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Drescher VS, Chen H-L & Romsos DR Corticotropin-Releasing Hormone Decreases Feeding, Oxygen Consumption and Activity of Genetically Obese (ob/ob) and Lean Mice. J. Nutr. (1994) doi: 10.1093/jn/124.4.524. [DOI] [PubMed] [Google Scholar]
- 131.Koob GF & Zorrilla EP Neurobiological mechanisms of addiction: Focus on corticotropin-releasing factor. Current Opinion in Investigational Drugs (2010). [PMC free article] [PubMed] [Google Scholar]
- 132.Spanagel R, Noori HR & Heilig M Stress and alcohol interactions: Animal studies and clinical significance. Trends in Neurosciences (2014) doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Refojo D et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science (80-. ). (2011) doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]