Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin's lymphoma (NHL), representing 30% of all lymphoma cases. Within the first 2-3 years following immunochemotherapy, 30-40% of patients will experience a relapse or a refractory disease, thereby exhibiting a poor prognosis. High-dose immunotherapy followed by autologous stem cell transplantation is the standard care for relapsed/refractory (RR) patients with DLBCL. However, >60% of patients are ineligible for a transplant, presenting a therapeutic challenge. Chimeric antigen receptor (CAR) T-cell therapy has shown promising efficacy in patients with DLBCL, including those with R/R disease. The present study conducted a meta-analysis that showed highly favorable outcomes [objective response rate (ORR): 69%; complete remission (CR): 49%] in B-cell NHL patients (n=419) who were treated with second-generation CAR T cells. The response rate varied in different types of B-cell NHL. In 306 patients with R/R DLBCL eligible for rate evaluation, the ORR and CR rate mean estimates were 68% [95% confidence interval (CI), 55-79%] and 46% (95% CI, 38-54%), respectively. Thus, the findings indicated that immunotherapy with CAR T cells has improved outcomes for patients with R/R DLBCL and other subtypes of B-cell NHL compared with standard chemotherapy regimens. The study revealed that grade ≥3 anemia (34%) and thrombocytopenia (30%) were the most common adverse effects of CAR T-cell therapy. Incidence of grade ≥3 cytokine release syndrome and neurotoxicity associated with CAR T-cell therapy was effectively managed.
Keywords: B-cell non-Hodgkin's lymphoma, diffuse large B-cell lymphoma, chimeric antigen receptor T-cell therapy, chimeric antigen receptor T cells, chimeric antigen receptor T-cell efficacy, chimeric antigen receptor T-cell safety
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin's lymphoma (NHL), representing 30% of all lymphoma cases (1). The combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone is the first line immunochemotherapy used in the treatment of DLBCL, with cure rates of 60-70% (2-4). However, 30-40% of these patients will experience a relapse or refractory disease within the first 2-3 years following immunochemotherapy, thus exhibiting a poor prognosis (5,6). Early relapses (≤1 year) and late relapses (>5 years) may also occur, with incidence rates of 10-15 and 3%, respectively (5,7).
High-dose immunotherapy followed by autologous stem cell transplantation (ASCT) is the standard treatment for patients with relapsed/refractory (RR) DLBCL that are <65 years and without major comorbidities; however, >60% of patients are ineligible for transplant, presenting a therapeutic challenge (8).
Promising immunotherapy approaches, including chimeric antigen receptor (CAR) T-cell therapy, have boosted the possibility of novel treatment options for patients with DLBCL (2). CAR T-cells are a form of immunotherapy in which immune cells are genetically engineered to target an antigen present on tumor cells so that they seek out those cells specifically; these T-cells then initiate an active and sustained immune response against the target cells (9).
Following years of research and development, the Food and Drug Administration (FDA) has already approved two CAR T-cell products. In October 2017, axicabtagene ciloleucel, marketed as Yescarta, became the first CAR T-cell therapy to be approved for patients with R/R NHL (10). Findings from phase II of the ZUMA-1 study revealed that the highest objective response rate (ORR) achieved using the therapy was 82%, and the highest complete remission (CR) rate was 54% (11). On a 12-month follow-up, the durable ORR was found to be 42%, and the durable CR rate was 40%. In May 2018, tisagenlecleucel was also approved for the treatment of large B-cell lymphoma, based on the phase II JULIET study; in the study, the highest reported ORR and CR rate were 52 and 40%, respectively (12,13). Based on a European Hematology Association presentation, the durable ORR and CR rate are postulated to be 34 and 29%, respectively (14). A third CAR T-cell therapy, lisocabtagene maraleucel has also shown promise in a phase II study, which is also expected to lead to FDA approval (15). In the phase II TRANSCEND study, at the dose level being explored for FDA submission, the highest ORR and CR rate were 80 and 59%, respectively; at 6 months, the durable ORR was 47% and the durable CR rate was 41% (15).
CAR T cells have thus shown promising efficacy in patients with DLBCL, including those with R/R disease; however, this therapy is also associated with unexpected toxicities that can be life-threatening, including cytokine release syndrome (CRS) and neurotoxicity (16). Therefore, the challenges in DLBCL management are to reduce toxicity, prolong disease-free survival and determine factors that can predict relapse of DLBCL following CAR T-cell therapy.
The aim of the present study was to evaluate the general outcomes of CAR T-cell therapy in B-cell NHL, including the ORR and CR rate, progression-free survival (PFS), overall survival (OS) and adverse effects.
Materials and methods
Meta-analysis
The meta-analysis was designed in accordance with the principles set by the PRISMA checklist (17). Inclusion criteria specified all clinical studies between 2010 and 2018 in which adult patients with DLBCL received the second generation of anti-CD19 or anti-CD20 CAR T-cell therapy. Ongoing clinical trials without reported outcomes and clinical trials with first-generation CAR T-cell therapy were excluded.
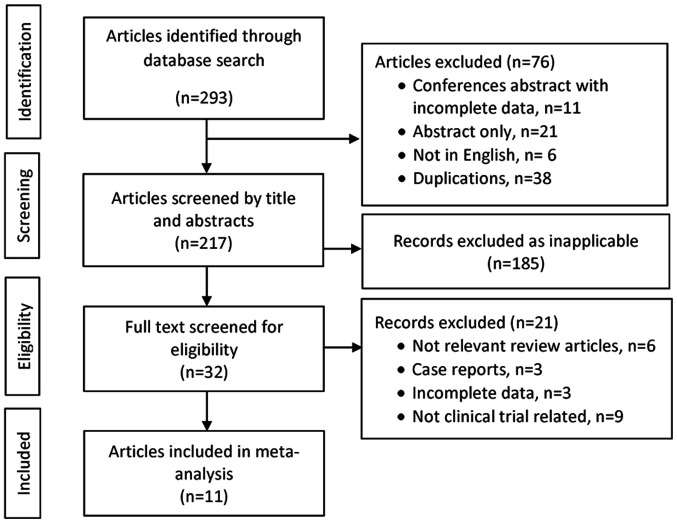
The literature search was performed using the following electronic medical bibliographic databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (www.scopus.com), and Web of Science (https://www.webofknowledge.com). Relevant oncology conference proceedings were also searched. Terms used included ‘anti-CD19’, ‘anti-CD20’, ‘diffuse large B-cell lymphoma’, ‘DLBCL’, ‘CAR T-cells’ and ‘chimeric antigen receptor T-cells’. The references of the retrieved articles and previous review articles were reviewed manually to obtain additional articles. Two investigators independently screened the retrieved titles and abstracts; the full texts were screened if the articles met the inclusion criteria. The full texts of these selected articles were obtained and evaluated by all investigators to confirm eligibility for inclusion (Fig. 1).
Figure 1.
Flow diagram of the study selection process.
Data were extracted using a structured template, and disagreements were resolved by consensus during the processes of screening and data extraction. For each study included, the following information was obtained: Author and year; phase of the study; patient population; CAR construct and signaling; dose of infused CAR T-cells; conditioning or lymphodepleting chemotherapy; origin type of the CAR T cells (autologous vs. donor-derived/allogeneic); outcomes; survival; and adverse effects. Second-generation CAR T-cell therapies in phase I and phase II clinical trials were selected for the final analysis. The primary outcome was ORR, while the secondary outcome was CR. Other secondary outcomes were PFS and OS. The toxicity data were analyzed in two main categories: Grade 3-4 CRS and severe neurotoxicity.
Statistical analysis
The meta-analysis was performed using Comprehensive Meta-Analysis software (version 3.3.070; BioStat, Inc.) due to the small sample size in most of the studies included (18). The pooled odds ratios (event rate) estimates of ORR, CR and adverse events with 95% confidence intervals (CI) were obtained using the random-effects model. Statistical heterogeneity of the trials' results was assessed via graphical inspections of the forest plots and by calculating a Chi-squared (χ2) test for heterogeneity with a significance level of P<0.10.
Results
Clinical trial and patient clinical characteristics
The initial search identified 293 potentially relevant studies, and from those, a total of 11 clinical trials including 441 patients with B-cell lymphoma were included in the final analysis. Of these, 292 (66%) patients had de novo R/R DLBCL, 73 (17%) patients had transformed DLBCL from follicular lymphoma (FL), and 15 (3%) had transformed from chronic lymphocytic leukemia (CLL) or marginal zone lymphoma (MZL). Furthermore, 25 (6%) had FL, 18 (4%) had primary mediastinal large B-cell lymphoma (PMBCL), 14 (3%) had mantle cell lymphoma (MCL), and the remaining 4 patients had other B-cell lymphomas (1%). Tables I-III present the characteristics and clinical outcomes of CAR T-cell therapy in the studies analyzed (11,13,15,19-30).
Table I.
Characteristics of second generation CAR T-cell clinical trials.
| Author, year | Seq. no. | Project name | Clinical trial phases | Construct name | Anti- | Co-stimulatory domain | Origin type of the CAR T cell | Mode of transduction | Dose | Lymphodepleting | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locke et al, 2017 | 1 | ZUMA 1 Trial (a) | Phase 1 | KTE-C19 | CD19 | CD28 | Autologous | Retroviral vector | 1-2x106 CAR T cells/kg (patients >100 kg: 2.0x108 fixed dose) | Cy (500 mg/m2) and Flu (30 mg/m2) for 3 days | (19) |
| Neelapu et al, 2017 | 2 | ZUMA 1 Trial (b) | Phase 2 (cohort no. 1) | KTE-C19 | CD19 | CD28 | Autologous | Retroviral vector | 2.0x106 CAR T cells/kg | Flu (30 mg/m2) and Cy (500 mg/m2) on days -5, -4 and -3 | (11) |
| Schuster et al, 2017 and 2019 | 3 | JULIET | Phase 2 | CTL019 | CD19 | 4-1BB | Autologous | Lentiviral vector | Median, 3.1x108 transduced cells (range, 0.1-6.0x108 transduced cells) | 73% received Flu (25 mg/m2) + Cy (250 mg/m2/day) for 3 days; 20% received bendamustine (90 mg/m2/day) for 2 days | (13,20,21) |
| Abramson et al, 2017 and 2018 | 4 | TRANSCEND | Phase 1 | JCAR017 | CD19 | 4-1BB Comprised of CD8 and CD4 1:1 ratio | Autologous | Lentiviral vector | DL1 (singleinfusion): 5.0x107 CAR T cells DL1 (double infusion): 5.0x107 CAR T cells DL2 (single infusion): 1.0x108 CAR T cells | Flu (30 mg/m2) and Cy (300 mg/m2) for 3 days | (15,22) |
| Schuster et al, 2017 | 5 | University of Pennsylvania | Phase 2 | CTL019 | CD19 | 4-1BB CD3ζ and CD28 | Autologous | Lentiviral vector | 5.79x106 (range, 3.08x106-8.87x106) | Bendamustine, Cy | (23) |
| Turtle et al, 2016 | 6 | Fred Hutch | Phase 1 | huJCAR014 | CD19 | 4-1BB Comprised of CD8 and CD4 1:1 ratio | Autologous | Lentiviral vector | 2x105-2x107 cells/kg | Cy (60 mg/kg) once + etoposide or Cy (60 mg/kg) once + Flu (25 mg/m2) for 3 days | (24) |
| Wang et al, 2014 | 7 | NA | Phase 1 | CD 20 | 4-1BB CD137-CD3ζ | Autologous | Lentiviral vector | Not Recorded | Cy, vincristine, doxorubicin, etoposide and carboplatin cytarabine | (25) | |
| Wang et al, 2016 | 8 | NHL2 | Phase 1 | CD 19 | CD28 | Autologous | Lentiviral vector | 5x107-2x108 | Autologous stem-cell transplantation | (26) | |
| Brudno et al, 2016 | 9 | NA | Phase 1 | Allogeneic | CD19 | CD28-CD3ζ | Allogenic CD3ζ-28 | Retroviral vector | 0.7x106-8.2x106 | None | (27) |
| Brudno et al, 2016 | 10 | NA | Phase 1 | HuCAR-19 | CD19 | CD28-CD3ζ | Allogenic | Lentiviral vector | 0.4-8.2x106 cells/kg | Cy (300 mg/m2) daily for 3 days + Flu (30 mg/m2) daily for 3 days | (28) |
| Kochenderfer et al, 2015 | 11 | NA | Phase 1 | CD19 | CD28-CD3ζ | Autologous | Retroviral vector | 1-2.5x106 cells/kg | Bendamustine; Bendamustine/Rituximab; Pentostatin/Cy | (29) | |
| Kochenderfer et al, 2017 | 12 | NA | Phase 1 | CD19 | CD 28 | Autologous | Retroviral vector | 1-2x106 CAR T cells/kg | Cy (300 mg/m2 or 500 mg/m2) intravenously daily for 3 days, and Flu (30 mg/m2) daily for 3 days (30) | (30) |
CAR, chimeric antigen receptor; DL, dose level; Cy, cyclophosphamide; Flu, fludarabine.
Table II.
Chimeric antigen receptor T-cell clinical trial outcomes.
| Author, year | Seq. no. | Project name | Median age | N | Histological subtypes | Median follow-up | B-cell NHL treatment outcome | DLBCL treatment outcome | Duration of response | PFS/OS | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locke et al, 2017 | 1 | ZUMA 1 Trial (a) | 59 | 7 | DLBCL (n=7) | 9 months | ORR, 71% (n=5/7); | ORR, 71% (n=5/7); | Not reported CR, 57% (n=4/7) CR, 57% (n=4/7); ongoing CR, 42% at 1 year of follow-up (n=3/7) | Not reported | (19) |
| Neelapu et al, 2017 | 2 | ZUMA 1 Trial (b) | 58 | 101 | DLBCL (n=77), PMBCL (n=8), transformed FL (n=16) | 27 months | ORR, 83% (n=84/101); CR, 58% (n=59/101) | ORR, 82% (n=63/77); CR, 49% (n=38/77); PR, 32% (n=25/77) | 11.1 months for CR is not reached | Median PFS, 5.9 months; PFS, 60% at 12 months; PFS, 35% at 24 months Median OS 12.8 months is not reached; OS, 60% at 12 months; OS, 50.5% at 24 months | (11) |
| Schuster et al, 2017 and 2019 | 3 | JULIET | 56 | 111 | R/R DLBCL (n=88), transformed FL (n=21), others (n=2) | 28.6 months | ORR, 52% (n=48/93); CR, 40% (n=37/93) | ORR, 52% (n=48/93); CR, 40% (n=37/93) | Not reached | Median PFS not reached; PFS, 65% at 12 months Median OS, 8.3 months; OS, 40% at 12 months | (13,20,21) |
| Abramson et al, 2017 and 2018 | 4 | TRANSCEND | 61 | 102 | De novo DLBCL (n=63), transformed from FL (n=23), transformed from other (MZL, CLL) (n=12), FL grade 3B (n=1), PMBCL (n=3) | 8 months | ORR, 75% (n=73/102); CR, 52% (n=53/102) | ORR, 80% (n=58/73); CR, 55% (n=40/73) | 9.2 months | PFS not reported Median OS, 10.4 months; OS, 59% at 12 months | (15,22) |
| Schuster et al, 2017 | 5 | University of Pennsylvania | 59 | 28 | DLBCL (n=14), FL (n=14) | 28.6 months | ORR, 64% (n=18/28); CR, 57% (n=16/28) | ORR, 50% (n=7/14); CR, 43% (n=6/14) PFS was 3.2 months not reached, and 43% PFS at median follow-up (95% CI, 18-66%) | Not reached | Median PFS, 3.2 months for DLBCL; PFS, 43% at 24 months Median OS, 22 months; OS, 47% at 24 months | (23) |
| Turtle et al, 2016 | 6 | Fred Hutch | 58 | 32 | De novo aggressive B-cell lymphoma (n=11), transformed large B-cell lymphoma (n=11), FL (n=6), MCL (n=4) | ORR, 63% (n=19/30); CR, 33% (n=10/30) | De novo aggressive B-cell lymphoma: ORR, 64% (n=7/11) De novo aggressive B-cell lymphoma: CR, 18% (n=2/11) Transformed large B-cell lymphoma: ORR, 70% (n=7/10) Transformed large B-cell lymphoma: CR, 60% (n= 6/10) | Median PFS follow-up for Cy and Cy/Flu, 1.5 and 5.8 months, respectively Median OS follow-up times for Cy and Cy/Flu, 25 months and 6.3 months, respectively | (24) | ||
| Wang et al, 2014 | 7 | NA | 62 | 7 | R/R DLBCL (n=7) | Not reported | ORR, 71% (n=5/7); CRR, 28% (n=2/7); PR, 43% (n=3/7); SD, 14% (n=1/7); PD, 14% (n=1/7) | ORR, 71% (5/7); CRR, 28% (2/7); PR, 43% (n=3/7); SD, 14% (n=1/7); PD, 14% (n=1/7) | Not reported | Not reported | (25) |
| Wang et al, 2016 | 8 | NHL2 | 8 | DBLCL (n=4), MCL (n=4) | 12.3 months | Best ORR, 100% (n=8/8); best CR, 100% (n=8/8) | Best ORR, 100% (n=4/4); best CR, 100% (n=4/4) | Not reported | PFS, 75% at 12 months | (26) | |
| Brudno et al, 2016 | 9 | NA | Not reported | 9 | DLBCL (n=3), transformed FL (n=1), FL (n=2), MCL (n=1), B-cell lymphoma unclassified (n=1), Burkitt lymphoma (n=1) | Not reported | ORR, 86% (n=8/9); CR, 22% (n=2/9) | ORR, 67% (n=2/3); CR, 33% (n=1/3) | Not reported | Not reported | (27) |
| Brudno et al, 2016 | 10 | NA | 48 | 10 (Total of 20, 10 excluded for ALL and CLL) | DLBCL (n=4), MCL (n=5), transformed FL to DLBCL (n=1), ALL (n=5), CLL (n=5) | Not reported | ORR for NHL, 20% (n=2/10); CR, 10% (n=1/10) | ORR, 25% (n=1/4); CR, 25% (n=1/4) | Not reported | Not reported for NHL For all 20 patients: EFS, 39% at 6 months; 39%, at 12 months OS, 77% at 12 months | (28) |
| Kochenderfer et al, 2015 | 11 | NA | 47 | 11 (total of 15, 4 excluded for CLL) | DLBCL NOS (n=4), PMBCL (n=4), transformed DLBCL from CLL (n=1), indolent lymphomas (n=2) | Not reported | For NHL excluding CLL: ORR, 89% (n=8/9); for evaluable NHL: CR, 56% (n=5/9) | ORR, 100% (n= 3/3) for evaluable DLBCL; CR, 67% (n=2/3) for evaluable DLBCL | 11 Months for NHL | Not reported | (29) |
| Kochenderfer et al, 2017 | 12 | NA | 47 | 7 | DLBCL NOS (n=3), PMBCL (n=3), DLBCL from CLL (n=1) | ORR, 86% (n=6/7); CR, 71% (n=5/7) | ORR, 100 % (n=3/3); CR, 100% (n=3/3) | Previously reported | (30) |
ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NHL, non-Hodgkin's lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NOS, not otherwise specified; PMBCL, primary mediastinal large B-cell lymphoma; R/R, relapsed/refractory; CI, confidence interval; CR, complete remission; ORR, objective response rate; PD, progressing disease; PR, partial response; SD, stable disease; EFS, event-free survival; PFS, progression-free survival; OS, overall survival.
Table III.
Chimeric antigen receptor T-cell clinical trial toxicity profile.
| Author, year | Seq. no. | Project name | Toxicity: CRS and neurotoxicity | Other toxicities, grade 3 or higher | (Refs.) |
|---|---|---|---|---|---|
| Locke et al, 2017 | 1 | ZUMA 1 Trial (a) | Grade 3-4 CRS, 11% (n=12/108) Neurotoxicity, 32% (n=35/108) | Febrile neutropenia: Grade 3, 31% (n=33/108); grade 4, 2% (n=2/108) Neutropenia: Grade 3, 9% (n=10/108); grade 4, 30% (n=32/108) Anemia: Grade 3, 43% (n=46/108); grade 4, 3% (n=3/108) Thrombocytopenia: Grade 3, 10% (n=11/108); grade 4, 14% (n=15/108) Intracranial hemorrhage: Grade 3, 30%; grade 4, 0%; grade 5, 14% (n=1/7) Hypocalcemia: Grade 3, 6% (n=7/108); grade 4, 0% Hyponatremia: Grade 3, 11% (n=12/108); Grade 4, 0% Hypophosphatemia: Grade 3, 17% (n=18/108); Grade 4, 1% (n=1/108) Hypotension: Grade 3, 13% (n=14/108); Grade 4, 0% Fatigue: Grade 3, 3% (n=3/108); Grade 4, 0% Treatment-related death, 2% (n=2/108) | (19) |
| Neelapu et al, 2017 | 2 | ZUMA 1 Trial (b) | Grade 3-4 CRS, 22% Neurotoxicity, 12% | Febrile neutropenia, 15% Infection, 20% Cytopenia, 22% Tumor lysis syndrome, 1% | (11) |
| Schuster et al, 2017 and 2019 | 3 | JULIET | Grade 3-4, 1% Grade 3-4 neurotoxicity, 12% | Not reported | (13,20,21) |
| Abramson et al, 2017 and 2018 | 4 | TRANSCEND | Grade 3-4, 18% (n=5/28) Grade 3-4 neurotoxicity, 11% (n=3/28) | Febrile neutropenia, 11% (n=3/28) Anemia, 11% (n=3/28) Atrial fibrillation, 4% (1/28) Intra-abdominal hemorrhage, 4% (n=1/28) Hypotension, 11% (n=3/28) Hypocalcemia, 4% (n=1/28) Hypercalcemia, 4% (n=1/28) Hyponatremia, 0% Hypomagnesemia, 0% | (15,22) |
| Schuster et al, 2017 | 5 | University of Pennsylvania | Severe CRS, 13% (n=4/32) Grade 3-4 neurotoxicity, 28% (n=9/32) | Not reported | (23) |
| Turtle et al, 2016 | 6 | Fred Hutch | Grade 3 CRS, 14% (n=1/7), Grade 4 CRS, 0% Grade 3-4 neurotoxicity, 0% Grade 3-4 alimentary tract hemorrhage, 29% (n=2/7) | Grade 4 infusion associated acute toxicities, 14% (n=1/7) Tumor lysis syndrome, 14% (n=1/7) Lung dysfunction, 14% (n=1/7) Serous cavity effusion, 14% (n=1/7) | (24) |
| Wang et al, 2014 | 7 | NA | Grade 3-4 CRS, 0% (n=0/8) Neurotoxicity, 0% (n=0/8) | Hematological toxicities G4, 100% (n=8/8) Non hematological toxicity G3, 88% (n=7/8) | (25) |
| Wang et al, 2016 | 8 | NHL2 | Grade 3-4 CRS, 38% (n=3/8) Neurotoxicity, 13% (n=1/8) | Not reported | (26) |
| Brudno et al, 2016 | 9 | NA | Grade 3-4 CRS, 25% (n=1/4) | For NHL patients excluding leukemia patients: Grade 3-4 anemia, 10% (n=1/10) Grade 3-4 neutropenia, 20% (n=2/10) Grade 3-4 thrombocytopenia, 10% (n=1/10) Grade 3-4 AST/ALT elevation, 10% (n=1/10) | (27) |
| Brudno et al, 2016 | 10 | NA | Grade 3-4 CRS, 40% (n=6/15) Grade 3-4 neurotoxicity, 40% (n=6/15) | Hypotension, 27% (n=4/15) Infection, 53% (n=8/15) Acute renal failure, 7% | (28) |
| Kochenderfer et al, 2015 | 11 | NA | CRS Neurotoxicity | Previously reported | (29) |
| Kochenderfer et al, 2017 | 12 | NA | Grade 3-4 CRS, 11% (n=12/108) Neurotoxicity, 32% (n=35/108) | Febrile neutropenia: Grade 3, 31% (n=33/108); grade 4, 2% (n=2/108) Neutropenia: Grade 3, 9% (n=10/108); grade 4, 30% (n=32/108) Anemia: Grade 3, 43% (n=46/108); grade 4 3% (n=3/108) Thrombocytopenia: Grade 3, 10% (n=11/108); grade 4, 14% (n=15/108) Intracranial hemorrhage: Grade 3, 30%; grade 4, 0%; grade 5, 14% (n=1/7) Hypocalcemia: Grade 3, 6% (n=7/108); grade 4, 0% Hyponatremia: Grade 3, 11% (n=12/108); grade 4, 0% Hypophosphatemia: Grade 3, 17% (n=18/108); grade 4, 1% (n=1/108) Hypotension: Grade 3, 13% (n=14/108); grade 4 0% Fatigue: Grade 3, 3% (n=3/108); grade 4, 0% Treatment-related death, 2% (n=2/108) | (30) |
CRS, cytokine release system; ALT, alanine transaminase; AST, aspartate transaminase; NHL, non-Hodgkin's lymphoma.
Efficacy
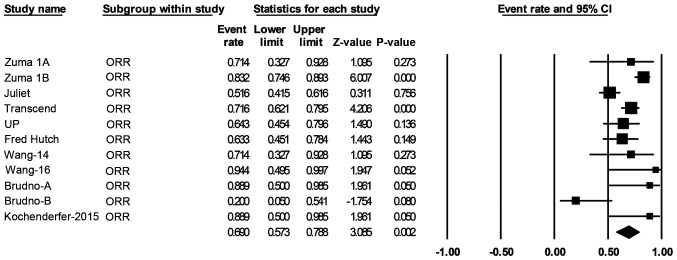
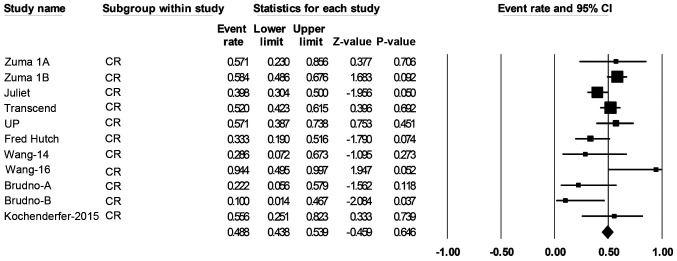
Over a median follow-up time of 19.6 months, response data were available for 419 of the patients with B-cell NHL. The pooled ORR (95% CI) was 69% (57-79%; Fig. 2), and the pooled CR rate (95% CI) was 49% (44-52%; Fig. 3).
Figure 2.
Forest plot of the ORR of patients with any B-cell lymphoma. Squares represent the event rates (square size reflects the study-specific statistical weight); horizontal lines represent the 95% CI; and diamonds represent the pooled estimate based on a random-effects model. ORR, objective response rate; CI, confidence interval.
Figure 3.
Forest plot of the CR rate of patients with any B-cell lymphoma. Squares represent the event rates (square size reflects the study-specific statistical weight); horizontal lines represent the 95% CI; and diamonds represent the pooled estimate based on a random-effects model. CR, complete remission; CI, confidence interval.
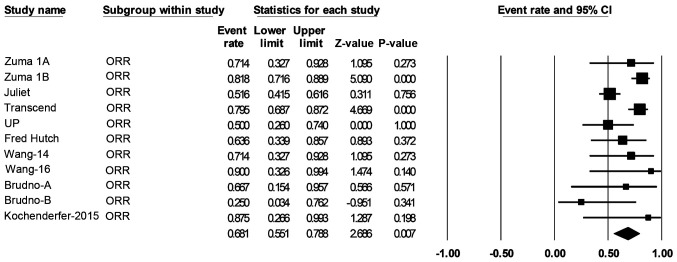
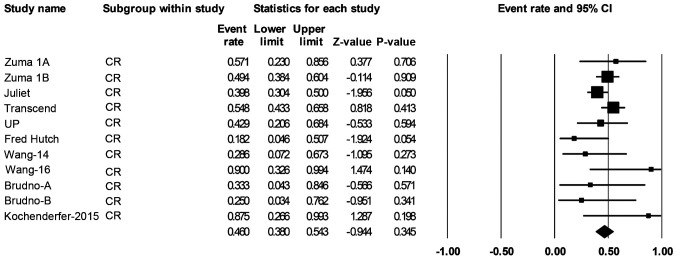
A total of 306 patients with de novo or transformed DLBCL were eligible for response rate evaluation. The ORR was 68% (55-79%; Fig. 4) and the CR rate was 46% (38-54%; Fig. 5).
Figure 4.
Forest plot of the ORR of patients with diffuse large B-cell lymphoma. Squares represent the event rates (square size reflects the study-specific statistical weight); horizontal lines represent the 95% CI; and diamonds represent the pooled estimate based on a random-effects model. ORR, objective response rate; CI, confidence interval.
Figure 5.
Forest plot of the CR rate of patients with large B-cell lymphoma. Squares represent the event rates (square size reflects the study-specific statistical weight); horizontal lines represent the 95% CI; and diamonds represent the pooled estimate based on a random-effects model. CR, complete remission; CI, confidence interval
The PFS was reported for 234 patients with B-cell lymphoma from the 11 clinical trials, and at 12 months, the PFS was 43% (95% CI, 35-75%). The median and mean PFS durations were 4.5 and 4.1 months (95% CI, 1.5-5.9 months), respectively (data not shown).
The OS was reported for 317 patients, and at 12 months, it was 58% (95% CI, 49-60%). The median and mean OS durations were 13.2 and 14.2 months (95% CI, 8.3-22.2 months), respectively (data not shown).
Safety
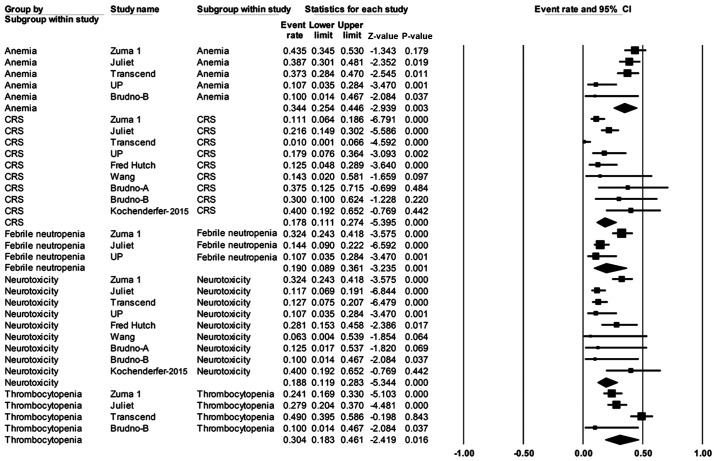
Safety was evaluated for 421 patients (Table III). The most frequently reported grade ≥3 adverse effects were anemia in 34% of patients (95% CI, 25-45%), thrombocytopenia at 30% (95% CI, 18-46%), and febrile neutropenia at 19% (95% CI, 9-36%). The risks of grade ≥3 CRS and neurotoxicity in patients were 18% (95% CI, 11-27%) and 19% (95% CI, 12-28%), respectively (Fig. 6).
Figure 6.
Forest plot of the rates of adverse events (grade ≥3) in patients with any B-cell lymphoma. Squares represent the event rates (square size reflects the study-specific statistical weight); horizontal lines represent the 95% CI; and diamonds represent the pooled estimate based on random-effects model. CRS, cytokine release syndrome; CI, confidence interval.
Heterogeneity
Statistical heterogeneity was observed among the 11 clinical trials in several outcomes, including ORR for patients with B-cell NHL (P=0.002; Fig. 2), ORR for patients with DLBCL (P=0.007; Fig. 4), and adverse events such as CRS (P=0.000), neurotoxicity (P=0.000), febrile neutropenia (P=0.001), anemia (P=0.003) and thrombocytopenia (P=0.016; Fig. 6).
Discussion
The efficacy of CAR T-cell immunotherapy has improved notably over the last decade. To date, three generations of CAR T-cells have been constructed; of these, the second and third generations of CAR T-cells show superior clinical outcomes relative to the first generation (31). It has been reported that first-generation CAR T-cells show decreased immune activation, limited efficacy and short duration of persistence, providing no evidence of clinical benefit for the treatment of B-cell NHL (32-34).
The present meta-analysis showed highly favorable clinical outcomes in patients with B-cell NHL that were treated with second-generation CAR T-cells. The results for 419 patients in 11 trials showed an ORR and CR rate mean estimate of 69% (95% CI, 57-79%) and 49% (95% CI, 44-52%), respectively. The response rates to CAR T-cells varied between different types of B-cell NHL. In 306 patients with R/R DLBCL eligible for rate evaluation, the ORR and CR rate mean estimates were 68% (95% CI, 55-79%) and 46% (95% CI, 38-54%), respectively; these results are comparable to the results reported on patients analyzed in the SCHOLAR-1 study, which showed an ORR of 26% and a CR rate of 7% with standard systemic therapy (35). Thus, the present findings suggested that CAR T-cell immunotherapy has significantly improved treatment outcomes for patients with R/R DLBCL, as well as other B-cell NHL subtypes. Comparisons between the reported outcomes in clinical trials included in the present study are difficult due to the clinical heterogeneity in the variables between clinical trials, including differences in patient populations, B-cell NHL subtypes disease specific variables, CAR T-cell methods, follow-up times and duration. Additionally, it has been suggested that the differences in clinical outcome could be due to clinical factors such as the CAR construct and signaling, conditioning or lymphodepleting chemotherapy, prior ASCT, prior treatments or other dissimilarities that will require further investigation (36-39). Given the consequences of clinical heterogeneity or methodological dissimilarities among CAR T-cell clinical trials included in this study, statistical heterogeneity was also observed for several outcomes, such as ORR and adverse events. Thus, a systematic review of literature is warranted following the present meta-analysis to summarize the evidence of relevant clinical factors that may have clinical utility in predicting CAR T-cell therapy clinical outcomes. Furthermore, with an increased number of clinical studies, detailed associations between clinical factors and clinical outcomes with CAR T-cell therapy will be uncovered further in the future.
The high response rates from second-generation CAR T-cells observed in the present analysis come with challenges posed by adverse events and toxicities of treatment. Evidence suggests that these adverse events tend to occur rapidly within the first few weeks of treatment and can cause potentially life-threatening complications (28,29). In 419 patients with B-cell NHL evaluated for safety, it was observed that grade ≥3 anemia (34%; 95% CI, 25-45%) and thrombocytopenia (30%; 95% CI, 18-46%) were the most common adverse effects of CAR T-cell therapy. Additionally, grade ≥3 CRS and neurotoxicity were estimated in 18% (95% CI, 11-27%) and 19% (95% CI, 12-28%) of the patients, respectively. In the present analysis, incidence of CRS and neurotoxicity varied greatly in trials. The study by Kochenderfer et al (29) reported the highest rates of grade 3 or higher CRS and neurotoxicity, which was 40% (95% CI, 19-65%). Based on a previous report, administration of interleukin (IL)-2 is associated with significant neurotoxicity in patients treated with CAR T-cells (40). Although IL-2 was not administered to patients in their study, neurological toxicity still occurred in certain patients. A potential factor to consider is that all patients had received cyclophosphamide and fludarabine lymphodepletion. Of note, all patients recovered completely from their neurological toxicities (29). In the Fred Hutchinson Cancer Research Center CAR T-cell clinical trial, grade ≥3 CRS and neurotoxicity were observed in 13% (95% CI, 5-29%) and 28% (95% CI, 15-46%) of patients, respectively, and these were predominantly observed in patients who had received cyclophosphamide and fludarabine lymphodepletion and higher CAR T-cell dose (24). A reduction in the CAR T-cell dose in subsequent patients achieved ORR and CR rates of 82 and 64%, respectively. In TRANSCEND trial, however, dose level was not associated with CRS or neurotoxicity (39). Of note, the relatively high CRS and neurotoxicity rates observed in single center studies are due to relatively small sample size; additionally, two of the trials are allogeneic CAR T-cells in origin (24,27,28).
Following the expansion of CAR T-cell clinical trials, the therapeutic procedures and treatment outcomes markedly improved. In the analysis of three front-running multi-center CAR T-cell clinical studies, highly comparable rates of grade ≥3 CRS and neurotoxicity were observed. In the ZUMA-1 trial, grade ≥3 CRS and neurotoxicity were observed in 11 and 32% of patients, respectively; despite the high rate of grade ≥3 neurotoxicity, patients were effectively managed and with extended follow-up, there were no new unexpected serious adverse events and no new-onset neurological events associated with the CAR T-cells (11,19). In the JULIET trial, grade ≥3 CRS and neurotoxicity were observed in 22 and 12% of patients, respectively; all cases of severe CRS were reversible, and no deaths were reported (13,20,21). In the analysis of the TRANSCEND trial, lower rates of toxicities were observed, with grade ≥3 CRS occurring in only 1% of patients, whereas neurotoxicity presented in 13%; additionally, no deaths from CRS or neurotoxicity were reported in this trial (15,22). In conclusion, the present meta-analysis reported on a large number of patients with B-cell NHL treated with second-generation CAR T-cells. The study showed a high clinical response rate to CAR T-cell therapy among patients with B-cell NHL, particularly with DLBCL, compared with standard chemotherapy regimens. Incidence of CRS and neurotoxicity associated with CAR T-cell therapy were effectively managed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Authors' contributions
MAM was involved in the conception and design of the study, conducted data collection, analysis and interpretation, and drafted and critically revised the manuscript, assuming general responsibility and guaranteeing the scientific integrity of the study. MAF was involved in drafting the study, conducting data collection, analysis and interpretation, and critically revising the manuscript. EI participated in statistical analysis and interpretation, critical revision, and helped to draft and finalize the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: The microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli JJ, Sutherland J, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;223:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B. Rituximab in the treatment of diffuse large B-cell lymphomas. Semin Oncol. 2002;29:30–35. doi: 10.1053/sonc.2002.30153. [DOI] [PubMed] [Google Scholar]
- 4.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 5.Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. 2018;31:209–216. doi: 10.1016/j.beha.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Damaj G, Bernard M, Legouill S, Cartron G, Le Mevel A, Dubus C, Berthou C, Colombat P, Milpied N, Marolleau JP, et al. Late relapse of localized high-grade non-hodgkin's lymphoma: Clinical and biological features. Blood. 2008;112:2603–2603. [Google Scholar]
- 7.Larouche JF, Berger F, Chassagne-Clément C, Ffrench M, Callet-Bauchu E, Sebban C, Ghesquières H, Broussais-Guillaumot F, Salles G, Coiffier B. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: Clinical characteristics and outcome. J Clin Oncol. 2010;28:2094–2100. doi: 10.1200/JCO.2009.24.5860. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Medeiros LJ, Young KH. Cancer immunotherapy in diffuse large B-cell lymphoma. Front Oncol. 2018;10(351) doi: 10.3389/fonc.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin P. The use of CAR T cells in diffuse large B-cell lymphoma and mantle cell lymphoma. Clin Adv Hematol Oncol. 2017;15:247–249. [PubMed] [Google Scholar]
- 10. US Food and Drug Administration: FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. Accessed on November 13, 2018 at https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma. [Google Scholar]
- 11.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration: FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. Accessed on November 13, 2018 at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma. [Google Scholar]
- 13.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 14. Borchmann P, Tam CS, Jäger U, McGuirk JP, Holte H, Waller EK, Jaglowski SM, Bishop MR, Andreadis C, Foley SR, et al: An updated analysis of JULIET, a global pivotal Phase 2 trial of tisagenlecleucel in adult patients with relapsed or refractory (r/r) diffuse large b-cell lymphoma (DLBCL). Presented at 2018 EHA Congress (abstract S799), 2018. https://library.ehaweb.org/eha/2018/stockholm/214521/peter.borchmann.an.updated.analysis.of.juliet.a.global.pivotal.phase.2.trial.html. [Google Scholar]
- 15.Abramson JS, Gordon LI, Palomba ML, Lunning MA, Arnason JE, Forero-Torres A, Wang M, Maloney DG, Sehgal A, Andreadis C, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol. 2018;36(7505) [Google Scholar]
- 16.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et al. Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Primary analysis of Juliet: A global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma. Blood. 2017;130(577) [Google Scholar]
- 21.Schuster SJ, Bishop MR, Tam C, Waller EK, Borchmann P, McGuirk J, Jäger U, Jaglowski S, Andreadis C, Westin J, et al. Global pivotal phase 2 trial of the CD19-targeted therapy CTL019 in adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL)-an interim analysis. Hematol Oncol. 2017;35(27) [Google Scholar]
- 22.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Arnason JE, Wang M, Forero A, Maloney DG, Albertson T, Garcia J, et al. High durable CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001): Defined composition allows for dose-finding and definition of pivotal cohort. Blood. 2017;130(58) [Google Scholar]
- 23.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, et al. Immunotherapy of non-hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355ra116) doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang Wy, Han Qw, Liu Y, Dai Hr, Guo Yl, Bo J, Fan H, Zhang Y, Zhang Yj, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol. 2014;155:160–175. doi: 10.1016/j.clim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127:2980–2990. doi: 10.1182/blood-2015-12-686725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brudno JN, Shi V, Stroncek D, Pittaluga S, Kanakry JA, Curtis LM, Gea-Banacloche JC, Pavletic S, Bagheri MH, Rose JJ, et al. T cells expressing a novel fully-human anti-CD19 chimeric antigen receptor induce remissions of advanced lymphoma in a first-in-humans clinical trial. Blood. 2016;128(999) [Google Scholar]
- 28.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ, Hickstein DD, Lu TL, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-Refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA, McIntyre L, Bot A, Rossi J, Lam N, Rosenberg SA. Long-Duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25:2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al. Adoptive immunotherapy for indolent non-hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawalekar OU, O'Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J, Keith B, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JH, Brentjens RJ. Are all chimeric antigen receptors created equal? J Clin Oncol. 2015;33:651–653. doi: 10.1200/JCO.2014.57.5472. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqi T, Abramson JS, Palomba ML, Gordon LI, Lunning MA, Arnason JE, Wang M, Forero-Torres A, Maloney DG, Heipel M, et al. Correlation of patient characteristics and biomarkers with clinical outcomes of JCAR017 in R/R aggressive BNHL (TRANSCEND NHL 001 study) J Clin Oncol. 2018;36(5) [Google Scholar]
- 40.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.