Abstract
Purpose:
We explored the use of intraventricular 131I-8H9 targeting B7-H3 in patients with ETMR.
Methods:
Patients were enrolled in an IRB approved, phase 1, 3+3 dose escalation trial. Patients with CNS disease expressing the antibody target antigen B7-H3, were eligible. We report on a cohort of 3 patients with ETMR who were enrolled on the study. Three symptomatic children (ages 14 months, 3 and 3.5 years) had large parietal masses confirmed to be B7-H3-reactive ETMR. Patients received 2 mCi 131I-Omburtamab as a tracer followed by 1 or 2 therapeutic 131I-Omburtamab injections. Dosimetry was based on serial CSF, blood samplings and region of interest (ROI) on nuclear scans. Brain and spine MRIs and CSF cytology were done at baseline, 5 weeks after 131I-Omburtamab, and approximately every 3 months thereafter. Acute toxicities and survival were noted.
Results:
Patients received surgery, focal radiation, and high dose chemotherapy. Patients 1 and 2 received 131I-Omburtamab (80 and 53 mCi, respectively). Patient 3 had a local recurrence prior to 131I-Omburtamab treated with surgery, external beam radiation, chemotherapy, then 131I-Omburtamab (36 mCi). 131I-Omburtamab was well-tolerated. Mean dose delivered by 131I-Omburtamab was 68.4 cGy/mCi to CSF and 1.95 cGy/mCi to blood. Mean ROI doses were 230.4 (ventricular) and 58.2 (spinal) cGy/mCi. Patients 1 and 2 remain in remission 6.8 years and 2.3 years after diagnosis, respectively; patient 3 died of progressive disease 7 month after therapy (2 years after diagnosis).
Conclusions:
131I-Omburtamab appears safe with favorable dosimetry therapeutic index. When used as consolidation following surgery and chemoradiation therapy, 131I-Omburtamab may have therapeutic benefit for patients with ETMR.
Keywords: Embryonal tumor with multilayered rosettes; ETMR; 131I-Omburtamab, 131I-8H9; Radioimmunotherapy
Introduction
Embryonal Tumor with Multilayered Rosettes (ETMR), C19MC Amplified is a new category of embryonal tumors within the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS). Previously called Embryonal Tumor with Abundant Neuropil and True Rosettes, Ependymoblastoma, and Medulloepithelioma, these tumors exhibit similar molecular characteristics and clinical behavior.1
ETMR contains histopathologic features of neuroblastoma and ependymoblastoma, having undifferentiated neuroepithelial cells, neuropil, and ependymoblastic rosettes with a gene fusion between TTYH1 and C19MC and amplification of the microRNA cluster C19MC, identified by FISH analysis of the 19q13.42 locus.2 Histologically similar tumors without C19MC alteration are classified as ETMR, Not Otherwise Specified. ETMR demonstrates immunohistochemical reactivity of the RNA binding protein, LIN28A2.
Approximately 70 cases of ETMR have been reported, presenting in children less than 4 years old and having an aggressive clinical course with average overall survival (OS) of 12 months, rarely beyond 30 months1, 3–6. Despite standard surgery, chemotherapy, and radiotherapy (RT), relapse rates remains high. Salvage chemotherapy, bevacizumab, autologous stem cell rescue, reresection and re-irradiation have all been tried. In one case series 18% of 38 patients with ETMR had CNS metastasis at diagnosis, necessitating treatment for distant disease, which has led to the use of craniospinal irradiation (CSI) as a potentially curative modality6, 7. Due to the high risk of neurocognitive impairment that generally discourages physicians from prescribing CSI to patients under the age of 3 (which is precisely the age group of ETMR), other modalities of addressing CNS metastatic disease must be sought.8
We explored the use of radiolabeled intraventricular immunotherapy into the cerebrospinal fluid (CSF) for children with high risk or recurrent CNS malignancies on an institutional Memorial Sloan Ketterig Cancer Center (MSKCC) Institutional Review Board (IRB) study NCT00089245. Omburtamab is a murine IgG1 monoclonal antibody highly expressed on several CNS tumors but with limited normal tissue expression9. Omburtamab binds to the tumor antigen B7-H3 (CD276), an inhibitory ligand for natural killer cells and T cells10. 124I-Omburtamab was demonstrated to be safely delivered via Convection Enhanced Delivery to patients with Diffuse Interstitial Pontine Glioma in a Phase I dose escalation trial without any Grade 4 Adverse Events or deaths11. We assessed the role of intraventricular 131I- omburtamab for patients with ETMR.
Materials and methods
Patients with ETMR were enrolled on IRB approved study 03–133 (NCT00089245). Informed consent was obtained from patients or guardians. Eligibility criteria included B7-H3 reactivity by immunohistochemistry, stable neurologic status, no obstructive or symptomatic hydrocephalus, absolute neutrophil count >1000/μL, platelet count >50,000/μL, serum bilirubin <3.0mg/dL, and serum creatinine <2mg/dL, and adequate CSF flow, which was evaluated by pretreatment 111-indium diethylene triamine pentaacetic acid studies in Ommaya catheters.
Pre- and post-treatment evaluation included a detailed history, physical exam, complete blood count, comprehensive profile, thyroid function tests, and CSF for total protein, glucose, cell count, cytology. Magnetic resonance imaging (MRI) studies of the brain and spinal cord with and without gadolinium occurred within 3 weeks before and 1 month after 131I- omburtamab, followed by every few months.
Radiolabeling
Omburtamab clinical batches were tested for purity requirements, including absence of nucleic acids, murine viruses, bacteria, fungi, mycoplasma and pyrogens. Radiolabeling was performed at the MSKCC Radiochemistry and Molecular Imaging Probes Core Facility in compliance with the requirements of the Food and Drug Advisory (FDA) Investigational New Drug (IND) using iodination of ~1 mg of the antibody with up to 50 mCi of 131I (FDA IND #9351)12. The final drug product was quality control tested to ensure conformance to the acceptance specifications defined in the Chemistry, Manufacturing, and Controls section of the IND. Sterility testing by media inoculation was performed post-release.
Dosimetry estimates
Patients received an imaging dose of 2 mCi 131I-8H9, followed by CSF and blood sampling, and 3 single-photon emission computed tomography (SPECT) scans over 48 hours to assess dosimetry. Distribution and activity concentrations of radioactivity in the craniospinal axis, and radiation doses to normal tissues were determined. Radiation exposure of CSF, ventricles, spinal cord, normal brain and blood was based on the assumption of complete local absorption of the 131I beta radiation. Measured aliquots were counted to estimate the time-dependent activity concentrations and to yield the decayed area under the curves, representing the cumulative activity concentrations in the blood and CSF as previously described.13
131I-Omburtamab therapy
Patient were premedicated with oral SSKI drops, Cytomel, acetaminophen and low dose decadron prior to injections. In the presence of a Radiation Safety Officer, Nuclear Medicine and Pediatric Oncology clinician, patients received 50-to-80 mCi 131I- omburtamab based on age and corresponding CSF volume. Patients had CNS (head and upper spinal cord) imaged by 131I-8H9 scintigraphy approximately 3–6 hours, 18–24 hours and 44–49 hours after dosimetry dose injection of the first cycle. Whole body scans were performed at the same time post injection. Patients were observed several hours after injection, daily for 2 more days, then weekly for 3 weeks. Toxicity was defined by the Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0, observed over 5 weeks. MRI of the brain and the spine was performed at week 5 after treatment dose. Neurocognitive function and quality of life testing occurred starting at approximately 3 months after the last treatment dose.
Data availability
De-identified patient data will be shared at the discretion of the sponsor of the trial (YMabs, Therapeutics, Inc.) who is also the holder of the IND. Data in addition to that provided in this report that would be considered for sharing upon request are doses of drug administered, and pertinent radiographic images. No statistical analysis is planned for these 3 subjects of this report.
Patient 1
A 3 year old boy presented with headaches, ataxia and behavior changes. A left parietal mass measured 8.4 x. 6.5 x.7.2 cm with mass effect was emergently resected, confirmed to be ETMR (C19MC status unknown). There was no evidence of metastatic disease. He received 3 cycles of methotrexate, vincristine, etoposide, cyclophosphamide, and cisplatin followed by 3 cycles of consolidation (carboplatin and thiotepa) with autologous stem cell rescue (as per clinicaltrials.gov NCT00002515). Diffuse fungal lung and spleen lesions curtailed the full regimen. During treatment, the patient developed status epilepticus and MR showed local tumor recurrence. Tumor histology showed ganglion cell maturation of ETMR. He was then treated with serial injections of 131I-3F8 without complication followed by 131I-Omburtamab. After the 2 mCi 131I-Omburtamab dosimetry dose, he suffered from rotavirus, parainfluenza and grade 3 transaminitis related to infections so he was temporarily removed from the trial. The patient was treated with CSI and RT to the primary tumor bed (total dose = 54Gy), and resumed 131I-Omburtamab (2 mCi and 80 mCi) without incident.
Patient 2
A 3 year old girl presented with seizures; MRI brain revealed a large parietal lesion. Following complete resection, pathology confirmed ETMR; cytogenetic report showed amplification of the C19MC/MIR-371–373 region (19q13.42) by FISH analysis. She was treated with multi-agent chemotherapy including doxorubicin, cisplatin, vincristine, cyclophosphamide, etoposide, intraventricular methotrexate and focal proton beam RT (total dose =54Gy)14. Nine months after diagnosis she received 2 mCi and 50 mCi 131I-Omburtamab, treatment complicated by grade 4 prolonged thrombocytopenia.
Patient 3
A 3-year old boy presented with seizures from a right parietal tumor. He underwent a complete resection; CSF cytology was unremarkable; pathology showed ETMR, C19MC status unknown. He was treated with focal RT (45 Gy), multiagent chemotherapy (vincristine, etoposide, cyclophosphamide, and cisplatin, and twice with carboplatin and thiotepa with stem cell rescue. He suffered a local recurrence 11 months after diagnosis with further progression through retrieval chemotherapy. A second resection confirmed recurrent ETMR. One month later, he had a third local progression and again underwent subtotal resection and additional focal proton RT (36 CGE). He then received 2 and 50 mCi 131I-omburtamab. Post therapy MRIs demonstrated continued local progression of disease.
Results
Intraventricular 131I-Omburtamab was well tolerated in these 3 patients with ETMR.(Table 1). A high therapeutic index to CSF vs blood was achieved in all patents. Mean ROI doses were 230.4 to the ventricles (range 147.8–392 cGy/mCi) and 58.1 (range 43.1–81.7 cGy/mCi) to the spine. (Figure 1). At last follow-up, Patient 1 is 6.8 years after diagnosis and remains in remission; long term complications include cataracts, hypothyroidism, and growth hormone deficiency. Patient 2 is 2.3 years after diagnosis and remains in clinical and radiographic remission. Patient 3 continued to progress through therapy and died 7 months after therapy, 2 years after initial diagnosis.
Table 1.
Patient characteristics and results of treatment
| Age at diagnosis | Gender | Age at start of 131I-8H9 | Number of Relapses Prior to 131I-8H9 | Status when treated with 131I-8H9 | CSF cGy/mCi | Blood | ROI Ventricle | ROI Spine | Months to Relapse after 131I-8H9 | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 3y | Male | 4y 7mo | 0 | Radio-graphic/cytologic remission | 40.9 | 2.1 | 170 | 50.3 | - | NED |
| 3y | Female | 3y 10mo | 0 | Radio-graphic/cytologic remission | 22.5 | 3 | 147.8 | 81.7 | - | NED |
| 1y | Male | 2y 8 mo | 3 | Minimal post-opresidual | 179 | 2.3 | 392 | 57.6 | 1 | DOD after local relapse |
CSF, cerebrospinal fluid; 131-I-Omburtamab, iodinated 8H9 (Omburtamab); mo, months; OS, overall survival; ROI, region-of-interest; y, years; NED, no evaluable disease; DOD, died of disease.
Figure 1:
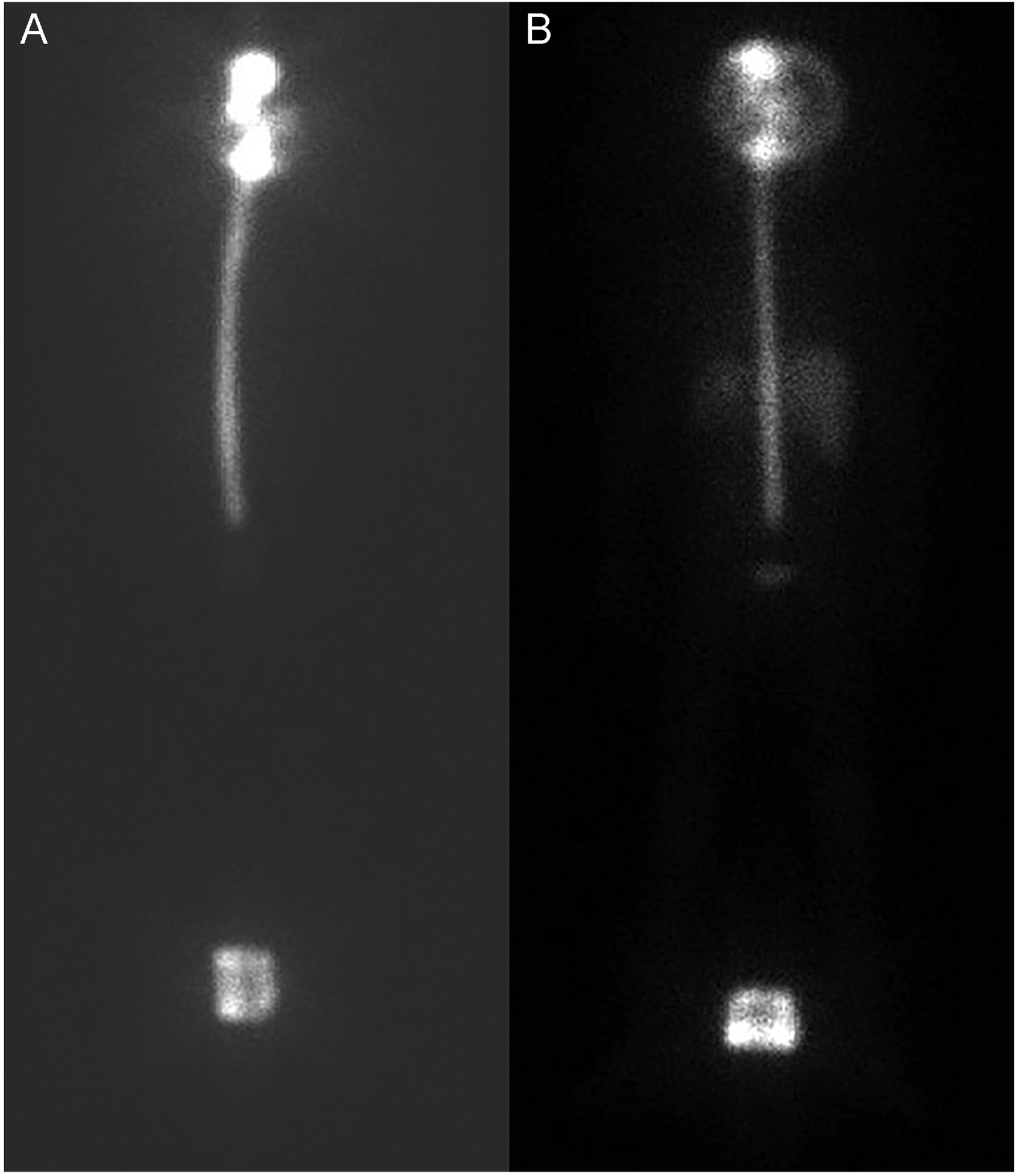
SPECT analysis at 4 (A) and 24 hours (B) after injection demonstrating activity of 131I-Omburtamab throughout the thecal sac.
Discussion
The cure of young children afflicted with ETMR remains a daunting challenge. Radiolabeled monoclonal antibodies targeting micrometastases are a promising therapy for brain tumors.
B7-H3 expression is significantly associated with poor outcome in several cancers, and is uniquely overexpressed in cancers compared to normal human tissues. Here we present mature data of a well-tolerated compartmental radiolabeled anti- B7-H3 monoclonal antibody for an incurable pediatric embryonal tumor. We describe the prolonged survival of 3 children treated with intraventricular 131I-Omburtamab, 2 of whom remain in clinical and radiographic remission at 6.8 and 2.3 years after completion of treatment. This is in stark contrast to published literature that shows an average OS of 12 months after diagnosis for patients with ETMR1, 3–6.
In this trial, there was no anticipated use of omburtamab in these patients, thus there were no limits of radiation doses of external beam RT before or after radioimmunotherapy. We have previously published the cumulative radiation doses administered to patients with other CNS tumors who received combined external beam RT and radioimmunotherapy. In that group, the radioimmunotherapy radiation is delivered by beta emission targeted 1–2mm. There does not appear to be a cumulative effect with the radiation delivered by combined external beam RT and radioimmunotherapy15. However we can, as cited, reference the radioimmunotherapy dose delivered to the CSF in cGy / mCi and CSI15.
We highlight the safety of radioimmunotherapy even in this young patient cohort. Specifically, there is an absence of radionecrosis seen in our three patients. Our prior retrospective analysis of patients with metastatic CNS neuroblastoma and medulloblastoma demonstrated the safety of using intrathecal radioimmunotherapy in patients who previously received radiotherapy without increasing the rate of radionecrosis, and neither of our patients has demonstrated evidence of radionecrosis15. Patient 1 was noted to have primary hypothyroidism diagnosed several years after completion of treatment. Given that he had 23Gy of radiation exposure to the hypothalamic pituitary axis from RT, it cannot be concluded that radioimmmunotherapy caused hypothyroidism. Neither patient 2 nor 3 had hypothyroidism following radioimmunotherapy. There were no changes on MRI that were attributable to receiving both RT and radioimmunotherapy.
Tumor cell cytotoxicity is attributed to the direct effect of 131I-radiation, although it is possible that a secondary mechanistic basis for successful eradication of microscopic tumor cells may in part be due to complement activation with the CSF space. Although there was interpatient variability in the dose delivered to the CSF, a high therapeutic ratio was achieved. Determining the optimal cGy delivered to the CSF by to fully eradicate micrometastatic deposits for this patient population and incorporating this into upfront treatment regimens is an ongoing focus of research.
Conclusions
131I-omburtamab appears safe with favorable dosimetry therapeutic index in ETMR. When used as consolidation following surgery and chemoradiation therapy, 131I-omburtamab may have therapeutic benefit for patients with ETMR.
Figure 2:
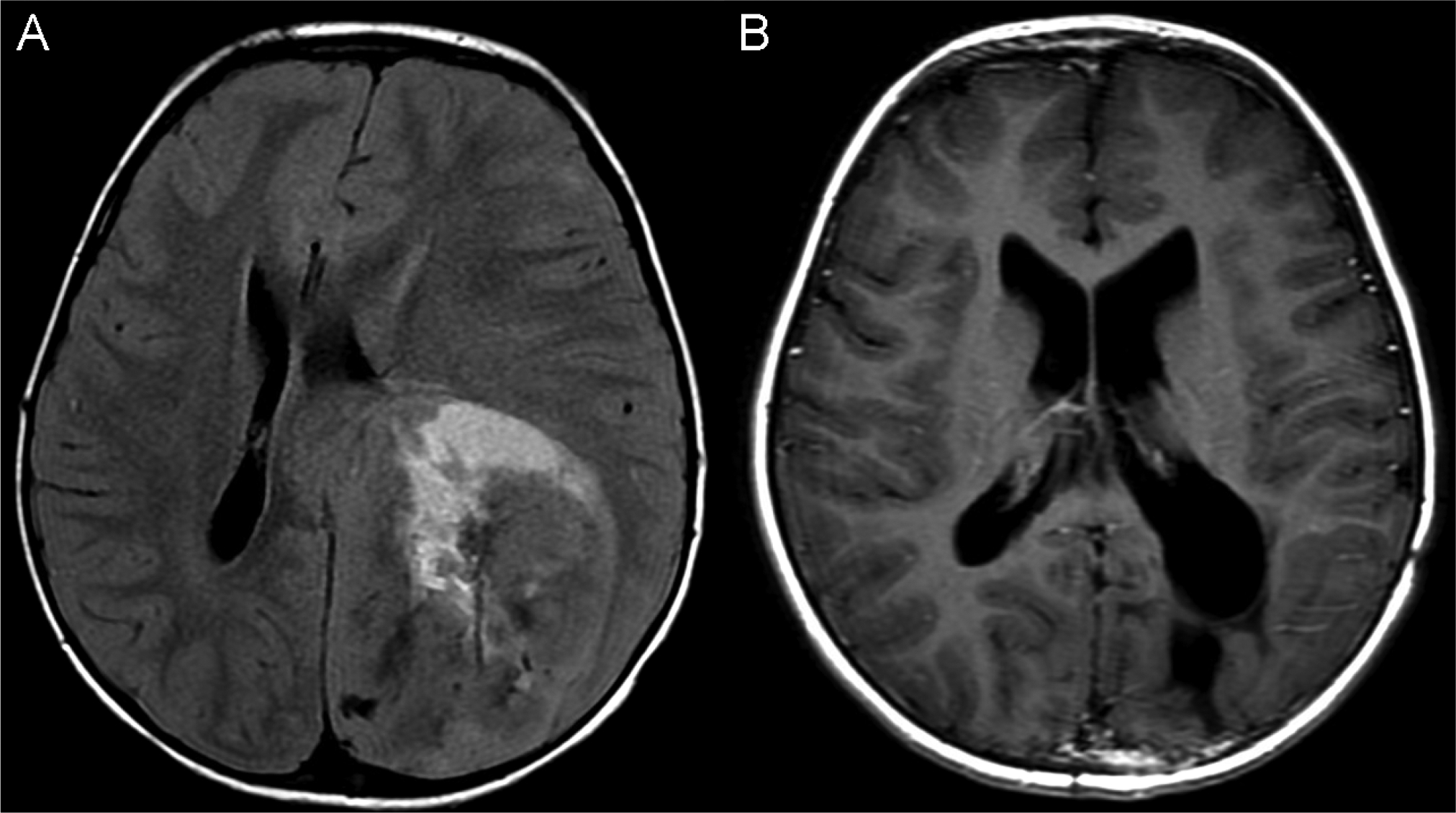
Patient 1 with tumor at diagnosis (A) and at most recent follow up (B).
Acknowledgements
We acknowledge support from the Memorial Sloan Kettering Cancer Center Support Grant (P30CA008748). We thank Mr. Joseph Olechnowicz for editorial assistance, Dr Serge Lyashchenko, HiJin Park, Jiong Wu for radiochemistry assistance. We are grateful to our patients and families for allowing us to participate in their clinical care.
Funding
The authors acknowledge support from the NIH Cancer Center Support Grant P30 CA008748
Footnotes
Conflict of interest: Dr. N.K. Cheung reports: receiving commercial research grants from Ymabs Therapeutics and Abpro-Labs Inc.; holding ownership interest/equity in Y-Mabs Therapeutics Inc., holding ownership interest/equity in Abpro-Labs, and owning stock options in Eureka Therapeutics. NKC is the inventor and owner of issued patents licensed by MSK to Ymabs Therapeutics, Biotec pharmacon, and Abpro-labs. NKC is a consultant/advisory board member for Abpro-Labs and Eureka Therapeutics. Dr. Pat Zanzonico is the inventor on a patent pending for systems and methods for determining optimum patient-specific antibody dose for tumor targeting. Dr. Kim Kramer is a consultant to Ymabs Therapeutics Inc. 8H9 is currently licensed to Ymabs Therapeutics. Data in this report was acquired prior to their sponsorship of this study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Research involving animal and human rights: There were no animals involved.
References
- 1.Ferri Niguez B, Martínez-Lage JF, Almagro MJ, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR): a new distinctive variety of pediatric PNET: a case-based update. Childs Nerv Syst 2010;26:1003–1008. [DOI] [PubMed] [Google Scholar]
- 2.Spence T, Sin-Chan P, Picard D, et al. CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol 2014;128:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozes P, Hauser P, Hortobágyi T, et al. Evaluation of the good tumor response of embryonal tumor with abundant neuropil and true rosettes (ETANTR). J Neurooncol 2016;126:99–105. [DOI] [PubMed] [Google Scholar]
- 4.Eberhart CG, Brat DJ, Cohen KJ, Burger PC. Pediatric neuroblastic brain tumors containing abundant neuropil and true rosettes. Pediatr Dev Pathol 2000;3:346–352. [DOI] [PubMed] [Google Scholar]
- 5.Manjila S, Ray A, Hu Y, Cai DX, Cohen ML, Cohen AR. Embryonal tumors with abundant neuropil and true rosettes: 2 illustrative cases and a review of the literature. Neurosurg Focus 2011;30:E2. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz M, Dufour C, Leblond P, et al. Embryonal tumors with multilayered rosettes in children: the SFCE experience. Childs Nerv Syst 2016;32:299–305. [DOI] [PubMed] [Google Scholar]
- 7.Jaramillo S, Grosshans DR, Philip N, et al. Radiation for ETMR: Literature review and case series of patients treated with proton therapy. Clin Transl Radiat Oncol 2019;15:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padovani L, André N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat Rev Neurol 2012;8:578–588. [DOI] [PubMed] [Google Scholar]
- 9.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001;61:4048–4054. [PubMed] [Google Scholar]
- 10.Ahmed M, Cheng M, Zhao Q, et al. Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. J Biol Chem 2015;290:30018–30029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souweidane MM, Kramer K, Pandit-Taskar N, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol 2018;19:1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miraldi FD, Nelson AD, Kraly C, et al. Diagnostic imaging of human neuroblastoma with radiolabeled antibody. Radiology 1986;161:413–418. [DOI] [PubMed] [Google Scholar]
- 13.Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol 2007;25:5465–5470. [DOI] [PubMed] [Google Scholar]
- 14.Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 2009;27:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer K, Pandit-Taskar N, Zanzonico P, et al. Low incidence of radionecrosis in children treated with conventional radiation therapy and intrathecal radioimmunotherapy. J Neurooncol 2015;123:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified patient data will be shared at the discretion of the sponsor of the trial (YMabs, Therapeutics, Inc.) who is also the holder of the IND. Data in addition to that provided in this report that would be considered for sharing upon request are doses of drug administered, and pertinent radiographic images. No statistical analysis is planned for these 3 subjects of this report.


