Abstract
Purpose
We sought to identify facility-level variation in the use of definitive therapy among men diagnosed with clinically localized low-risk prostate cancer and who are both older than 65 years of age and have a limited life expectancy (<10 years).
Materials and Methods
Using data from the National Cancer Data Base, we identified 24,077 men >65 years of age with <10-year life expectancy receiving definitive therapy at 1,172 facilities for biopsy-confirmed localized low-risk prostate cancer diagnosed between January 2004 and December 2013. A multilevel hierarchical mixed-effects logistic regression model was fitted to predict the odds of receiving definitive therapy.
Results
Overall, 18,178 (76%) men >65 years of age with a limited life expectancy and a diagnosis of low-risk prostate cancer received definitive therapy, although rates of therapy decreased significantly over time (p<0.001). Patients receiving definitive therapy were more often younger (≥80 vs. 66–69: OR 0.12, 95% CI 0.09–0.15; p<0.001) and White rather than Black (OR 0.86, 95% CI 0.75–0.98; p=0.03). Conversely, being uninsured (OR 0.37, 95% CI 0.21–0.63; p<0.001) and receiving care at an academic medical center (OR 0.36, 95% CI 0.28–0.46; p<0.001) conferred decreased odds of undergoing definitive therapy. The proportion of men undergoing definitive therapy ranged from 0.12% to 100% across facilities.
Conclusions
We found significant facility-level variation in rates of definitive therapy for men with localized prostate cancer and limited life expectancy. Health care providers and policy makers alike should be aware of the varying frequency with which this potentially low-value service is performed.
Keywords: prostatic neoplasms, health care quality
INTRODUCTION
Prostate cancer (PCa) represents the most frequently diagnosed non-cutaneous cancer and the second leading cause of cancer-related mortality among United States (US) men.1 Despite this, overall mortality remains low and many men diagnosed with PCa will die of other causes.2 Consequently, significant controversy has emerged surrounding both the overdiagnosis and subsequent overtreatment of PCa, prompting the emergence of viable treatment alternatives such as active surveillance, especially among men with limited life expectancy.3 While overall rates of radical prostatectomy appear to be decreasing since the 2012 United States Preventive Services Task Force’s (USPSTF) grade D recommendation against PSA screening, contemporary rates of reported overtreatment range from 5% to 46%.3–5
The aforementioned overtreatment trends are particularly glaring in the setting of looming payment reforms that will subject individual providers to a merit-based incentive payment system that aims to standardize care.6 National Comprehensive Cancer Network (NCCN) guidelines currently designate prostatectomy as an acceptable treatment option for men diagnosed with low-risk localized PCa.7 However, the combination of growing concern regarding overtreatment of localized disease and increasing emphasis on value-based care raises the possibility of active surveillance emerging as the only acceptable treatment option for low-risk PCa patients, particularly among men older than 69 years of age or with a life expectancy less than ten years. We therefore used the National Cancer Database (NCDB) to identify facility-level variation in the use of definitive therapy, defined as radical prostatectomy (RP), brachytherapy (BT) or external beam radiation (EBRT), among men diagnosed with clinically localized low-risk PCa and who are both older than 65 years of age and have a limited life expectancy (less than 10 years). Our guiding hypothesis is that this low-value practice varies according to several non-clinical factors such as the type of facility where care is provided and patient insurance status.
METHODS
Data Source and Study Population
The NCDB, a joint program of the Commission on Cancer (CoC) and the American Cancer Society, is a nationwide oncology database that contains information on patterns of cancer care and treatment outcomes. The NCDB collects data on newly diagnosed cancers since 1989 and includes information on more than 29 million cancers from >1,500 CoC-accredited programs in the US and Puerto Rico. The CoC accreditation process aims to ensure high quality patient care with a particular emphasis on patient-centered care. Approximately 40% of newly diagnosed PCa cases in the US are reported to the NCDB.8
All men >65 years of age with limited life expectancy and NCCN low-risk PCa (PSA <10ng/ml, Gleason Score ≤6 and clinical stage T1 to T2a) diagnosed between 2004 and 2013 were eligible for inclusion in our study. Men with a limited life expectancy (<10 years) were defined according to the method previously described by Daskivich et al.9 More specifically, men were considered to have a limited life expectancy if the cumulative incidence of other-cause mortality within 10 years exceeded 50%, which was generally satisfied if any of the following conditions were met: age 66 to 69 years with CCI ≥2, age 70 to 74 years with CCI ≥1, or age >75 years.
Cases with missing clinical T stage (cT), Gleason Score, and/or PSA as well as those who received definitive therapy other than RP, BT, or EBRT were excluded. TNM staging was established based on the American Joint Committee on Cancer Staging Manual (7th edition). Our selection criteria ultimately yielded 24,077 PCa assessable cases treated at 1,172 CoC accredited facilities.
Covariates
Baseline patient variables consisted of age at diagnosis, PSA at diagnosis, cT stage, year of diagnosis, race, percentage of adults within patient’s home zip code without a high school diploma quartiles, ZIP code™ level median income quartile, urban/rural/metropolitan status as defined by 2013 Rural-Urban Continuum Codes (https://www.ers.usda.gov/data-products/rural-urbancontinuum-codes), great circle distance (distance in miles between patient’s residence based on ZIP code centroid or city to street address of treating facility), and census geographical region.
Comorbidity burden at diagnosis was ascertained using the Charlson Comorbidity Index (CCI) and categorized into 0, 1, and ≥2 as specified by NCDB.10 More specifically, the CCI for each patient represents a weighted score derived from the sum of as many as ten reported ICD-9-CM or ICD-10 secondary diagnosis codes. Hospital volume was categorized according to case volume quartiles: very low (<20 cases), low (21–36 cases), high (37–66 cases), and very high (≥67cases). CoC facility type was categorized as Community Cancer Program (CCP), Comprehensive Community Cancer Program (CCCP), Academic/Research Program, Integrated Network Cancer Program, and Other.
Statistical analyses
Frequencies and proportions were reported for categorical variables, while median and interquartile ranges were reported for continuous variables. Bivariate differences in categorical and continuous variables between treatment groups were examined using the Rao-Scott chi-square and the Wilcoxon tests, respectively. Both approaches accounted for clustering within facilities.
A multilevel hierarchical mixed-effects logistic regression model with a random effect to capture potential clustering in each CoC facility was fitted to predict the odds of receiving definitive therapy (RP, EBRT or BT) for prostate cancer. Fixed covariates (fixed effects) included the life-expectancy cohorts, sociodemographic variables (race, education, income, urban/rural status, great circle distance), and facility features (type, census geographical region, hospital volume). Figures were created based on the adjusted probabilities of receiving definitive therapy.
Pseudo-R^2 values derived from the multilevel hierarchical mixed-effects logistic regression model were used to assess the relative contribution of patient and hospital variables to the odds of receiving definitive therapy. Sensitivity analyses were performed on high and very high-volume facilities in order to reduce potential confounding by outlier institutions treating a small number of patients.
All analyses were performed using STATA 14 (STATA Corp, College Station, TX, United States), with a two-sided significance level set at p<0.05. An institutional review board waiver from Brigham and Women’s Hospital was obtained prior to conducting this study.
RESULTS
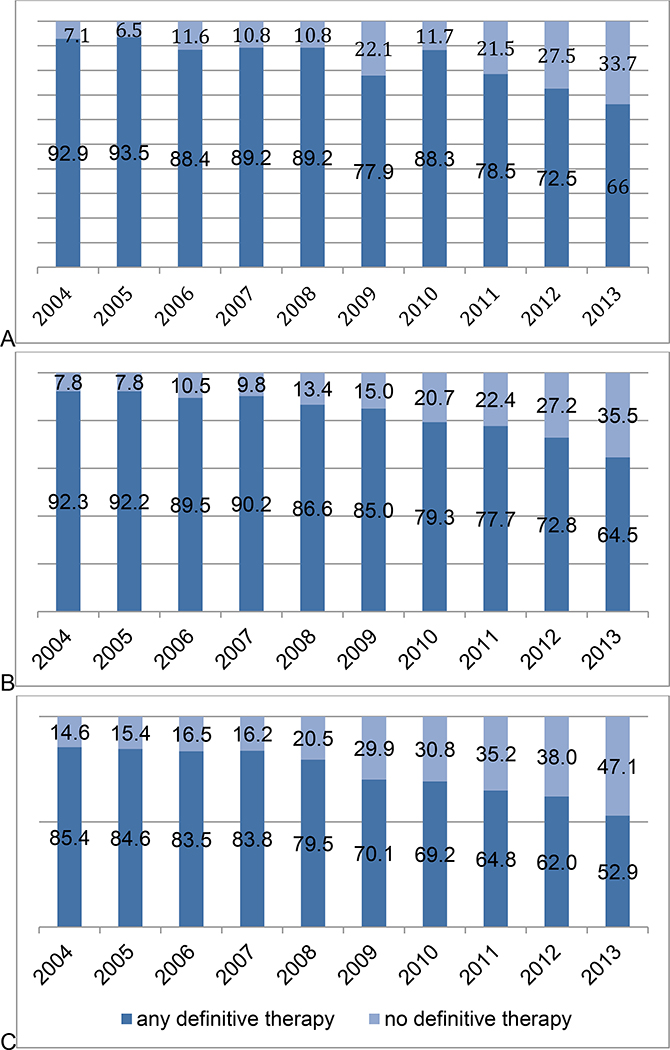
Baseline characteristics of the cohort are presented in Table 1. Overall, 18,178 (76%) men >65 years of age with a limited life expectancy and a diagnosis of NCCN low-risk PCa received definitive therapy in the form of RP, BT, or EBRT. However, rates of definitive therapy decreased significantly over the study period across all cohorts of men with limited life expectancy, particularly between 2010 and 2013 (Figure 1). More specifically, rates decreased by 19% (95% CI 10.7–27.9, p<0.001) for men age 66 to 69 years with CCI ≥2 (A), by 19.8% (95% CI 17.1–22.5, p<0.001) for men age 70 to 74 years with CCI ≥1, and by 15.2% (95% CI 12.2–18.2, p<0.001) for men 75–79 with any CCI (C) over the course of the study period. Those undergoing treatment were more frequently younger (p<0.001), White (p=0.01), diagnosed with PCa during the first half of the study period (p<0.001), and privately insured (p<0.001). Alternatively, those not undergoing definitive therapy more frequently received their care from an academic medical center (p<0.001).
Table 1.
Baseline sociodemographic and facility characteristics.
| Variables | Whole cohort n, (%) | No Treatment n, (%) | Treatment n, (%) | p-value |
|---|---|---|---|---|
| Continuous Variables | ||||
| Age, years (Mean, SD) | 76.24 (3.74) | 77.48 (4.11) | 75.85 (3.51) | <0.001 |
| PSA, ng/ml (Median, IQR) | 5.6 (4.4–7.2) | 5.8 (4.5–7.4) | 5.6 (4.4–7.2) | <0.001 |
| Categorical Variables | ||||
| Life Expectancy Groups | <0.001 | |||
| 66–69 & CCI=2 | 758 (3.15) | 136 (2.31) | 622 (3.42) | |
| 70–74 & CCI≥1 | 4,485 (18.63) | 755 (12.80) | 3,730 (20.52) | |
| 75–79 & all CCI | 15,097 (62.70) | 3,450 (58.48) | 11,647 (64.07) | |
| ≥80 & all CCI | 3,737 (15.52) | 1,558 (26.41) | 2,179 (11.99) | |
| Race | 0.0113 | |||
| White | 20,944 (86.99) | 5,040 (85.44) | 15,904 (87.49) | |
| Black | 2,218 (9.21) | 606 (10.27) | 1,612 (8.87) | |
| Other | 627 (2.60) | 153 (2.59) | 474 (2.61) | |
| Unknown | 288 (1.20) | 100 (1.70) | 188 (1.03) | |
| Year of Diagnosis | <0.001 | |||
| 2004 | 2,989 (12.41) | 509 (8.63) | 2,480 (13.64) | |
| 2005 | 2,877 (11.95) | 493 (8.36) | 2,384 (13.11) | |
| 2006 | 3,104 (12.89) | 579 (9.82) | 2,525 (13.89) | |
| 2007 | 3,154 (13.10) | 570 (9.66) | 2,584 (14.21) | |
| 2008 | 2,750 (11.42) | 632 (10.71) | 2,118 (11.65) | |
| 2009 | 2,292 (9.52) | 662 (11.22) | 1,630 (8.97) | |
| 2010 | 2,112 (8.77) | 648 (10.98) | 1,464 (8.05) | |
| 2011 | 2,072 (8.61) | 690 (11.70) | 1,382 (7.60) | |
| 2012 | 1,433 (5.95) | 538 (9.12) | 895 (4.92) | |
| 2013 | 1,294 (5.37) | 578 (9.80) | 716 (3.94) | |
| Clinical T Stage | 0.0001 | |||
| 1 | 20,508 (85.18) | 5,157 (87.42) | 15,351(84.45) | |
| 2 | 3,569 (14.82) | 742 (12.58) | 2,827 (15.55) | |
| Insurance Type | <0.001 | |||
| Private | 2,948 (12.24) | 665 (11.27) | 2,283 (12.56) | |
| Medicaid | 239 (0.99) | 83 (1.41) | 156 (0.86) | |
| Medicare | 20,234 (84.04) | 4,948 (83.88) | 15,286 (84.09) | |
| Other Government | 172 (0.71) | 31 (0.53) | 141 (0.78) | |
| Uninsured | 90 (0.37) | 42 (0.71) | 48 (0.26) | |
| Unknown | 394 (1.64) | 130 (2.20) | 264 (1.45) | |
| Income | 0.8551 | |||
| ≥$63,000 | 7,573 (31.45) | 1,816 (30.78) | 5,757 (31.67) | |
| $48,000–$62,999 | 6,636 (27.56) | 1,650 (27.97) | 4,986 (27.43) | |
| $38,000–$47,999 | 5,841 (24.26) | 1,421 (24.09) | 4,420 (24.32) | |
| ≤$37,999 | 4,005 (16.63) | 1,007 (17.07) | 2,998 (16.49) | |
| Unknown | 22 (0.09) | 5 (0.08) | 17 (0.09) | |
| Education Level* | 0.1838 | |||
| ≥21% | 3,495 (14.52) | 903 (15.31) | 2,592 (14.26) | |
| 13–20.9% | 5,903 (24.52) | 1,354 (22.95) | 4,549 (25.02) | |
| 7–12.9% | 8,421 (34.98) | 2,053 (34.80) | 6,368 (35.03) | |
| ≤6.9% | 6,246 (25.94) | 1,585 (26.87) | 4,661 (25.64) | |
| Unknown | 12 (0.05) | 4 (0.07) | 8 (0.04) | |
| Great Circle Distance, Miles** | 0.0933 | |||
| ≤12.4 | 15,081 (62.64) | 3,590 (60.86) | 11,491 (63.21) | |
| 12.5–49.9 | 7,103 (29.50) | 1,758 (29.80) | 5,345 (29.40) | |
| ≥50 | 1,893 (7.86) | 551 (9.34) | 1,342 (7.38) | |
| Urban/Rural Status | 0.8117 | |||
| Metro | 19,200 (79.74) | 4,665 (79.08) | 14,535 (79.96) | |
| Urban | 3,899 (16.19) | 991 (16.80) | 2,908 (16.00) | |
| Rural | 544 (2.26) | 135 (2.29) | 409 (2.25) | |
| Unknown | 434 (1.80) | 108 (1.83) | 326 (1.79) | |
| Facility Type | <0.001 | |||
| Comprehensive Community | 12,959 (53.82) | 2,547 (43.18) | 10,412 (57.28) | |
| Community Cancer Center | 3,157 (13.11) | 884 (14.99) | 2,273 (12.50) | |
| Integrated Network Cancer Program | 1,404 (5.83) | 262 (4.44) | 1,142 (6.28) | |
| Academic | 6,552 (27.21) | 2,206 (37.40) | 4,346 (23.91) | |
| Other | 5 (0.02) | 0 (0) | 5 (0.03) | |
| Facility Case Volume | 0.8814 | |||
| Very Low (≤20/year) | 6,187 (25.70) | 1,559 (26.43) | 4,628 (25.46) | |
| Low (21–36/year) | 5,896 (24.49) | 1,413 (23.95) | 4,483 (24.66) | |
| High (37–66/year) | 6,089 (25.29) | 1,444 (24.48) | 4,645 (25.55) | |
| Very High (≥67/year) | 5,905 (24.53) | 1,483 (25.14) | 4,422 (24.33) | |
| Facility Location | 0.1619 | |||
| New England | 1,547 (6.43) | 504 (8.54) | 1,043 (5.74) | |
| Middle Atlantic | 4,158 (17.27) | 894 (15.16) | 3,264 (17.96) | |
| South Atlantic | 5,259 (21.84) | 1,235 (20.94) | 4,024 (22.14) | |
| East North Central | 4,750 (19.73) | 1,286 (21.80) | 3,464 (19.06) | |
| East South Central | 1,734 (7.20) | 320 (5.42) | 1,414 (7.78) | |
| West North Central | 2,242 (9.31) | 573 (9.71) | 1,669 (9.18) | |
| West South Central | 1,309 (5.44) | 324 (5.49) | 985 (5.42) | |
| Mountain | 738 (3.07) | 193 (3.27) | 545 (3.00) | |
| Pacific | 2,340 (9.72) | 570 (9.66) | 1,770 (9.74) |
Percentage of adults within patient’s home zip code without a high school diploma quartiles.
Distance in miles between patient’s residence based on ZIP code centroid or city to street address of treating facility.
Figure 1.
Overall, rates of definitive therapy decreased significantly over the study period among (A) men aged 66 to 69 years with CCI ≥2 (p<0.001), (B) men aged 70 to 74 years with CCI ≥1 (p<0.001), and (C) men 75–79 with any CCI (p<0.001).
After adjustment, several clinical and non-clinical variables predicted the receipt of definitive therapy (Table 2). For example, patients receiving definitive therapy were more often younger (≥80 vs. 66–69: OR 0.12, 95% CI 0.09–0.15; p<0.001), presenting with high clinical T stage (OR 1.46, 95% CI 1.31–1.63; p<0.001), and receiving care in the East South Central versus New England regions (OR 3.72, 95% CI 2.20–6.29; p<0.001). Conversely, being Black rather than White (OR 0.86, 95% CI 0.75–0.98; p=0.03), uninsured (OR 0.37, 95% CI 0.21–0.63; p<0.001) and receiving care at an academic medical center (OR 0.35, 95% CI 0.28–0.45; p<0.001) conferred decreased odds of undergoing definitive therapy.
Table 2.
Multivariable logistic regression analysis predicting receipt of definitive therapy in patients with low-risk prostate and limited life expectancy.
| OR | 95% CI | p-value | |
|---|---|---|---|
| Continuous Variables | |||
| PSA, ng/ml | 1.01 | 1.00–1.03 | 0.118 |
| Categorical Variables | |||
| Life Expectancy | |||
| 66–69 & CCI2 | reference | ||
| 70–74 & CCI≥1 | 0.76 | 0.60–0.96 | 0.023 |
| 75–79 & all CCI | 0.35 | 0.28–0.44 | <0.001 |
| ≥80 & all CCI | 0.12 | 0.09–0.15 | <0.001 |
| Race | |||
| White | reference | ||
| Black | 0.86 | 0.75–0.98 | 0.027 |
| Other | 0.97 | 0.76–1.24 | 0.831 |
| Unknown | 0.68 | 0.50–0.93 | 0.015 |
| Year of Diagnosis | |||
| 2004 | reference | ||
| 2005 | 0.93 | 0.79–1.09 | 0.367 |
| 2006 | 0.87 | 0.75–1.02 | 0.090 |
| 2007 | 0.87 | 0.74–1.01 | 0.072 |
| 2008 | 0.61 | 0.52–0.72 | <0.001 |
| 2009 | 0.42 | 0.36–0.50 | <0.001 |
| 2010 | 0.38 | 0.32–0.44 | <0.001 |
| 2011 | 0.33 | 0.28–0.38 | <0.001 |
| 2012 | 0.25 | 0.21–0.30 | <0.001 |
| 2013 | 0.18 | 0.15–0.22 | <0.001 |
| Clinical T Stage | |||
| 1 | reference | ||
| 2 | 1.46 | 1.31–1.63 | <0.001 |
| Insurance Type | |||
| Private | reference | ||
| Medicaid | 0.87 | 0.61–1.24 | 0.440 |
| Medicare | 0.89 | 0.79–1.00 | 0.046 |
| Other government | 2.00 | 1.23–3.24 | 0.005 |
| Not insured | 0.37 | 0.21–0.63 | <0.001 |
| Unknown | 0.65 | 0.49–0.87 | 0.003 |
| Income | |||
| ≥$63,000 | reference | ||
| $48–62,999 | 0.91 | 0.81–1.02 | 0.119 |
| $38–47,999 | 0.95 | 0.83–1.09 | 0.456 |
| ≤$37,999 | 0.87 | 0.73–1.03 | 0.109 |
| Unknown | 4.44 | 0.43–45.40 | 0.209 |
| Education Level | |||
| (≥21%) | reference | ||
| 13% – 20.9% | 1.13 | 0.98–1.29 | 0.087 |
| 7% – 12.9% | 1.00 | 0.86–1.17 | 0.951 |
| ≤6.9% | 0.98 | 0.82–1.16 | 0.796 |
| Unknown | 0.19 | 0.01–2.98 | 0.234 |
| Great Circle Distance, Miles | |||
| ≤12.4 | reference | ||
| 12.5–49.9 | 1.10 | 1.01–1.21 | 0.028 |
| ≥50 | 1.34 | 1.14–1.58 | <0.001 |
| Urban/Rural Status | |||
| Metro | reference | ||
| Urban | 1.12 | 0.98–1.27 | 0.087 |
| Rural | 1.12 | 0.86–1.47 | 0.404 |
| Unknown | 1.06 | 0.80–1.41 | 0.515 |
| Facility Type | |||
| Comprehensive Community Cancer Center | reference | ||
| Community Cancer center | 0.52 | 0.41–0.65 | <0.001 |
| Integrated network cancer | 0.84 | 0.54–1.32 | 0.460 |
| Academic | 0.35 | 0.28–0.45 | <0.001 |
| Other | 3.13 | 0–5.50 | 0.999 |
| Facility Case Volume | |||
| Very Low (≤20/year) | reference | ||
| Low (21–36/year) | 1.01 | 0.79–1.29 | 0.914 |
| High (37–66/year) | 0.97 | 0.72–1.31 | 0.854 |
| Very High (≥67/year) | 1.10 | 0.75–1.62 | 0.614 |
| Facility Location | |||
| New England | reference | ||
| Middle Atlantic | 3.07 | 2.00–4.73 | <0.001 |
| South Atlantic | 2.15 | 1.43–3.22 | <0.001 |
| East North Central | 2.02 | 1.34–3.04 | 0.001 |
| East South Central | 3.72 | 2.20–6.29 | <0.001 |
| West North Central | 2.79 | 1.76–4.43 | <0.001 |
| West South Central | 2.17 | 1.31–3.59 | 0.003 |
| Mountain | 3.36 | 1.97–5.75 | <0.001 |
| Pacific | 2.37 | 1.49–3.72 | <0.001 |
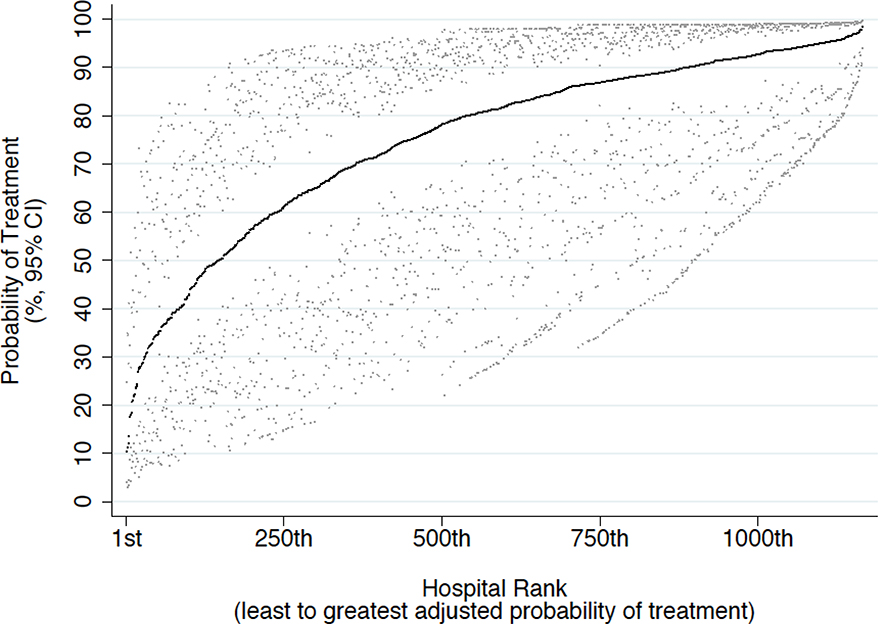
Significant facility-level variation in the rates of definitive therapy among men with limited life expectancy was noted, with the proportion of men undergoing definitive therapy ranging from 0.12% to 100% among the 1,172 institutions included in our analysis (Figure 2). Pseudo-R2 values derived from our multilevel hierarchical mixed effects logistic regression model (Table 3) revealed that patient-level factors explained 56% of the variation in receiving definitive therapy. Facility-level features such as facility type, location, and treatment volume contributed 10% of the variation.
Figure 2.
The overall adjusted facility-level probability of receiving definitive therapy among 24,077 patients with limited life expectancy treated at 1,172 Commission on Cancer facilities between 2012 and 2013 was 74% (95% CI 6089%). Facilities are sorted in ascending order of probability of treatment.
Table 3.
Partial R2 Values Derived from Mixed Effects Model: Factors Associated with the Receipt of Definitive Therapy Among Men ≥66 Years of Age with Low-risk PCa and Limited Life Expectancy (n=24,077) Across 1,165 Facilities (National Cancer Data Base, 2004–2013)
| Variable | P-value | Partial R2 |
|---|---|---|
| Overall Model | <0.001 | 0.7203 |
| Patient-level Variables | <0.001 | 0.5629 |
| Life Expectancy Group | <0.001 | 0.4425 |
| Race | 0.0240 | 0.0080 |
| Clinical T Stage | <0.001 | 0.0387 |
| PSA | 0.1221 | 0.0020 |
| Year of Diagnosis | <0.001 | 0.3948 |
| Distance from treating facility | 0.0012 | 0.0114 |
| Insurance Type | <0.001 | 0.0262 |
| Income | 0.2402 | 0.0047 |
| Education | 0.1092 | 0.0063 |
| Facility-level Variables | <0.001 | 0.0965 |
| Region | 0.9624 | 0.0002 |
| Facility Type | <0.001 | 0.0637 |
| Facility Location | <0.001 | 0.0323 |
| Facility Case Volume | 0.9557 | 0.0003 |
DISCUSSION
Significant controversy has emerged surrounding both the diagnosis and treatment of PCa in the years following the 2012 USPSTF grade D recommendation against PSA-based screening. Special attention has been placed on the evaluation and treatment of men older than 69 and/or with limited life expectancy given that the risks associated with therapy may outweigh any clinical benefits.11 While overall rates of both PSA testing and radical prostatectomy have decreased following the USPSTF’s 2012 guideline recommendation, self-reported rates of PSA screening among men with a limited life expectancy remain high and there is evidence suggesting that these men ultimately undergo definitive therapy.12, 13 Given recent public and private efforts aimed at eliminating low-value clinical practices, it is critical that providers and policymakers alike gain insight into both the incidence of and factors contributing to the continued use of low-value services.14, 15 We therefore used the NCDB to investigate facility-level variation in rates of definitive therapy for localized PCa among men with limited life expectancy. Our study has several important findings.
First, we found that 76% of men greater than 65 years of age with limited life expectancy and a diagnosis of NCCN low-risk PCa received definitive therapy in the form of either radiation or surgery, although it is noteworthy that rates of therapy did decrease dramatically between 2010 and 2013. Notably, 12% of men aged greater than 80 years underwent some form of definitive therapy for low-risk PCa. These findings are similar to those reported by Daskavich et al., who used the Surveillance, Epidemiology, and End Results-Medicare database (SEER) to identify 50,049 men ≥66 years of age with limited life expectancy undergoing aggressive therapy for low/intermediate risk PCa between 1991 and 2007.9 They found that among men with limited life expectancy aged 66 to 69 years, 70 to 74 years, 75 to 79 years, and ≥80 years, aggressive treatment was received by 68%, 69%, 57%, and 24%, respectively. More recently, Borza et al. used a 20% sample of Medicare data to demonstrate that 25% of men ≥66 years of age with a greater than 78% chance of 10-year non-cancer mortality and with newly diagnosed PCa underwent curative prostate cancer treatment.16 Of note, pathological data was not available for that study. Taken together, our findings suggest that the prevalence of this low-value practice, while still performed, appears to be declining in the setting of recent value-based purchasing efforts and literature supporting the efficacy and safety of treatment alternatives, such as active surveillance.6, 17
Several non-clinical factors, such as facility features, also influenced the odds of receiving definitive therapy, and accounted for 10% of the variation observed in our study. Notably, men with limited life expectancy receiving care at academic hospitals had decreased odds of undergoing definitive therapy. This finding is consistent with recent literature demonstrating superior performance among academic medical centers compared to community centers with regards to rates of suboptimal or contraindicated urologic health services, which may represent a form of supplier-induced demand.18 For example, using the NCDB, Loppenberg et al. found that men newly diagnosed with low-risk PCa receiving care at academic medical centers had over twice the odds of being placed on active surveillance relative to men receiving care at a comprehensive community cancer center.19 Such trends appear to extend beyond the realm of urologic oncology, with academic centers or practices with an academic affiliation typically demonstrating superior performance relative to community centers with regards to limiting the number of low value services offered in general.20
We also found that several clinical factors predicted receipt of definitive therapy. More specifically, younger men, Whites, and those with higher clinical T stage experienced greater odds of undergoing definitive therapy. It is unsurprising that younger men and those with higher clinical T stage experienced greater odds of undergoing definitive therapy as these men have the greatest odds of disease progression and cancer-related mortality over the course of their lives, and in theory stand to benefit from earlier intervention.21, 22 Such patient-level predictors ultimately explained 56% of the variation observed in our study. Collectively, these results suggest that counseling patients on the concept of competing risks remains difficult, particularly when they are faced with a new cancer diagnosis that they desire treatment for. Contemporary literature suggests that improved access to standardized patient education and support materials may help resolve the aforementioned patient-provider conflicts while simultaneously preserving patients’ values, which will grow in importance as policies aimed at standardizing care are implemented over the coming years.23
Our findings are timely given looming payment reforms that emphasize value-based care redesign. Beginning in 2019, health care provider organizations participating in the Medicare Shared Savings Program will be responsible for any costs exceeding 48 predefined episodes of care payment bundles (hospitalization following radical prostatectomy is not currently included in this list).24 Similar policies will also impact outpatient care. Under the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), individual providers will be subject to a merit-based incentive payment system that aims to standardize care.6 It is conceivable that urologists may eventually be faced with a payment environment in which definitive therapy in men with limited life expectancy is no longer reimbursed and/or penalized.11
Our findings must be considered within the statistical limitations of our study design. As with any retrospective cohort study, our findings are subject to the influence of unaccounted for confounders. Regarding patient selection, we identified men with limited life expectancy using a technique previously validated using SEER data, and it is conceivable that inherent differences between the NCDB and SEER cohorts effected the specificity of our selection criteria.25 Along these lines, while SEER contains “population-level” data, NCDB is specific to care received at hospitals, and therefore may be subject to selection bias.
Furthermore, debate exists over the accuracy of comorbidity indices extrapolated from claims data.26 Additionally, we were unable to identify individuals that were initially placed on AS and subsequently failed/progressed to treatment, which may partially account for the seemingly high percentage of men with limited life expectancy undergoing definitive therapy. Similarly, we were unable to determine which men were receiving palliative therapy, although this seems unlikely given that we restricted our cohort to men with low-risk PCa.
CONCLUSIONS
Using a nationwide hospital-based database, we found that a high proportion of men with low-risk PCa and limited life expectancy underwent definitive therapy, although rates of therapy decreased significantly over the study period. Rates of definitive therapy varied according to both patient and facility-level variables. Health care providers and policymakers alike should be aware of the varying frequency with which this potentially low-value service is performed, particularly in the setting of looming value-based payment reform efforts and growing concern over PCa overdiagnosis and overtreatment.
Supplementary Material
Acknowledgements
Quoc-Dien Trinh is supported by the Brigham Research Institute Fund to Sustain Research Excellence, the Bruce A. Beal and Robert L. Beal Surgical Fellowship, the Genentech Bio-Oncology Career Development Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology, a Health Services Research pilot test grant from the Defense Health Agency, the Clay Hamlin Young Investigator Award from the Prostate Cancer Foundation, and an unrestricted educational grant from the Vattikuti Urology Institute. David F. Friedlander is supported by a National Institutes of Health T32 training grant.
KEY OF ABBREVIATIONS
- PCa
prostate cancer
- CoC
Commission on Cancer
- SEER
Surveillance, Epidemiology and End Results
- NCDB
National Cancer Data Base
- US
United States
- CCI
Charlson-Deyo comorbidity index
- IQR
interquartile range
- NCCN
National Comprehensive Cancer Network
- PSA
prostate-specific antigen
Footnotes
Disclosure: The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Quoc-Dien Trinh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin, 65: 5, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Welch HG, Black WC: Overdiagnosis in cancer. J Natl Cancer Inst, 102: 605, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Loeb S, Bjurlin MA, Nicholson J et al. : Overdiagnosis and overtreatment of prostate cancer. Eur Urol, 65: 1046, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpern JA, Shoag JE, Artis AS et al. : National Trends in Prostate Biopsy and Radical Prostatectomy Volumes Following the US Preventive Services Task Force Guidelines Against Prostate-Specific Antigen Screening. JAMA Surg, 152: 192, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Huland H, Graefen M: Changing Trends in Surgical Management of Prostate Cancer: The End of Overtreatment? Eur Urol, 68: 175, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Printz C: MACRA paves way for changes in reimbursements: Physicians hopeful law will lead to more value-based care. Cancer, 121: 2103, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Armstrong AJ, Bahnson RR et al. : Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw, 14: 19, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Miller DC, Hafez KS, Stewart A et al. : Prostate carcinoma presentation, diagnosis, and staging: an update form the National Cancer Data Base. Cancer, 98: 1169, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Daskivich TJ, Lai J, Dick AW et al. : Variation in treatment associated with life expectancy in a population-based cohort of men with early-stage prostate cancer. Cancer, 120: 3642, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson M, Szatrowski TP, Peterson J et al. : Validation of a combined comorbidity index. J Clin Epidemiol, 47: 1245, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Bibbins-Domingo K, Grossman DC, Curry SJ: The US Preventive Services Task Force 2017 Draft Recommendation Statement on Screening for Prostate Cancer: An Invitation to Review and Comment. Jama, 317: 1949, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Daskivich TJ, Chamie K, Kwan L et al. : Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer, 117: 2058, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Fedewa SA, Ma J et al. : Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. Jama, 314: 2054, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Burwell SM: Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med, 372: 897, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Cassel CK, Guest JA: Choosing wisely: helping physicians and patients make smart decisions about their care. Jama, 307: 1801, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Borza T, Kaufman SR, Shahinian VB et al. : Sharp Decline In Prostate Cancer Treatment Among Men In The General Population, But Not Among Diagnosed Men. Health Aff (Millwood), 36: 108, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Nyame YA, Almassi N, Haywood SC et al. : Intermediate-Term Outcomes for Men with Very Low/Low and Intermediate/High Risk Prostate Cancer Managed by Active Surveillance. J Urol, 198: 591, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Smith R: Why medicine is overweight. Don’t forget inconvenient truth of supplier induced demand. Bmj, 340: c3334, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Loppenberg B, Friedlander DF, Krasnova A et al. : Variation in the use of active surveillance for low-risk prostate cancer. Cancer, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Rocque G, Blayney DW, Jahanzeb M et al. : Choosing Wisely in Oncology: Are We Ready For Value-Based Care? J Oncol Pract, 13: e935, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Cary KC, Cowan JE, Sanford M et al. : Predictors of pathologic progression on biopsy among men on active surveillance for localized prostate cancer: the value of the pattern of surveillance biopsies. Eur Urol, 66: 337, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Godtman RA, Holmberg E, Khatami A et al. : Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol, 63: 101, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Berry DL, Halpenny B, Hong F et al. : The Personal Patient ProfileProstate, Decision Support for Men With Localized Prostate Cancer: A Multi-center Randomized Trial. Urologic oncology, 31: 1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bundled Payments for Care Improvement (BPCI) initiative: general information. CMS.gov, vol. 2018, 2017 [Google Scholar]
- 25.Mettlin CJ, Menck HR, Winchester DP et al. : A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer, 79: 2052, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Potosky AL, Legler JM et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol, 53: 1258, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.