Abstract
Age-related hearing loss (ARHL), clinically referred to as presbycusis, is one of the three most prevalent chronic medical conditions of our elderly, with the majority of persons over the age of 60 suffering from some degree of ARHL. The progressive loss of auditory sensitivity and perceptual capability results in significant declines in workplace productivity, quality of life, cognition and abilities to communicate effectively. Aldosterone is a mineralocorticoid hormone produced in the adrenal glands and plays a role in the maintenance of key ion pumps, including the Na-K+-Cl co-transporter 1 or NKCC1, which is involved in homeostatic maintenance of the endocochlear potential. Previously we reported that aldosterone (1 μM) increases NKCC1 protein expression in vitro and that this up-regulation of NKCC1 was not dose-dependent (dosing range from 1 nM to 100 μM). In the current study we measured behavioral and electrophysiological hearing function in middle-aged mice following long-term systemic treatment with aldosterone. We also confirmed that blood pressure remained stable during treatment and that NKCC1 protein expression was upregulated. Pre-pulse inhibition of the acoustic startle response was used as a functional measure of hearing, and the auditory brainstem response was used as an objective measure of peripheral sensitivity. Long-term treatment with aldosterone improved both behavioral and physiological measures of hearing (ABR thresholds). These results are the first to demonstrate a protective effect of aldosterone on age-related hearing loss and pave the way for translational drug development, using aldosterone as a key component to prevent or slow down the progression of ARHL.
Keywords: Aging, aldosterone, Hearing loss, startle reflex, Drug development
Age-related hearing loss (ARHL), or presbycusis, is one of the top three, major chronic medical conditions in elderly people, along with cardiovascular disease and arthritis. The ARHL loss of auditory sensitivity and speech perceptual capability has an extremely high prevalence, with the majority of persons over the age of 60 suffering from ARHL, so hundreds of millions of people worldwide. The psychological sequelae accompanying ARHL are associated with depression, anxiety, social isolation, loneliness and can even be life threatening (Dalton, et al. 2003; Kramer et al. 2002; Strawbridge et al. 2000; Viljanen et al., 2009). Furthermore, presbycusis has been associated with more rapid cognitive decline in the elderly (Lin et al. 2011; Lindenberger et al. 2009; Van et al. 2007). The perceptual difficulties in suprathreshold hearing faced by older listeners likely develop not only from impaired cochlear function, but also from age-related physiological changes in the parts of the brain used for hearing – central auditory system (Frisina and Frisina, 1997; Takeda et al. 1992; Syka, 2002; Willott, 1991 & 1996). Virtually all components of the nervous system suffer from numerous age-related declines such as a loss of neurons and atrophy of their processes (e.g., axons, dendrites, and synapses) that degrade auditory processing in neural circuits. Age-linked systemic changes in hormonal, cardiovascular, and metabolic functions provide additional challenges (Aspinall, 2003; Frisina et al. 2006; Guimaraes et al., 2006; Tadros et al. 2005; Willott & Turner, 1999). The auditory system is, of course, no exception in its susceptibility to these and other biological changes with age.
A key player in ARHL is the decline in function of cochlear stria vascularis (SV) cells located in the lateral wall of the inner ear. Within the SV there are specific ion channels and pumps, which control the concentrations of Na+ and K+ for the endolymph of scala media. Two of these important ion channels are Na+-K+-2Cl− co-transporter (NKCC1) and Na+/K+-ATPase, which are found in many physiological systems (Anderson, and Cala, 2006; Ding et al. 2014; Herbert et al. 2004, Garg et al 2007; Dowd and Forbush, 2003; Wall et al, 2006). NKCC1 is a co-transporter protein that moves Na+, K+, and Cl− into and out of cells (Pedersen et al. 2006). NKCC1 levels and the flow of the ions regulated by it functionally decline with age. In the auditory system, the cochlea depends heavily on the presence of NKCC1 transporters, specifically in the basolateral plasma membrane of marginal cells of the SV where endolymph is produced (Weaver et al. 2004). So, NKCC1 is critical for maintaining the endocochlear potential (EP), which powers auditory sensory transduction. ARHL, which can be caused by age-related degeneration of the SV, is linked to age-related reduction in the EP or “cochlear transduction battery” (Schmiedt et al. 2002; Lang et al. 2010).
The mineralocorticoid steroid hormone aldosterone is released from the adrenal cortex and can control NKCC1 and Na+/K+-ATPase via changes in mRNA/protein synthesis in the inner ear (Pitovski et al. 1993a; Pitovski et al. 1993b) and brain (Grillo et al. 1997). Further evidence for aldosterone as being beneficial for auditory processing comes from the pioneering studies of Trune and colleagues. They demonstrated that oral administration of aldosterone can reverse hearing loss in autoimmune mice, while administration of spironolactone (an aldosterone antagonist) blocked this effect (Trune and Kempton 2001; Trune et al. 2006; Trune et al. 2000).
Serum aldosterone levels decrease with age in humans (Brudiex et al. 1995; Kau et al. 1999; Hegstad et al., 1983; Bauer, 1993) and other mammals, including mice (Brudiex et al., 1995; Kau et al., 1999; Magdich, 1980; Wang et al., 2004). Although a direct clinical effect of aldosterone on age-related hearing loss has yet to be demonstrated in a prospective study, a correlation does exist between low serum aldosterone and severity of presbycusis in otherwise healthy elderly human subjects (Tadros et al., 2005). In addition, in vitro application of aldosterone to a human cell line (HT-29) revealed that aldosterone regulates NKCC1 activity and protein expression levels, quite sensitively and rapidly (Ding et al., 2014).
A biotherapeutic that can modulate NKCC1 protein expression opens the door for therapeutic interventions for diseases involving the dysregulation or depletion of NKCC1 or Na+/K+-ATPase. A prime example would be the age-related down-regulation of these ion channels observed in the cochlear lateral wall. Along with declines in serum levels of aldosterone in aging CBA mice (Zhu et al., 2011), we have discovered that NKCC1 and Na+/K+-ATPase expression levels decline with age in the CBA mouse cochlea, including the stria vascularis of the lateral wall (Zhu et al., 2012; 2013; 2014; Ding et al., 2012; 2013). These age changes suggest that aldosterone could be used as a therapeutic intervention for ARHL.
The acoustic startle reflex (ASR), a sensory-motor response that serves as a quantifiable measurement of arousal, was utilized in the present investigation to assess the effects of long-term aldosterone treatment on hearing function. In addition, we measured peripheral hearing sensitivity using auditory brainstem response audiometry. The ASR is an efficient behavioral measure to assess hearing in animal models and has been used extensively to examine the effects of various genetic mutations on hearing (e.g., Allen et al., 2008). The ASR manifests behaviorally as a rapid contraction of skeletal muscles in rodents (Hoffman and Ison, 1980). Rodents are typically placed on a platform where sensors transduce the motion generated by the reflex. Pre-pulse inhibition (PPI) is a reduction in the startle response observed when a stimulus placed prior to the startle elicitor is perceived by the animal, regardless of the sensory modality. The ASR can be inhibited by a pre-pulse stimulus presented before (~50–100 ms) the startle elicitor, for example a tone burst or silent gap in an ongoing noise can serve as a startle-eliciting stimulus (Ison and Hammond, 1971; Ison, 1982).
The goal of the present study was to determine if long-term, systemic, treatment with aldosterone can improve hearing function in a mouse model of ARHL. We hypothesized that chronic slow-release aldosterone treatment will improve hearing sensitivity thereby increasing the salience of pre-pulse stimuli without modifying the startle input-output function for the middle-aged CBA/CaJ mouse model of ARHL. ABR assessments were used to confirm the efficacy of the aldosterone treatment on peripheral auditory function.
Methods
Subjects
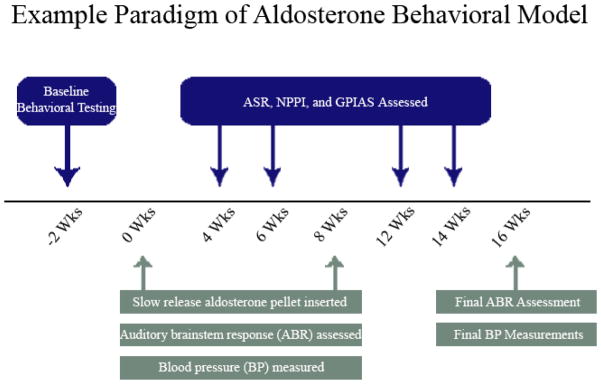
A total of 18 CBA/CaJ inbred mice were used, and were the same group utilized in our companion study (Frisina et al. Submitted). Animals were bred at the University of South Florida Vivarium with breeders obtained from Jackson Labs (Bar Harbor, ME). All mice were between 15–18 months of age at the time of baseline testing. Mice were randomly assigned to 2 groups, either control (n = 10) or treatment (n = 8). The treatment group was systemically administered 1.67 μg per day of D-aldosterone, via extended release pellets (Innovative Research of America, Sarasota, FL) implanted subcutaneously via syringe injection in a pocket of skin behind the shoulders while the mouse was under ketamine/xylazine (100/10 mg/kg) anesthesia. This dose and route of administration was chosen based on data showing that this dose reversed the reduction of serum aldosterone levels in older mice (Zhu et al., 2011). Control animals received a placebo pellet, inserted using the same technique as the treatment group. Baseline tests were followed by tests at 4 and 6 weeks. A second pellet was inserted at 8 weeks, and tests were done at 12 and 14 weeks post-hormone pellet treatment (Figure 1).
Figure 1.
General schematic of the study showing baseline testing, aldosterone administration, ABR assessment, blood pressure assessment, and all behavioral testing. Baseline testing was completed 2 weeks prior to the initiation of treatment via slow release, subcutaneous pellets. Behavioral testing commenced at 4 weeks and continued throughout study, while electrophysiological assessment was completed at baseline and 2 weeks after the last behavioral test.
Blood Pressure Measurements
In order to measure cardiovascular health mice were placed in a restraining tube for 15 min for 3 consecutive days to acclimate them to having their blood pressure (BP) measured using the Kent Scientific CODA™ tail-cuff blood pressure system. The animal was either placed in the holder by picking up the tail, or the animal entered freely. The rear hatch to the holder was carefully secured, and care taken to avoid pinching the tail or any other body parts while securing the rear hatch. The mouse was allowed to rest at least 5-minutes to acclimate to the holder.
Startle Apparatus
Custom 3-D printed platforms housing piezoelectric transducers, located inside one of four identical sound attenuated chambers (40.6 × 40.6 × 40.6cm), lined with sound dampening foam were used to collect startle reflex data. Each wire mesh cage (9.53 × 3.81 × 4.13cm) was cleaned with Clidox (concentration ratio 1:18:1), rinsed with tap water, and dried after use, and fresh cages were used for each individual animal to avoid any odor contamination. Acoustic stimuli were generated by Tucker-Davis Technologies (Alachua, FL) System III RP2 processors and SA1 amplifiers. Tone and noise stimuli were presented through Fostex model FT17H speakers mounted 25cm from the base of the wire mesh cage. All acoustic signals were calibrated using a ½″ free-field ACO Pacific (Belmont, CA) microphone (model 7047, frequency response ±2 dB from 2 Hz to 100 kHz) connected to a Quest Electronics (Oconomowoc, WI) sound-level meter (model 1800). Spectral analysis was performed with a ¼″ free-field Larson Davis (Depew, NY) microphone (model 2520, frequency response ±2 dB from 10 Hz to 100 kHz) and preamp (model 2221) sent to a Hewlett Packard 35665A Spectrum Analyzer (Palo Alto, CA). All calibrations were performed to approximate the position and conditions of the animals’ ears in any given behavioral testing chamber. Startle response signals were acquired using a TDT RX8 processor. Startle stimulus and reflex measurement were controlled using custom programing in MATLAB software (version 2012b) on a Dell Optiplex 790 PC running Windows 7. All experimental trials were presented in variable intervals in order to avoid habituation of the ASR.
General Procedure for ASR and PPI Testing
Mice were transported in their home cages to the adjoining behavior testing room approximately 30 min before testing. Each animal was handled for at least 1 min before being placed inside the wire mesh cage (9.53 × 3.81 × 4.13 cm), which allows free movement during testing while maintaining the animal over the center of the transducer. Once secured inside the wire mesh cages, prior to the presentation of the acoustic stimuli, animals were given 5 min to acclimate to the testing environment. As shown in Figure 1 animals were tested at four time points over the course of 4 months at 4, 6, 12, and 14 weeks. One test point was comprised of 3 sessions, over 6 days. ASR, noise PPI, and gap-prepulse inhibition of the acoustic startle reflex (GPIAS) were performed at all test points, each separated by 10 minutes.
Data Acquisition
All startle reflex data consisted of voltage output (mV) from the transducers. The maximum voltage amplitude was automatically detected for each individual trial, and the first peak (local maxima found through differential analysis) within 25% of this amplitude was identified. The analysis window was then set to this point and included voltage excursions for an additional 70 ms. If no peak was identified within 25% of the max, the time (and start of analysis) was set to the point at which the maximum peak occurred. The analysis algorithm ensured that the beginning of this analysis time frame was never less than 0 ms relative to the startle stimulus. If the maximum peak amplitude was less than 2 mV for the startle-eliciting stimulus, that single trial was removed, as it indicates the animal was not engaged in the test (Longnecker & Galazyuk, 2012).
ASR
A standard startle elicitor was used, consisting of broadband noise bursts, 20 ms in duration and presented with no carrier noise, at various inter-trial intervals (ITI) of 10 to 20 seconds (Ison, et al., 1998). Startle elicitors ranged from 55 to 115 dB SPL in 10 dB increments, and 10 trials were run at each intensity (70 trials total). The ASR was measured using standard methods and stimuli were presented through speakers located 30 cm directly above the transducer platform, upon which the subject stood.
PPI
In the noise PPI paradigm, each trial consisted of a low to moderate level pre-pulse and a 110 dB SPL startle elicitor, with an ISI of 30 ms. The ITI varied from 15 to 30 seconds throughout the paradigm. Pre-pulse stimuli were broadband noise bursts (20 ms duration; 1 ms rise-fall time) presented at 0, 20, 40, 55, and 75 dB SPL. Twenty trials were run at each pre-pulse intensity.
In the GPIAS paradigm, each trial consisted of a silent gap in carrier noise and a 110 dB SPL startle elicitor, with an ISI of 30 ms. The ITI varied from 10 to 20 seconds. The silent gaps were embedded in was broadband noise presented at 70 dB SPL. Silent gaps were 0, 1, 2, 4, 6, 8, 12, 15, and 30 ms in duration. Ten trials were run at each gap length. All broadband stimuli had filter cutoffs of 1,000 and 50,000 Hz, and trials were randomized throughout each paradigm.
Data Reduction
The ASR data were quantified by taking the arithmetical means derived from the electrical waveform output of all included trials for each animal collected in a 300 ms time window around the startle stimulus. The maximum startle amplitude after the startle stimulus was the primary measure used. Group means were then calculated from these data. PPI was based on percent reduction of ASR max peak amplitude when the pre-pulse was present, calculated with the formula, (1- (ASR with pre-pulse/ASR without pre-pulse)) X 100; therefore a larger value indicated greater inhibition of the ASR. Each behavioral data point was comprised of the average response across 3 sessions of testing that occurred every other day for each subject.
Auditory Brainstem Response Audiometry
Mice were divided into two groups: treatment (n=5) and control (n=5), and had clearly visualized, healthy tympanic membranes. Prior to recording the auditory brainstem response (ABR) mice were anesthetized with a mixture of ketamine/xylazine (120 and 10 mg/kg body weight, respectively, IP injection). The ABR testing procedures were similar to our previous reports (Lowe and Walton, 2015; Zhu et al., 2007, Frisina et al. 2007). Briefly, ABR sessions were carried out in a soundproof acoustic chamber, with body temperature maintained at 37°C with a heating pad. Prior to recording, the stimulus probe was placed near the tympanic membrane with the aid of an operating microscope. Needle electrodes were inserted at the vertex (non-inverted) and in the muscle posterior to the left pinna (inverted), with a ground inserted under the contralateral pinna. ABR waveforms were evoked with 5 ms tone pips (0.5-ms rise-fall times) with a cos2 envelope, delivered at a rate of 21/sec though electrostatic speakers (TDT EC1) connected by 4 cm tubes to the opening of the external ear canal. The response was amplified (10,000 X), filtered (0.1–3 kHz), and averaged using the BioSig (TDT, Gainesville, FL) data-acquisition system. A total of 200 responses were averaged (with stimulus polarity alternated), using an ‘artifact reject’ algorithm, whereby response waveforms were discarded when peak-to-peak amplitude exceeded 7 μV, to prevent contamination by muscle activity. Intensity was varied in 5 dB steps starting at 80 dB and decreasing to at least 20 dB below threshold for a specific test frequency. Each intensity was replicated and threshold was defined as the lowest intensity at which a response was replicated, as determined by two experimenters blind to the experimental condition. The recording sessions occurred every 2 months after the initial evaluation (baseline) for a period of 4 months.
NKCC1 Protein Expression
NKCC1 protein expression was measured using western blot assay similar to the method described in our previous report (Ding et al., 2013). A subset of three young adult, middle-aged control and ALD-treated CBA/CaJ mice were sacrificed by decapitation. The cochleae were quickly dissected from the temporal bone, and transferred into ice-cold DPBS (HyClone Lab Inc., Logan, UT) on ice to dissect the lateral wall using a Zeiss stereomicroscope, then tissue lysates were prepared in RIPA buffer (Pierce 89901; Thermo Scientific, Waltham, MA) with protease inhibitor cocktail (78430; Thermo Scientific). The samples were homogenized in buffer, followed by centrifugation at 2,000 rpm for 10 min at 4°C. Supernatants were subjected to Western blot analysis by loading 200 μg of protein per lane, after the protein concentrations were determined by Bradford protein assay. Proteins were fractionated by SDS-PAGE gel electrophoresis and transferred to a PVDF blotting membrane (Whatman, Piscataway, NJ). The blot was incubated with primary antibodies against β-actin and Na-K-2Cl cotransport protein (Cell Signaling, Danvers, MA); primary antibodies were utilized in a diluted concentration (1:1,000). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Cell Signaling).
Statistical Analysis
ASR data were obtained from the raw maximum output of the transducers (mV), consisting of the arithmetical means of each trial, for each animal in each session. PPI data were calculated likewise, using the averages at each pre-pulse intensity, relative to the no-pre-pulse trials. The analysis was performed using SPSS (IBM Corporation, Somers, NY) using a repeated measures ANOVA. Each stimulus intensity represented a measure, the testing session represented the within-subjects variable, and treatment was the between subjects variable. Post-hoc testing using Fischer’s least significant differences test, with α = 0.05 used to detect between-subject effects at various stimulus intensity levels.
Results
Systemic aldosterone treatment improves hearing function
ASR
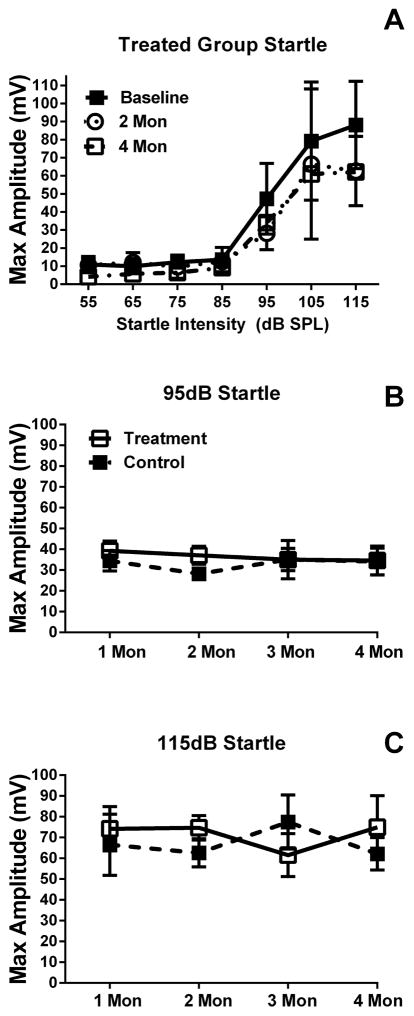
Significant differences in mean startle amplitudes were found in the ASR at each testing point (Figure 3), with the 95 dB SPL startle amplitude being lower than both the 105 and 115 dB SPL stimuli; baseline, F(2,18) = 52.62, p<0.01, 4 weeks F(2,18) = 27.97, p<0.01, 6 weeks F(2,18) = 10.19, p<0.01, after first-pellet insertion, and four weeks, F(2,18) = 13.32, p<0.01, and 6 weeks after second pellet insertion, F(2,18) = 20.79, p<0.01. There was no significant difference between treatment and control for ASR at any intensity, or any test point, or for interactions at any of the testing points.
Figure 3.
Mean startle input/output functions and comparison of maximum startle amplitudes for treated (1st and 2nd pellet) and untreated (baseline) mice. Panel A shows mean (SEM) startle amplitudes from the aldosterone treated group across the two test time points (2 and 4 months). Pane B and C show that no significant differences in startle amplitude to suprathreshold stimuli were found at any test time point between treated and control animals..
Noise PPI
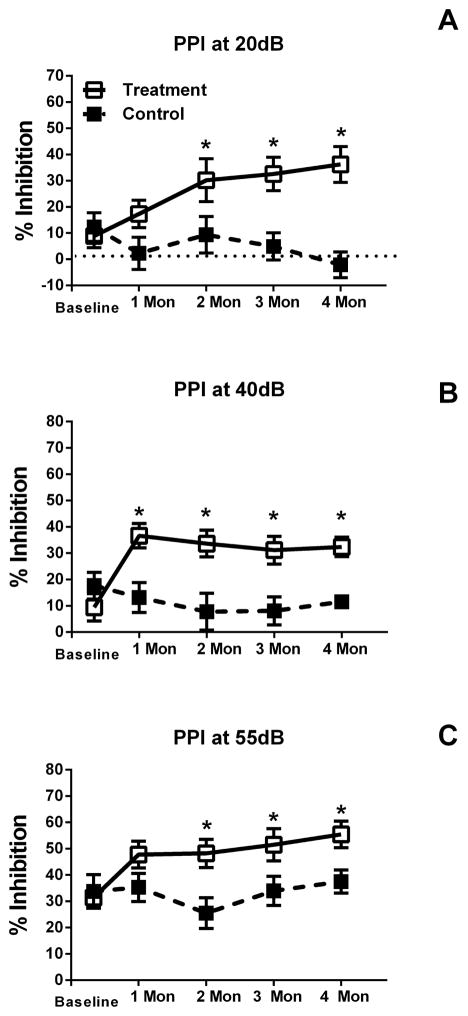
In contrast to the startle amplitude data, repeated measures ANOVA revealed significant effects of pre-pulse intensity on ASR inhibition. The 55 dB pre-pulse condition showed increased salience for treated mice as compared to untreated mice, F(4,28) = 3.03, p = 0.03, and no other intensities were found to have significant effects. Significant interactions between pre-pulse intensity and treatment were found for the 20 dB pre-pulse intensity, F(4,28) = 2.77, p<0.05. Post hoc analysis revealed a 25% increase in inhibition with treatment beginning at 6 weeks post first-pellet, and 4 and 6 weeks post-second pellet. A significant interaction was also found between 40 dB and treatment, F(4,28) = 4.10, p = 0.01. Post hoc analysis here also revealed a 25% increase in inhibition with treatment starting at 4 weeks post first-pellet and continuing through each testing point. Although there were no interactions between 55 dB and 75 dB and treatment, post hoc analysis at 55 dB showed a significant 20% increase in inhibition in the treatment group at 6 weeks post first-pellet treatment and 4 and 6 weeks post second-pellet. Aldosterone treated animals were found to have greater PPI as compared to the control group at 55 dB (* designates p<0.05 in the top row of Figure 3), indicating greater salience to the acoustic signal.
PPI to Gap Stimuli
Analysis of gap ASR inhibition showed significant effects of gap duration at baseline, F(7,42) = 3.77, p<0.01 and 4 weeks F(7,42) = 2.98, p = 0.01, but no effect of treatment on gap perception. These effects indicated that longer gap durations resulted in increased inhibition as gap duration increased and there was also no significant effect of treatment or group-by-gap duration interaction effects (Figure 3).
ABRs
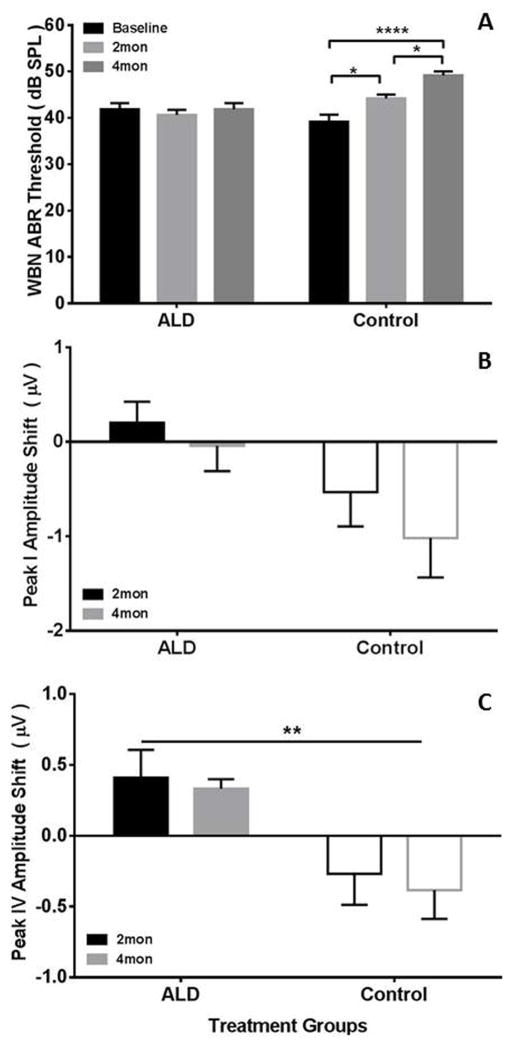
ABR thresholds to wideband noise stimuli having similar spectral composition as the noise PPI stimulus, were measured prior to the initiation of aldosterone treatment (baseline) and at 2 and 4 months during treatment (Figure 6A). Mice treated with aldosterone showed stable noise thresholds (± 1 dB) over the course of treatment while thresholds for untreated mice systematically increased from 39 dB to 49 dB, F(1,12)= 4.46, p=.05. In order to assess the peripheral excitatory drive to the central auditory system we measured the peak amplitude of P1 and P4 of the ABR elicited by the 80 dB SPL WBN. Figure 6B shows the P1 amplitude shift between baseline and the 2 and 4 month treatment time points. Initial baseline P1 amplitudes were comparable across groups, 2.66 μV for control versus 2.63 μV for treated. Results of the ANOVA showed no significant effects. However, aldosterone treatment effects were significant, F(1,12)= 4.74, p =0.05; and post-treatment time effects showed a trend, F(1,12)=3.40, p =0.09, indicating that the auditory processing between baseline and 4 months was systematically improved, i.e., larger amplitudes for treated mice, as compared to control mice. To assess the effects of treatment on central auditory function we measured P4 amplitude (Fig 6C). Significant beneficial effects of aldosterone treatment, F(1,12)=10.10, p=0.008, were found. Similar to P1 there was no significant difference in baseline P4 amplitudes, but after treatment at both the 2 and 4 month test points, a positive shift was recorded compared to an overall negative shift in control mice of the P4 amplitude.
Figure 6.
The therapeutic effects of aldosterone treatment on auditory brainstem response metrics at 2 and 4 months following the initiation of treatment. (A) Wideband noise mean thresholds measured before treatment and at 2 and 4 months following treatment. Aldosterone treated mice showed stable thresholds, whereas control mice continued to display threshold increases characteristic of age-related hearing loss. (B) P1 amplitude shifts following 2 and 4 months of treatment show declines in the control group relative to the aldosterone-treated animals. (C) P4 amplitude shift following 2 and 4 months aldosterone treatment reveal significant preservation of amplitudes in the aldosterone-treated mice. Positive voltage shifts in B and C indicate greater ABR peak amplitudes. Error bars represent S.E.M., ANOVA results: *p<0.05; **p<0.01; ****p<0.001.
Aldosterone Treatment Up-Regulates NKCC1
NKCC1 Protein Expression
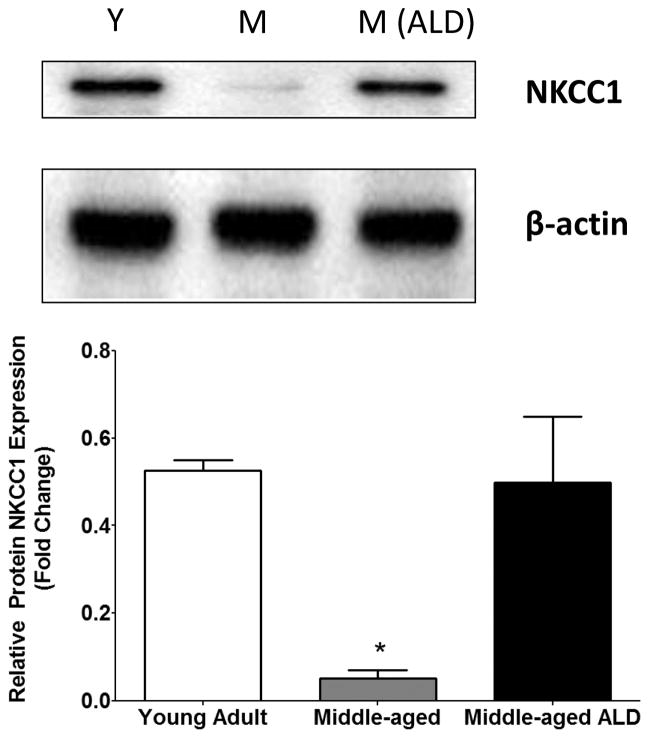
Strong NKCC1 protein expression was observed from the cochlear lateral wall from young mice and there was over a two-fold decrease in NKCC1 protein expression observed in middle-aged mice. The data are based on representative of the triplicates for each subject group. A one-way ANOVA indicated a significant main effect of ALD treatment by group, [F(2,6)=9.16, p=0.015] and Bonferroni multiple comparison post-hoc tests showed a significant decrease in expression in samples from young adult (Y) as compared to middle-aged (M), t=3.81, df=2), p<0.05. Following long-term aldosterone treatment NKCC1 expression in middle-aged mice NKCC1 was up-regulated and the age-related decline seen in the lateral wall was prevented. Furthermore, the level of protein expression found in the stria approximated that observed in young adult mice.
Discussion
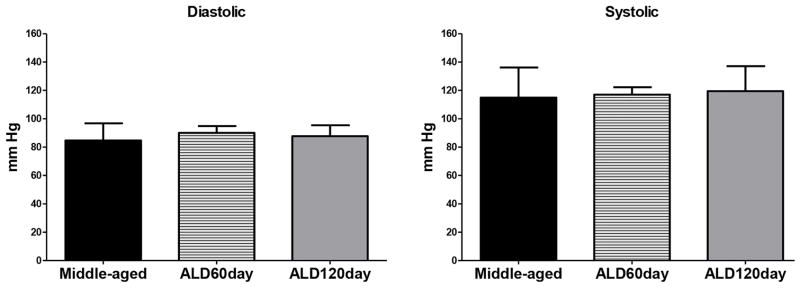
Various targets exist for the prevention of age-related hearing loss and for the first time we report that long-term aldosterone treatment improves behavioral and electrophysiological measures of hearing and results in increased excitatory drive. We hypothesized that this would be the case as previous research demonstrated aldosterone serum levels in human subjects correlate significantly with pure-tone hearing thresholds (Tadros et al., 2005). Furthermore, aldosterone treatment significantly improves cochlear function as measured by the ABR in autoimmune deficient mice, and spironolactone (an aldosterone antagonist) blocks the therapeutic effect (Trune et al., 2006). Frisina et al. (2016, Submitted) provide further evidence of the therapeutic effects of aldosterone showing increased spiral ganglion survival, upregulation of mineralocorticoid receptors, and blockage of apoptotic pathways. One potential limitation and possible adverse side effect of long-term ALD treatment is hypertension, therefore we measured blood pressure of the mice at the midway point of the treatments and just before the serum ALD level was assayed at the endpoint of the study. Mean systolic (~120 mm Hg) and diastolic (~85 mm Hg) pressures remained stable relative to the baseline, and over the treatment periods of 60 and 120 days in both the control and treated groups. These results indicate that long-term ALD treatment does not induce hypertension, a potential negative side effect, using the therapeutic dosing regimen of the present experiment.
Our results are the first to demonstrate a positive behavioral effect of aldosterone on hearing as measured by the salience of noise pre-pulses and modification of the ASR in mice. The increase in PPI indicates increased salience for low-level noise signals and supports the hypothesis that aldosterone improves peripheral hearing function. The importance of this behavioral assay ties together our previous in vitro work showing the upregulation of a key Na+-K+ ion channel, NKCC1, in the cochlear lateral wall; and the improvement reported here in peripheral hearing thresholds and stable ABR peak 1 and 4 amplitudes following long term aldosterone treatment as a possible mechanism to slow down the progression of age-related hearing loss, a result also supported by Zhu et al. (2014). Specifically, ABR peak amplitudes decreased at both time points in untreated mice, whereas P1 and P4 amplitudes remained stable in mice treated with aldosterone. Finally, we show that in mice treated with ALD, NKCC1 is up-regulated, consistent with the idea that increased NKCC1 channel expression is a possible cochlear mechanism for the beneficial effects of ALD treatment on hearing thresholds and spiral ganglion neuron survival. The beneficial effects might also impact ribbon synapse function, and subsequent neuronal survival, leading to stronger excitatory drive (Kujawa and Liberman, 2009). More importantly, in a subsequent study by Yevgeniya et al. (2013), the authors report a correlation between synaptic losses and aging that can be predicted by P1 amplitudes in CBA mice. The increase in P4, which is generated in the auditory midbrain, may be direct reflection of the increased P1-auditory nerve output, consistent with the increased excitatory drive.
One issue to be addressed is whether the startle elicitor, against which PPI is measured, might be altered by aldosterone treatment; as such an interaction might confound PPI measurements. The ASR obtained from the aldosterone-treated and control CBA mice illustrated in the bottom row of Figure 3 shows that chronic aldosterone treatment does not affect basic noise-burst elicited ASR, for any of the stimulus intensities. Thus, we believe that pre-pulse inhibition is a valid method to evaluate aldosterone treatment effects without concerns about the ASR probe responses.
In summary, aldosterone treatment in aged animals rescues certain aspects of presbycusis and the therapeutic result is possibly due to the cellular stabilization of NKCC1 protein structures in cochlear lateral wall cells, such as marginal cells of the stria vascularis. This study opens the door to potential therapeutic applications of aldosterone for people developing age-related hearing loss and furthers our understanding of the cellular mechanisms of sound processing in the aging auditory system.
Figure 2.
Mean diastolic and systolic blood pressure measurements taken at baseline (middle-aged), at 2 months (ALD 60 day), and at 4 months (ALD 120 day) following aldosterone pellet implantation. No significant effect on blood pressure was found on diastolic or systolic blood pressure.
Figure 4.
Noise burst pre-pulse inhibition at various pre-pulse intensities for treated (open squares) and untreated or control (filled squares) mice across the 4-month period. Panel A shows inhibition levels for a 20 dB pre-pulse, the 40 dB pre-pulse (Panel B) and 55 dB pre-pulse (Panel C). Significant increases in inhibition emerged in the treated group at the 6-week testing point, and continued throughout testing. A similar pattern was seen for the 55 dB pre-pulse shown in Panel C, with expected overall inhibition increases due to the increasing pre-pulse intensity. Significant difference in mean % PPI is indicated by the (*).
Figure 5.
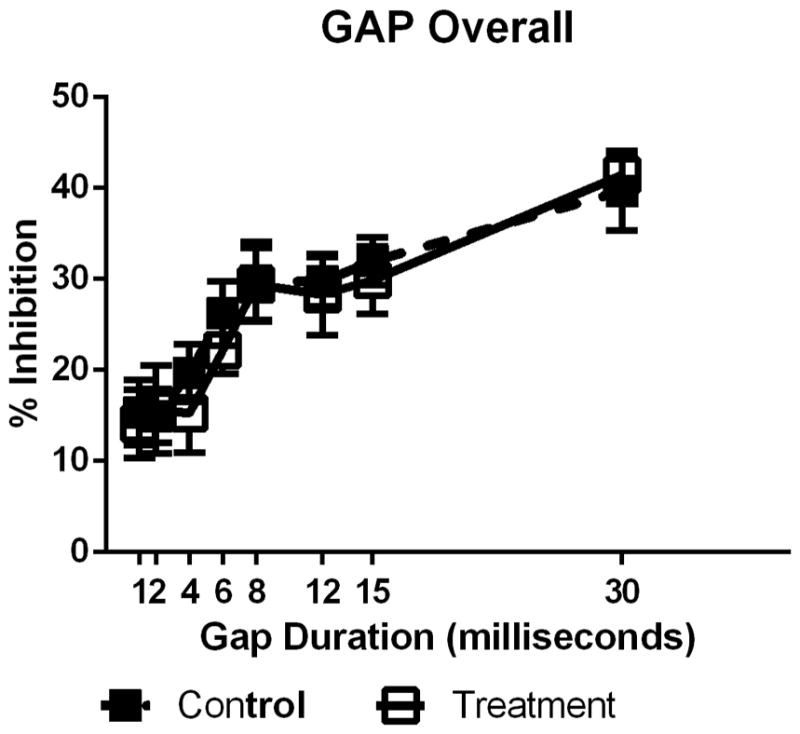
Example of PPI at 4 weeks of treatment for a pre-pulse having silent gap embedded 60 ms before the SES and gap durations which varied from 1 to 30 ms for treated (open squares) and untreated or control (filled squares) mice. Increases in gap duration results in a systematic increase in PPI. No significant effects of treatment were found for any gap duration at any test point.
Figure 7.
ALD inhibits age-related NKCC1 down regulation in the cochlear lateral wall. Lateral walls of cochlea from young adult (Y: 3–4 month), middle age (M: 22–24 month) and middle age following 4 months of ALD treatment (M- ALD). NKCC1 protein expressions were detected using western blots and the expressed levels are relative to β-actin expression, an equal loading control. The expression was determined quantitatively using densitometry (NIH Image J) as shown in the bar graph. The data are representative of the triplicates for each subject group. Results are means ±SD. One Way ANOVA indicated a significant main effect of treatment: F(2,6)=9.16, p=0.015; Bonferroni multiple comparison post-hoc test: young adult (Y) vs. middle age (M), t=3.81, df=2), p<0.05; middle age(M) vs. middle age ALD treatment (M, ALD), t=3.59, df=2, p<0.05 (*).
Highlights.
We systemically treated mice with the hormone aldosterone for up to 4 months
Hearing was assessed via the acoustic startle response and auditory brainstem response audiometry
Improvement in auditory sensitivity as well as excitatory drive was observed for mice treated with aldosterone but not for untreated
Acknowledgments
The authors thank Shannon Salvog for administrative support and copy editing. We would also like to thank Drs. Adam Dziorny and Daniel Stolzberg for the behavioral data collection and ABR data analysis software packages. This work was supported by NIH Grant P01 AG009524 from the National Institute on Aging, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PD, Schmuck N, Ison JR, Walton JP. Kv1.1 channel subunits are not necessary for high temporal acuity in behavioral and electrophysiological gap detection. Hearing Research. 2008;246(1):52–58. doi: 10.1016/j.heares.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall R. Age-related changes in the function of T cells. Microscopy Research and Technique. 2003;62(6):508–513. doi: 10.1002/jemt.10412. [DOI] [PubMed] [Google Scholar]
- Bauer JH. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging. 1993;3(3):238–245. doi: 10.2165/00002512-199303030-00005. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. The Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Ding B, Frisina RD, Zhu X, Frisina DR, Walton JP. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. Aldosterone increases the protein expression of Na-K-Cl cotransporter (NKCC1) via an ubiquitination mechanism. Program No. 367.10. Online. [Google Scholar]
- Ding B, Frisina RD, Zhu X, Sakai Y, Sokolowski B, Walton JP. Direct control of Na(+)-K(+)-2Cl(−)-cotransport protein (NKCC1) expression with aldosterone. American Journal of Physiology: Cell Physiology. 2014;306(1):C66–75. doi: 10.1152/ajpcell.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Frisina RD, Zhu X, Walton JP. Hormonal modulation of a key sodium-potassium co-transporter (NKCC1) that maintains the endocochlear potential through the SGK1-Nedd4-2 Pathway. Aging & Speech Communication; 5th International & Interdisciplinary Research Conference Abstract.2013. [Google Scholar]
- Ding B, Zhu X, Frisina RD, Walton JP. Age related Na+/K+-ATPase isoform gene and protein expression changes in the cochlear stria vascularis. Assoc Res Otolaryngol Abs. 2013:304. [Google Scholar]
- Dowd BFX, Forbush B. PASK (Proline-Alanine-rich STE20-related Kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) The Journal of Biological Chemistry. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hearing Research. 1997;106(1–2):95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Newman SR, Zhu X. Auditory efferent activation in CBA mice exceeds that of C57s for varying levels of noise. The Journal of the Acoustical Society of America. 2007;121(1):EL29–EL34. doi: 10.1121/1.2401226. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Ding B, Zhu X, Walton JP. Age-related hearing loss: Prevention of threshold decline and apoptosis in spiral ganglion neurons. Aging. 2015 doi: 10.18632/aging.101045. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P, Martin CF, Elms SC, Gordon FJ, Wall SM, Garland CJ, O’Neill C. Effect of the Na-K-2Cl cotransporter NKCC1 on systemic blood pressure and smooth muscle tone. American Journal of Physiology: Heart and Circulatory Physiology. 2007;292:H2100–H2105. doi: 10.1152/ajpheart.01402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C, Piroli G, Lima A, McEwen BS, De Nicola AF. Aldosterone up-regulates mRNA for the alpha3 and beta1 isoforms of (Na,K)-ATPase in several brain regions from adrenalectomized rats. Brain Research. 1997;767(1):120–127. doi: 10.1016/S0006-8993(97)00541-6. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Frisina ST, Mapes F, Tadros SF, Frisina DR, Frisina RD. Progestin Negatively Affects Hearing in Aged Women. Proceedings of the National Academy of Sciences – PNAS. 2006;103(38):14246–14249. doi: 10.1073/pnas.0606891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegstad R, Brown RD, Jiang N, Pai K, Weinshilboum RM, Strong C, Wisgerhof M. Aging and aldosterone. American Journal of Medicine. 1983;74(3):442–448. doi: 10.1016/0002-9343(83)90971-3. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological Review. 1980;87(2):175–189. doi: 10.1037/0033-295X.87.2.175. [DOI] [PubMed] [Google Scholar]
- Ison JR. Temporal acuity in auditory function in the rat: Reflex inhibition by brief gaps in noise. Journal of Coparative and Physiological Psychology. 1982;96(6):945–954. doi: 10.1037/0735-7036.96.6.945. [DOI] [PubMed] [Google Scholar]
- Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: A study of the acoustic startle reflex and its inhibition by brief decrements in noise level. The Journal of the Acoustical Society of America. 1998;104:1696–1704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of the startle reflex in the rat by changes in the auditory and visual environments. Journal of Comparative and Physiological Psychology. 1971;75(3):435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Kau MM, Chen JJ, Wang SW, Cho WL, Wang PS. Age-related impairment of aldosterone secretion in zona glomerulosa cells of ovariectomized rats. Journal of Investigative Medicine. 1999;47(8):425–432. [PubMed] [Google Scholar]
- Kramer SE, Kapteyn TS, Kuik DJ, Deeg DJ. The association of hearing impairment and chronic diseases with psychosocial health status in older age. Journal of Aging and Health. 2002;14(1):122–137. doi: 10.1177/089826430201400107. [DOI] [PubMed] [Google Scholar]
- Lang H, Jyothi V, Smythe NM, Dubno JR, Schulte BA, Schmiedt RA. Chronic reduction of endocochlear potential reduces auditory nerve activity: Further confirmation of an animal model of metabolic presbyacusis. The Journal of the Association for Research in Otolaryngology. 2010;11:419–434. doi: 10.1007/s10162-010-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing Loss and Cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychology and Aging. 2009;24(1):1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- Lowe AS, Walton JP. Alterations in Peripheral and Central Components of the Auditory Brainstem Response: A Neural Assay of Tinnitus. PLoS One. 2015;10(2):e0117228. doi: 10.1371/journal.pone.0117228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdich LV. Age and the effect of adrenocorticotropic hormone on aldosterone secretion in rats. Biulleten’ Eksperimental’noi Biologii I Meditsiny. 1980;89(7):19–20. [PubMed] [Google Scholar]
- Pedersen SF, O’Donnell ME, Anderson SE, Cala PM. Physiology and pathophysiology of Na(+)/H(+) exchange and Na(+)-K(+)-2Cl(−) cotransport in the heart, brain, and blood. Regulatory, Integrative, and Comparative Physiology. 2006;291:R1–R25. doi: 10.1152/ajpregu.00782.2005. [DOI] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Drescher DG. High affinity aldosterone binding sites (type I receptors) in the mammalian inner ear. Hearing Research. 1993a;69(1–2):10–14. doi: 10.1016/0378-5955(93)90088-I. [DOI] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Kerr TP, Drescher DG. Aldosterone mediates an increase in [3H]ouabain binding at Na+, K(+)-ATPase sites in the mammalian inner ear. Brain Research. 1993b;601(1–2):273–278. doi: 10.1016/0006-8993(93)91720-D. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura H, Schulte BA. Effects of furosemide applied chronically to the round window: A model of metabolic presbyacusis. The Journal of Neuroscience. 2002;22(21):9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: A longitudinal analysis. The Gerontologist. 2000;40(3):320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. The American Physiological Society. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. High serum aldosterone correlates with lower hearing thresholds in aged humans: A possible protective hormone against presbycusis. Hearing Research. 2005;209(1–2):10–18. doi: 10.1016/j.heares.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Kessi M. Aldosterone (mineralocorticoid) equivalent to prednisolone (glucocorticoid) in reversing hearing loss in MRL/MpJ-Fas1pr autoimmune mice. Laryngoscope. 2000;110(11):1902–1906. doi: 10.1097/00005537-200011000-00025. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB. Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Fas(lpr) autoimmune mice. Hearing Research. 2001;155(1–2):9–20. doi: 10.1016/S0378-5955(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hearing Research. 2006;212(1–2):22–32. doi: 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Van EE, Van CG, Van LL. The complexity of age-related hearing impairment: Contributing environmental and genetic factors. Audiology and Neuro-otology. 2007;12(6):345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- Viljanen A, Kaprio J, Pyykko I, Sorri M, Pajala S, Kauppinen M, Rantanen T. Hearing as a predictor of falls and postural balance in older female twins. The Journals of Gerontology, Biological Sciences and Medical Sciences. 2009;64A(2):312–317. doi: 10.1093/18erona/gin015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, … Kim Y. Hypotension in NKCC1 null mice: Role of the kidneys. American Journal of Physiology: Renal Phygiology. 2006;290:F409–F416. doi: 10.1152/ajprenal.00309.2005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Clement S, Gabbiani B, Horisberger JD, Burnier M, Rossier BC, Hummler E. Chronic hyperaldosteronism in a transgenic mouse model fails to induce cardiac remodeling and fibrosis under a normal-salt diet. American Journal of Physiology: Renal Physiology. 2004;286(6):F1178–1184. doi: 10.1152/ajprenal.00386.2003. [DOI] [PubMed] [Google Scholar]
- Weaver DC, Harden D, Dworetzky SI, Robertson B, Knox RJ. A thallium-sensitive, fluorescence-based assay for detective and characterizing potassium channel modulators in mammalian cells. Journal of Biomolecular Screening. 2004;9:671–677. doi: 10.1177/1087057104268749. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the Auditory System: Anatomy, Physiology, and Psychophysics. San Diego, CA: Singular; 1991. [Google Scholar]
- Willott JF. Anatomic and physiologic aging: A behavioral neuroscience perspective. Journal of the American Academy of Audiology. 1996;7(3):141–151. [PubMed] [Google Scholar]
- Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hearing Research. 1999;135(1–2):78–88. doi: 10.1016/S0378-5955(99)0094-5. [DOI] [PubMed] [Google Scholar]
- Yevgeniya S, Kumud L, Liberman M, Kujawa SG. Age-Related Cochlear Synaptopathy: An Early-Onset Contributor to Auditory Functional Decline. The Journal of Neuroscience. 33(34):133686–133695. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ding B, Walton JP, Frisina RD. Age-associated changes of Na, K-ATPase subunit isoform distribution and expression in the CBA/CaJ mouse cochlea. Assoc Res Otolaryngol Abs. 2013:628. [Google Scholar]
- Zhu X, Ding B, Walton JP, Frisina RD. Aldosterone Reduces Spiral Ganglion Neuron Loss in Middle Age CBA/CaJ Mice. Assoc Res Otolaryngol Abs. 2014:14. [Google Scholar]
- Zhu X, Ding B, Walton JP, Frisina RD. 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2014. Aldosterone neuroprotection via mineralocorticoid receptors in the cochlea of aging CBA/CaJ mice. Program No. 722.08. Online. [Google Scholar]
- Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, Frisina RD. Auditory efferent feedback system deficits precede age-related hearing loss: Contralateral suppression of Otoacoustic emissions in mice. The Journal of Comparative Neurology. 2007;503(5):593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]
- Zhu X, Walton JP, Ding B, Frisina RD. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. Sodium-potassium-chloride cotransporter (NKCC1) expression declines with age in the CBA/CaJ mouse cochlea. Program No. 363.04. Online. [Google Scholar]
- Zhu X, Walton JP, Ding B, Peterson B, Frisina RD. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. Serum aldosterone levels decrease in old mice with age-related hearing loss. Program No. 476.03. Online. [Google Scholar]