The coronavirus disease 2019 (COVID-19) pandemic has magnified the importance of delivering high-quality healthcare while preserving limited or depleted resources. This is particularly true in cardiac surgery, which depends on the same equipment, hospital capacity, and personnel that have been re-directed to COVID-19 care. Enhanced recovery programs promote standardized, consistent perioperative care, with an emphasis on incorporating evidence-based measures to optimize the patient experience, improve outcomes, and utilize resources efficiently. Developing and implementing a program in the current COVID-19 environment is a daunting task. The Society for Enhanced Recovery After Cardiac Surgery (ERAS Cardiac), representing an international multidisciplinary group of experts, provides the rationale, supportive evidence, and a proposed outline for a sustainable modified program within the constraints of the COVID-19 pandemic. It is feasible to launch in the current healthcare climate and is designed to preserve resources, reduce case backlog, and protect patient and provider safety while improving patient care and preserving institutional quality metrics. The program can also create the foundation for future growth.
COVID-19: Challenges for the Cardiac Surgical Community
SARS-CoV-2 and the COVID-19 pandemic have turned healthcare systems worldwide upside-down, and hospitals are adjusting the volume of nonurgent surgical cases according to local COVID-19 prevalence rates.1 , 2 In the face of active disease surges or resurgences, many hospitals are postponing all nonemergent cardiac surgery to redirect scarce resources to the care of patients with severe viral illness. This includes rationing personal protective equipment, establishing additional intensive care unit (ICU) capacity often in novel spaces, sequestering ventilators, and redeploying personnel. Hospitals are at risk of being overwhelmed as demand for care exceeds available resources. In locations where infection rates are lower, the throughput of elective and semiurgent procedures may nevertheless be maintained at a lower level in the effort to preserve reserve capacity in the event of an acute surge. In a recent survey of cardiacsurgery centers, the median reduction in case volume was between 50% to 75% over the first months of the pandemic.3 The forced deferral of necessary care has resulted in a backlog of patients, leading to new potential risks of increased morbidity and mortality secondary to longer wait times.4 , 5
As the acute burden of coronavirus illness subsides and cities and countries enter the recovery phase, hospitals will feel intense external and internal pressure to “catch up” on their deferred procedures while maintaining preparedness for new resurgences.6 The results of modeling of the increased capacity required to work through the cardiac surgical backlog is daunting. Even if surgical volumes were increased to 150% of pre-COVID levels, it has been estimated it could take 2-to-3 months to clear the backlog. A 120% increase could take 8 months.5
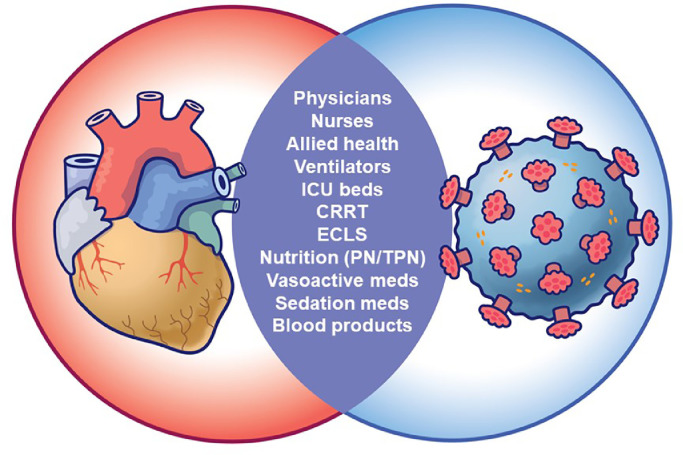
Compounding this issue is the unique challenge of the uncertain and unpredictable availability of several key resources shared between cardiac surgical and COVID-19 patients (Fig 1 ). The supply of healthcare workers with cardiac surgical expertise (many of whom were the first to be redeployed), hospital and ICU beds, ventilators, and critical care/resuscitative medications, supplies, and equipment are all necessary to perform cardiac surgery and are at risk of shortages. Noncardiac surgical patients, although also affected by the COVID-19 pandemic, do not share the same degree of resource competition. Clearly, there will be limited capacity to provide timely, effective, and optimal cardiac surgical care by simply “working harder.” Success will invariably depend on each institution's ability to identify and correct suboptimal or inefficient entrenched practices.
Fig 1.
Cardiac surgery and COVID-19 patients compete for many shared resources. This list, although not exhaustive, demonstrates the high stakes (often zero-sum) overlap in resource demands between the 2. It highlights the critical importance of increasing cardiac-surgical quality and efficiency during this pandemic. CRRT, continuous renal replacement therapy; ECLS, extracorporeal life support; ICU, intensive care unit; PN, parenteral nutrition; TPN, total parenteral nutrition.
Cardiac Enhanced Recovery in the Era of COVID-19
The enhanced recovery paradigm has consistently focused on the reduction of clinical variation in the delivery of perioperative care, with an emphasis on incorporating evidence-based measures to optimize the patient experience, improve outcomes and maximize efficient use of resources.7, 8, 9, 10 The reported results of cardiac-enhanced recovery programs (ERPs) are promising and include measures in several areas that directly address the COVID-19 pandemic's effects on the healthcare system. There have been demonstrated improvements in patient-centered outcomes, lower costs, reduced intubation times, and shorter ICU and hospital lengths of stay at multiple cardiac centers (Table 1 ).11, 12, 13, 14, 15 The potential benefits of ERPs demonstrated before COVID have become magnified as nonemergency surgeries across the surgical spectrum resume; hence, their inclusion in the American College of Surgeons, American Society of Anesthesiologists, Association of periOperative Registered Nurses, and American Hospital Association joint statement on resuming elective surgery.16 “Good enough” is no longer sufficient and time is of the essence. Healthcare teams that adapt the fastest will have the greatest chance for success.
Table 1.
Improved Outcome Measures Demonstrated in Published Results From Cardiac-Enhanced Recovery Programs.
| Improved Outcome Measure | Fleming11 | Grant12 | Li13 | Williams14 | Zaouter15 |
|---|---|---|---|---|---|
| Reduced hospital LOS | No | Yes | No | Yes | Yes |
| Reduced ICU LOS | N/R | No | Yes | Yes | Yes |
| Less complications | Yes | N/R | Yes | N/R | No |
| Earlier extubation | N/R | Yes | Yes | No | No |
| Improved analgesia | Yes | N/R | N/R | N/R | Yes |
| Improved GI function | N/R | N/R | Yes | Yes | Yes |
| Decreased cost | N/R | N/R | Yes | N/R | N/R |
| Reduced opioid use | Yes | N/R | N/R | Yes | No |
| Reduced duration of vasoactive support | N/R | N/R | Yes | N/R | N/R |
NOTE. This table represents a general summary. Listed outcome categories were not defined identically in each referenced publication.
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; LOS, length of stay; N/R, not reported.
Unfortunately, as attractive as ERPs may seem, the overwhelming pressures of the current COVID-19 environment are at odds with successful implementation of new programs. Previous publications have outlined the ideal strategy for implementing a comprehensive cardiac ERP.17, 18, 19, 20, 21 The recommended process generally consumes a high degree of time, resources, effort, enthusiasm, and acceptance of change in current practices at start-up, with the offsetting gains realized once adoption is complete. Meanwhile, hospitals are full and resources are stretched thin. Cardiac surgical programs are still learning, in real time, how to triage cases optimally, screen patients for SARS-CoV-2, allocate personal protective equipment safely yet judiciously, and balance the ethical dilemmas of these choices.22, 23, 24, 25, 26 Members of the healthcare team are working harder, longer, with less autonomy, and in more stressful situations than before, with detrimental effects on mental health and quality of life.27, 28, 29
A Modified Start-up Plan for Cardiac ERP During the Pandemic
Enhanced recovery aligns with the new, magnified urgency to deliver healthcare more efficiently. How can the positive impact of an ERP be balanced with the challenges inherent to its implementation? ERAS Cardiac (www.erascardiac.org) is a multidisciplinary, nonprofit organization dedicated to optimizing perioperative care of cardiac surgical patients through collaborative discovery, analysis, expert consensus, and dissemination of best practices. ERAS Cardiac has recently published evidence-based guidelines outlining key components for consideration in a cardiac ERP, with benefits and level of evidence graded by the guideline committee.30 It is unlikely that addressing all aspects of perioperative care will be feasible in the current healthcare climate. However, a focused program specifically tailored to address and relieve key resource constraints secondary to the COVID-19 pandemic is still achievable and will yield meaningful benefit.
Based on available evidence and collective expertise, The ERAS Cardiac Society has developed a proposed modified ERP for implementation during the COVID-19 pandemic. Because most nonemergency cardiac surgery patients are not expected to have co-existing COVID-19 illness, the benefit of the proposed ERP will focus on (1) preserving resources for the care of COVID-19 patients, (2) increasing efficiency to help address caseload backlog, and (3) improving the safety of patients and staff by reducing duration of in-hospital stay and risk of readmission. The development, implementation, and expected benefits of the program fit within any phase of COVID-19—preparation for surge, surge, recovery, and possible resurgence. These programs are equally applicable to academic and community hospitals and to high- and low-volume centers. Divided into 6 steps that mirror a traditional approach to ERP development, the proposed program includes modifications to each step tailored to fit within the context of the COVID-19 pandemic, with the initial completion and implementation (steps 1-4) realistically achievable within 4-to-6 weeks (Fig 2 ).
Fig 2.
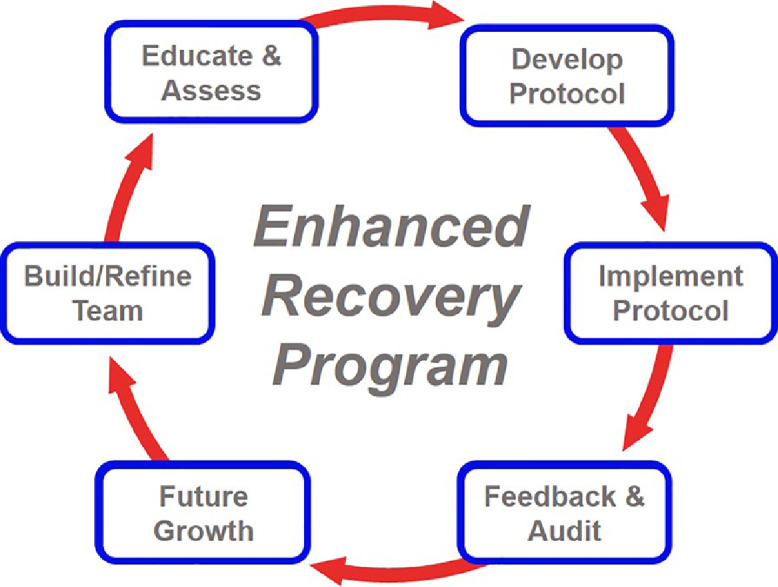
The standard iterative process of an enhanced recovery program. After an initial team and protocol are built, the program cycles through the listed steps. Continually refining, adapting, and evolving, the end goal is always optimal patient outcomes and efficient healthcare delivery. Program implementation during COVID-19 will follow the same steps, but with modifications related to the direct and indirect impact of the pandemic on patients and the healthcare system.
Step 1: Build/Refine the Team
A bottom-up approach, with early input from all stakeholders, will have a higher likelihood of success. This is particularly true in the current environment, in which rapid, top-down dissemination of ever-changing policies has been required to address the dynamic COVID-19 crisis in a timely manner. This same approach in an ERP may result in a sense of lost autonomy and lack of input among many of the same healthcare providers who must participate in the implementation. Ideally, to achieve engagement, the team should be built with representation from a broad base of stakeholders and encompass all groups who will be impacted by and benefit from the proposed program. This includes patient/caregiver representation wherever possible. Although a typical program should strive to be as inclusive as possible, COVID-19 team building will need to be more strategic, incorporating the minimum number of team members that will still allow successful implementation (Table 2 ). For a COVID-19 cardiac ERP, team members can be designated as essential (ERP failure is likely without them) or valuable (will add value and should be included whenever possible). The remaining potential stakeholders, who are either not directly involved in the COVID-19 modified ERP or are unlikely to be feasible for immediate inclusion, should be added once the ERP grows in future iterations. Local representatives from the hospital, city, or regional COVID-19 data-analysis, policy, or response teams are a unique group during the pandemic that may provide valuable information about anticipated resource utilization trends, as well as where the ERP fits within current COVID-19 policy.
Table 2.
Examples of Members for Consideration When Building an ERAS Cardiac Team During the COVID-19 Pandemic.
| ERAS Cardiac Team Prioritization | Team Members |
|---|---|
|
Essential The program will likely fail without inclusion. |
Anesthesiologist Cardiothoracic surgeon Critical care physician Hospital administration Nurse clinicians/educators |
|
Valuable Implementation can occur without, but impact will be greater with their involvement. Include if feasible. |
Advanced practitioners COVID-19 response team members Information technology Noncardiac ERP team leaders Patient/caregiver representatives Respiratory therapist |
Abbreviations: COVID-19, coronavirus disease 2019; ERP, enhanced recovery program.
Step 2: Educate the Team and Assess Current Status
Once the team has been established, program design can commence with an introductory meeting (likely virtual). The 4 key objectives for this meeting are based on those of a traditional ERP but with specific modifications for COVID-19. The first is to educate team members about the concepts and benefits of enhanced recovery and summarize the history, principles, and demonstrated benefits of both cardiac and noncardiac ERPs. The second is to identify the best timing for ERP implementation, assess local and regional pandemic status—current and anticipated—and review prediction models, expected surgical volume, and current guidelines for safe delivery of cardiac surgical care during COVID-19.4 , 6 , 22 , 23 , 31 The third is to identify the strengths and weaknesses in current cardiac surgery programs. This should be done in an open, honest, objective, and dispassionate manner based on real institutional historic data. The Society of Thoracic Surgeons (STS) database can provide easily obtainable benchmarks. Although this process would be the same as in a traditional program, the self-assessment should incorporate consideration of local systemic impacts and constraints secondary to COVID-19, as well as focus on outcomes most related to preserving shared resources and reducing length of stay. Finally, options for protocol interventions should be introduced (see step 3 for examples), with team members’ input and feedback encouraged. The protocol will not be finalized during this meeting, but initiating the discussion on interventions early will facilitate the next step—protocol design.
Step 3: Plan and Develop the ERP Protocol
The ERAS Cardiac Society's published guidelines offer several potential targets and interventions that could be considered for cardiac surgery ERPs.30 Under ideal circumstances, institutions will include as many of the recommendations as possible to allow for the greatest impact through accumulation of marginal gains, ease of implementation through “bundles,” and the highest chance of achieving synergism between the interventions. This approach is not practical for most institutions in the present healthcare environment.
For the COVID-19 ERP modified approach, interventions should be prioritized based on those that are most likely to achieve the above-stated goals of preserving resources, addressing caseload backlog, and improving the safety of the hospital environment within the current COVID-19 environment. The best interventions will meet the following criteria: least required cost, lowest possible impact on current workflows, and minimal complexity for implementation.
Using the ERAS Cardiac guidelines as a starting point, the authors have selected 14 of the 22 original recommendations that they believe best meet criteria for consideration in a concise, focused, COVID-19 modified ERP (Table 3 ).
Table 3.
Proposed Interventions for a Modified Cardiac Enhanced Recovery Program to be Implemented During the COVID-19 Pandemic.
| Intervention | Level of Evidence | Expected Benefit in COVID-19 | Additional Cost | Impact on Workflow | Implementation Complexity | |
|---|---|---|---|---|---|---|
| Preoperative | Smoking and alcohol cessation for 3 weeks before surgery | Moderate | Medium | Low | Low | Low |
| Encourage clear-fluid intake up to 4-hours before surgery | Low | Small | Low | Medium | Low | |
| Provide a liquid carbohydrate beverage 4 hours before surgery | Low | Small | Medium | Medium | Low | |
| Use a surgical-site infection reduction bundle | Moderate | Large | Medium | High | Medium | |
| Intraoperative | Intraoperative multimodal opioid-sparing analgesia | Moderate | Large | Medium | Medium | Medium |
| Administer an intraoperative antifibrinolytic | High | Large | Low | Low | Low | |
| Maintain intraoperative glucose levels below 180 mg/dL (10 mmol/L) | Moderate | Large | Low | Low | Low | |
| Avoid hyperthermia (>37.9°C) or excessively rapid rates during re-warming on cardiopulmonary bypass | Moderate | Large | Low | Medium | Low | |
| Avoid persistent hypothermia (<35°C) postoperatively | Moderate | Large | Low | Medium | Low | |
| Postoperative | Postoperative multimodal opioid-sparing analgesia | Moderate | Large | Medium | Medium | High |
| Optimize strategies to ensure extubation as early as safely possible | Moderate | Large | Low | High | High | |
| Maintain postoperative glucose levels below 180 mg/dL (10 mmol/L) | Moderate | Large | Low | Medium | Medium | |
| Promote early mobilization and removal of tubes, drains, and lines | Moderate | Large | Low | High | High | |
| Ensure chemical thromboprophylaxis is initiated for all patients when appropriate | Moderate | Medium | Low | Low | Medium | |
Adapted from guidelines published by the ERAS Cardiac Society.30
Abbreviations: COVID-19, coronavirus disease 2019.
Compared with the measures not included, these 14 were expected to yield the greatest ratio of benefit compared with effort, cost, and complexity, specifically in the COVID-19 hospital environment. These selections were based on current evidence, including the results from existing published results for cardiac ERPs (Table 1), as well as targets for improvement that would have a higher impact during COVID-19 (such as reduced ventilator time and ICU length of stay). Table 3 compares the 14 selected interventions to each other in terms of important features to consider when deciding whether to include them in an ERP. Any intervention in Table 3 will be less costly, easier to implement, and more likely to yield meaningful benefit during COVID-19 than any of the original guideline recommendations that were not included. The list provided is not exhaustive but offers a sound, evidence-based starting point. Any final protocol will vary depending on the needs of and resources available to an individual institution, as identified in step 2.
Step 4: Implement the ERP Protocol
Traditional ERP implementation starts with a period of pre-launch “socialization,” wherein team members begin discussing the concept of cardiac ERPs with colleagues, including current status (good and bad), expected improvements in local outcomes, types of protocol interventions, anticipated changes in workflow, and how to provide feedback. This would be followed by a program launch and activation of the protocol's interventions. Implementation education would usually be carried out in a myriad of formats including one-on-one, informal small group, formalized teaching sessions, business meetings, question/answer (Q & A) sessions, and so on.
During COVID-19, these processes will require substantial modification. Socialization will need to be brief, with less breadth and depth. The list of COVID-19 modified team members from step 1 can assist with focusing the socialization efforts. There will also be a greater reliance on electronic media, such as virtual meetings, group emails, updates on an institution's website, and social media platforms. If required, program changes may need to be phased in sequentially to accommodate limitations on resources, time, team member availability, and acceptable workflow changes during COVID-19.
Step 5: Post-implementation Feedback and Audit
Seeking feedback from all members of the cardiac surgical team and the patients themselves is essential, even during COVID-19. It is imperative for institutions to have an open, available communication platform that ensures comments, suggestions, and complaints are funneled to the cardiac ERP team for discussion and action. Audit is the foundation of enhanced recovery, providing measurements of protocol adherence and patient outcomes.8 , 21 After implementation, an audit will assist in identifying areas of poor protocol adherence, root cause analysis, and adjustment of the protocol or further healthcare provider education as needed. Auditing key outcomes will spotlight “wins” while also directing attention to areas that need greater attention and improvement. A fully functioning, de novo audit system may not be feasible given current COVID-related surge and resource constraints. In the interim, most institutions have access to some form of auditing system, such as the Society of Thoracic Surgeons National Database, which can be used to track selected outcomes.32 Nonetheless, it is recommended that there is a plan for inclusion of an ERP-specific audit system as soon as feasible after the pandemic.
Step 6: Iterative Program Planning and Future Growth
Enhanced recovery fundamentally opposes complacency. Even a fully comprehensive ERAS Cardiac Program, designed and implemented under ideal circumstances, should undergo review and update within 12- to-24 months; enhanced recovery is thus not a static change but an iterative process. New evidence, updated guidelines and standards of practice, innovations in healthcare delivery, and program evolution guided by patient's feedback will challenge a mature program to either adapt or risk long-term failure.19 , 33 In addition to the immediate benefits of developing an active ERP now—even if modified for COVID-19 considerations—a foundation will have been created for future growth once the bulk of the pandemic limitations have subsided. The establishment of a network of engaged team members and the introduction of ERPs within each institution's culture will benefit all stakeholders. Improvements in key metrics, including patient experience and staff morale, will provide momentum, credibility, and encourage buy-in for additional developments.
How Can the COVID-19 Response Identify Future Directions in Patient Care and Cardiac ERPs?
The global COVID-19 pandemic has altered the landscape of healthcare delivery, including abrupt changes to practices that were once considered near-immutable “standards of care.” Although the immediacy of these changes was born of necessity to protect patients, families, and healthcare providers, some of them may remain permanent or highlight areas for future optimization of perioperative care. Institutions would benefit from actively and collectively assessing changes to perioperative strategies implemented during the COVID-19 pandemic and maintaining or expanding them where appropriate. For example, an array of preoperative investigations has typically been included in standard preparation for cardiac surgery. Historically, the decision to reconsider ordering these tests were made with a strong consideration of cost.34 However, COVID-19 calls for reduction in out-of-home travel and in-person contact between patients and providers in order to protect one another against potential SARS-CoV-2 exposure. This has caused providers to view these tests through a different lens, often resulting in elimination of tests, such as basic labs or chest radiographs, when normal results previously had been obtained within an acceptable time-interval before surgery. Although this shift in priorities was forced upon the system, it could form the basis for redesigning order sets to ensure they are standardized, necessary, and provide patient-centered value. The prepandemic interest in integrating technology into patient care has gained additional momentum, particularly in the areas of virtual medicine and mobile monitoring.35, 36 Additional examples include creation of parallel recovery pathways for low-risk patients, improved discharge planning, and seeking new “low-hanging fruit” to reduce complications.37, 38, 39, 40, 41 Surviving the various phases of the COVID-19 pandemic requires additional emphasis on pursuing methods to improve care, reducing lengths of stay, preventing readmissions, utiliz-ing resources effectively, and decreasing complications. COVID-19 has been a harsh teacher, but clinicians ought to capitalize on lessons learned from these challenging times to catalyze innovation and thoughtful discourse about the future of caring for the cardiac surgical patient.
Conclusion
Institutional status quo survives because the outcomes achieved are sufficient for the current environment, providing little incentive for the examination of the potential for improvement. The COVID-19 pandemic has changed the calculus by imposing enormous peril to humanity that has necessitated abrupt and unprecedented disruption to institutional systems. How surgical programs adapt to the uncertainties and continuing fallout from the COVID-19 pandemic is critical. Proactive actions, rather than reactions, will ensure that the healthcare system can come back, providing even better and more efficient patient care than before.
ERPs have proven over time to be reproducible mechanisms to reduce ICU and hospital resource utilization, decrease costs, and improve outcomes. These guiding principals foster a drive toward standardized, patient-centric, innovative, and evidence-based care to supplant tradition, dogma, and habit. A cardiac ERP, designed specifically for implementation within the COVID-19 environment, can help achieve an institution's optimal potential and build a dynamic platform for development and growth going forward.
Whereas some may have previously considered enhanced recovery a luxury, in these times it is more of a necessity.
Acknowledgments
Jeanne McAdara PhD provided expert medical editing assistance. Figure 1 is courtesy of Mark Cromwell.
Conflict of Interest
All of the authors are non-remunerated executive board members of the ERAS Cardiac Society, a non-profit organization. R.C.A. additional disclosures: unrestricted educational grant from Pfizer Canada Inc and honoraria from Mallinckrodt Pharmaceuticals, Abbott Nutrition, and Edwards Lifesciences.
References
- 1.Rosenbaum L. The untold toll - the pandemic's effects on patients without covid-19. N Engl J Med. 2020;382:2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 2.Engelman DT, Lother S, George I. Adult cardiac surgery and the COVID-19 pandemic: Aggressive infection mitigation strategies are necessary in the operating room and surgical recovery. Ann Thorac Surg. 2020;110:707–711. doi: 10.1016/j.athoracsur.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudino M, Chikwe J, Hameed I. Response of cardiac surgery units to COVID-19: An internationally-based quantitative survey. Circulation. 2020;142:300–302. doi: 10.1161/CIRCULATIONAHA.120.047865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood DA, Mahmud E, Thourani V.H. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: Guidance from North American Society Leadership. Ann Thorac Surg. 2020;36:971–976. doi: 10.1016/j.athoracsur.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salenger R, Etchill EW, Ad N. The surge after the surge: Cardiac surgery post-COVID-19. Ann Thorac Sur. 2020 doi: 10.1016/j.athoracsur.2020.04.018. Accessed August 1, 2020. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parolari A, di Mauro M, Bonalumi G. Safety for all: Coronavirus disease 2019 pandemic and cardiac surgery: A roadmap to 'phase' 2. Eur J Cardiothorac Surg. 2020;58:213–216. doi: 10.1093/ejcts/ezaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joliat GR, Ljungqvist O, Wasylak T. Beyond surgery: Clinical and economic impact of enhanced recovery after surgery programs. BMC Health Serv Res. 2018;18:1008. doi: 10.1186/s12913-018-3824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 9.Gregory AJ, Grant MC, Manning MW. Enhanced Recovery After Cardiac Surgery (ERAS Cardiac) recommendations: An important first step-but there is much work to be done. J Cardiothorac Vasc Anesth. 2020;34:39–47. doi: 10.1053/j.jvca.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Noss C, Prusinkiewicz C, Nelson G. Enhanced recovery for cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32:2760–2770. doi: 10.1053/j.jvca.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Fleming IO, Garratt C, Guha R. Aggregation of marginal gains in cardiac surgery: Feasibility of a perioperative care bundle for enhanced recovery in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2016;30:665–670. doi: 10.1053/j.jvca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Grant MC, Isada T, Ruzankin P. Results from an enhanced recovery program for cardiac surgery. J Thorac Cardiovasc Surg. 2020;159 doi: 10.1016/j.jtcvs.2019.05.035. 1393-402.e1397. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Zhang J, Gan TJ. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: A randomized clinical trial. Eur J Cardiothorac Surg. 2018;54:491–497. doi: 10.1093/ejcts/ezy100. [DOI] [PubMed] [Google Scholar]
- 14.Williams JB, McConnell G, Allender JE. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) program. J Thorac Cardiovasc Surg. 2019;157:1881–1888. doi: 10.1016/j.jtcvs.2018.10.164. [DOI] [PubMed] [Google Scholar]
- 15.Zaouter C, Oses P, Assatourian S. Reduced length of hospital stay for cardiac surgery-implementing an optimized perioperative pathway: Prospective evaluation of an enhanced recovery after surgery program designed for mini-invasive aortic valve replacement. J Cardiothorac Vasc Anesth. 2019;33:3010–3019. doi: 10.1053/j.jvca.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Anesthesiologists (ASA), American College of Surgeons (ACS), and Association of periOperative Registered Nurses (AORN) 2020. Joint statement: Roadmap for resuming elective surgery after COVID-19 pandemic.https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/joint-statement-on-elective-surgery-after-covid-19-pandemic Available at: Accessed Aug 4, 2020. [Google Scholar]
- 17.Graham ID, Logan J, Harrison MB. Lost in knowledge translation: Time for a map? J Contin Educ Health Prof. 2006;26:13–24. doi: 10.1002/chp.47. [DOI] [PubMed] [Google Scholar]
- 18.Gramlich LM, Sheppard CE, Wasylak T. Implementation of enhanced recovery after surgery: A strategy to transform surgical care across a health system. Implement Sci. 2017;12:67. doi: 10.1186/s13012-017-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramlich L, Nelson G, Nelson A. Moving enhanced recovery after surgery from implementation to sustainability across a health system: A qualitative assessment of leadership perspectives. BMC Health Serv Res. 2020;20:361. doi: 10.1186/s12913-020-05227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu SY, Lai Y, Dalia AA. Implementing a cardiac enhanced recovery after surgery protocol: Nuts and bolts. J Cardiothorac Vasc Anesth. 2019 doi: 10.1053/j.jvca.2019.12.022. Accessed August 4, 2020. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Salenger R, Morton-Bailey V, Grant M. Cardiac enhanced recovery after surgery: A guide to team building and successful implementation. Semin Thorac Cardiovasc Surg. 2020;32:187–196. doi: 10.1053/j.semtcvs.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Patel V, Jimenez E, Cornwell L. Cardiac surgery during the coronavirus disease 2019 pandemic: Perioperative considerations and triage recommendations. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan A, Arora RC, Adams C. Cardiac surgery in Canada during the COVID-19 pandemic: A guidance statement from the Canadian Society of Cardiac Surgeons. Can J Cardiol. 2020;36:952–955. doi: 10.1016/j.cjca.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn M, Sheehan M, Hordern J. 'Your country needs you': The ethics of allocating staff to high-risk clinical roles in the management of patients with COVID-19. J Med Ethics. 2020;46:436–440. doi: 10.1136/medethics-2020-106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emanuel EJ, Persad G, Upshur R. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 26.Kramer JB, Brown DE, Kopar PK. Ethics in the time of coronavirus: Recommendations in the COVID-19 pandemic. J Am Coll Surg. 2020;230:1114–1118. doi: 10.1016/j.jamcollsurg.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo M, Guo L, Yu M. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and general public - a systematic review and meta-analysis. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappa S, Ntella V, Giannakas T. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chew NWS, Lee GKH, Tan BYQ. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelman DT, Ben Ali W, Williams JB. Guidelines for perioperative care in cardiac surgery: Enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154:755–766. doi: 10.1001/jamasurg.2019.1153. [DOI] [PubMed] [Google Scholar]
- 31.Haft JW, Atluri P, Alawadi G. Adult cardiac surgery during the COVID-19 pandemic: A tiered patient triage guidance statement. Ann Thorac Surg. 2020;110:697–700. doi: 10.1016/j.athoracsur.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs JP, Shahian DM, D'Agostino RS. The Society of Thoracic Surgeons national database 2018 annual report. Ann Thorac Surg. 2018;106:1603–1611. doi: 10.1016/j.athoracsur.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Baxter RD, Fann JI, DiMaio JM. Digital health primer for cardiothoracic surgeons. Ann Thorac Surg. 2020;110:364–372. doi: 10.1016/j.athoracsur.2020.02.072. [DOI] [PubMed] [Google Scholar]
- 34.Beliveau L, Buddenhagen D, Moore B. Decreasing resource utilization without compromising care through minimizing preoperative laboratories. Am Surg. 2018;84:1185–1189. [PubMed] [Google Scholar]
- 35.Wosik J, Fudim M, Cameron B. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27:957–962. doi: 10.1093/jamia/ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khairat S, Meng C, Xu Y. Interpreting COVID-19 and virtual care trends: Cohort study. JMIR Public Health Surveill. 2020;6:e18811. doi: 10.2196/18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott BK, Miller GT, Fonda SJ. Advanced digital health technologies for COVID-19 and future emergencies. Telemed J E Health. 2020 doi: 10.1089/tmj.2020.0140. Available at: https://www.liebertpub.com/doi/10.1089/TMJ.2020.0140. Accessed Aug 4, 2020. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Engelman DT, Uddin QK, Crisafi C. Commentary: Low hanging fruit-reducing hospital-acquired pressure injuries associated with cardiac surgery. J Thorac Cardiovasc Surg. 2020;160:164–166. doi: 10.1016/j.jtcvs.2019.12.101. [DOI] [PubMed] [Google Scholar]
- 39.Probst S, Cech C, Haentschel D. A specialized post anaesthetic care unit improves fast-track management in cardiac surgery: A prospective randomized trial. Crit Care. 2014;18:468. doi: 10.1186/s13054-014-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonçalves-Bradley DC, Lannin NA, Clemson LM. Discharge planning from hospital. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD000313.pub5. 2016:Cd000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rushton M, Howarth M, Grant MJ. Person-centred discharge education following coronary artery bypass graft: A critical review. J Clin Nurs. 2017;26:5206–5215. doi: 10.1111/jocn.14071. [DOI] [PubMed] [Google Scholar]