Abstract
Background
A cytokine storm conceivably contributes to manifestations of corona virus disease (COVID-19). Inflammatory cytokines such as interleukin-6 (IL-6) cause acute liver injury while serum detectability indicates systemic inflammation.
Aims
We explored a link between systemic IL-6, related acute phase proteins and liver injury in hospitalized COVID-19 patients.
Methods
655 patients with suspected COVID-19 were screened in the emergency department at the University Hospital of Innsbruck, Austria, between February and April 2020. 96 patients (∼15%) were hospitalized with COVID-19. 15 patients required intensive-care treatment (ICT). Plasma aminotransferases, alkaline phosphatase, bilirubin, and gamma glutamyl transferase, as well as IL-6, C-reactive protein (CRP), ferritin and lactate dehydrogenase (LDH) were determined by standard clinical assays.
Results
Of all hospitalized COVID-19 patients, 41 (42%) showed elevated aspartate aminotransferase (AST) concentration. COVID-19 patients with elevated AST exhibited significantly higher IL-6 (p < 0.001), ferritin (p < 0.001), LDH (p < 0.001) and CRP (p < 0.05) serum concentrations compared to patients with normal AST. Liver injury correlated with systemic IL-6 (p < 0.001), CRP (p < 0.001), ferritin (p < 0.001) and LDH (p < 0.001) concentration. In COVID-19 patients requiring ICT, correlations were more pronounced.
Conclusion
Systemic inflammation could be a fuel for hepatic injury in COVID-19.
Keywords: Acute phase protein, COVID-19, Cytokines, Interleukin-6, Liver damage, SARS-CoV2
1. Introduction
In December 2019, a series of patients with pneumonia caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported from Wuhan, Hubei province in China [1]. Since then, Coronavirus Disease 2019 (COVID-19) has spread globally with more than 6.300.000 infections and, by date, more than 370.00 deaths worldwide. The typical symptoms of SARS-CoV-2 infection, such as fever, cough, sore throat, or dyspnea, are well recognized and have been widely described [2], [3], [4], [5], [6] while also organs beyond the respiratory tract may be affected [7,8].
SARS-CoV-2 is a beta-coronavirus that is closely related to severe acute respiratory syndrome corona virus (SARS-CoV) [9]. Both viruses use the angiotensin-converting enzyme–related carboxypeptidase (ACE2) as receptor to gain entry into mammalian cells [10]. Previous studies found that ACE2 expression is related to the severity of acute respiratory distress syndrome (ARDS) caused by SARS-CoV infection, and mediates the production of cytokines in ARDS [11,12]. Massive release of pro-inflammatory cytokines results in a cytokine storm (also termed cytokine release syndrome (CRS)) which is characterized by elevated C-reactive protein (CRP), interleukin 6 (IL-6), lactate dehydrogenase (LDH) and ferritin concentration that is accompanied by organ dysfunction (such as ARDS, progressive liver damage and liver failure). As such, the systemic release of pro-inflammatory cytokines seems to be a driver of disease progression in COVID-19 [13], [14], [15].
The impact of SARS-CoV-2 on liver disease is poorly understood [16,17]. Several studies reported clinical features of liver injury in COVID-19 patients [3], [4], [5], [6],[18], [19], [20] with elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT) in 14% to 53% of COVID-19 patients [3,5,6] which could be an indicator for severe pneumonia [21]. Similarly, delayed hospital admission after illness onset was associated with increased risk of liver injury in patients with COVID-19 [22]. Notably, hepatic infiltration of lymphocytes, centrilobular sinusoidal dilation and patchy necrosis could be observed in COVID-19 patients [23,24], and SARS-CoV-2 might directly bind to ACE2-expressing cholangiocytes [25]. However, the origin of liver injury remains unresolved and could be related to systemic inflammation, SARS-CoV-2 infection or drug administration [26].
IL-6 is a potent cytokine with diverse functions during hepatic inflammation and regeneration [27]. IL-6 serves inflammatory (danger) signaling and (because of its half life) better indicates systemic inflammation when compared to other cytokines, such as Interleukin-1 beta (IL-1β) or TNF-alpha (TNF-α) [28]. The aim of this study was to explore a link between systemic inflammation and liver injury in COVID-19.
2. Methods
2.1. Patients
The study was performed at the University Hospital of Innsbruck, Austria, the only referral hospital in Tyrol, Austria. From February 26th, 2020 to April 21st, 2020, 655 patients with suspected COVID-19 were evaluated. 96 patients were diagnosed with COVID-19 based on the World Health Organization interim guidance [29]. 81 patients were hospitalized without the need for intensive care, while 15 patients required intensive care treatment on admission day. None of the included individuals was admitted to an intensive care unit (ICU) 3 months prior to study inclusion. 8 of 96 patients showed evidence of hepatic steatosis by ultrasonography (6/8 diagnosed with metabolic associated liver disease [30]) 3 to 12 month prior to hospitalization. No other chronic liver disease (such as chronic viral hepatitis, alcoholic liver disease, immune-mediated liver disorders or hemochromatosis) were documented in any patient and all patients had normal liver enzymes documented in previous biochemical studies (8.9 months ± 6.1months prior to hospitalization) . None of the patients received any antibiotic or antiviral therapy in the past 3 months. Fever was defined as body temperature ≥ 37.3°C and dyspnea was defined by a respiratory rate ≥ 20 breaths/minute and resting finger oxygen saturation ≤ 93% [31]. Gastrointestinal symptoms were recorded based on medical history taken at hospital admission. Diarrhea was defined by loose stools > 3 times per day, vomiting was defined by ≥ 1 per day. All reported parameters were collected on admission day.
2.2. SARS-CoV2- analysis
Laboratory confirmation of SARS-CoV-2 infection was performed using previously described real-time polymerase chain reaction [4] as recommended by the Centers for Disease Control and Prevention (DeKalb, GA).
2.3. Liver parameters
Elevated liver tests were defined by the AST ≥ 50 Units/liter (U/l), ALT ≥ 50 U/l, gamma-glutamyl transferase (GGT) ≥ 71 U/l, alkaline phosphatase (AP) ≥ 129 U/l and total bilirubin ≥ 1.28 U/l assessed on admission day. In COVID-19 consensus on liver injury classification by biochemical means is lacking. Therefore, patients displaying AST concentrations (≥ 50 U/l) was considered to be increased (as in other clinical situations).
2.4. IL-6 and acute phase proteins
IL-6, CRP, LDH and ferritin were determined by Cobas 8000, (Roche, Basel, Switzerland) according to the manufacturer´s specification. The lower detection limit of the assay for IL-6 is < 5 nanograms/mililter (ng/ml), for CRP 0,05 miligrams/deciliter (mg/dl) and 20 micrograms/liter (µg/l) for ferritin. The established cut-off value for CRP is 0,50 mg/dl, for LDH 100 U/l and for ferritin 400 µg/l.
2.5. Creatininkinase (CK), Troponin T and creatinine (Crea)
CK, Troponin T and Crea were determined by Cobas 8000, (Roche, Basel, Switzerland) according to the manufacturer´s specification. The lower detection limit of the assay for CK is < 10U/l, for Troponin T i5 nanograms/deciliter (ng/dl) and 0,3 micrograms/deciliter (mgd/l) for Crea. The established cut-off value for CK is 190 U/l, for Troponin T is 50 ng/dl and 1,2 mg/dl for Crea.
2.6. Data analysis
Data were expressed as mean ± standard error of mean or as median with first and third quartiles. For comparing quantitative variables, the Student's t-test or the non-parametric Mann–Whitney U or Wilcoxon signed-rank test were used as appropriate. Normality of distribution was determined by Kolmogorov-Smirnov test. The correlation analysis was estimated using the Spearman's p coefficient A p-value <0.05 was considered as statistically significant. All statistical analyses were performed using SPSS Statistics v.22 (IBM, Chicago, IL) and GraphPad PRISM 5 (La Jolla, CA).
2.7. Ethical consideration
The study protocol was approved by the institutional ethics commission with an amendment to AN2017-0016 369/4.21 and informed consent was obtained, if applicable, from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by institutional ethics commission.
3. Results
3.1. Clinical characteristics of hospitalized COVID-19 patients
The mean age of patients included into our study was 60.69 years with 37.5% (n=36) females. Patients characteristics are listed in Table 1 . Most COVID-19 patients reported fever (75.8%), cough (88.9%), muscle pain (66.7%) and fatigue (67.8%), whereas dyspnea was noted in 50% of patients. Gastrointestinal symptoms like nausea, vomiting, and diarrhea were present in 15.6%, 7.8% and 17.8%, respectively. Importantly, 61.8% of patients showed typical radiologic findings in imaging studies (chest X-ray or computed tomography). Most patients reported chronic diseases like hypertension (50.5%), type 2 diabetes (19.8%), pulmonary disease (11%) and 13.4% reported a history of smoking. During hospitalization (i.e. after liver enzyme testing) 66.3% of patients received antibiotic-therapy with cephalosporines in all cases to cover secondary infections, and 42.7% of patients received anti-viral therapy with favipiravir. Important to note, only 3 patients received antibiotic therapy and none received anti-viral therapy ahead of admission. On admission day, 32 non-ICU patients (39.5%) displayed elevated AST, 60% of patients at the ICU showed elevated AST, and no patient showed clinical or biochemical features of fulminant hepatitis (Table 1). Skeletal muscle, myocardium, and kidney might all be sources of AST, therefore AST levels were correlated with CK (non significant (ns), data not shown), Troponin T (ns, data not shown) and Crea (ns, data not shown). ALT was elevated in only 5 patients on admission day. These findings are going in line with other studies, suggesting ALT elevation appears at a later timepoint during COVID-19 [21,22,26]. None of the patients showed elevated Bili, GGT and AP at a such early stage of disease. ICU patients displayed higher frequencies of fever (p=0.033), dyspnea (p=0.014) and imaging findings (p=0.012) at admission, compared to non ICU patients (Table 1). During hospitalization none of the non ICU-patients was transferred to the ICU. The length of hospital stay was significantly longer in ICU patients (p<0.001) and mortality rate was significantly higher in ICU patients (p<0.001) (Table 1). Patients admitted to the ICU showed significantly higher levels of AST (p=0.02), CRP (p<0.001), LDH (p<0.001), procalcitonin (PTC, p<0.001), ferritin (p<0.001) and IL-6 (p=0.003) (Table 2 ). Furthermore maximum AST (p<0.001) and ALT (p<0.001) during hospital stay were significantly higher and peaked significantly later during disease course (p<0.001) in ICU patients (Table 2).
Table 1.
Patient characteristics in Covid-19 patients admitted to general ward (non ICU) or ICU.
| Overall |
Non ICU |
ICU |
non ICU vs ICU | ||||
|---|---|---|---|---|---|---|---|
| n | N | n | p | ||||
| Age [years] | 60.69 ± 18.915 | 96 | 60.98 ± 20.12 | 81 | 59.13 ± 10.54 | 15 | ns |
| Females (%) | 36 (37.5) | 96 | 32 (39.5) | 81 | 4 (26.7) | 15 | ns |
| Smoker (%) | 11 (13.4) | 89 | 9 (11.8) | 76 | 2 (15.4) | 13 | ns |
| Allergies (%) | 21 (23.3) | 91 | 17 (22.1) | 77 | 4 (30.8) | 13 | ns |
| Malignant disease (%) | 9 (9.9) | 91 | 9 (11.5) | 78 | 0 (0) | 13 | ns |
| Hypertension (%) | 46 (50.5) | 91 | 38 (48.7) | 78 | 8 (61.5) | 13 | ns |
| Diabetes (%) | 18 (19.8) | 91 | 15 (19.2) | 78 | 3 (23.1) | 13 | ns |
| Chronic liver disease*,** (%) | 8 (8.8) | 91 | 5 (6.4) | 78 | 3 (23.1) | 13 | ns |
| Heart disease (%) | 20 (122.2) | 90 | 18 (23.4) | 77 | 2 (15.3) | 13 | ns |
| Pulmonary disease (%) | 10 (11) | 91 | 7 (9) | 78 | 3 (12.1) | 13 | ns |
| Immunosuppression (%) | 3 (3.3) | 90 | 1 (1.3) | 77 | 2 (15.4) | 13 | ns |
| Fever >37,3°C (%) | 69 (75.8) | 91 | 56 (71.8) | 78 | 13 (100) | 13 | 0.033 |
| Dyspnea (%) | 45 (50) | 90 | 34 (44.2) | 77 | 11 (84.6) | 13 | 0.014 |
| Cough (%) | 80 (88.9) | 90 | 68 (88.3) | 77 | 12 (92.3) | 13 | ns |
| Sputum (%) | 24 (26.7) | 90 | 22 (28.6) | 77 | 2 (13.3) | 13 | ns |
| Hemoptysis (%) | 5 (5.6) | 89 | 5 (6.5) | 77 | 0 (0) | 13 | ns |
| Sore throat (%) | 26 (28.9) | 90 | 23 (29.9) | 77 | 3 (23.1) | 13 | ns |
| Nasal obstruction (%) | 18 (20) | 90 | 15 (19.5) | 77 | 3 (23.1) | 13 | ns |
| Muscle ache (%) | 60 (66.7) | 90 | 50 (64.9) | 77 | 10 (76.9) | 13 | ns |
| Fatigue (%) | 61 (67.8) | 90 | 51 (66.2) | 77 | 10 (76.9) | 13 | ns |
| Nausea (%) | 14 (15.6) | 90 | 13 (16.9) | 77 | 1 (7.7) | 13 | ns |
| Vomiting (%) | 7 (7.8) | 90 | 7 (9.1) | 77 | 0 (0) | 13 | ns |
| Diarrhea (%) | 16 (17.8) | 90 | 14 (18.2) | 77 | 2 (15.4) | 13 | ns |
| Imaging finding (%) | 55 (61.8) | 89 | 43 (56.6) | 76 | 12 (92.3) | 13 | 0.012 |
| Antibiotic therapy on admisson day (%) | 14 (15.7) | 89 | 11 (14.5) | 76 | 3 (23.1) | 13 | ns |
| Antiviral therapy on admisson day (%) | 1 (1.2) | 86 | 1 (1.4) | 73 | 0 (0) | 13 | ns |
| Antibiotic therapy during hospital stay (%) | 59 (66.3) | 89 | 46 (60.5) | 76 | 13 (100) | 13 | 0.04 |
| Antiviral therapy during hospital stay (%) | 30 (34.5) | 87 | 17 (23) | 74 | 13 (100) | 13 | <0.001 |
| Elevated AST | 41 (42.7) | 96 | 32 (39.5) | 81 | 9 (60) | 15 | ns |
| Hospital stay (days) | 19.4 ± 7.2 | 96 | 8.7 ± 6.3 | 81 | 36.2 ± 15.7 | 15 | <0.001 |
| ICU stay (days) | 24 ± 13.2 | 96 | 0 | 81 | 24 ± 13.2 | 15 | <0.001 |
| Mortality | 7 (7.3) | 96 | 5 (6.2) | 81 | 2 (15.4) | 15 | 0.04 |
**MAFLD (n=6) **chronic hepatitis B infection (n=2).
Patient characteristics in Covid-19 patients admitted to general ward (non ICU) or ICU. Data are expressed as case numbers (percentage) or mean ± standard deviation. AST, aspartate aminotransferase; ICU, intensive care unit; MAFLD, metabolic associated fatty liver disease.
Table 2.
Laboratory parameters in Covid-19 patients admitted to general ward (non ICU) or ICU.
| Non ICU |
ICU |
||||
|---|---|---|---|---|---|
| n | N | p | |||
| Bilirubin [mg/dl] | 0.45 (0.3-0.67) | 81 | 0.641 ± 0.30 | 15 | ns |
| AST [U/l] | 34 (23-56.5) | 81 | 54.73 ± 18.40 | 15 | 0.02 |
| ALT [U/l] | 25 (17-42) | 81 | 44.93 ± 24.83 | 15 | ns |
| Maximum AST [U/l] | 57.4 ± 44.8 | 81 | 179.9 ± 121.1 | 15 | <0.001 |
| Maximum ALT [U/l] | 59.1 ± 32.9 | 81 | 178.3 ± 116.9 | 15 | <0.001 |
| Maximum AST after hospital admission (days) | 3.1 ± 2.0 | 81 | 11.7 ± 5.6 | 15 | <0.001 |
| Maximum ALT after hospital admission (days) | 3.9 ± 2.1 | 81 | 15.5 ± 6.9 | 15 | <0.001 |
| Liver stiffness (kPa) | 4.8 ± 2.7 | 25 | 0 | 15 | – |
| CK [U/l] | 111.3 ± 81.5 | 81 | 107.6 ± 52.1 | 15 | ns |
| Troponin T [U/l] | 16.8 ± 9.2 | 81 | 10.9 ± 6.2 | 15 | ns |
| Creatinine [mg/dl] | 0.85 ± 0.28 | 81 | 0.84 ± 0.21 | 15 | ns |
| LDH [U/l] | 256.6 ± 95.9 | 81 | 439.2 ± 176.2 | 15 | <0.001 |
| GGT [U/l] | 38 (25-67) | 81 | 47 (27-82) | 15 | ns |
| AP [U/l] | 61 (47-72.5) | 81 | 60 (50-69) | 15 | ns |
| CRP [mg/dl] | 3.28 (0.58-7.35) | 81 | 12.982 ± 9.33 | 15 | <0.001 |
| PCT [µg/l] | 0.08 (0.6-0.23) | 81 | 0.28 (0.2-0.7) | 15 | <0.001 |
| IL-6 [ng/l] | 18.6 (5.3-68.3) | 81 | 92.2 (18-171.6) | 15 | 0.003 |
| Leukocytes [109/l] | 5.821 ± 1.94 | 81 | 7.033 ± 3.25 | 15 | ns |
| Hb [g/l] | 133.26 ± 17.31 | 81 | 131 ± 21.86 | 15 | ns |
| Platelets [109/l] | 220 (171-294.5) | 81 | 227.67 ± 92.77 | 15 | ns |
| Prothrombin time [%] | 1 (0.9-1) | 81 | 1.02 ± 0.11 | 15 | ns |
| Ferritin [µg/l] | 525 (217-982) | 81 | 1650.67 ± 1187.12 | 15 | <0.001 |
Laboratory parameters of Covid-19 patients admitted to ICU or general ward (non ICU). Quantitative data were expressed as means ± standard deviation if normally distributed. For non-normally distributed variables, data are shown as medians with first and third quartiles. AP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; kPa, Kilopascal; CK, creatinine kinase; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; Hb, hemoglobin; ICU, intensive care unit; IL-6, interleukin 6; LDH, lactate dehydrogenase; mg/dl, milligrams/deciliter; µg/l, micrograms/liter; PCT, procalcitonin; U/l, Units/liter.
3.2. Liver damage correlates with IL-6 and acute phase proteins
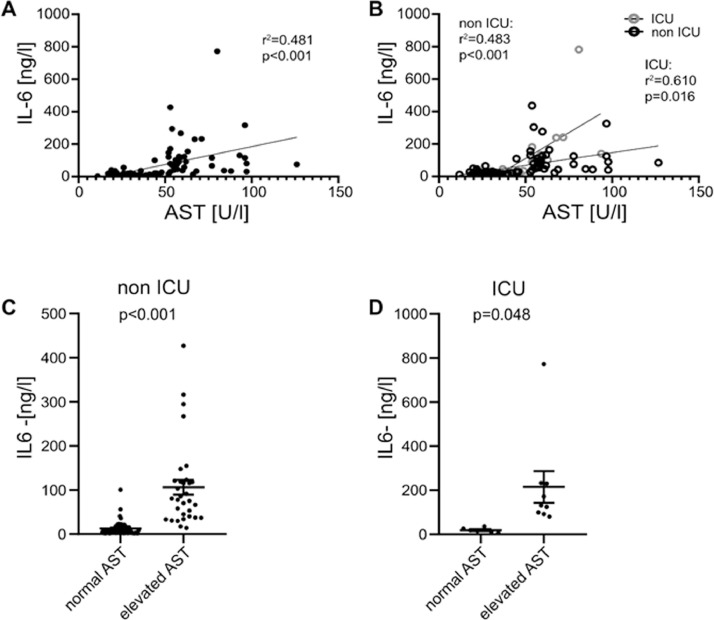
IL-6 positively correlated with AST in all patients (r 2=0.481, p<0.001, Fig. 1 A). In both ICU and non-ICU patients a positive correlation could be observed (Fig. 1B) with more pronounced effects in ICU patients (r 2=0.610, p=0.016). Splitting our cohort in individuals with increased (≥ 50 U/l) and normal (<50 U/l) AST concentration, we could observe higher IL-6 levels in non ICU patients (p<0.001, Fig. 1C) as well as ICU patients (p=0.048, Fig. 1D) with elevated AST. IL-6 didn´t correlate with ALT, GGT, Bilirubin and AP on admission day.
Fig. 1.
IL-6 correlates with AST and is increased in patients with impaired liver function. (A) IL-6 and AST levels correlate in all hospitalized patients. (B) IL-6 levels correlate with AST concentration in non ICU and ICU patients. Displayed are IL-6 concentrations in patients with normal AST and elevated AST in (C) non ICU and (D) ICU patients. Data are shown as mean ± SEM, n: (C-D). *p<0.05; **p<0.01; ***p<0.001 according to Spearman correlation or Student's t-test. AST, aspartate aminotransferase; ICU, intensive care unit; IL-6, interleukin 6.
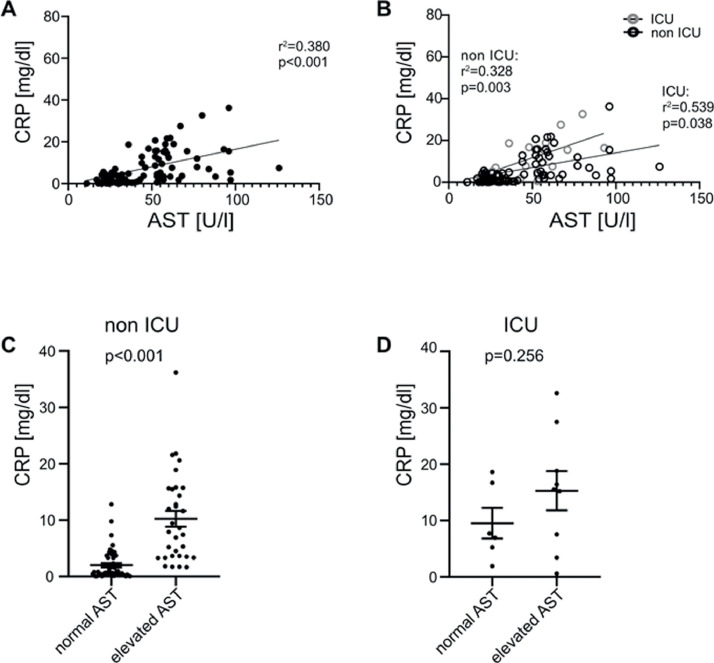
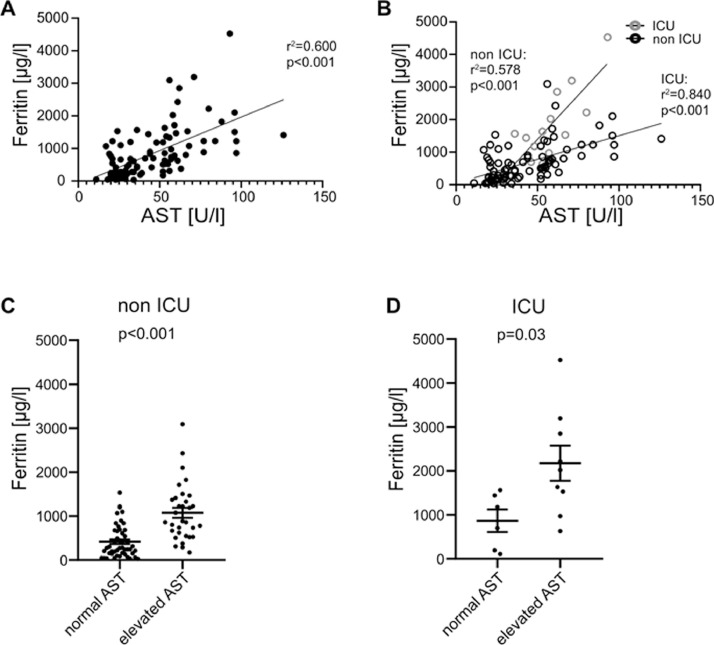
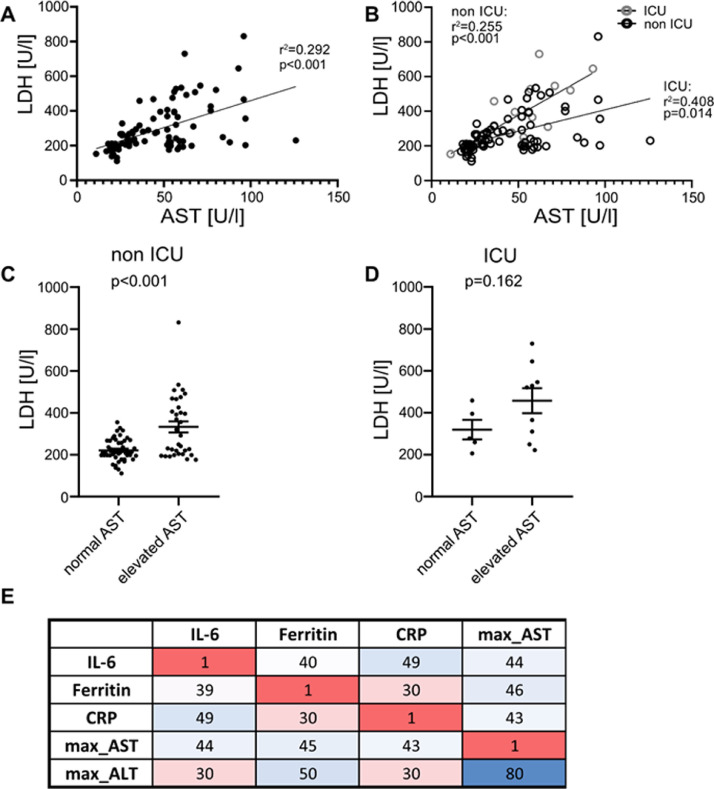
Likewise, CRP correlated with AST in all hospitalized patients (r2=0.38, p<0.001, Fig. 2 A). Both ICU (r 2=0.539, p=0.038, Fig. 2B) and non ICU (r 2=0.328, p=0.003, Fig. 2B) patients displayed a correlation with CRP and AST at admission. In individuals with liver damage, CRP levels were higher in non-ICU patients (p<0.001, Fig. 2C), whereas we could not observe any significant difference in patients at the ICU (p=0.256, Fig. 2D). Similarly, ferritin concentration correlated with AST (r 2=0.6, p<0.001, Fig. 3 A), although in ICU patients this effect was more distinct (r 2=0.84, p<0.001, Fig. 3B) compared to non-ICU patients (r 2=0.578, p<0.001, Fig. 3B). In both non-ICU and ICU patients, individuals with elevated AST seem to have higher concentrations of ferritin (Fig. 3C and D). Furthermore, LDH concentration correlated with AST (r 2=0.6, p<0.001, Fig. 4 A), although in ICU patients this effect was more distinct (r 2=0.84, p<0.001, Fig. 4B) compared to non-ICU patients (r 2=0.578, p<0.001, Fig. 4B). In Non-ICU patients LDH levels were higher in individuals with increased AST (p<0.01, Fig. 4C), whereas ICU patients with altered AST only tended to be higher (p=0.162, Fig. 4D). Furthermore, maximum AST and ALT levels correlated directly with IL-6, Ferritin and CRP in non-ICU patients (Fig. 4E). Furthermore, we performed a longitudinal comparison of AST, ALT and Bilirubin. Liver parameters were more pronounced in patients with higher IL-6 levels. Data are now presented in Supplementary Fig. 1. We compared outcome data with IL-6 and acute phase protein levels. IL-6 was positively correlated with duration of hospital stay (r 2=0.109, p≤0.01 Supp. Fig. 2A), ICU stay (r 2=0.162, p≤0.001 Supp. Fig. 2A), days of mechanical ventilation (r 2=0.146, p≤0.001 Supp. Fig. 2A), and negatively correlated with mean arterial pressure (r 2=0.082, p≤0.01 Supp. Fig. 2A). CRP also showed a significant correlation whereas LDH, showed no significant correlation with mean arterial pressure. Supp. Fig. 2b and c). Ferritin was not significantly correlated to patient´s outcome (data not shown).
Fig. 2.
CRP correlates with AST and is increased in patients with impaired liver function. (A) CRP and AST levels correlate in all hospitalized patients. (B) CRP levels correlate with AST concentration in non ICU and ICU patients. Displayed are CRP concentrations in patients with normal AST and elevated AST in (C) non ICU and (D) ICU patients. Data are shown as mean ± SEM, n: (C-D). *p<0.05; **p<0.01; ***p<0.001 according to Spearman correlation or Student's t-test. AST, aspartate aminotransferase; CRP, C-reactive protein; ICU, intensive care unit.
Fig. 3.
Ferritin correlates with AST and is increased in patients with impaired liver function. (A) Ferritin and AST levels correlate in all hospitalized patients. (B) Ferritin levels correlate with AST concentration in non ICU and ICU patients. Displayed are ferritin concentrations in patients with normal AST and elevated AST in (C) non ICU and (D) ICU patients. Data are shown as mean ± SEM, n: (C-D). *p<0.05; **p<0.01; ***p<0.001 according to Spearman correlation or Student's t-test. AST, aspartate aminotransferase; ICU, intensive care unit.
Fig. 4.
LDH correlates with AST and is increased in patients with impaired liver function. (A) LDH and AST levels correlate in all hospitalized patients. (B) LDH levels correlate with AST concentration in non ICU and ICU patients. Displayed are LDH concentrations in patients with normal AST and elevated AST in (C) non ICU and (D) ICU patients. (E) Correlation heat map of biochemical parameters in all hospitalized patients. Tile colours code for magnitude of the correlation with darker shades indicating stronger correlation. The first two decimal digits of r are displayed. Only significant correlations are shown. Data are shown as mean ± SEM, n: (C-D). *p<0.05; **p<0.01; ***p<0.001 according to Spearman correlation or Student's t-test. LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ICU, intensive care unit.
4. Discussion
Previous studies reported signs of liver injury by biochemical and histologic means in COVID-19 patients [4,19,[21], [22], [23]]. However, the origin of hepatic damage in COVID-19 is poorly understood and potentially involves systemic inflammation, viral replication or drug-induced liver injury. Today, we lack evidence for SARS-CoV-2 replication in the liver and our study renders drug-induced liver injury as cause of elevated liver enzymes very unlikely. We rather found a direct correlation between systemic inflammation (indicated by IL-6, CRP and ferritin) and liver damage. IL-6 production may stem from immune cells [32] fibroblasts, endothelial cells [33] and hepatocytes [27,34] which orchestrates an hepatic acute phase response [32,35,36]. While IL-6 signaling impinges on hepatic regeneration [27], clinical studies (that for example tested the effect of IL-6 administration in cancer patients) demonstrated a critical role of this pathway in hepatic injury and hepatotoxicity [37], [38], [39]. In line with a critical impact of systemic inflammation and specifically IL-6 on liver injury, we noted a direct correlation between acute phase proteins and IL-6 in the serum of COVID-19 patients with elevated AST. Based on previous reports and our study, with the limitation of the Cross-sectional design, we propose that the systemic inflammatory response to SARS-CoV-2 infection to COVID-19 patients serves as a fuel of hepatic injury. In line with this, our findings appeared more pronounced in COVID-19 patients with a more severe CRS (i.e. that required intensive care measures). Vice versa, tocilizumab, a recombinant humanized monoclonal antibody targeting the human IL-6 receptor, reversed liver injury during CRS that was induced by non-infectious means (i.e. CAR-T cell therapy) [40,41].
While experimental evidence suggests that blockade of an inflammatory response in SARS-CoV-2-related CRS improves the course of infection [42,43], clinical trials are pending. In the previous MERS-CoV epidemic, IL-6 and other inflammatory cytokines, such as IL-2 have been identified as key players during infection and trigger hepatic injury [44,45]. In COVID-19 patients, elevated IL-6 concentrations (amongst others) have been described [46] and a cytokine signature was indeed associated with loss of lung function, lung injury and outcome [47]. As such, it appears that SARS-CoV-2 potently triggers a systemic cytokine response that is detrimental for some patients (as in CRS) while factors that control this response remain undetermined. This cytokine response, but probably not viral burden, seems to play the key role in disease severity and patient outcome [48].
Conflict of interest
All authors declare no conflict of interest.
Funding
This study is supported by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community), an R&D K-Centre (COMET program - Competence Centers for Excellent Technologies) funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs and the federal states Tyrol, Salzburg and Vienna.
Acknowledgments
No funders were involved in the study concept, design, conduct, data analyses, writing of this manuscript, or in the decision to submit this work for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dld.2020.08.004.
Appendix. Supplementary materials
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effenberger M., Grabherr F., Mayr L. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020;69:1543–1544. doi: 10.1136/gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan S., Altayar O., Siddique S.M. AGA institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.05.001. 320-334.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wit E., van Doremalen N., Falzarano D. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockx B., Baas T., Zornetzer G.A. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L., Ding Y., Zhang Q. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 15.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27 doi: 10.1016/j.chom.2020.04.009. 992-1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie H., Zhao J., Lian N. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 19.Lescure F.X., Bouadma L., Nguyen D. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Zheng L., Liu L. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020 doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 21.Cai Q., Huang D., Yu H. Characteristics of liver tests in COVID-19 patients. J Hepatol. 2020;73:712–713. doi: 10.1016/j.jhep.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi X., Liu C., Jiang Z. Multicenter analysis of clinical characteristics and outcome of COVID-19 patients with liver injury. J Hepatol. 2020;73:455–458. doi: 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley B., Lucas S.B., Youd E. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 24.Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai X., Hu L., Zhang Y. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 [Google Scholar]
- 26.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. 2016;64:1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Netea M.G., Balkwill F., Chonchol M. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed on January 31, 2020. 2020.
- 30.Eslam M., Newsome P.N., Anstee Q.M. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Zhou L., Yang Y. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–ee2. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauldie J., Richards C., Harnish D. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bode J.G., Albrecht U., Häussinger D. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2012;91:496–505. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Fielding C.A., Jones G.W., McLoughlin R.M. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40:40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 36.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 37.Banks R.E., Forbes M.A., Patel P.M. Subcutaneous administration of recombinant glycosylated interleukin 6 in patients with cancer: pharmacokinetics, pharmacodynamics and immunomodulatory effects. Cytokine. 2000;12:388–396. doi: 10.1006/cyto.1999.0556. [DOI] [PubMed] [Google Scholar]
- 38.Weber J., Gunn H., Yang J. A phase I trial of intravenous interleukin-6 in patients with advanced cancer. J Immunother Emphasis Tumor Immunol. 1994;15:292–302. doi: 10.1097/00002371-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Yamaji K., Ochiai Y., Ohnishi K. Up-regulation of hepatic heme oxygenase-1 expression by locally induced interleukin-6 in rats administered carbon tetrachloride intraperitoneally. Toxicol Lett. 2008;179:124–129. doi: 10.1016/j.toxlet.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., Wu Z., Li J.W. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brudno J.N., Kochenderfer J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B., Li M., Zhou Z. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fehr A.R., Channappanavar R., Perlman S. Middle east respiratory syndrome: emergence of a pathogenic human coronavirus. Annu Rev Med. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu L., Liu J., Lu M. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., Tan Y., Ling Y. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.