Abstract
Background:
Estrogens and calcium regulate vascular health, but caused adverse cardiovascular events in randomized trials.
Objectives:
Whether phytoestrogenic soy isoflavones modulate the physiological effects of calcium on blood pressure was explored.
Design:
A double-blind, randomized study assigned 99 premenopausal women to 136.6 mg isoflavones (as aglycone equivalents) and 98 to placebo for 5 days per week for up to 2 years. Blood pressure, serum calcium and urinary excretion of daidzein (DE) and genistein (GE) were measured repeatedly before and during treatment.
Results:
Isoflavones did not affect blood pressure per intake dose assignment (i.e. intention-to-treat, N=197), but significantly affected blood pressure per measured urinary excretion of isoflavones (i.e. per protocol analysis, N=166). Isoflavones inversely moderated calcium effects on systolic blood pressure (SBP) (interaction term β-estimates: −3.1 for DE, −12.86 for GE, all P<0.05), and decreased diastolic blood pressure (DBP) (β-estimates: −0.84 for DE, −2.82 for GE, all P<0.05) after controlling for calcium. The net intervention effects between maximum and no isoflavone excretion were −17.7 and +13.8 mmHg changes of SBP, respectively, at serum calcium of 10.61 and 8.0 mg/dL, and about 2.6 mmHg decrease of DBP.
Conclusions:
Moderation by isoflavones of the physiological effect of calcium tend to normalize SBP, and this effect is most significant when calcium concentrations are at the upper and lower limits of physiological norm. Isoflavones decrease DBP independent of calcium levels. Further studies are needed to assess the impact of this novel micronutrient effect on blood pressure homeostasis and cardiovascular health.
Keywords: Daidzein, genistein, blood pressure homeostasis, selective estrogen receptor modulator, micronutrients
Introduction
Blood pressure is a strong, consistent, continuous, and independent predictor of risk for age-specific mortality from vascular, cardiac and renal diseases at all ages [1]. Rapid reductions in vascular diseases have been documented in randomized trials even after a few years of antihypertensive treatment [2]. Therefore, treatment of high blood pressure is recommended for primary and secondary prevention of cardiovascular disease [3].
In women, blood pressure from adolescence to menopause is lower than in age-matched men, but then rises progressively with older age [4]. Observational studies suggest that replacement of ovarian steroids after menopause is beneficial for preventing cardiovascular disease (CVD) [5], but this was not supported by large randomized controlled trials, which actually found that hormone replacement increased risk for stroke, myocardial infarction, and pulmonary embolism [6,7]. Similar adverse events were also observed in several randomized trials when calcium supplements were given as monotherapy to prevent osteoporosis [8–11]. Concern about these risks [12,13] prompted additional research for alternative estrogenic agents.
Phytoestrogens that are abundant in soy and other legumes have established estrogenicity [14,15] and might serve as alternative estrogenic agents in women. However, observational studies [16] and randomized clinical trials measuring effects of soy and isoflavones on surrogate biomarkers for CVD [17–23], osteoporosis [24], and menopausal symptoms [25] have shown mixed results [26]. These mixed results may be due to a failure to account for large inter-individual differences in the metabolism of isoflavones [27–30]. Additionally, estrogens have complex direct and indirect effects on calcium homeostasis. For example, estrogen deficiency after menopause decreases renal calcium reabsorption [31] and administered estrogens enhance the beneficial effects of calcium supplements on bone [32]. Estrogen and calcium are both important for the function of endothelial cells and myocytes, and the homeostasis of blood pressure [5]. Our previous randomized clinical trial of soy isoflavones as alternative selective estrogen modulators in premenopausal women found isoflavones significantly increased serum calcium levels [33]. Using the same study samples, we investigated (i) whether soy isoflavones affected blood pressure, and (ii) whether the relationship is affected by serum calcium concentrations. To account for individual differences in metabolism and adherence, isoflavone effects were analyzed both as an intention-to-treat (i.e. by intake dose) and a per-protocol basis (i.e. by measured urinary excretion of isoflavones). These isoflavone effects were studied in premenopausal women, because populations consuming legumes usually do so life-long and not just after menopause.
Subjects and Methods
A single-site, parallel, two-arm, repeated measures, randomized, double blind, and placebo-controlled study examined effects of isoflavones on vascular health [33]. Inclusion criteria were healthy 30–42 year old premenopausal women who were not pregnant, not breast feeding, and had not taken hormonal contraceptive agents (oral, injection, or patch) or exogenous hormones in the previous 6 months, and were not on medically prescribed diets or medications chronically (other than occasional antibiotics and NSAIDs). They had regular monthly menstrual cycles, and no personal or family history of breast cancer. They had no history of breast augmentation, reduction, or lifting and had normal screening mammograms. The study was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB), and written informed consent was obtained from all subjects. Primary outcomes included breast density, bone density and blood pressure. Effects on serum electrolytes were pre-specified secondary outcomes, as reported [33]. Trial outcomes remained unchanged and there was no interim analysis.
The characteristics of the intervention agents have been described [33,34]. Briefly, each isoflavone pill contained 246 mg of Novasoy™ that contained 68.3 mg of aglycone equivalent of daidzein (30 mg), genistein (30 mg), and glycitein (8.3 gm) of which 90 mol-% was as the glycosides (daidzin, genistin, and glycitin). Each placebo pill contained 246 mg of a carbohydrate filler. Both pills were identical in appearance and weight (1000 mg per tablet), and also included 60 mg sorbitol, 3 mg magnesium stearate, and 676 mg dicalcium phosphate and 15 mg of riboflavin for measuring adherence. These were kindly provided at no cost by Archer Daniel Midland Co. (Decatur, IL).
Figure 1 outlines the study design and protocol. There were four baseline visits, i.e. two paired visits during the luteal phase of two separate menstrual cycles that were not more than 6 months apart. During baseline visits, health status was assessed by study physicians based on clinical history and physical, gynecological, mammographic examinations and blood test results. Only qualified healthy subjects as determined by the investigative team were dispensed blinded isoflavone or placebo pills by the UTMB Research Pharmacy according to a pre-generated randomization list. The study statistician generated this randomization list using the PLAN procedure in SAS©. Randomization was in blocks of six to ensure equal sizes of the two study groups and of the three sub-groups in each study group. At each follow-up visit, subjects were dispensed the next three-month supply of pills as blister packs that for each day, provided two assigned study pills and one prenatal vitamin pill (Rugby Prenavite Prenatal Formula, Swanson Health Products, Duluth, GA). Subjects ingested all three pills from a blister pack daily for five days per week for up to two years. Time of the day and days of the week for pill ingestion was at the discretion of each study subject.
Figure 1:
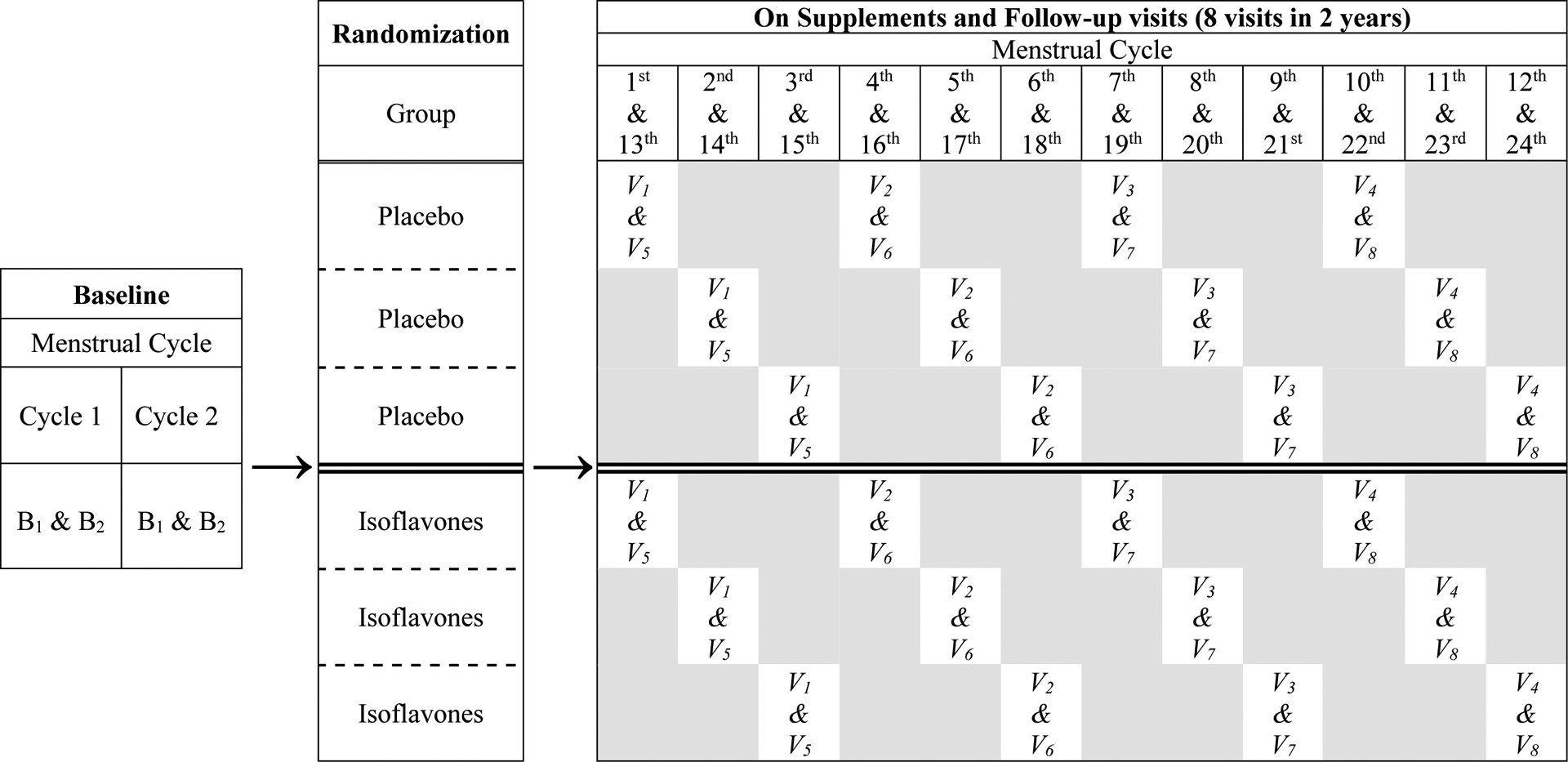
Study design and protocol: There were 2 baseline visits (B1 & B2) 2 days apart in each one of the two screening menstrual cycles. After randomization and during supplements, follow-up visits (V1 to V8) occurred every 3 menstrual cycles apart for 2 years. Subjects in each treatment arm were randomized into 3 subgroups, so the first follow-up visit was after being on supplement for either 1, 2, or 3 menstrual cycles. All study visits occurred during the luteal phase of the menstrual cycle. On each visit, subjects brought a 12-hr urine collection, provided fasting blood samples, and were measured for blood pressure, weight, and height by study nurse. Mammograms, breast magnetic resonance images, and bone density scans were acquired once before and annually after supplements coincided with a study visit.
Treatment started on the second day of the menstrual period that immediately followed the 4th baseline visit. During treatment, follow-up visits occurred approximately once every three menstrual cycles (i.e. roughly seasonally) except for the first treatment visit, which occurred after one, two, or three menstrual cycles after starting treatment depending on the scheduling sub-group to which they were randomized. This scheduling scheme allowed staggering study visits and studying kinetics of treatment effect for every menstrual cycle during treatment. The subjects reported to the study team at each first day of menstruation, which allowed scheduling follow-up visits to occur 20 to 24 days later, during the luteal phase of that cycle. The subjects, research staff, and investigators remained blinded to the treatment assignments.
At each baseline and follow-up visit, subjects arrived after an overnight fast and brought with them a 12-hr urine collection, which were stored at −20°C until analyzed for riboflavin, daidzein and genistein, as described [34]. Fasting blood samples were drawn at each visit between 8:00 and 10:00 a.m. and analyzed for blood chemistry, including serum calcium, by the certified UTMB hospital clinical laboratory using VITROS® 5.1 FS (Ortho-Clinical Diagnostics, Rochester, NY). Blood pressure was measured once soon after the subjects arrived at the clinical research unit by trained clinical research nurses, with a Dinamap Plus™, Model # 9710 vital signs monitor (Critikon, Tampa, FL), usually in the right arm and in the sitting position. The blood pressure cuff size was according to the size of the subject’s arm, and was usually ‘adult regular’. Anthropometrics (including BMI) were measured at each visit. Demographic and reproductive information was obtained at the first visit using a self-administered questionnaire.
Statistical analyses
Baseline characteristics of the subjects were summarized for each study group using means and standard deviations (SDs) for continuous variables and compared by independent t-tests; and frequencies and percentages for categorical variables and compared by chi-squared tests. With an estimated attrition rate of 15%, 100 subjects per arm would provide 85 subjects who would complete the study. Using estimates of the SDs ranging from 10 to 15 for blood pressure (Table 1), we would have 80% power to detect 0.5 SD change in mean blood pressure, i.e. ≥4.5–7 mmHg, using a two group t-test with a 0.05 two-sided significance level.
Table 1.
Baseline characteristics and number of study visits of 197 female subjects randomized to treatment with placebo or soy isoflavones *, †, ‡
| Variables | Placebo | Isoflavone | ||
|---|---|---|---|---|
| n0 | Mean ± SD; % | n1 | Mean ± SD; % | |
| Age at study consent (yr) | 98 | 37.35 ± 3.45 | 99 | 37.58 ± 2.95 |
| Race/Ethnicity (%) | 98 | 49.75 | 99 | 50.25 |
| Hispanic | 39 | 39.80 | 51 | 51.52 |
| African American | 12 | 12.24 | 14 | 14.14 |
| White | 47 | 47.96 | 34 | 34.34 |
| At study site | ||||
| Body weight (kg) | 98 | 74.99 ± 17.33 | 99 | 73.46 ± 16.62 |
| Height (cm) | 98 | 160.21 ± 6.01 | 99 | 160.69 ± 7.63 |
| BMI (kg/m2) | 98 | 29.17 ± 6.33 | 99 | 28.45 ± 6.05 |
| Waist circumference (cm) | 97 | 90.83 ± 13.79 | 99 | 89.60 ± 13.14 |
| Hip circumference (cm) | 97 | 109.02 ± 12.95 | 99 | 108.10 ± 11.40 |
| Waist-to-hip circumferenceratio | 97 | 0.83 ± 0.06 | 99 | 0.83 ± 0.06 |
| Systolic blood pressure (mmHg) | 98 | 119.27 ± 14.85 | 99 | 115.55 ± 14.07 |
| Diastolic blood pressure (mmHg) | 98 | 71.51 ± 9.08 | 99 | ;69.47 ± 9.09 |
| Urinary level | ||||
| Riboflavin concentration (μg/mL) | 91 | 0.66 ± 1.00 | 93 | 0.82 ± 1.72 |
| Riboflavin excretion (mg/h) | 91 | 0.05 ± 0.08 | 93 | 0.06 ± 0.01 |
| Daidzeinexcretion (mg/h) | 90 | 0.06 ± 0.03 | 89 | 0.07 ± 0.04 |
| Genistein excretion (mg/h) | 90 | 0.08 ± 0.05 | 89 | 0.08 ± 0.04 |
| Serum level | ||||
| Total protein (g/dL) | 98 | 7.54 ± 0.50 | 98 | 7.50 ± 0.45 |
| Albumin (g/dL) | 98 | 4.28 ± 0.34 | 99 | 4.28 ± 0.26 |
| Calcium (mg/dL) | 98 | 9.01 ± 0.35 | 99 | 9.05 ± 0.28 |
| Chloride (mEq/L) | 98 | 105.27 ± 1.91 | 99 | 105.49 ± 1.85 |
| Potassium (mEq/L) | 98 | 4.04 ± 0.22 | 99 | 4.07 ± 0.24 |
| Sodium (mEq/L) | 98 | 140.50 ± 1.47 | 99 | 140.67 ± 1.50 |
| Study visits (n) | 98 | 8.60 ± 3.00 | 99 | 8.70 ± 3.10 |
Variables were average of 4 baseline screening visits except for race/ethnicity. Study visitswere mean number of baseline and completed follow-up visit.
Means and standard deviations (SDs) for numerical variables and the percentage for categorical variables are stratified by groups.
For race/ethnicity, the P value between groups was 0.15 (Fisher’s exact test). For numerical variables, all P values between groups were greater than 0.05.
Outcomes were systolic blood pressure (SBP) and diastolic blood pressure (DBP). Exposure predictors were isoflavone treatment assignment (categorical data, yes/no) or urinary excretion of daidzein and genistein (continuous data, mg/h). Linear mixed effects (LME) models, accounting for inter-subject heterogeneity and intra-subject dependence on repeated measures, were applied. The first strategy was an ‘intention-to-treat’ analysis that assessed the association between treatment assignment and changes in SBP or DBP during the treatment period by including an interaction term between months of treatment and type of treatment assignment. The second strategy was a per protocol analysis that considered only the measured urinary excretion rates (mg/h) of daidzein and/or genistein and ignored treatment assignment, as exposure variables. Analyses used the number of days on assigned treatment to measure exposure time and assess the effect of time on blood pressure. Models were also adjusted for race/ethnicity, age at entry to the study, and BMI at each study visit. If interaction terms were significant, the Johnson-Neyman procedure [35] was used to probe the interactions of two continuous variables to fully explicate the nature of these conditional relationships. To facilitate interaction-term result interpretation, data for urinary excretion of isoflavones and the moderating serum electrolyte levels were mean-centered for statistical model analyses. Other variables were not mean-centered. The model fit of the LME models was assessed using the conditional Akaike information criterion (CAIC) [36,37]. The corresponding assumption on LME models and the identification of potential outliers or influential points were also inspected through residual analysis. All tests of statistical significance were two-sided with a P < 0.05 indicating a significant difference. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Enrollment, treatment assignment and retention (Figure 2) [33].
Figure 2:
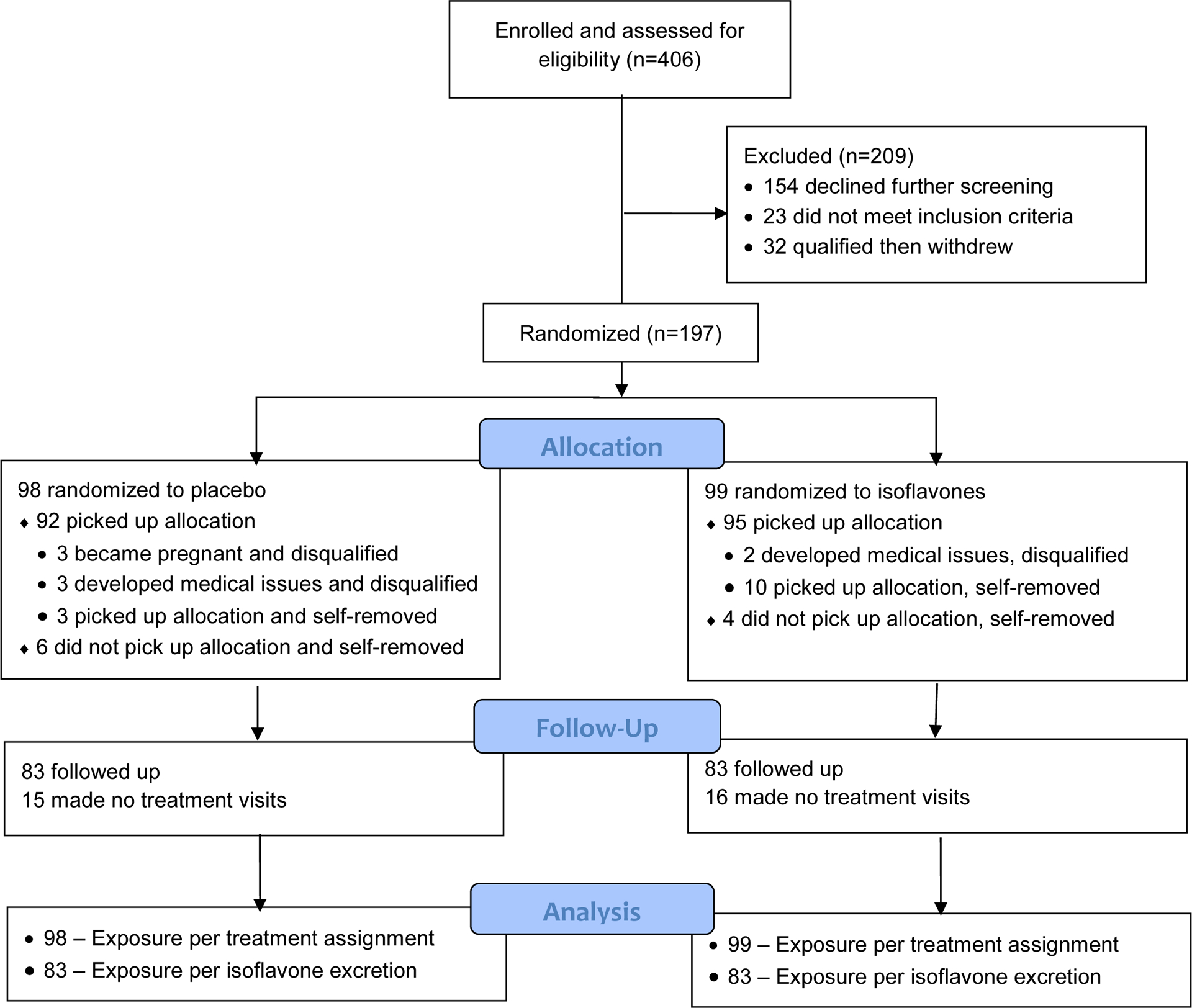
Flow diagram of a blinded randomized trial comparing effects of soy isoflavones and placebo on blood pressure showing the enrollment of 30- to 42-year-old female subjects, allocation to treatment, follow-up for up to 2 years, and data analysis, as described [33].
Baseline characteristics, including blood pressure, basal dietary exposure to daidzein and genistein as measured in urine, and serum calcium, were balanced between treatment groups as randomized for all 197 subjects (Table 1) and for 166 subjects with follow-up visits (31 drop-outs) (not shown). Each subject had 4 baseline visits and from 0 to 8 treatment phase visits, and the average number of these study visits was a proxy for duration of study participation, which was balanced as randomized. No subjects were removed due to serious side effects. The enrollment period between January 2003 and August 2012 was longer than expected due to a large number of dropouts, and also to interruption of the study by Hurricane Ike in 2008. The trial ended after research funding was exhausted [33].
Intention-to-treat analyses:
These analyses included all 197 randomized subjects (Table 2). Isoflavone treatment did not affect blood pressure compared to placebo, because interaction terms between treatment assignment and years on supplements were not significant for outcomes studied in models either unadjusted (Model 1) or adjusted for serum calcium (Models 2–4). However, a positive association between serum calcium and SBP was noted (Models 3–4, all P≤0.05).
Table 2.
Results of multiple regression models of effect of treatment with isoflavones on systolic and diastolic blood pressure in 197 premenopausal women assessed by intention-to-treat analysis
| Systolic Blood Pressure * | Diastolic Blood Pressure * | ||||
|---|---|---|---|---|---|
| Effect | β-Estimate (SE) | P value | β-Estimate (SE) | P value | |
| Model 1† | Treatment×Month | −0.040 (0.071) | 0.57 | 0.023 (0.041) | 0.57 |
| Model 2‡ | Treatment×Month | −0.036 (0.071) | 0.61 | 0.029 (0.041) | 0.48 |
| Calcium | 0.869 (0.856) | 0.31 | 0.787 (0.539) | 0.15 | |
| Model 3§ | Treatment×Month | −0.018 (0.071) | 0.80 | 0.030 (0.041) | 0.46 |
| Calcium | 2.494 (1.278) | 0.051 | 0.936 (0.797) | 0.24 | |
| Treatment×Calcium | −2.943 (1.719) | 0.09 | −0.274 (1.082) | 0.80 | |
| Model 4‖ | Treatment×Month | −0.023 (0.070) | 0.75 | 0.024 (0.041) | 0.56 |
| Calcium | 2.740 (1.258) | 0.030 | 1.051 (0.791) | 0.18 | |
| Treatment×Calcium | −2.779 (1.695) | 0.10 | −0.221 (1.073) | 0.84 | |
Regression coefficient (β-Estimate), corresponding standard error (SE), and P value for effect indicated.
Model 1 included treatment (Treatment), number of months on treatment (Month), and the interaction term of Treatment×Month measured at each sampling.
Model 2 included the variables in model 1 and mean-centered calcium.
Model 3 included the variables in model 2 and the interaction term of Treatment×Calcium. Consult results of Tables 3–4 for rationale for testing Treatment×Calcium.
Model 4 included the variables in model 3 and age at entry to the study, race, and BMI measured at the time of each blood sampling.
Both the placebo and isoflavone pills contained riboflavin and subjects were restricted from taking other vitamins not provided by the study. As previously described [33,34], excretion of riboflavin at concentrations ≥ 1.42 μg/ml (Youden index) in a 12-hour urine collection was used to categorize each subject as adherent or non-adherent in taking study pills within 12 hours preceding each follow-up visit. The Youden index defines a data point that yields the optimal value of sensitivity and specificity from a receiver operating curve. Main and interactive effects remained non-significant after additional adjustment for adherence in all models (β-estimates for the interaction term of treatment and time of Model 1 were −0.03 and 0.04 for SBP and DBP, respectively, all P>0.05).
Based on a simultaneous presence of riboflavin and isoflavones in urine, an ‘as-observed treatment/exposure assignment’ was designated for all urine collections in 166 subjects, as previously described [34]. The ‘as-observed treatment assignment’ was consistent with the randomized assignment in 143 subjects, which excluded 3 ‘non-adherent’ subjects, due to absence of all three compounds in their urine samples, 20 placebo subjects with unexpected high levels of all three compounds indicating unexpected multiple exposures to isoflavone pills in error [34], and 4 isoflavone subjects who received a batch of placebo pills in error. When the intention-to-treat model analyses were restricted to these 143 subjects, there was again no statistically significant treatment effect of isoflavones on blood pressure (results not shown).
Per protocol analyses of influence of variation in urinary excretion of isoflavones on blood pressure.
This analysis focused on the 166 subjects with follow-up visits. The intake-dose ratio of the two main isoflavones, daidzein and genistein in the isoflavone pills was 1:1. However, the excretion ratios of daidzein to genistein in the follow-up urine samples varied from 0.9 to 8.9 among adherent subjects assigned to isoflavone group. To examine the effects of this metabolic variation, measured urinary excretion of isoflavones at baseline (average of four measures) and at each individual follow-up visit were used as exposure predictor instead of treatment assignment in statistical models.
Isoflavone exposure, measured as daidzein excretion (DE), genistein excretion (GE), their sum excretion (DE + GE) or net difference in excretion (DE – GE), had no significant effects on DBP in unadjusted model (Model 1, Table 3). However, after adjustment for serum calcium concentrations (Models 2–3, Table 3), excretion rates (average of a 12 hr urine collection) of isoflavones were inversely correlated with DBP. DBP lowering effects increased by 20.9% for DE, 11.8% for GE, 18.8% for DE + GE, and 25% for DE – GE, when comparing the coefficients of Model 2 and Model 1. There were no statistically significant interactions between isoflavone excretion and serum calcium on DBP as an outcome (Model 4, Table 3). Serum calcium was significantly associated with DBP in all models. The CAIC showed that models with DE, GE, and DE + GE as predictors (Models 2–3, Table 3) were slightly better (all P<0.05) than DE – GE (P=0.052 to 0.068) [36,37]. Based on Model 2 (Table 3) and controlling for serum calcium concentration and days of exposure, the mean decreases in DBP was up to 2.6 mmHg when comparing between adherent isoflavone subjects with maximum isoflavone excretion and placebo subjects with minimum isoflavone excretion.
Table 3.
Results of linear mixed effects models of the amounts of excretion of isoflavones (daidzein and genistein) and serum calcium levels on diastolic blood pressure in 166 adherent premenopausal women
| Model 1a,b | Model 2a,b | Model 3a,b | Model 4a,b | |
|---|---|---|---|---|
| Effect | Isoflavones | Isoflavones, Calcium |
Isoflavones, Calcium, Age at entry, Race, BMI |
Model 3, Isoflavones×Calcium |
| Daidzein Exposure | ||||
| Daidzein (DE) | −0.695 (0.421)c | −0.841 (0.424)c | −0.886 (0.419)c | −0.851 (0.420)c |
| 0.10d | 0.048d | 0.035d | 0.043d | |
| Calcium | -e | 1.097 (0.553) | 1.289 (0.548) | 1.320 (0.548) |
| - | 0.048 | 0.019 | 0.016 | |
| DE×Calcium | - | - | - | −1.004 (0.884) |
| - | - | - | 0.26 | |
| Genistein Exposure | ||||
| Genistein (GE) | −2.524 (1.423) | −2.821 (1.425) | −2.987 (1.409) | −2.996 (1.408) |
| 0.08 | 0.048 | 0.034 | 0.034 | |
| Calcium | - | 1.074 (0.552) | 1.266 (0.547) | 1.323 (0.548) |
| - | 0.052 | 0.021 | 0.016 | |
| GE×Calcium | - | - | - | −3.959 (2.995) |
| - | - | - | 0.19 | |
| Sum Exposure | ||||
| DE + GE | −0.575 (0.333) | −0.683 (0.334) | −0.720 (0.332) | −0.700 (0.332) |
| 0.09 | 0.042 | 0.030 | 0.035 | |
| Calcium | - | 1.099 (0.553) | 1.291 (0.548) | 1.331 (0.548) |
| - | 0.047 | 0.019 | 0.016 | |
| (DE + GE)×Calcium | - | - | - | −0.851 (0.699) |
| - | - | - | 0.22 | |
| Difference Exposure | ||||
| DE – GE | −0.814 (0.551) | −1.018 (0.557) | −1.070 (0.551) | −1.010 (0.554) |
| 0.14 | 0.07 | 0.052 | 0.07 | |
| Calcium | - | 1.084 (0.553) | 1.275 (0.548) | 1.294 (0.548) |
| - | 0.050 | 0.020 | 0.019 | |
| (DE - GE)×Calcium | - | - | - | −1.135 (1.166) |
| - | - | - | 0.33 |
Isoflavone effects included mean-centered daidzein excretion (DE), genistein excretion (GE), sum excretion (DE + GE), or difference excretion (DE – GE) controlling for calcium. Overall mean (minimum, maximum) for isoflavones in mg/h (N=166 subjects) were 0.385 (0.003, 3.028) for DE, 0.167 (0.003, 0.9340) for GE, 0.551 (0.006, 3.817) for DE + GE, and 0.218 (−0.440, 2.239) for DE – GE, and for calcium was 9.146 (8, 10.6) mg/dL.
Models 1–4 included days on supplement of isoflavones, but results of effects of days on supplements, age at entry, race and BMI are not shown.
Estimate of regression coefficient, β, and corresponding standard error (SE) for effect indicated.
P value for the significance of the effect indicated.
Effect (variable) not included in models.
Isoflavone excretion was not significantly associated with SBP, in models unadjusted or adjusted for serum calcium (Models 1 and 2, respectively, Table 4). However, the interaction terms between isoflavones (DE, GE, DE + GE, or DE – GE) and serum calcium were significantly and inversely associated with SBP (Models 3–4). The CAIC on models with interaction terms (Models 3–4) indicated that GE was the best predictor of SBP (GE > (DE + GE) > DE > (DE – GE)) [36,37]. Nonetheless, all models with interaction terms adequately explained variation in SBP.
Table 4.
Results of linear mixed effects models of the amounts of excretion of the isoflavones (daidzein and genistein) on systolic blood pressure and the influence of serum calcium levels in 166 premenopausal women
| Model 1a,b | Model 2a,b | Model 3a,b | Model 4a,b | |||
|---|---|---|---|---|---|---|
| Effect | Isoflavones | Isoflavones, Calcium |
Isoflavones, Calcium, Isoflavones×Calcium |
Model 3, Age at entry, Race, BMI |
||
| Daidzein Exposure | ||||||
| Daidzein (DE) | 0.371 (0.689)c | 0.250 (0.697)c | 0.352 (0.697)c | 0.245 (0.680)c | ||
| 0.59d | 0.72d | 0.61d | 0.72d | |||
| Calcium | -e | 0.814 (0.896) | 0.889 (0.895) | 1.349 (0.878) | ||
| - | 0.36 | 0.32 | 0.13 | |||
| DE×Calcium | - | - | −3.099 (1.459) | −3.362 (1.439) | ||
| - | - | 0.034 | 0.020 | |||
| Genistein Exposure | ||||||
| Genistein (GE) | 0.206 (2.323) | −0.041 (2.332) | −0.098 (2.324) | −0.686 (2.270) | ||
| 0.93 | 0.99 | 0.97 | 0.76 | |||
| Calcium | - | 0.855 (0.894) | 0.999 (0.892) | 1.468 (0.876) | ||
| - | 0.34 | 0.26 | 0.09 | |||
| GE×Calcium | - | - | −12.859 (4.933) | −13.627 (4.863) | ||
| - | - | 0.009 | 0.005 | |||
| Sum Exposure | ||||||
| DE + GE | 0.244 (0.546) | 0.154 (0.551) | 0.213 (0.550) | 0.113 (0.537) | ||
| 0.66 | 0.78 | 0.70 | 0.83 | |||
| Calcium | - | 0.823 (0.896) | 0.918 (0.894) | 1.382 (0.878) | ||
| - | 0.36 | 0.31 | 0.12 | |||
| (DE + GE)×Calcium | - | - | −2.635 (1.154) | −2.841 (1.138) | ||
| - | - | 0.023 | 0.013 | |||
| Difference Exposure | ||||||
| DE – GE | 0.606 (0.902) | 0.437 (0.914) | 0.612 (0.918) | 0.520 (0.895) | ||
| 0.50 | 0.63 | 0.51 | 0.56 | |||
| Calcium | - | 0.801 (0.896) | 0.844 (0.895) | 1.296 (0.878) | ||
| - | 0.37 | 0.35 | 0.14 | |||
| (DE – GE)×Caclium | - | - | −3.466 (1.922) | −3.810 (1.897) | ||
| - | - | 0.07 | 0.045 | |||
Isoflavone effects included mean-centered daidzein excretion (DE), genistein excretion (GE), sum excretion (DE + GE), or difference excretion (DE – GE), serum calcium and the interaction term of isoflavone and calcium. Consult footnote of Table 3 for means used to center data.
Models 1–4 included days on supplement of isoflavones, but results of effects of days on supplements, age at entry to the study, race, and BMI are not shown.
Estimate of regression coefficient, β, and corresponding standard error (SE) for effect indicated.
P value for the significance of the effect indicated.
Effect (variable) not studied in models.
A significant interaction term implies that serum calcium and isoflavones moderate the effects of each other on SBP. A negative interaction term indicates that elevated isoflavones (DE and GE) decrease SBP when serum calcium levels are high, but increase (restore) SBP when serum calcium levels are low. A significant interaction also implies that there are certain serum calcium concentrations that isoflavones (focal predictors) have no effects on SBP. Their interaction on changes of SBP (using Model 3 in Table 4 as an example) was further explored using the Johnson-Neyman technique [35]. Example plots of simple slopes (Figures 3A–B) show that the effects of DE and GE on SBP varied with serum calcium concentrations. Plots of simple slopes between DE + GE and DE – GE and serum calcium were similar (not shown). When DE is the focal predictor (Figure 3C), both upper and lower confidence bands (95% CI) for all slope estimates at serum calcium ≤ −0.6737 mg/dL are greater than zero (i.e. positive slopes). (Note: the negative value of serum calcium level is due to mean-centered data transformation.) When GE is the focal predictor (Figure 3D), the regions of statistical significance for simple slopes are: (i) significantly greater than 0 when serum calcium concentrations are ≤−0.5499 mg/dL, (ii) significantly less than 0 when serum calcium concentrations are ≥0.528 mg/dL or (iii) not statistically different from 0 when serum calcium concentrations are between these values. When DE + GE is the focal predictor (Figure 3E), there are also three regions of different statistical significances for the regression slope estimates separated by serum calcium concentrations ≤-0.5774 mg/dL (positive slopes) and ≥1.0712 mg/dL (negative slopes), and between these values (insignificant slopes). When the focal predictor is DE – GE (Figure 3F), regions of significance were not found for any serum calcium concentrations observed in our data set.
Figure 3:
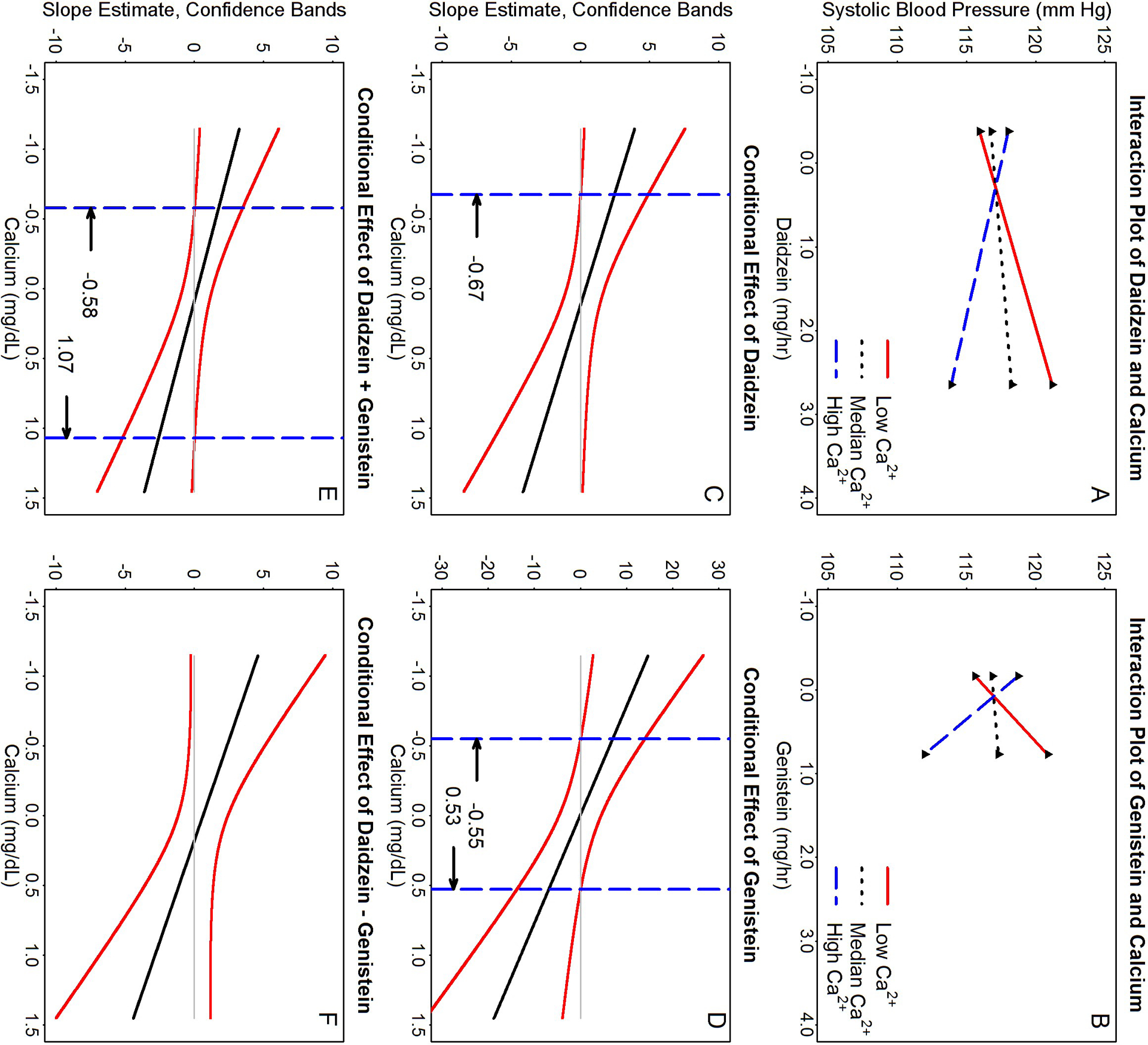
Probing the interactive effects of isoflavone(s) and calcium on systolic blood pressure (SBP) (using Model 3 of Table 4 as an example) by the Johnson-Neyman technique [35]. Panels A-B are plots of simple slopes for focal predictor isoflavones and SBP as a function of serum calcium at the 10th (Low Ca2+), 50th (Median Ca2+), and 90th (High Ca2+) percentiles of values found in our study samples. Panels C to F show the regions of significance and 95% confidence bands for the regression slope estimates for the conditional relation between SBP and isoflavone concentrations as a function of serum calcium. The dashed vertical lines (----- in panels C-F) indicate calcium thresholds separating regions of statistical significances. Panels A & C for daidzein (DE) as predictor; B & D for genistein (GE); E for sum excretion (DE + GE); and F for difference in excretion (DE – GE). Note that the lengths of all graph lines correspond to ranges of data found in our study samples. Simple slopes for DE + GE and DE – GE are not shown.
The intention-to-treat Model 3 of Table 2 for SBP, was reanalyzed by categorizing serum calcium levels into three calcium conditioning effect regions assessed using GE (Figure 3D). The means ± standard error (SE), sample sizes (n), and net treatment effects (Δ between placebo and isoflavones) for SBP were (i) for serum calcium ≤ −0.55 mg/dL: 116.10±2.39 (n=18 placebo), 115.81±2.46 mmHg (n=17 isoflavones), and Δ=−0.30 mmHg (P=0.93) (positive slope); (ii) for serum calcium between −0.55 to 0.53 mg/dL: 118.66±1.45, 116.44±1.44 mmHg (both n=83), and Δ=−2.23 mmHg (P=0.28) (insignificant slope); and (iii) for serum calcium ≥0.53 mg/dL: 120.99±1.93 (n=34 placebo), 114.89±1.89 mmHg (n=37 isoflavones), and Δ=−6.10 mmHg (P=0.03) (negative slope).
Table 5 shows how estimates of the intervention effect for SBP vary with both levels of isoflavones and serum calcium (using GE in model 2 of Table 4 as an example). When serum calcium is at the 100th percentile for the group, its effects on SBP are −13.07 mmHg and 4.67 mmHg, when GE is at 100th and 0th percentiles, respectively, resulting in a net intervention effect on SBP of −17.74 mmHg (a decrease). In contrast when serum calcium concentration is at the 0th percentile of the group, the net intervention effect between maximum vs minimum GE is an increase in SBP of 13.81 mmHg. As also shown in Table 5, when urinary GE is, for example, at the 100th percentile, effects on SBP change from −13.07 to 10.16 mmHg as serum calcium level changes from the 100th to 0th percentiles.
Table 5.
Estimated intervention effects of isoflavones conditioned on serum calcium levels on systolic blood pressure during treatment of women with isoflavones using amounts of genistein excreted in urine, as an example
| Effects on systolic blood pressure (mmHg)* | |||||
|---|---|---|---|---|---|
| Genistein (GE), mg/h (percentile) | Calcium, mg/dL (percentile) | ||||
| 1.46 (100th) | 0.56 (90th) | −0.05 (50th) | −0.45 (10th) | −1.15 (0th) | |
| 0.77 (100th) | −13.07 | −5.06 | 0.37 | 3.93 | 10.16 |
| 0.22 (90th) | −2.69 | −1.05 | 0.07 | 0.80 | 2.08 |
| −0.06 (50th) | 2.59 | 1.00 | −0.08 | −0.79 | −2.03 |
| −0.14 (10th) | 4.10 | 1.58 | −0.13 | −1.25 | −3.21 |
| −0.17 (0th) | 4.67 | 1.80 | −0.14 | −1.42 | −3.65 |
| Intervention effect† | −17.74 | −6.86 | 0.51 | 5.35 | 13.81 |
Discussion
In this study, the assigned intake dose of isoflavone supplementation did not affect SBP or DBP by intention to treat analyses. However, urinary excretion levels of the two main soy isoflavones, daidzein and genistein, strongly predicted changes in SBP and DBP and importantly these effects on blood pressure were also modified inversely by serum calcium levels. Serum calcium moderated the effects of isoflavones on SBP and suppressed some, but not all effects of isoflavones on DBP.
Calcium is essential for life. A well-established physiological effect of calcium is that higher serum calcium levels lead to higher blood pressure [38]. This is consistent with our data showing that serum calcium concentrations were positively associated with DBP in all models with β-estimates significant for most models. In contrast, urinary output of isoflavones was inversely correlated with DBP (Table 3, negative slopes). Thus, serum calcium and isoflavones have opposite effects on DBP. As reported previously, isoflavones were found to increase serum calcium concentrations in the same study subjects [33], which explains why controlling for serum calcium in the models (Models 2–3, Table 3) strengthened the effects of isoflavones on DBP by up to 30%.Controlling for serum calcium levels at baseline (which were balanced between the two study groups, Table 1) and after treatment [33] did not completely negate the effects of isoflavones on decreasing DBP, suggesting a pathway for isoflavone effects on DBP that is independent of serum calcium, perhaps involving estrogen receptors. While these changes are small, they may be cumulatively significant over periods of continued isoflavone ingestion and may have a long term role in preventing CVD.
We have shown that isoflavones interact with serum calcium to affect SBP. The negative β-estimate of the interaction term implies that isoflavones counteract the physiological effects of calcium on SBP, so that when serum calcium levels are higher than the group median, isoflavones decrease SBP and when serum calcium levels are lower than median, isoflavones increase or restore SBP (Table 5 and Figure 3). The nature of a significant interaction also implies that there is a no-effect zone of their interaction. This no effect zone is found to be close to the group mean for serum calcium, and appears to be the narrowest for GE and absent for DE – GE as exposure predictors. Thus, GE is more efficient in moderating the physiological effect of calcium on SBP than DE + GE or DE. Our prior observation that isoflavones increase serum calcium levels [33] suggests that the isoflavones-SBP dose-response curve is more likely to occur around the high serum calcium effect zone, where SBP is more likely to be decreased by isoflavone exposure.
There are a number of possible explanations for failure to detect isoflavone treatment effects on blood pressure by intention-to-treat analysis in this (Table 2) and other studies [21,22,39,40]. Firstly, differences in isoflavone bioavailability inclusive of adherence and metabolism as noted here and by others [27–30] can have substantial effects on blood pressure and this was not considered in prior studies [21,22,39,40]. Even if adherence rates are similar and balanced between the treatment groups, as in this study, patterns of variation in adherence and metabolism differed within- and between-subjects, which are harder to adjust for in intention-to-treat models [33,34]. Secondly, thresholds of serum calcium required to significantly moderate isoflavone effects on SBP differed for the excretion of individual, sum or difference of the two isoflavones suggesting that daidzein and genistein act synergistically (DE + GE) but also somewhat antagonistically (DE – GE) to influence blood pressure. Model fit statistics showed that that the two isoflavones have different potencies. GE is the best-fit predictor, followed by DE + GE (testing for synergism), and then by DE. DE – GE (testing for antagonism) was the least fit predictor of treatment effects suggesting that DE dominates the effect. Such differences in activity of daidzein and genistein in our current study are consistent with preclinical study results (reviewed in [41,42]), and make differential bioavailability a more critical factor to consider in statistical models. We have considered controlling for the ratio of DE to GE in the intention-to-treat model. But this approach was inappropriate, because DE to GE ratios cannot be calculated in urine samples from placebo subjects. Lastly, even though the physiological role of serum calcium in regulating blood pressure is well-known, serum calcium has not been considered in statistical modeling of the effects of estrogens or isoflavones and CVD.
The significant interaction term of isoflavones and serum calcium as a negative predictor of SBP in this study can be explained as follows. In preclinical models, calcium is central to the regulation of vascular tone and reactivity, and thus, blood pressure. Myogenic responses in blood vessels occur by both endothelium-dependent and independent pathways [5]. The former depends on endothelium-derived relaxing factors, and particularly nitric oxide (NO), to maintain vascular tone. The synthesis of NO requires activation of endothelial nitric oxide synthase (eNOS), which occurs when it dissociates from membrane caveolae. This dissociation is calcium dependent [5,43]. 17β-Estradiol [43] and isoflavones (both daidzein and genistein) [44,45] have been shown to modulate eNOS by inducing a rapid non-genomic and membrane receptor-mediated influx of calcium, leading to NO production and a decrease in blood pressure; this process is not affected by anti-estrogens [5,40,46]. In the endothelium-independent pathway, calcium influx and efflux through L-type calcium channels and other transporters regulate myocyte contraction and blood pressure in a manner opposite to that induced by NO [5,39,47,48]. Both 17β-estradiol [5,49] and isoflavones have been shown to have direct effects on these ion channels and transporters [50–52] and therefore calcium concentration in the myocytes. Thus, isoflavones (as 17β-estradiol) modulate calcium levels in both myocytes and endothelial cells with opposite directional changes on SBP. Since our models show that the interaction term is a negative predictor of SBP, it suggests that effects of isoflavones on the endothelium-dependent pathway (i.e. through NO production) may be the dominant pathway.
As previously described [33], this study had a number of strengths, including the study of premenopausal women to prevent future postmenopausal complications, a high quality study design (randomized, double blind, placebo controlled, and Figure 1), repeated measurement of riboflavin excretion to assess adherence and measurement of isoflavone excretion to assess bioavailable exposure to both major isoflavones. Weaknesses included a high dropout rate, although this was balanced between treatment groups and comparable to that in many other studies including the Women’s Health Initiative [6,7]. The double-blind study design prevented ongoing quality control of all batches of dispensed pills and detection of dispensing errors. However, monitoring urinary excretion of isoflavones allowed direct assessment of isoflavone exposure and detection of dispensing errors, dietary isoflavone exposure, nonadherence, and variation in excretion. Use of urinary excretion of isoflavones as predictors in the analysis reduced any impact of dispensing errors. Collecting 12-hour urine samples rather than obtaining single time-point blood samples was a strength for assessing exposure to isoflavones, given their short half-lives [28]. Tissue levels of isoflavones are likely to be more specific and sensitive as exposure markers, but tissue sampling for analytical measurement is not practical. Other limitations were that riboflavin was used to assess adherence for only the day of sampling; and the use of a mixture of genistein, daidzein, and glycitein limited comparisons of their individual potencies. We chose to not analyze the contributions of glycitein (a minor isoflavone component) or equol (a daidzein metabolite) due to anticipated collinearity with daidzein.
Conclusions
Exposure to soy isoflavones, measured as amounts excreted in urine, appears to moderate (modulate) the physiological effect of calcium on blood pressure, an effect not evident by dose assignment. Daidzein and genistein differ in potency in moderating effects of calcium on blood pressure homeostasis. Our novel findings suggest that dietary isoflavones participate in calcium and blood pressure homeostasis. These observations may help to explain why calcium, when provided along with other micronutrients in foods, is associated with fewer adverse cardiovascular effects such as stroke and thromboembolism than when provided as calcium monotherapy [11,19]. Our findings also support previous epidemiological observations of low risk for CVD with soy consumption. Therefore, soy consumption should be helpful for population health. Health benefits of isoflavones requires further studies.
Acknowledgements
The authors wish to acknowledge the nursing staff of the Institute for Translational Sciences-Clinical Research Center (ITS-CRC). We are also very grateful for the women who volunteered as subjects in this study for up to 2 years.
Sources of Support
This research was supported by grants from the National Institute of Health (NIH) R01 CA095545 and CA065628. This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, NIH and by NIEHS 2 P30 ES06676.
Abbreviations:
- CVD
cardiovascular disease
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- DE
daidzein excretion
- GE
genistein excretion
- CAIC
conditional Akaike information criterion
- SD
standard deviation
- SE
standard error
- LME
linear mixed effects
Footnotes
Financial and non-financial competing interests:
All authors declare that they have no competing interests.
Trial registration: www.clinicaltrials.gov identifier: NCT00204490.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Collaboration PS (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360 (9349):1903–1913 [DOI] [PubMed] [Google Scholar]
- 2.PROGRESS Collaborative Group (2001) Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358 (9287):1033–1041. doi: 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 3.McInnes GT (2005) Lowering blood pressure for cardiovascular risk reduction. J Hypertens Suppl 23 (1):S3–8 [DOI] [PubMed] [Google Scholar]
- 4.Dubey RK, Oparil S, Imthurn B, Jackson EK (2002) Sex hormones and hypertension. Cardiovasc Res 53 (3):688–708 [DOI] [PubMed] [Google Scholar]
- 5.Orshal JM, Khalil RA (2004) Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286 (2):R233–249. doi: 10.1152/ajpregu.00338.2003 [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB (2013) Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 310 (13):1353–1368. doi: 10.1001/jama.2013.278040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossouw JE, Manson JE, Kaunitz AM, Anderson GL (2013) Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol 121 (1):172–176. doi:http://10.1097/AOG.0b013e31827a08c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR (2011) Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis JR, Calver J, Zhu K, Flicker L, Prince RL (2011) Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res 26 (1):35–41. doi: 10.1002/jbmr.176 [DOI] [PubMed] [Google Scholar]
- 10.Mao P-J, Zhang C, Tang L, Xian Y-Q, Li Y-S, Wang W-D, Zhu X-H, Qiu H-L, He J, Zhou Y-H (2013) Effect of calcium or vitamin D supplementation on vascular outcomes: A meta-analysis of randomized controlled trials. International Journal of Cardiology 169 (2):106–111. doi: 10.1016/j.ijcard.2013.08.055 [DOI] [PubMed] [Google Scholar]
- 11.Reid IR, Birstow SM, Bolland MJ (2017) Calcium and Cardiovascular Disease. Endocrinol Metab (Seoul) 32 (3):339–349. doi: 10.3803/EnM.2017.32.3.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harman SM, Naftolin F, Brinton EA, Judelson DR (2005) Is the estrogen controversy over? Deconstructing the Women’s Health Initiative study: a critical evaluation of the evidence. Ann N Y Acad Sci 1052:43–56. doi: 10.1196/annals.1347.004 [DOI] [PubMed] [Google Scholar]
- 13.Choi SD, Steinberg EM, Lee HH, Naftolin F (2011) The Timing Hypothesis remains a valid explanation of differential cardioprotective effects of menopausal hormone treatment. Menopause 18 (2):230–236 [PubMed] [Google Scholar]
- 14.Bennetts HW, Underwood EJ, Shier FL (1946) A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J 22:2–12 [DOI] [PubMed] [Google Scholar]
- 15.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138 (3):863–870 [DOI] [PubMed] [Google Scholar]
- 16.de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF (2002) Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S.women: the Framingham study. Journal of Nutrition 132 (2):276–282 [DOI] [PubMed] [Google Scholar]
- 17.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT (2005) Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr 81 (1):189–195 [DOI] [PubMed] [Google Scholar]
- 18.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M, Committee AHAN (2006) Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 113 (7):1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052 [DOI] [PubMed] [Google Scholar]
- 19.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, Association AH (2006) Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47 (2):296–308. doi: 10.1161/01.HYP.0000202568.01167.B6 [DOI] [PubMed] [Google Scholar]
- 20.Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Mazzaferro S, D’Anna R, Cannata ML, Gaudio A, Frisina A, Frisina N, Corrado F, Cancellieri F, Lubrano C, Bonaiuto M, Adamo EB, Squadrito F (2007) Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study In: J Clin Endocrinol Metab, vol 92 vol 8. United States, pp 3068–3075. doi: 10.1210/jc.2006-2295 [DOI] [PubMed] [Google Scholar]
- 21.Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, Wang P, Melby MK, Hooper L, Kurzer MS, Mizuno S, Ishimi Y, Watanabe S (2010) Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens 28 (10):1971–1982. doi: 10.1097/HJH.0b013e32833c6edb [DOI] [PubMed] [Google Scholar]
- 22.Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, Hui RT (2012) Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 22 (6):463–470. doi: 10.1016/j.numecd.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 23.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A (2008) Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 88 (1):38–50 [DOI] [PubMed] [Google Scholar]
- 24.Zheng X, Lee SK, Chun OK (2016) Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J Med Food 19 (1):1–14. doi: 10.1089/jmf.2015.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A (2009) Efficacy of isoflavones in relieving vasomotor menopausal symptoms - A systematic review. Mol Nutr Food Res 53 (9):1084–1097. doi: 10.1002/mnfr.200800552 [DOI] [PubMed] [Google Scholar]
- 26.Messina M (2010) Insights gained from 20 years of soy research. J Nutr 140 (12):2289S–2295S. doi: 10.3945/jn.110.124107 [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S (1994) Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. Journal of Nutrition 124 (6):825–832 [DOI] [PubMed] [Google Scholar]
- 28.Lu LJ, Lin SN, Grady JJ, Nagamani M, Anderson KE (1996) Altered kinetics and extent of urinary daidzein and genistein excretion in women during chronic soya exposure. Nutr Cancer 26 (3):289–302. doi: 10.1080/01635589609514485 [DOI] [PubMed] [Google Scholar]
- 29.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD (2006) Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr 136 (1):45–51 [DOI] [PubMed] [Google Scholar]
- 30.van der Velpen V, Hollman PC, van Nielen M, Schouten EG, Mensink M, van’t Veer P, Geelen A (2014) Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur J Clin Nutr 68 (10):1141–1147. doi: 10.1038/ejcn.2014.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dick IM, Devine A, Beilby J, Prince RL (2005) Effects of endogenous estrogen on renal calcium and phosphate handling in elderly women. Am J Physiol Endocrinol Metab 288 (2):E430–435. doi: 10.1152/ajpendo.00140.2004 [DOI] [PubMed] [Google Scholar]
- 32.Robbins JA, Aragaki A, Crandall CJ, Manson JE, Carbone L, Jackson R, Lewis CE, Johnson KC, Sarto G, Stefanick ML, Wactawski-Wende J (2014) Women’s Health Initiative clinical trials: interaction of calcium and vitamin D with hormone therapy. Menopause 21 (2):116–123. doi: 10.1097/GME.0b013e3182963901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L-JW, Chen N-W, Nayeem F, Ramanujam VMS, Kuo Y-F, Brunder DG, Nagamani M, Anderson KE (2018) Novel effects of phytoestrogenic soy isoflavones on serum calcium and chloride in premenopausal women: A 2-year double-blind, randomized, placebo-controlled study. Clinical Nutrition 37 (6, Part A):1862–1870. doi: 10.1016/j.clnu.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramanujam VS, Nayeem F, Anderson KE, Kuo YF, Chen NW, Ju H, Lu LW (2017) Riboflavin as an independent and accurate biomarker for adherence in a randomized double-blind and placebo-controlled clinical trial. Biomarkers 22 (6):508–516. doi: 10.1080/1354750x.2016.1269201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer DJ, Curran PJ (2005) Probing Interactions in Fixed and Multilevel Regression: Inferential and Graphical Techniques. Multivariate Behav Res 40 (3):373–400. doi: 10.1207/s15327906mbr4003_5 [DOI] [PubMed] [Google Scholar]
- 36.Vaida F, Blanchard S (2005) Conditional Akaike information for mixed-effects models. Biometrika 92 (2):351–370. doi: 10.1093/biomet/92.2.351 [DOI] [Google Scholar]
- 37.Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology 65 (1):13–21. doi: 10.1007/s00265-010-1037-6 [DOI] [Google Scholar]
- 38.Kamycheva E, Jorde R, Haug E, Sager G, Sundsfjord J (2005) Effects of acute hypercalcaemia on blood pressure in subjects with and without parathyroid hormone secretion. Acta Physiol Scand 184 (2):113–119. doi: 10.1111/j.1365-201X.2005.01436.x [DOI] [PubMed] [Google Scholar]
- 39.Hall WL, Rimbach G, Williams CM (2005) Isoflavones and endothelial function. Nutr Res Rev 18 (1):130–144. doi: 10.1079/NRR2005101 [DOI] [PubMed] [Google Scholar]
- 40.Mann GE, Rowlands DJ, Li FY, de Winter P, Siow RC (2007) Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc Res 75 (2):261–274. doi: 10.1016/j.cardiores.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 41.Cederroth CR, Nef S (2009) Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol 304 (1–2):30–42. doi: 10.1016/j.mce.2009.02.027 [DOI] [PubMed] [Google Scholar]
- 42.Cano A, García-Pérez MA, Tarín JJ (2010) Isoflavones and cardiovascular disease. Maturitas 67 (3):219–226. doi: 10.1016/j.maturitas.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 43.Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE (1999) Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 96 (6):2788–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra SK, Abbot SE, Choudhury Z, Cheng M, Khatab N, Maycock NJ, Zavery A, Aaronson PI (2000) Endothelium-dependent relaxation of rat aorta and main pulmonary artery by the phytoestrogens genistein and daidzein. Cardiovasc Res 46 (3):539–546 [DOI] [PubMed] [Google Scholar]
- 45.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ (2001) The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation 103 (2):258–262 [DOI] [PubMed] [Google Scholar]
- 46.Chambliss KL, Shaul PW (2002) Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23 (5):665–686. doi: 10.1210/er.2001-0045 [DOI] [PubMed] [Google Scholar]
- 47.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV (2006) Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc 34 (1–2):67–79 [PubMed] [Google Scholar]
- 48.Schubert R, Lidington D, Bolz SS (2008) The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res 77 (1):8–18. doi: 10.1016/j.cardiores.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 49.Jiang C, Poole-Wilson PA, Sarrel PM, Mochizuki S, Collins P, MacLeod KT (1992) Effect of 17 beta-oestradiol on contraction, Ca2+ current and intracellular free Ca2+ in guinea-pig isolated cardiac myocytes. Br J Pharmacol 106 (3):739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE (1998) Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB J 12 (7):613–619 [DOI] [PubMed] [Google Scholar]
- 51.Figtree GA, Griffiths H, Lu YQ, Webb CM, MacLeod K, Collins P (2000) Plant-derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J Am Coll Cardiol 35 (7):1977–1985 [DOI] [PubMed] [Google Scholar]
- 52.Nevala R, Paukku K, Korpela R, Vapaatalo H (2001) Calcium-sensitive potassium channel inhibitors antagonize genistein- and daidzein-induced arterial relaxation in vitro. Life Sciences 69 (12):1407–1417. doi: 10.1016/S0024-3205(01)01233-4 [DOI] [PubMed] [Google Scholar]


