Abstract
The explosive spread of SARS-CoV-2 suggests that a vaccine will be required to end this global pandemic. Progress in SARS-CoV-2 vaccine development to date has been faster than for any other pathogen in history. Multiple SARS-CoV-2 vaccine candidates have been evaluated in preclinical models and are currently in clinical trials. In this Perspective, we discuss three topics that are critical for SARS-CoV-2 vaccine development: antigen selection and engineering, preclinical challenge studies in non-human primate models, and immune correlates of protection.
Keywords: SARS-CoV-2, COVID-19, vaccine
A vaccine will likely be required to end the SARS-CoV-2 pandemic. In this Perspective, Dagotto, Yu, and Barouch summarize the ongoing preclinical studies on SARS-Cov2 vaccine candidates and discuss standards for antigen selection and immune correlates of protection.
Introduction
SARS-CoV-2 was initially identified as the etiologic agent responsible for a cluster of severe pneumonia cases in Wuhan, China in December 2019 (Chan et al., 2020; Huang et al., 2020; Li et al., 2020; Wu et al., 2020; Zhu et al., 2020c). On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a global pandemic, and the disease was named COVID-19. In response to this pandemic, industry, government, philanthropy, non-governmental organizations, and academia are collaborating to develop therapeutic and prophylactic countermeasures, including vaccines.
SARS-CoV-2 is a member of the Coronaviridae family, joining severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) as coronaviruses causing severe disease in humans. These pathogens are single-stranded, positive-sense RNA viruses and encode four main structural proteins, spike (S), envelope (E), membrane (M), and nucleocapsid (N), as well as multiple non-structural proteins (Srinivasan et al., 2020). Similar to SARS-CoV, the SARS-CoV-2 S protein binds angiotensin-converting enzyme 2 (ACE2) as its primary host receptor to mediate viral entry (Hoffmann et al., 2020), and S is the main target for neutralizing antibodies.
SARS-CoV-2 has proven to be highly transmissible, including from asymptomatic and presymptomatic individuals (Arons et al., 2020; McMichael et al., 2020). Currently, at least six vaccine candidates have been tested in non-human primates (NHPs) and have reported either partial or complete protection (Corbett et al., 2020; Gao et al., 2020; Mercado et al., 2020; van Doremalen et al., 2020; Wang et al., 2020; Yu et al., 2020). Moreover, multiple promising vaccine candidates have entered clinical trials, and early phase clinical trial data have also been reported (Folegatti et al., 2020; Jackson et al., 2020; Mulligan et al., 2020; Zhu et al., 2020a). In this Perspective, we focus on three core issues related to SARS-CoV-2 vaccine development: antigen selection and engineering, preclinical challenge studies, and immune correlates of protection. A discussion of clinical development and deployment strategies for SARS-CoV-2 vaccines, while also important, is beyond the scope of this manuscript.
Antigen Selection and Engineering
Coronaviruses Encode Multiple Structural and Non-structural Proteins that Could Potentially Serve as Immunogens for a SARS-CoV-2 Vaccine
The best characterized proteins are S, N, M, and E. S has most commonly been utilized in coronavirus vaccine studies, due to its pivotal role in mediating viral entry into cells (Song et al., 2019). Mature S is a trimeric class I fusion protein located on the surface of the virion. Many coronaviruses proteolytically process S into the S1 and S2 domains. The S1 fragment contains the receptor binding domain (RBD) and the S2 fragment contains the fusion peptide, which are responsible for receptor binding and cell fusion, respectively. For SARS-CoV, S has been demonstrated to be the primary target of neutralizing antibodies. In mouse models of SARS-CoV, passive transfer and vaccine studies have shown that S-specific antibodies confer protective immunity (Enjuanes et al., 2008; Yang et al., 2004). For SARS-CoV-2, studies with monoclonal antibodies have shown that SARS-CoV-2-infected humans develop robust neutralizing antibody responses against S and in particular the RBD (Baum et al., 2020; Hansen et al., 2020; Ju et al., 2020; Rogers et al., 2020; Shi et al., 2020; Zost et al., 2020).
In addition to S, early studies with SARS-CoV suggested that most infected individuals developed an antibody response to N (Pei et al., 2005). N-protein-immunized BALB/c mice also induced CD4+ and CD8+ T cells (Liu et al., 2006). However, vaccination with vaccines expressing N resulted in no protection against SARS-CoV challenge as well as enhanced infection, which was characterized by increased pulmonary eosinophil infiltration (Deming et al., 2006). Passive transfer of anti-N antibodies did not generate an enhanced response, leading the authors to believe that the increased severity was linked to a T cell response (Deming et al., 2006). This was shown upon vaccination with VV expressing S, M, N, and E, as well as VV expressing N, causing the authors to trace back the response to nucleocapsid as an immunogen. Similarly, BALB/c mice vaccinated with a vaccinia virus (VV) expressing N showed enhanced viral infection upon SARS-CoV challenge, which was associated with a Th2 response with pulmonary infiltration of neutrophils, eosinophils, and lymphocytes (Yasui et al., 2008).
The M and E proteins have garnered less interest as vaccine targets due to lower immunogenicity (Du et al., 2008a), although SARS-CoV patient sera has been shown to be reactive to M peptides (Wang et al., 2003). Of the less-studied proteins, Orf3a has been shown to be capable of raising a neutralizing polyclonal antibody response in rabbits (Akerström et al., 2006).
Currently, Most Vaccines for SARS-CoV-2 Are Focused on S
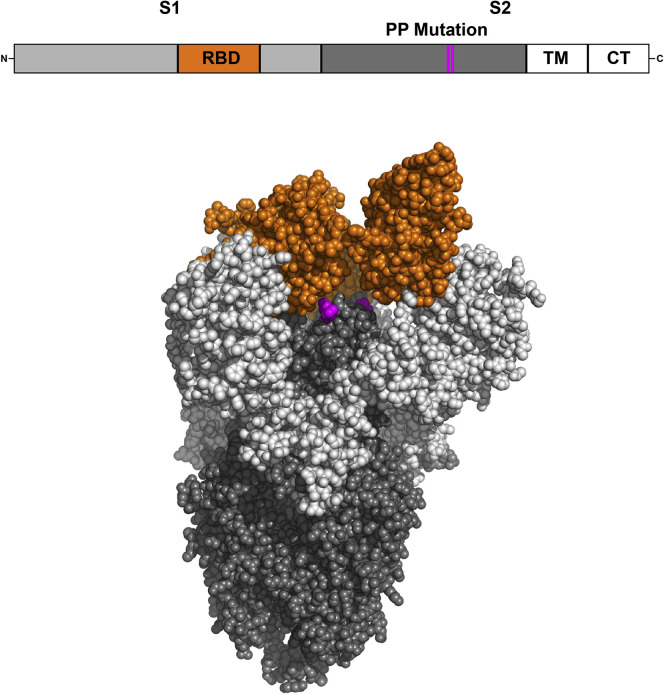
To target the SARS-CoV-2 S protein in its native prefusion form, antigen stabilization strategies have been used (Figure 1 ). For MERS-CoV and SARS-CoV, introduction of two prolines in the S2 subunit effectively stabilized the S in its prefusion conformation (Pallesen et al., 2017). The prefusion-stabilized MERS-CoV S generated higher neutralizing antibody titers when compared to native S trimer (Pallesen et al., 2017). This mutation prevented conformational changes of S in the presence of its receptor, ACE2, or trypsin (Kirchdoerfer et al., 2018). This stabilization method has also been demonstrated to stabilize the SARS-CoV-2 S (Wrapp et al., 2020) and has been applied to multiple SARS-CoV-2 vaccine candidates (Corbett et al., 2020; Jackson et al., 2020; Mercado et al., 2020). Unlike SARS-CoV, but similar to MERS-CoV, SARS-CoV-2 includes a furin cleavage site between the S1 and S2 domains. For MERS-CoV, researchers mutated the furin cleavage site to enhance homogeneity and stability (Walls et al., 2019). Some investigators have also added a foldon trimerization tag to the C terminus (Walls et al., 2020), and deletion of the cytoplasmic tail of the SARS-CoV S has been shown to increase neutralizing antibody titers (Yang et al., 2004). Current vaccine candidates in clinical trials are also exploring the inclusion of a tissue plasminogen activator leader sequence (tPA) with S (Folegatti et al., 2020; Mercado et al., 2020; Zhu et al., 2020a; Zhu et al., 2020). The tPA leader can increase immunogen secretion and elicit increased humoral immune responses in influenza (Luo et al., 2008) and HIV vaccines (Wallace et al., 2013), although cellular immunogenicity was only increased in the influenza vaccine.
Figure 1.
SARS-CoV-2 Spike
Graphical representation of the SARS-CoV-2 S protein sequence and crystal structure of SARS-CoV-2 S protein ectodomain (PDB: 6VSB) (Wrapp et al., 2020) created by using PyMol software. The transmembrane domain and cytoplasmic tail were not crystallized. TM, transmembrane domain; CT, cytoplasmic tail.
The RBD alone has also been explored as an immunogen. In preclinical studies in mice, rabbits, and monkeys, vaccination with modified vaccinia Ankara (MVA) expressing the full-length SARS-CoV S induced neutralizing antibodies that targeted RBD (Chen et al., 2005). Moreover, depletion of RBD-specific antibodies significantly reduced convalescent plasma neutralizing capabilities (He et al., 2005), consistent with the concept that RBD is the primary target of S-specific neutralizing antibodies. When rabbits were immunized with a variety of SARS-CoV-2 S immunogens, including RBD, S1, S2, and modified variants, RBD was found to elicit five-fold higher affinity antibodies than the other immunogens (Ravichandran et al., 2020). Sprague-Dawley rats immunized with SARS-CoV-2 RBD also elicited a strong neutralizing antibody response (Quinlan et al., 2020). RBD immunogens have been combined with a foldon trimerization tag in the BNT162b1 COVID-19 RNA vaccine candidate (Mulligan et al., 2020).
We recently compared a series of SARS-CoV-2 S immunogens in the context of both DNA vaccines and Ad26 vectors (Mercado et al., 2020; Yu et al., 2020). DNA vaccines encoding the full-length S elicited higher neutralizing antibody titers than did DNA vaccines encoding several S deletion mutants and also afforded improved protection against SARS-CoV-2 challenge in rhesus macaques (Yu et al., 2020). Ad26 vectors were also generated encoding a series of S variants, and the full-length S with the PP-stabilizing mutations proved the most immunogenic and afforded the best protection against SARS-CoV-2 challenge in rhesus macaques (Mercado et al., 2020).
Preclinical Challenge Studies in NHPs
An optimal model for SARS-CoV-2 infection studies would involve an animal species permissive to viral replication and that develops pathologic and clinical features consistent with the human disease. Clinical manifestations of COVID-19 in humans are usually mild but can include cough, fever, pneumonia, and occasionally respiratory failure and death (Yang et al., 2020). NHPs have significant genetic homology to humans and are often useful models for infectious diseases, although cost and availability can be limiting. In both rhesus and cynomolgus macaques, virus shedding could be detected in nasal, throat, and rectal swabs and in bronchoalveolar lavage for approximately 2 weeks after infection with SARS-CoV-2 using either intratracheal and intranasal infection or intratracheal, intranasal, ocular, and oral infection (Chandrashekar et al., 2020; Munster et al., 2020; Rockx et al., 2020). Early data suggest the utility of both macaque species as animal models for SARS-CoV-2 infection, although respiratory disease has been reported to be mild. The potential utility of African green monkeys as a model is also being explored. We recently demonstrated that SARS-CoV-2 infected macaques were also robustly protected against re-challenge, demonstrating natural protective immunity (Chandrashekar et al., 2020).
At least six SARS-CoV-2 vaccine challenge studies in macaques have been published at the time of this writing (Corbett et al., 2020; Gao et al., 2020; Mercado et al., 2020; van Doremalen et al., 2020; Wang et al., 2020; Yu et al., 2020) (Table 1 ). These vaccine studies in NHPs have included inactivated vaccines (PiCoVacc [Gao et al., 2020], BBIBP-CorV [Wang et al., 2020]), DNA vaccines (Yu et al., 2020), RNA vaccines (mRNA-1273 [Corbett et al., 2020]), and adenovirus-based vaccines (ChAdOx1 [van Doremalen et al., 2020], Ad26 [Mercado et al., 2020]). PiCoVacc and BBIBP-CorV are based on SARS-CoV-2 CN2 and SARS-CoV-2 HB02 strains, respectively. The viruses were grown in Vero cells and inactivated by using β-propiolactone and were evaluated as two- or three-shot immunization regimens (Gao et al., 2020; Wang et al., 2020). The DNA and mRNA-1273 vaccines encoded stabilized S immunogens and were tested as two-shot immunization regimens (Yu et al., 2020; Corbett et al., 2020). The ChAdOx1 vaccine expressed a codon-optimized full-length S with a human tPA leader sequence and was tested as a single-shot and a two-shot vaccine regimen (van Doremalen et al., 2020). The optimal Ad26 vaccine expressed a prefusion-stabilized S immunogen and was tested as a single-shot vaccine (Mercado et al., 2020).
Table 1.
NHP Challenge Studies of SARS-CoV-2 Vaccine Candidates
| Vaccine Name | Vaccine Type | Vaccine Immunogen | Vaccine Dose | Number of Injections | Challenge Route | Reference |
|---|---|---|---|---|---|---|
| PiCoVacc | inactivated | whole virus | 3 or 6 μg | 3 | intratracheal | Gao et al., 2020 |
| BBIBP-CorV | inactivated | whole virus | 2 or 8 μg | 2 | intratracheal | Wang et al., 2020 |
| DNA-S | DNA | engineered S | 5 mg | 2 | intranasal and intratracheal | Yu et al., 2020 |
| mRNA-1273 | RNA | engineered S | 10 or 100 μg | 2 | intranasal and intratracheal | Corbett et al., 2020 |
| ChAdOx1 nCoV-19 | adenoviral vector | tPA-S | 2.5x1010 VP | 1 or 2 | intranasal, intratracheal, ocular, and oral | van Doremalen et al., 2020 |
| Ad26.COV2.S (Ad26-S.PP) | adenoviral vector | engineered S | 1x1011 VP | 1 | intranasal and intratracheal | Mercado et al., 2020 |
After vaccination, macaques were challenged by SARS-CoV-2 by the intratracheal, intranasal, oral, and/or ocular routes. Efficacy was determined in these studies by assessment of viral loads in the upper and lower respiratory tracts. Most studies are now focusing on analysis of subgenomic RNA rather than genomic RNA (Wölfel et al., 2020), because subgenomic RNA is believed to be more reflective of replicating virus, rather than input challenge virus. The inactivated vaccine PiCoVacc resulted in reduced viral loads in throat swabs (Gao et al., 2020). The inactivated vaccine BBIBP-CorV also decreased viral loads in throat swabs (Wang et al., 2020). The DNA vaccine encoding the full-length S resulted in >3.1 and >3.7 log10 reductions in median subgenomic RNA levels in BAL and nasal swabs, respectively (Yu et al., 2020). The mRNA-1273 vaccine resulted in undetectable subgenomic RNA in lungs in all but one macaque, and more rapid clearance of virus from nasal swabs (Corbett et al., 2020). The optimal Ad26-S.PP vaccine resulted in undetectable subgenomic RNA in lungs and only one breakthrough in nasal swabs (Mercado et al., 2020). The ChAdOx1 vaccine resulted in reduced subgenomic RNA in BAL but no decrease in nasal swabs (van Doremalen et al., 2020).
The different challenge doses, strains, routes, and assays that were utilized make direct comparisons among these studies difficult. Nevertheless, these studies provide a substantial amount of preclinical data demonstrating protective efficacy of multiple vaccine candidates in NHPs. These data help inform the ongoing clinical development programs for these vaccines. A limitation of all these studies is that macaques do not develop severe disease, respiratory failure, or death. Small animal models are currently being developed that could model severe disease, including hamsters and transgenic mice.
Immune Correlates of Protection
Determining immune correlates of protection (CoP) for SARS-CoV-2 will be critical for guiding vaccine development. Currently, mechanistic CoP for SARS-CoV-2 have not yet been fully determined, although several studies point to the importance of both humoral and cellular immunity.
Humoral Immunity
Neutralizing antibodies (nAbs) represent a commonly studied immune correlate of protection. Early human challenge studies reported that volunteers with higher pre-existing anti-CoV 229E nAb titers (> 5) demonstrated lower proportions of virus isolation and upper respiratory infection than those with low neutralizing titers (≤5) (Bradburne et al., 1967). Pre-challenge serum antibody titers were also negatively associated with upper respiratory infections, nasal virus shedding, and disease severity (Barrow et al., 1990; Callow, 1985). Passive administration of convalescent sera, purified IgG, or monoclonal antibodies have also been shown to suppress SARS-CoV challenge/infection and associated disease progression in mice, hamsters, ferrets, and humans (Cheng et al., 2005; Roberts et al., 2006; Subbarao et al., 2004; Sui et al., 2005; Yuan et al., 2015).
For SARS-CoV-2, neutralizing monoclonal antibodies isolated from convalescent COVID-19 patients have been shown to inhibit SARS-CoV-2 infection in both prophylactic and therapeutic settings in rhesus macaques and hamsters (Rogers et al., 2020; Shi et al., 2020; Zost et al., 2020). We also reported that DNA vaccines and Ad26-based vaccines induced nAbs that strongly correlated with a reduction of viral loads in rhesus macaques (Mercado et al., 2020; Yu et al., 2020). SARS-CoV-2 inactivated virus vaccines and mRNA vaccines also induced nAbs and conferred protection in macaques (Corbett et al., 2020; Gao et al., 2020; Wang et al., 2020). Collectively, these studies suggest that nAb titers could serve as a useful biomarker for evaluating SARS-CoV-2 vaccines in both preclinical and clinical studies, although these correlates need to be confirmed in humans.
A question that has been raised is whether sub-neutralizing levels of antibodies could have detrimental effects. A prior study revealed that a vaccinia vector-based vaccine expressing feline coronavirus S induced low titers of neutralizing antibodies, which led to early cat mortality upon challenge (Vennema et al., 1990). Consistent with this observation, several studies observed enhanced respiratory disease (ERD) in response to SARS vaccines when antibodies had suboptimal potency or low binding affinity (Jaume et al., 2011; Wan et al., 2020; Wang et al., 2014). A major effort in the field will therefore be the development of animal models for ERD for SARS-CoV-2.
In addition to neutralization, emerging evidence suggests that certain antibody Fc-mediated functionscould also contribute to protective efficacy (Yu et al., 2020), including antibody-dependent complement deposition (ADCD), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent NK cell activation (ADNKA) (Zohar and Alter, 2020). Mucosal immunity is also likely important for protection, because coronavirus infection occurs in the respiratory tract and potentially in the gastrointestinal tract (Xiao et al., 2020). Pulmonary immunoglobulin (Ig)A was observed to be inversely associated with MERS-CoV infectivity in humans (Muth et al., 2015), and animal studies have highlighted the potential role of mucosal immunity in defending SARS-CoV infection (Du et al., 2008b; Huang et al., 2009). Given that intranasal administration could induce stronger mucosal immunity in the respiratory tract than parenteral routes, it could prove useful to evaluate intranasal routes for vaccines (Roper and Rehm, 2009).
Cellular Immunity
Cellular immunity also appears important in the control of coronavirus infections. Current evidence suggests that not all patients develop protective humoral immune responses, and asymptomatic patients or individuals with mild disease typically develop robust T cell responses (Mathew et al., 2020; Sekine et al., 2020). In a mouse model, SARS-CoV-specific CD8+ T cell numbers correlated with virus clearance and increased survival (Zhao et al., 2010). In addition, memory CD4+ T cells were associated with protective immunity against MERS-CoV (Zhao et al., 2016). Recent studies have reported that neutralizing antibody titers correlated with SARS-CoV-2-specific T cell responses (Ni et al., 2020) and that IgG and IgA antibody titers correlated with S-specific CD4+ T cell responses (Grifoni et al., 2020), suggesting that T cells could indirectly modulate the virus infection by orchestrating antibody production. However, in DNA vaccine studies in mice and NHPs, T cell responses did not correlate with protection (Yang et al., 2004; Yu et al., 2020). Nevertheless, severe patients tend to have a higher frequency of polyfunctional CD4 cells expressing interferon (IFN)γ, interleukin (IL)-2, and tumor necrosis factor alpha (TNF-α) (Sekine et al., 2020; Thieme et al., 2020), although it is possible that these high-frequency T cells could simply represent long-term viral exposure (Altmann and Boyton, 2020).
Innate Immunity
There is limited data on innate immune correlates of protection. Existing pathogenesis studies suggest that the excessive production of proinflammatory cytokines and chemokines (cytokine storm) is associated with poor clinical outcome (Channappanavar and Perlman, 2017). IFN-I and IFN-III have shown to be able to suppress SARS-CoV-2 infection in vitro (Sallard et al., 2020; Stanifer et al., 2020) and could represent innate immune responses that assist in controlling SARS-CoV-2 infection. Blocking IFN-I signaling in mice has been shown to increase viral load and mortality for SARS-CoV and MERS-CoV (Channappanavar et al., 2019; Frieman et al., 2010). Retrospective cohort studies have also suggested that induction of IFNs correlated with disease severity and viral load in both SARS and MERS patients (Cameron et al., 2007; Kim et al., 2016). It is possible that early induction promotes viral clearance, whereas delayed IFN responses can cause viral persistence, inflammation, and immunopathology (Park and Iwasaki, 2020).
The potential off-target effects of BCG, historically used as a vaccine for tuberculosis, and other live attenuated organisms are also being explored for SARS-CoV-2 (Curtis et al., 2020). Trained immunity is regarded as immunological memory in the innate immune system and likely mediated by epigenetic modifications (Netea et al., 2016). If non-specific trained innate immunity protects against SARS-CoV-2, this would provide an intriguing alternate to antigen-specific vaccination.
Conclusions
A vaccine will likely be required to end the global SARS-CoV-2 pandemic (Lurie et al., 2020). A successful vaccine will need to be safe, effective, durable, and deployable to large populations. There are currently at least 40 SARS-CoV-2 vaccine clinical trials ongoing. Most vaccine strategies aim to generate S-specific neutralizing antibodies, and preclinical studies have shown substantial protection against SARS-CoV-2 challenge in NHPs. Over the next several months, additional preclinical efficacy studies as well as studies on enhanced respiratory disease will likely be reported, and multiple large-scale clinical efficacy trials will be conducted.
Acknowledgments
We acknowledge support from the Ragon Institute of MGH, MIT, and Harvard; Mark and Lisa Schwartz Foundation; Massachusetts Consortium on Pathogen Readiness (MassCPR); Bill & Melinda Gates Foundation (INV-006131); Janssen Vaccines & Prevention BV; and the National Institutes of Health (OD024917, AI129797, AI124377, AI128751, and AI126603).
Declaration of Interests
Correspondence and requests for materials should be addressed to D.H.B. (dbarouch@bidmc.harvard.edu). D.H.B. is a co-inventor on provisional vaccine patents (62/969,008; 62/994,630) that have been licensed.
References
- Akerström S., Tan Y.J., Mirazimi A. Amino acids 15-28 in the ectodomain of SARS coronavirus 3a protein induces neutralizing antibodies. FEBS Lett. 2006;580:3799–3803. doi: 10.1016/j.febslet.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann D.M., Boyton R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020;5:eabd6160. doi: 10.1126/sciimmunol.abd6160. [DOI] [PubMed] [Google Scholar]
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., Taylor J., Spicer K., Bardossy A.C., Oakley L.P., et al. Public Health–Seattle and King County and CDC COVID-19 Investigation Team Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow G.I., Higgins P.G., al-Nakib W., Smith A.P., Wenham R.B.M., Tyrrell D.A.J. The effect of intranasal nedocromil sodium on viral upper respiratory tract infections in human volunteers. Clin. Exp. Allergy. 1990;20:45–51. doi: 10.1111/j.1365-2222.1990.tb02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020:eabd0831. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A.F., Bynoe M.L., Tyrrell D.A. Effects of a “new” human respiratory virus in volunteers. BMJ. 1967;3:767–769. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow K.A. Effect of specific humoral immunity and some non-specific factors on resistance of volunteers to respiratory coronavirus infection. J. Hyg. (Lond.) 1985;95:173–189. doi: 10.1017/s0022172400062410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., et al. Canadian SARS Research Network Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L., Tostanoski L.H., Yu J., Maliga Z., Nekorchuk M., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020:eabc4776. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., McCray P.B., Jr., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhang L., Qin C., Ba L., Yi C.E., Zhang F., Wei Q., He T., Yu W., Yu J., et al. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J. Virol. 2005;79:2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. European journal of clinical microbiology & infectious diseases. European Society of Clinical Microbiology. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M., et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N., Sparrow A., Ghebreyesus T.A., Netea M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395:1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming D., Sheahan T., Heise M., Yount B., Davis N., Sims A., Suthar M., Harkema J., Whitmore A., Pickles R., et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Jiang S., Zheng B.-J. Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today (Barc) 2008;44:63–73. doi: 10.1358/dot.2008.44.1.1131830. [DOI] [PubMed] [Google Scholar]
- Du L., Zhao G., Lin Y., Sui H., Chan C., Ma S., He Y., Jiang S., Wu C., Yuen K.-Y., et al. Intranasal vaccination of recombinant adeno-associated virus encoding receptor-binding domain of severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein induces strong mucosal immune responses and provides long-term protection against SARS-CoV infection. J. Immunol. 2008;180:948–956. doi: 10.4049/jimmunol.180.2.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Dediego M.L., Alvarez E., Deming D., Sheahan T., Baric R. Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease. Virus Res. 2008;133:45–62. doi: 10.1016/j.virusres.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Oxford COVID Vaccine Trial Group Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31604-4. S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M.B., Chen J., Morrison T.E., Whitmore A., Funkhouser W., Ward J.M., Lamirande E.W., Roberts A., Heise M., Subbarao K., Baric R.S. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I, II and III interferon receptor independent mechanism. PLoS Pathog. 2010;6:e1000849. doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020:eabd0827. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhu Q., Liu S., Zhou Y., Yang B., Li J., Jiang S. Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines. Virology. 2005;334:74–82. doi: 10.1016/j.virol.2005.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Lu B., Yu W., Fang Q., Liu L., Zhuang K., Shen T., Wang H., Tian P., Zhang L., Chen Z. A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS ONE. 2009;4:e4180. doi: 10.1371/journal.pone.0004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. mRNA-1273 Study Group An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaume M., Yip M.S., Cheung C.Y., Leung H.L., Li P.H., Kien F., Dutry I., Callendret B., Escriou N., Altmeyer R., et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Kim E.S., Choe P.G., Park W.B., Oh H.S., Kim E.J., Nam E.Y., Na S.H., Kim M., Song K.H., Bang J.H., et al. Clinical Progression and Cytokine Profiles of Middle East Respiratory Syndrome Coronavirus Infection. J. Korean Med. Sci. 2016;31:1717–1725. doi: 10.3346/jkms.2016.31.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Wang N., Pallesen J., Wrapp D., Turner H.L., Cottrell C.A., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci. Rep. 2018;8:15701. doi: 10.1038/s41598-018-34171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.-J., Leng C.-H., Lien S.P., Chi H.-Y., Huang C.-Y., Lin C.-L., Lian W.-C., Chen C.-J., Hsieh S.-L., Chong P. Immunological characterizations of the nucleocapsid protein based SARS vaccine candidates. Vaccine. 2006;24:3100–3108. doi: 10.1016/j.vaccine.2006.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Tao P., Li J., Zhou S., Guo D., Pan Z. Immunization with plasmid DNA encoding influenza A virus nucleoprotein fused to a tissue plasminogen activator signal sequence elicits strong immune responses and protection against H5N1 challenge in mice. J. Virol. Methods. 2008;154:121–127. doi: 10.1016/j.jviromet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., Kuri-Cervantes L., Pampena M.B., D’Andrea K., et al. UPenn COVID Processing Unit Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., Lewis J., Baer A., Kawakami V., Lukoff M.D., et al. Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020 doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S.P., Neuzil K., Raabe V., Bailey R., Swanson K.A., et al. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report. medRxiv. 2020 doi: 10.1101/2020.06.30.20142570. [DOI] [Google Scholar]
- Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Pérez-Pérez L., Schulz J., Meade-White K., Okumura A., Callison J., Brumbaugh B., et al. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2. bioRxiv. 2020 doi: 10.1038/s41586-020-2324-7. 2020.03.21.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth D., Corman V.M., Meyer B., Assiri A., Al-Masri M., Farah M., Steinhagen K., Lattwein E., Al-Tawfiq J.A., Albarrak A., et al. Infectious Middle East Respiratory Syndrome Coronavirus Excretion and Serotype Variability Based on Live Virus Isolates from Patients in Saudi Arabia. J. Clin. Microbiol. 2015;53:2951–2955. doi: 10.1128/JCM.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Joosten L.A.B., Latz E., Mills K.H.G., Natoli G., Stunnenberg H.G., O’Neill L.A.J., Xavier R.J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H., Wei P., Ge J., Gou M., Li X., et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52:971–977.e3. doi: 10.1016/j.immuni.2020.04.023. e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A., Iwasaki A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H., Liu J., Cheng Y., Sun C., Wang C., Lu Y., Ding J., Zhou J., Xiang H. Expression of SARS-coronavirus nucleocapsid protein in Escherichia coli and Lactococcus lactis for serodiagnosis and mucosal vaccination. Appl. Microbiol. Biotechnol. 2005;68:220–227. doi: 10.1007/s00253-004-1869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan B.D., Mou H., Zhang L., Guo Y., He W., Ojha A., Parcells M.S., Luo G., Li W., Zhong G., et al. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. bioRxiv. 2020 doi: 10.1101/2020.04.10.036418. [DOI] [Google Scholar]
- Ravichandran S., Coyle E.M., Klenow L., Tang J., Grubbs G., Liu S., Wang T., Golding H., Khurana S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci. Transl. Med. 2020;12:eabc3539. doi: 10.1126/scitranslmed.abc3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Thomas W.D., Guarner J., Lamirande E.W., Babcock G.J., Greenough T.C., Vogel L., Hayes N., Sullivan J.L., Zaki S., et al. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J. Infect. Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A., et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., Limbo O., Smith C., Song G., Woehl J., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020:eabc7520. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev. Vaccines. 2009;8:887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. bioRxiv. 2020 doi: 10.1101/2020.06.29.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Cui H., Gao Z., Liu M., Lu S., Mkandawire W., Narykov O., Sun M., Korkin D. Structural Genomics of SARS-CoV-2 Indicates Evolutionary Conserved Functional Regions of Viral Proteins. Viruses. 2020;12:360. doi: 10.3390/v12040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer M.L., Kee C., Cortese M., Zumaran C.M., Triana S., Mukenhirn M., Kraeusslich H.-G., Alexandrov T., Bartenschlager R., Boulant S. Critical Role of Type III Interferon in Controlling SARS-CoV-2 Infection in Human Intestinal Epithelial Cells. Cell Rep. 2020;32:107863. doi: 10.1016/j.celrep.2020.107863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K., Packard M., Shieh W.J., Zaki S., Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Marasco W.A. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 2005;79:5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme C.J., Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A., Seibert F.S., Hoelzer B., Konik M.J., Brenner T., Tempfer C., et al. The SARS-CoV-2 T-cell immunity is directed against the spike, membrane, and nucleocapsid protein and associated with COVID 19 severity. medRxiv. 2020 doi: 10.1101/2020.05.13.20100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020 doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., de Groot R.J., Harbour D.A., Dalderup M., Gruffydd-Jones T., Horzinek M.C., Spaan W.J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J. Virol. 1990;64:1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A., West K., Rothman A.L., Ennis F.A., Lu S., Wang S. Post-translational intracellular trafficking determines the type of immune response elicited by DNA vaccines expressing Gag antigen of Human Immunodeficiency Virus Type 1 (HIV-1) Hum. Vaccin. Immunother. 2013;9:2095–2102. doi: 10.4161/hv.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Xiong X., Park Y.-J., Tortorici M.A., Snijder J., Quispe J., Cameroni E., Gopal R., Dai M., Lanzavecchia A., et al. Unexpected Receptor Functional Mimicry Elucidates Activation of Coronavirus Fusion. Cell. 2019;176:1026–1039.e15. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020;94:94. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wen J., Li J., Yin J., Zhu Q., Wang H., Yang Y., Qin E., You B., Li W., et al. Assessment of immunoreactive synthetic peptides from the structural proteins of severe acute respiratory syndrome coronavirus. Clin. Chem. 2003;49:1989–1996. doi: 10.1373/clinchem.2003.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.F., Tseng S.P., Yen C.H., Yang J.Y., Tsao C.H., Shen C.W., Chen K.H., Liu F.T., Liu W.T., Chen Y.M., Huang J.C. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020 doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S., Kase R., Sekiguchi S., Morita K., Hishima T., et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020:eabc6284. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Yang H., Qi H., Cui J., Hua W., Li C., Pang Z., Zheng W., Cui G. IL-9 antibody injection suppresses the inflammation in colitis mice. Biochem. Biophys. Res. Commun. 2015;468:921–926. doi: 10.1016/j.bbrc.2015.11.057. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhao J., Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J. Virol. 2010;84:9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K., Agnihothram S., Baric R.S., David C.S., Perlman S. Airway Memory CD4(+) T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31605-6. S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., Wu S.-P., Wang B.-S., Wang Z., Wang L., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar T., Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat. Rev. Immunol. 2020;20:392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S., et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]