Abstract
Recently, a petition was offered to the European Commission calling for an immediate ban on animal testing. Although a Europe-wide moratorium on the use of animals in science is not yet possible, there has been a push by the non-scientific community and politicians for a rapid transition to animal-free innovations. Although there are benefits for both animal welfare and researchers, advances on alternative methods have not progressed enough to be able to replace animal research in the foreseeable future. This trend has led first and foremost to a substantial increase in the administrative burden and hurdles required to make timely advances in research and treatments for human and animal diseases. The current COVID-19 pandemic clearly highlights how much we actually rely on animal research. COVID-19 affects several organs and systems, and the various animal-free alternatives currently available do not come close to this complexity. In this Essay, we therefore argue that the use of animals is essential for the advancement of human and veterinary health.
In this Essay, Genzel et al. make the case for animal research in light of the COVID-19 pandemic.
Main Text
With the implementation of the European Directive to protect animals used in scientific procedures [1] around 10 years ago, the European Union set high ambitions regarding the protection of animals for research purposes. This directive focused on the development and implementation of the ‘3Rs’ (reduction, refinement and replacement), transparency (public information about the use of animals) and their harmonization across Europe. The implementation of this directive into national legislation has revived intense political discussions in many countries. For example, the Dutch government has expressed its ambition that the Netherlands “lead the way in the international transition with animal-free innovations” [2]. Further, in response [3] to a petition to immediately ban animal research, the European Commission has stated that more investments will be made in the development of alternatives, the goal of which is to ultimately replace all use of animals in research. The European Union directive, combined with political pressure, has mainly resulted in a substantial increase in the administrative burden associated with scientific studies. This burden comes in multiple forms: financial, since more approvals and amendments are needed, which cost money to submit; time, since ethical approval takes longer to be processed and is required for even small adjustments to experiments; and effort, with more stress involved in achieving ethical approval. The consequence is that scientific quality declines, due to the inability to repeat and refine experimental designs. Furthermore, this has made timely advances in research and insights informing new treatments for human and animal diseases more challenging. Although the aim to progress towards research without animals is laudable, we argue that the use of animals is essential for the advancement of human and veterinary health, and will likely be so for the foreseeable future. This necessity is now clearly highlighted by the ongoing COVID-19 pandemic.
Need for biomedical research
The human population is facing an unprecedented threat. Unless we are prepared to accept many more COVID-19 victims — in addition to the continuing restrictions on our daily lives due to social distancing and quarantine measures — the current crisis can only be solved by the development of an effective vaccine and/or antiviral and adjunctive drug therapies. This will require biomedical research, of which animal studies are an indispensable part.
The European Animal Research Association website features an interactive map of all animal research currently being conducted to find a remedy for COVID-19. This map highlights the various animal species being used to obtain fundamental knowledge about the disease and to test therapeutic approaches and vaccines. Different animal models bring value depending on the specific scientific question being addressed [4] and enable us to respond swiftly and efficiently to major societal challenges such as COVID-19. Further, to increase collaboration and the exchange of knowledge, the World Health Organization arranges a video conference on this topic of nearly 100 scientists, regulators, and funders every week. Highlighting this use of animals for COVID-19 research confronts the public and politicians with the essential value they provide to progress in biomedical research. The scientific community is moving towards greater openness and transparency, and the present crisis has created a new context to discuss the continuous need for animal research.
Biomedical scientists use a wide spectrum of complementary models, including molecular methods, cell and tissue culture, human challenge models, human biobank material, and mathematical modelling. However, currently there is no integrated model to be able to completely replace animal research to study the complex functions of the body. The comprehensive statistics [5] of the European Union on the numbers and species of animals used in the various areas of research is useful for a more constructive debate.
The Netherlands is performing well in the development of alternatives for animal research (for example, organoids or organ-on-a-chip approaches, early clinical technologies, computer simulations). These alternatives have been developed thanks to fundamental knowledge gained through animal research. Although these alternatives are valuable for the study of certain aspects of a biological process, they cannot provide complete answers to biomedical questions. For the understanding of complex bodily functions, and for drug and vaccine testing, a whole living organism is often required. After all, organs do not function in isolation, but interact with other body systems (through microvascular networks, blood supply and lymph drainage) and the environment (for example, stress, social environment, sensory stimulation, diet, exercise, etc.), which cannot be mimicked in vitro. Furthermore, a drug that is effective in organoids derived from a specific organ can have other, possibly adverse, effects on other organs. It is therefore challenging to predict how a compound that is effective in an organoid would work in a body with properly interacting or diseased organs.
In the case of COVID-19, and the virus that causes it, SARS-CoV-2, the indispensability of animal research is clear. The interaction between COVID-19 and other diseases that may increase risk for death (including diabetes and heart disease) cannot be investigated in organoids. An organoid cannot model the immune system or inter-organ metabolic homeostasis mediated by, for example, hormones, neural networks and gut microbiota [6], let alone behavior and cognition. Additionally, although adaptive immunity to SARS-CoV-2 can be predicted by a variety of in vitro methods, it can only be validated in animals. This is essential for vaccine development.
Animals that are essential in COVID-19 research include ferrets, non-human primates, pigs and rodents [4,7]. These animals are used to understand transmission and to identify potential treatments. For example, a recent study investigated the transmission route of SARS-CoV-2 using ferrets, which experience an upper respiratory tract infection and long-term shedding of the virus, similar to humans [8]. Scientists demonstrated that SARS-CoV-2 is transmitted by both direct and indirect contact between ferrets, suggesting that it can be transmitted via the air, and provides a model to test if this transmission is droplet or aerosol based. Further, inoculation with SARS-CoV-2 in cynomolgus macaques led to lung pathology similar to that observed in humans. Like humans, the animals shed the virus from their upper respiratory tract [9]. As another example, remdesivir, an antiviral drug that was developed and tested using animals to treat Ebola infection, was found to effectively reduce symptoms of SARS-CoV-2 infection in rhesus macaques, resulting in transient lower respiratory tract disease [10]. Remdesivir is now being tested in clinical trials with initially positive results.
Animals are of particular importance for testing the efficacy, safety, and the mode of action of new vaccines. This is not trivial, as we learned from previous experiences. For example, a study on a SARS-CoV-1 vaccine candidate in 2004 found that some vaccinated ferrets developed hepatitis, rather than protection against the virus [11]. The risk of antibody-dependent enhancement of disease by a vaccine is also a risk that should not be underestimated [12]. Clearly, treating the whole human population with a vaccine that has unforeseen side-effects may be even more damaging than COVID-19 itself. To prevent this risk, preclinical development of a vaccine includes animal testing. Thanks in part to animal research, modern vaccines against polio, measles, tuberculosis, meningitis, human papillomavirus and Ebola have been successfully developed, saving millions of lives [13,14].
Finally, animals are important for understanding how immunity to pathogens develops after infection. Although controlled human infection with SARS-CoV-2 could potentially accelerate vaccine testing [15], this approach raises ethical concerns as there is not yet sufficient knowledge on the development of COVID-19 disease to ensure the safety of human subjects. Non-human primate research in China has revealed that rhesus macaques develop protective immunity to SARS-CoV-2 after infection [16]. However, it is still unclear if — and for how long — this immunity is protective and whether it would also protect against subsequent exposure to different variants of the virus. To answer this question, we need to continue such experiments with non-human primates. Yet despite their essential contribution to biomedicine, research on non-human primates has been scaled down in The Netherlands and many other European countries.
Mice and rats, which are currently the most widely used animal species in biomedical research, are less suitable to study the immune response to SARS-CoV-2 as they lack mediators important in antiviral responses, for example, interleukin (IL)-32 and IL-37. Furthermore, due to structural differences between the mouse and human ACE2 receptor, which SARS-CoV-2 uses to enter cells, coronaviruses do not infect wild-type mice and rats. To overcome this, a transgenic mouse strain has been developed that expresses the human ACE2. Studies showed that hACE2-transgenic mice could mimic the human clinical features in a dose-dependent manner, and did not survive a very high dose of SARS-CoV-1 [17]. Although the hACE2-transgenic mouse is available at a few commercial breeders, its availability is currently too limited for the many animal facilities involved in COVID-19 research [4].
Additional complexity
Although SARS-CoV-2 primarily infects the airways, it can independently cause intestinal problems [18], a loss of smell and taste [19], neurological symptoms [19], acute hemorrhagic necrotizing encephalopathy [20], gut microbiome dysbiosis [6], and thromboembolic stroke [21] in patients. Appropriate animal models that mimic these disease processes are needed to understand and treat them. For example, in the case of thromboembolic stroke, blood clotting cannot be modeled in organoids due to their lack of a vasculature, but the intact murine pulmonary vasculature can be monitored with micro-computed tomography. Additionally, using non-invasive neuroimaging techniques, therapeutic effects on thromboembolic stroke can be investigated longitudinally in animals to determine different therapeutic time windows.
Social isolation due to quarantine measures and anxiety or worries about the COVID-19 pandemic can combine with other biological and environmental factors to aggravate mental health problems. Although environmental factors are very diverse in humans and can have huge confounding effects (for example, depending on socioeconomic status, or hospitalization with or without intensive care treatment), animal studies allow neuroscientists to investigate the effects of social isolation and interventions on brain function and behavior of COVID-19 patients in a well-controlled manner.
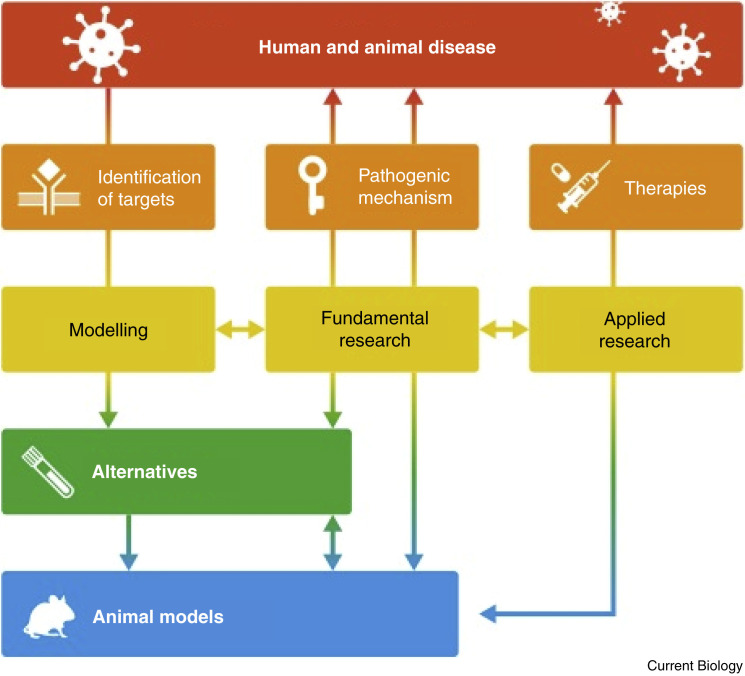
Animal research is thus indispensable to find solutions for COVID-19. This is also the case for many other biomedical domains, including (but not limited to) inflammatory, autoimmune and metabolic diseases, cardiovascular diseases, brain disorders, cancer and ageing. In addition, animal studies are needed to explore the biology of many as yet poorly studied wild animal species. Figure 1 sets out the role of animal research in tackling human and animal diseases.
Figure 1.
Research model for pathogenic mechanism and development of therapeutic interactions.
Alternatives and animal models are developed based on similarity in mechanisms and drug effects in humans and models. The interaction between disease, alternatives and animal models has a positive effect on the refinement and reduction of the animal models and unraveling the underlying pathogenic mechanisms. Due to the complexity of most diseases, validation of candidate treatments can only be performed by the use of animal models.
Some lessons learned from COVID-19 and conclusions to guide political leaders
Most ethical reviews have been accelerated for COVID-19 research. However, the general trend is that the paperwork needed to start an animal experiment demands more and more time and effort. European regulations require two types of ethical permission: an initial general project license covering a longer period (maximally five years) and, subsequently, an approval of the actual experiment by local review bodies. In several European countries, including The Netherlands, rules have been implemented that leave very little room for experimental flexibility and require substantial detail at the first (project) level, rather than at the second (experimental) level, where new scientific insights and all options for reduction and refinement can be considered. The resulting inflexibility and often too extreme restrictions reduce the ability to respond swiftly to new (often unexpected) results and emerging biomedical threats, making good research truly impossible. Scientifically rigorous research and development require changes, updates, and refinements of experimental protocols in an intelligent manner, in order to focus on unexpected intermediate results rather than wasting critical time on writing new protocols.
Issuing general ethical permits, as intended by the European legislation, would significantly speed up our ability to respond to pandemics and other diseases, and help people whose lives depend on scientific discoveries, while maintaining adequate oversight of animal experimentation and ethics. Because researchers work at the forefront of biomedicine, constantly responding to emerging biomedical issues and implementing state-of-the-art approaches, rules should focus more on welfare and less on experimental details and administration. This would allow researchers to quickly change gears when needed, saving time, money and perhaps even valuable animal resources.
As a second lesson, the focus on animal replacement in The Netherlands, initially intended for routine screening, is now generalized to all biomedical research. Concomitantly, the medical domain of the Dutch Research Council directed funding to animal-free innovations. While increased funding for animal-free innovations is certainly a positive development, funding for animal research remains necessary to secure our future health, as alternatives will never fully replace animal research.
In summary, the current COVID-19 crisis highlights the reality that animal research remains essential to find solutions for human and animal health in relation to many different disorders. The current trend we see in The Netherlands — to scale down animal research in a non-scientific based manner, and to continuously increase the administrative burden and costs on institutions and researchers using animals — is also present in many other European countries. This time cannot be spent on research and increases costs for society. While we fully support the implementation of the 3Rs in principle, we critically question the extent to which this trend is being implemented, because it impacts our future health. Furthermore, while animals are treated with extreme care in European countries, the current bureaucracy will drive animal research to countries where ethical standards may be lower.
We trust that governments, policy makers and animal activists realize that animal studies are an indispensable part of fundamental and applied research and also essential for the development of new medical treatments. Instead of the current trend to curtail animal research, we should, while maintaining optimal care for each experimental animal, all work together to make animal research as efficient and effective as possible such that rapid responses to newly emerging health threats can be achieved.
Acknowledgements
We thank Tansu Celikel, Guillen Fernandez, Otto Boerman, EARA, Guus Smit, Onno Meijer, Ricardo Fodde, Michael Cohen, Francesco Battaglia, Nael Nadif Kasri, Liya Ma, Freyja Olafsdottir, Joanes Grandjean, Erik Storkebaum, Gerard Martens, and Vereniging Innovative Geneesmiddelen for supporting this article.
References
- 1.https://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm
- 2.https://www.transitieproefdiervrijeinnovatie.nl/documenten/rapporten/18/06/01/tpi-philosophy-and-approach
- 3.https://www.europarl.europa.eu/doceo/document/PETI-CM-653736_EN.pdf
- 4.Cohen J. From mice to monkeys, animals studied for coronavirus answers. Science. 2020;368:221–222. doi: 10.1126/science.368.6488.221. [DOI] [PubMed] [Google Scholar]
- 5.https://www.eara.eu/post/research-sector-welcomes-the-publication-of-eu-wide-figures-on-the-number-of-animals-used-in-science
- 6.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A., Cheung C.P., Chen N. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hozain A.E., O’Neill J.D., Pinezich M.R., Tipograf Y., Donocoff R., Cunningham K.M., Tumen A., Fung K., Ukita R., Simpson M.T. Xenogeneic cross-circulation for extracorporeal recovery of injured human lungs. Nat. Med. 2020;26:1102–1113. doi: 10.1038/s41591-020-0971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y.I., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Chang J.H., Kim E.J., Lee S., Casel M.A.B. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., van Doremalen N., Leighton I., Yinda C.K., Pérez-Pérez L. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.04.15.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020;20:339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdts V., Littel-van den Hurk S., Griebel P.J., Babiuk L.A. Use of animal models in the development of human vaccines. Future Microbiol. 2007;2:667–675. doi: 10.2217/17460913.2.6.667. [DOI] [PubMed] [Google Scholar]
- 14.Stanley D.A., Honko A.N., Asiedu C., Trefry J.C., Lau-Kilby A.W., Johnson J.C., Hensley L., Ammendola V., Abbate A., Grazioli F. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014;20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 15.Eyal N., Lipsitch M., Smith P.G. Human challenge studies to accelerate coronavirus vaccine licensure. J. Infect. Dis. 2020;221:1752–1756. doi: 10.1093/infdis/jiaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao L., Deng W., Gao H., Xiao C., Liu J., Xue J., Lv Q., Liu J., Yu P., Xu Y. Reinfection could not occur in SARS-Cov-2 infected rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.03.13.990226. [DOI] [Google Scholar]
- 17.McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G. Features of 20,133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyiadji, N., Shahin, G., Noujaim, D., Stone, M., Patel, S., and Griffith, B. (2020). COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. 296, E119–E120. [DOI] [PMC free article] [PubMed]
- 21.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]