Abstract
A striking feature of COVID-19 is the high frequency of thrombosis, particularly in patients who require admission to intensive care unit because of respiratory complications (pneumonia/adult respiratory distress syndrome). The spectrum of thrombotic events is wide, including in situ pulmonary thrombosis, deep-vein thrombosis and associated pulmonary embolism, as well as arterial thrombotic events (stroke, myocardial infarction, limb artery thrombosis). Unusual thrombotic events have also been reported, e.g., cerebral venous sinus thrombosis, mesenteric artery and vein thrombosis. Several hematology abnormalities have been observed in COVID-19 patients, including lymphopenia, neutrophilia, thrombocytopenia (usually mild), thrombocytosis, elevated prothrombin time and partial thromboplastin times (the latter abnormality often indicating lupus anticoagulant phenomenon), hyperfibrinogenemia, elevated von Willebrand factor levels, and elevated fibrin d-dimer. Many of these abnormal hematologic parameters—even as early as the time of initial hospital admission—indicate adverse prognosis, including greater frequency of progression to severe respiratory illness and death. Progression to overt disseminated intravascular coagulation in fatal COVID-19 has been reported in some studies, but not observed in others. We compare and contrast COVID-19 hypercoagulability, and associated increased risk of venous and arterial thrombosis, from the perspective of heparin-induced thrombocytopenia (HIT), including the dilemma of providing thromboprophylaxis and treatment recommendations when available data are limited to observational studies. The frequent use of heparin—both low-molecular-weight and unfractionated—in preventing and treating COVID-19 thrombosis, means that vigilance for HIT occurrence is required in this patient population.
Keywords: COVID-19, Disseminated intravascular coagulation, Heparin, Thrombocytopenia, Thrombosis
Graphical abstract
1. COVID-19 as a hypercoagulable state
The emergence of the novel coronavirus, SARS-CoV-2 (abbreviation for severe acute respiratory syndrome coronavirus 2), in late 2019, and the resulting illness, COVID-19 (coronavirus disease, 2019), was declared a pandemic by the World Health Organization on March 11, 2020. At the time of writing (August 14, 2020), cases diagnosed world-wide have exceeded 21,000,000, with over 750,000 deaths (both values representing underestimates due to incomplete case ascertainment) [1]. Although clinical manifestations of COVID-19 are protean, the major clinical picture of pneumonia and adult respiratory distress syndrome (ARDS) is generally believed to account for the majority of deaths. However, it is increasingly apparent that there is a high frequency of hemostatic abnormalities, and thrombotic events, in COVID-19, with emerging consensus that this novel virus induces a hypercoagulable state beyond that expected in the “typical” critically ill patient. It is also likely that significant mortality is secondary to pulmonary thrombotic events, either local (in situ pulmonary thrombosis) or embolic (pulmonary embolism [PE]).
This review summarizes clinical and laboratory features of COVID-19 hypercoagulability. The viewpoint is from the perspectives of the two authors, one of whom has studied another hypercoagulable disorder—heparin-induced thrombocytopenia (HIT)—for over 30 years (T.E.W.), and the other with a longstanding interest in anticoagulation (S.K). As in HIT, the challenge is to make appropriate thromboprophylaxis and treatment recommendations when available data are limited and largely observational.
SARS-CoV-2 is a positive-sense single-stranded RNA virus, i.e., its genetic material functions both as genome and messenger RNA that directs host ribosomes. The virus gains entry to human cells via its surface spike protein, which binds to host receptor angiotensin converting enzyme 2 (ACE2), highly expressed on human endothelial cells, among other cells (pneumocytes of the alveolar epithelium, renal tubular epithelium, hepatocytes, enterocytes, cardiomyocytes). Targeting vascular endothelium likely plays an important part in the prothrombotic diathesis of COVID-19, through endotheliilitis (endothelialitis), i.e., viral invasion of endothelial cells and resulting accumulation of inflammatory cells (host inflammatory response) [[2], [3], [4]].
2. COVID-19: comparison with HIT and severe sepsis
Table 1 lists some comparisons between COVID-19 and HIT. Both severe COVID-19 and HIT occur in a minority of at-risk patients (those infected with SARS-CoV-2 and those exposed to heparin, respectively). Both feature a hypercoagulable state, including high frequency of thrombosis, and occurrence of unusual thrombotic events. Both feature abnormalities in blood cell counts (leukocytes, platelets), coagulation values indicating likely hemostasis activation—elevations in prothrombin time (PT) and fibrin d-dimer—and potential for “high-fibrinogen disseminated intravascular coagulation (DIC)”. One major distinction: while it is now widely accepted that treatment of acute HIT requires therapeutic-dose anticoagulation (even if no evidence of thrombosis is apparent) [5], the dosing of anticoagulation needed to prevent thrombosis in COVID-19 is controversial.
Table 1.
Comparison of COVID-19 and HIT.
| COVID-19 | HIT | |
|---|---|---|
| Similarities | ||
| Risk of severe disease | 1–5% (?) infected patients develop severe disease | 1–5% heparin-exposed patients develop HIT |
| High frequency of thrombosis | ~40–50% of ICU patients | ~40–50% thrombosis rate |
| Higher frequency of thrombosis with greater disease severity | Thrombosis rate higher in ICU versus ward patients | Thrombosis rate higher in patients with more severe thrombocytopenia |
| Venous versus arterial thrombosis | Venous predominance | Venous predominance |
| Arterial thrombosis hierarchy | Stroke > MI > limb | Limb > stroke > MI |
| Occurrence of unusual thrombi | Yes (e.g., CVST, mesenteric artery or vein) | Yes (adrenal, CVST, mesenteric artery or vein, etc.) |
| Endothelial activation | Yes | Yes |
| Neutrophilia | Yes | Yes |
| Leukocyte activation | Yes | Yes |
| Differences | ||
| Prominent thrombocytopenia | No (mild thrombocytopenia common); moderate to severe thrombocytopenia occurs in some patients with fatal COVID-19 | Yes (>50% platelet fall in ~90% of patients with HIT; median platelet count nadir, 60–70 × 109/L) |
| In situ pulmonary thrombosis | Common | Uncommon |
| ARDS picture | Common | No |
| Pathological criterion indicating risk for thrombosis | No distinct marker for risk for thrombosis | Platelet-activating HIT antibodies detectable by platelet activation assay |
| Thromboprophylaxis and treatment consensus | No consensus re: anticoagulant dosing | Therapeutic-dose anticoagulation generally recommended (even in the absence of documented thrombosis) |
Abbr.: ARDS, adult respiratory distress syndrome; COVID-19, coronavirus disease, 2019; CVST, cerebral venous sinus thrombosis; ICU, intensive care unit; HIT, heparin-induced thrombocytopenia; MI, myocardial infarction.
“Pancellular” activation in HIT involves platelets [6], monocytes [7], neutrophils [8,9], and endothelium [10,11]. Most striking in HIT is “strong” platelet activation—including formation of procoagulant platelet-derived microparticles [12]—that occurs when HIT antibodies recognize platelet factor 4 (PF4)/polyanion and activate platelets through their FcγIIa receptors [13]. PF4 binds to monocytes, with resulting tissue factor expression [14]. Netosis of neutrophils is triggered by HIT antibodies [8,9]. Finally, numerous prothrombotic consequences of HIT antibody binding to endothelium has been reported, including perturbed protein C activation [15] and formation of von Willebrand factor (VWF) strings [16,17].
COVID-19 also has features of pancellular activation. As noted, SARS-CoV-2 invades endothelial cells, producing endotheliitis and an associated inflammatory response. Neutrophil infiltration occurs in the lungs [18], producing high levels of neutrophil extracellular traps (NETs) in many patients with COVID-19 [19]. Mild thrombocytopenia often occurs in patients with severe COVID-19 (discussed subsequently), and it is possible this represents platelet activation response, with or without associated activation of hemostasis.
Severe COVID-19 usually results in admission to the intensive care unit (ICU), and so comparisons with severe sepsis and (non-COVID-19) ARDS is apropos. Whereas DIC occurs frequently in sepsis [20], the frequency and clinical impact of DIC in COVID-19 is controversial. Systemic inflammation, including fibrinolytic shut-down (elevated plasminogen activator inhibitor-1 levels)—common in sepsis [21]—is likely a feature too of COVID-19 [22]. Substantial proinflammatory features indicating “cytokine storm” occur in many patients, contributing also to a prothrombotic phenotype [23]. As discussed later, some critically ill COVID-19 patients develop a clinical picture reminiscent of symmetrical peripheral gangrene (SPG), a disorder of acral microthrombosis that occurs in a minority of patients with sepsis or HIT, particularly when certain risk factors (“shock liver”, warfarin) compromise levels of natural anticoagulant proteins [24].
3. Hematologic abnormalities in COVID-19: prognostic implications
Hematologic abnormalities of COVID-19 include leukocyte counts (neutrophilia, lymphopenia), platelet counts (thrombocytopenia, thrombocytosis), and markers of hemostasis (elevations in PT and partial thromboplastin time (PTT), fibrin d-dimer and other fibrin-specific markers). Moreover, various prognostic implications of these abnormalities have been demonstrated.
3.1. Leukocyte abnormalities
Guan and colleagues [25] reported on almost 1100 patients diagnosed with COVID-19 in China. They evaluated admission laboratory findings, comparing these between patients with non-severe (N = 926) and severe (N = 173) pulmonary disease, and further comparing admission laboratory values among patients who subsequently developed one or more of the following (versus those who did not): death, mechanical ventilation, admission to ICU. Zhou et al. [26] similarly evaluated various admission laboratory abnormalities as risk factors for mortality in 191 COVID-19 patients, 54 of whom died.
Guan et al. found lymphopenia (<1.5 × 109/L) in 83% of COVID-19 patients, with a higher frequency among patients with severe versus non-severe disease (96% vs 80%, respectively) as well as a higher frequency among those who died, required mechanical ventilation or admission to ICU (93% vs 83%, respectively) [25]. Zhou et al. observed that 76% of non-survivors had a lymphocyte count <0.8 × 109/L on admission, versus only 26% of survivors; further, absolute lymphocyte counts tended to rise among survivors whereas they tended to decline further in non-survivors [26]. Although neither study reported on neutrophil levels, others have reported a high frequency of neutrophilia (approximately one-third of patients) in COVID-19 [27]; further, progressive increase in absolute neutrophil counts often occurs in non-survivors [28]. Fu and colleagues [29] found a high neutrophil/lymphocyte ratio to be associated with severe disease to a greater extent than other proinflammatory markers such as C-reactive protein (CRP) or procalcitonin.
For comparison, although HIT is considered a platelet activation disorder, neutrophilia is common, particularly in patients who develop thrombosis [30]. Acute neutrophilia can also accompany rapid-onset HIT post-intravenous heparin bolus administration [30].
3.2. Thrombocytopenia and thrombocytosis
Mild thrombocytopenia is a common feature of COVID-19. Guan and coworkers [25] found the median (IQR) platelet count on admission to be 168 (132, 207) × 109/L, with one-third having a platelet count below 150 × 109/L. The frequency of thrombocytopenia was higher in patients with severe versus non-severe COVID-19 (58% vs 32%). Zhou et al. [26] also found the admission platelet count to be significantly lower in non-survivors versus survivors (166 [107, 229] vs 220 [168, 271] × 109/L, respectively; P < 0.0001). Almost 20% of COVID-19 patients had thrombocytopenia on admission in another study [27].
Among survivors, the platelet count tended to rise during the second hospital week in one study, whereas among non-survivors, thrombocytopenia tended to persist or worsen [31]. Thrombocytosis (platelet count >450 × 109/L) was seen in 10% of patients on admission in a study of 5 Boston (Massachusetts) hospitals [32].
HIT features high thrombotic risk despite an oftentimes mild to moderate degree of thrombocytopenia. For example, the median platelet count nadir in HIT is approximately 55 to 70 × 109/L [[33], [34], [35]], with a high proportion of patients (~30–50%) with platelet count nadirs >100 × 109/L or even >150 × 109/L developing thrombotic events [36]. Perhaps, as in HIT, COVID-19 associated platelet count declines—even within the “normal” range—could portend progressive hypercoagulability and high thrombotic risk.
3.3. Coagulation abnormalities
Guan and workers [25] reported on admission fibrin d-dimer levels, observing that 46% of COVID-19 patients had levels above the upper-limit of the reference range (0.5 mg/L); further, the frequency of elevated d-dimer was higher in patients with severe (versus non-severe) disease (60% vs 43%), and higher in patients with severe illness (69% vs 44%). Zhou et al. [26] also found elevated d-dimer levels on hospital admission, with this representing a prognostic marker: 93% of non-surviving patients had a d-dimer over 0.5 mg/L on admission versus only 57% of survivors; using a 1.0 mg/L d-dimer cut-off, the mortality difference was even greater (81% vs 24%, respectively), a difference highlighted in the paper's abstract. Significantly higher d-dimer levels in critically-ill vs other COVID-19 patients were reported by others [27,28], with Wang et al. [28] and Li et al. [37] noting progressive increase in d-dimer levels among non-survivors.
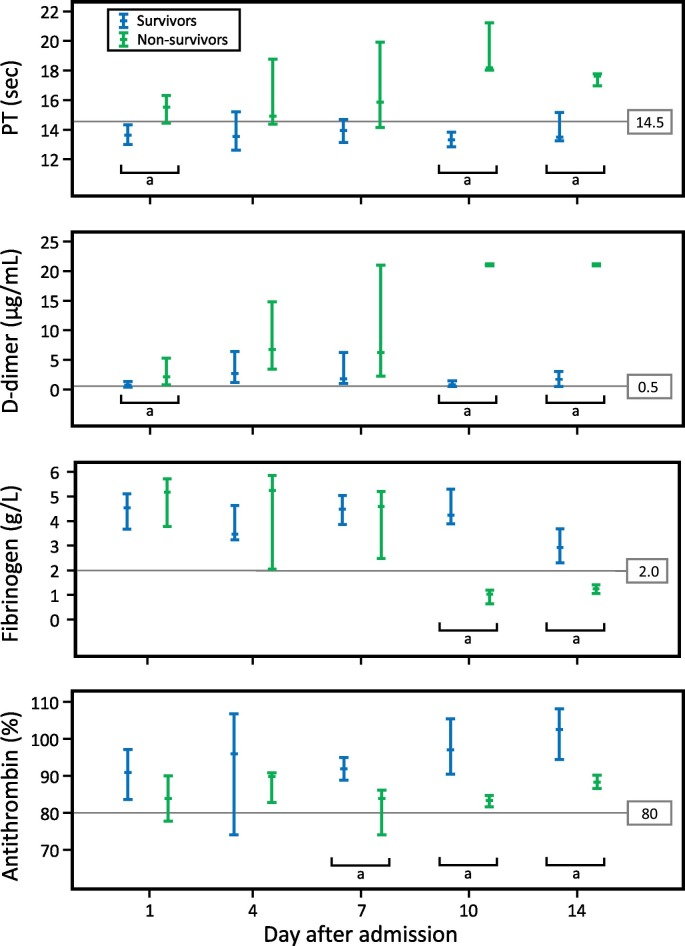
Tang and colleagues [38] analyzed various coagulation markers in their study of COVID-19 in Wuhan, China, including a comparison of initial values and subsequent changes in survivors versus non-survivors. Fig. 1 shows that d-dimer levels were higher on admission among non-survivors versus survivors (median [IQR]: 2.12 [0.77, 5.27] vs 0.61 [0.35, 1.29]; P = 0.001). Similar observations were made for fibrin degradation products.
Fig. 1.
Progression to overt DIC in patients with fatal COVID-19.
Timeline charts illustrate the changes in coagulation parameters in 183 patients with COVID-19 pneumonia (21 non-survivors, 162 survivors). The error bars show medians and 25% and 75% percentiles. The horizontal lines show the upper normal limits of prothrombin time (PT) and d-dimer, and the lower normal limits of fibrinogen and antithrombin activity.
aP < 0.05 for survivors versus non-survivors.
Reprinted from [38], with revisions, with permission.
Tang et al. [38] also found elevated PT values in approximately one-quarter of COVID-19 patients upon admission (Fig. 1). Remarkably, whereas approximately three-quarters of non-survivors had abnormal PTs on admission (in relation to normal range of 11.5 to 14.5 s), the opposite was true for survivors (median [IQR]: 15.5 [14.4–16.3] versus 13.6 [13.0–14.3], respectively; P < 0.001).
Tang et al. also found PTT levels to be prolonged (PTT reference range, 29.0–42.0 s), with trend to higher values in non-survivors versus survivors (median [IQR]: 44.8 [40.2, 51.0] vs 41.2 [36.9, 44.0], respectively; p = 0.096). Bowles and colleagues systematically investigated the cause for PTT prolongation in 35 COVID-19 patients [39]. Of 34 patients investigated for lupus anticoagulant, positive results were seen in 31 (91%). Further, factor XII levels tended to be low in COVID-19 patients (mean value, 55%), without any other factor deficiencies identified. Thus, as no bleeding tendency related to elevated PTT values would be expected, the authors argued that PTT prolongation per se should not be used to discourage anticoagulant prophylaxis or therapy. The authors were careful not to impute any prothrombotic implications to their findings.
Helms et al. also noted a high frequency of lupus anticoagulant in their COVID-19 patients admitted to ICU [40]. Harzallah and colleagues [41] found that 25/56 (45%) COVID-19 patients had lupus anticoagulant, with only 3 patients also testing positive for anticardiolipin or anti-β2-glycoprotein 1 antibodies. Heparin contamination did not appear to explain positive lupus anticoagulant testing [39,41]
Fibrinogen values are elevated—often markedly—in patients with COVID-19 [32,38,[42], [43], [44]]. This reflects the proinflammatory state, given that patients also have elevations in the other proinflammatory markers, procalcitonin [25,26,32], CRP [25,32], and ferritin [26,32]. Similarly, VWF levels are elevated in COVID-19 patients [40], often markedly [44]. ADAMTS13 levels tend to be normal [43] or mildly reduced [44], perhaps contributing to VWF-platelet microvascular thrombosis. Varatharajah and Rajah have speculated that endothelial-derived ultralarge VWF multimers could form large microthrombi “strings” comprised of platelets and large VWF complexes [45]. Pathologic evidence of complement deposition in lungs and skin suggests that vascular injury involves generalized activation of both alternative and lectin-based pathways [46].
Coagulation abnormalities are also a feature of severe HIT, including increase in PT [47], PTT [47,48], and fibrin d-dimer (with or without associated thrombosis) [49]. HIT-associated PTT prolongation in particular has therapeutic implications, as this phenomenon increases risk of “PTT confounding” with treatment failure resulting from systematic underdosing of PTT-adjusted anticoagulant therapy (argatroban, bivalirudin) [48]. Overt hypofibrinogenemia indicating decompensated DIC can also complicate HIT [47,50]. However, as discussed in the next section, typically elevated levels of fibrinogen in patients with HIT and COVID-19 complicate the diagnosis of DIC.
4. Does severe COVID-19 cause DIC?
A controversial issue is whether progressively severe COVID-19 causes DIC in the absence of another superimposed DIC trigger, such as complicating bacterial sepsis. d-dimer elevation alone does not necessarily indicate DIC, but can simply indicate presence of thrombosis, such as deep-vein thrombosis (DVT) or PE [51]. This is relevant given the association between COVID-19 and thrombosis (discussed subsequently). It has been suggested that progressive lung disease with associated alveolitis and in situ pulmonary microthrombosis could also explain elevated d-dimer in COVID-19 [52,53], perhaps as a result of high pulmonary levels of lung urokinase [54].
4.1. Progression to overt DIC in patients with fatal COVID-19
The most compelling study pointing to an association between COVID-19 and DIC is from Tang et al. [38] They reported a remarkable association between evolution to DIC in COVID-19 non-survivors versus survivors: whereas only 1/162 (0.6%) survivors met the International Society on Thrombosis and Haemostasis (ISTH) criteria for overt DIC [55], 15/21 (72%) non-survivors developed DIC. Fig. 1 shows progressive rise in PT and d-dimers, and decline in fibrinogen and antithrombin levels, in non-survivors versus survivors. Further, the DIC criterion, “thrombocytopenia” (yielding 1 point for platelet count fall to <100 × 109/L, and 2 points if <50 × 109/L) [55], was met by 12/21 (57%) non-survivors. Despite these striking findings, they do not rule out the possibility of superimposed bacterial sepsis (a common trigger of overt DIC), rather than COVID-19 progression per se.
Helms and colleagues' experience [40] argues against overt DIC occurring in many COVID-19 patients. They reported 150 COVID-19 patients in 4 French ICUs. Although their patients typically had elevated d-dimer levels, none met ISTH criteria for DIC. Moreover, these authors noted that COVID-19 d-dimer levels were typically lower than seen in matched patients with (non-COVID-19) ARDS. Similarly, Al-Samkiri [32] found only 3/400 (0.75%) COVID-19 patients met DIC criteria.
There are several practical difficulties in standardizing the definition of DIC. The PT prolongation criteria (in seconds) result in different INR categories in different hospital laboratories. Also, given numerous available d-dimer assays, standardization is problematic. One of the authors (T.E.W) uses d-dimer cutoffs of 2.0 and 10.0 mg/L to assign 2 points (moderate elevation, 2.0–9.9) or 3 points (≥10.0 mg/L), whereas Tang et al. [38] used cutoffs of 1.0 and 3.0 for assigning these categories. Nevertheless, DIC is usually characterized by marked consumption of coagulation factors—both procoagulant and anticoagulant—and this does not appear to occur in the majority of patients with COVID-19.
4.2. High-fibrinogen DIC
Clinicians often rule out DIC when fibrinogen values are normal or elevated. However, fibrinogen levels are often normal in patients who otherwise meet criteria for DIC [56]. Indeed, one author (T.E.W.) has observed “high-fibrinogen DIC” in some patients who develop symmetrical peripheral gangrene [57]. Such patients can have high fibrinogen levels on hospital admission reflecting several days of prodromal illness (e.g., initial Klebsiella pneumonia evolving to pneumosepsis). A similar phenomenon occurs in HIT—as approximately two-thirds of cases of HIT occur in postoperative patients [35,58]—featuring high postoperative fibrinogen values—occurrence of HIT can lead to fibrinogen consumption but with “normal” fibrinogen levels. Such “high-fibrinogen DIC” helps explain severe thrombotic events in patients with HIT and sepsis, and, potentially, in patients with COVID-19. However, progressive—usually marked—declines in platelet count are usually seen in patients with high-fibrinogen DIC associated with sepsis or HIT [35,57,58], and so absence of major platelet count declines in COVID-19 argues against this phenomenon.
5. Thrombosis in COVID-19
Thrombosis complicating COVID-19 is emerging as a major explanation for patient morbidity and mortality. Just as greater severity of HIT (judged by lower platelet count nadirs) corresponds to higher thrombosis frequency [33,34], so too with COVID-19, greater severity of illness (judged by need for ICU vs ward admission) is associated with greater frequency of thrombosis.
We identified 16 cohort studies (Table 2 ) [32,40,[59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73]] that quantified rates of thromboembolic disease during hospitalization, from which several observations emerge. Although stroke, myocardial infarction/acute coronary syndrome (MI/ACS) and limb gangrene are apparent, venous thromboembolism (VTE) dominates. All studies still had patients in hospital (1 study did not report) and therefore, the true rates of thromboembolic complications during hospitalization are not known. Some studies use cumulative rates adjusted for competing risk of death to estimate the true rate (although this could underestimate the true frequency of thrombosis if deaths were caused by unrecognized thromboembolism) [64]. The rate and type of VTE prophylaxis varies widely among the studies, with some reporting no prophylaxis, other utilizing standard-dose pharmacologic prophylaxis on the wards and intermediate-dose prophylaxis in the ICU, to others with a predominance of therapeutic-dose anticoagulation.
Table 2.
Proportion and rates of thromboembolic events in COVID-19.
| Study [reference] | Setting | Baseline anticoagulation | N | DVT screening | Hospitalized at analysis | Proportion with TE event | Cumulative rate of thromboembolic event | Thrombosis predictors | Adjusted analysis |
|---|---|---|---|---|---|---|---|---|---|
| Al-Samkari [32] | 5 hospitals in U.S. | Proph. (89%); interm./ther. (9%); none (3%) | 400 (ICU: 144 ward: 256) |
None | 37% | Total VTE: 24 (6%) ICU: 10% ward: 3.5% Total ATE: 11 (2.8%) ICU: 5.6% ward: 1.2% |
Not reported | d-dimer; platelet count; CRP | Yes |
| Criel [59] | 1 hospital in Belgium | Proph. (ward) Interm. (ICU) none (2%) |
82 (ICU: 30 ward: 52) |
All | 100% | Total DVT: 6 (7%) ICU DVT: 13% ward DVT: 4% |
Not reported | NR | NA |
| Cui [60] | 1 ICU in China | None | 81 | All | 11% | DVT: 20 (25%) | Not reported | Age; lymphopenia; PTT; d-dimer | No |
| Demelo-Rodríguez [61] | 1 hospital in Spain | Proph.; none (2%) | 156 | All | 100% | Total DVT: 23 (15%) 1 proximal DVT 22 distal DVT |
Not reported | d-dimer | Yes |
| Fraissé [62] | 1 hospital in France | Proph. (47%); ther. (53%) | 92 | None | 27% | Total TE: 39 in 37 pts. (40%) 19 PE 6 DVT 6 PE + DVT 2 stroke 1 ACS 2 limb ischemia 3 mesenteric ischemia |
Not reported | d-dimer; PT | No |
| Helms [40] | 4 ICUs in 4 French hospitals | Proph. (70%); ther. (30%) | 150 | Nonea | 67% | Total TE: 27 (18%) 25 PE 3 DVT 2 stroke/TIA 1 limb ischemia 1 mesenteric ischemia |
Not reported | OR 2.6 for TE events versus non-COVID-19 ARDS | Yes |
| Klok [63,64] | ICUs in 3 hospitals in The Netherlands | Proph./interm; ther. continued on admission (9%) | 184 | None | 35% | 65 PE 3 DVT 7 ATE |
Total (VTE + ATE): 57% (at 25 days); Adjusted for competing risk of death: 49% |
Age; PT; PTT; ther. anticoagulation | Yes |
| Llitjos [65] | 2 ICUs in France | Proph. (31%); ther. (69%) | 26 | All | 27% | Total VTE: 18 (69%) 12 DVT 6 PE |
Not reported | Ther. anticoagulation | No |
| Lodigiani [66] | 1 hospital in Italy | Proph./interm./ther.; none (25% of ward pts) |
388 (ICU: 61 ward: 327) |
None | 7%b | Total TE: 28 (7.7%) 16 VTE (10 with PE) 4 MI 9 stroke (1 stroke pt. also had PE) |
ICU: 27.6% (mdn, 18 days) Ward: 6.6% (mdn, 9 days) |
NR | NA |
| Maatman [67] | 3 ICUs in U.S. | Proph. | 109 | None | 6% | Total VTE: 31 (28%) 26 DVT 1 PE 4 DVT + PE |
Not reported | d-dimer; platelet count | No |
| Middeldorp [68] | 1 hospital in The Netherlands | Proph. (ward); proph./interm (ICU); ther. continued on admission (9.6%) | 198 (ICU: 75 ward: 123) |
Some in ICU | 8% | Total VTE: 39 (20%) Total ICU: 35 (47%) Total ward: 4 (3.3%) Total sympt. VTE: 25 (13%) ICU sympt. VTE: 21 (28%) Ward sympt. VTE 4 (3.3%) |
Total VTE: 42% Total ICU: 59% Ward sympt. VTE: NR At 21 days, adjusted for competing risk of death Total sympt. VTE: 25% ICU sympt. VTE: 34% Ward sympt. VTE: NR |
Neutrophil/ lymphocyte ratio; d-dimer; ther. anticoagulation |
Yes |
| Nahum [69] | 1 ICU in France | Proph. | 34 | All | 100% | Total DVT: 27 (79%) 9 Proximal DVT 23 Distal DVT |
NR | NR | NA |
| Ren [70] | 2 ICUs in China | Proph.; none (2%) | 48 | All | 100% | Total DVT: 41 (85.4%) 5 Proximal DVT 36 Distal DVT |
NR | NR | NA |
| Stoneham [71] | 2 hospitals in United Kingdom | NR | 274 | NR | NR | Total VTE: 21 (8%) 16 PE 5 DVT |
NR | WBC count; d-dimer; fibrinogen | Yes |
| Thomas [72] | 1 ICU in United Kingdom | Proph. | 63 | No | 44% | Total TE: 8 (13%) 5 PE 2 MI 1 DVT |
29% | NR | NA |
| Zhang [73] | 1 hospital in China | Proph.; none (63%) | 143 | All | 100% | Total DVT: 66 (46%) 23 Proximal DVT 43 Distal DVT |
NR | CURB-65 score; Padua score; d-dimer | Yes |
Abbr.: ATE, arterial thromboembolism; CT, computed tomography; DVT, deep-vein thrombosis; ICU, intensive care unit, Interm., intermediate-dose anticoagulation; NA, not applicable; NR, not reported; PE, pulmonary embolism; Proph., prophylactic-dose anticoagulation; pts., patients; sympt., symptomatic; TE, thromboembolic; Ther., therapeutic-dose anticoagulation; U.S., United States; VTE, venous thromboembolism.
Footnotes:
99 patients had computed tomography imaging per clinical suspicion.
TE proportions based on 362 patients discharged or died.
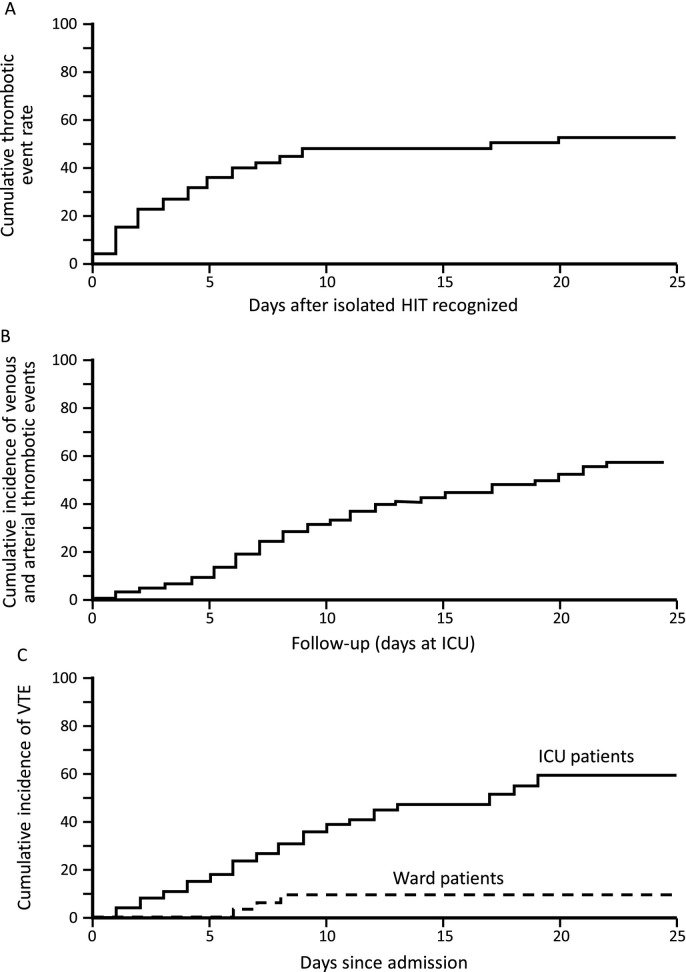
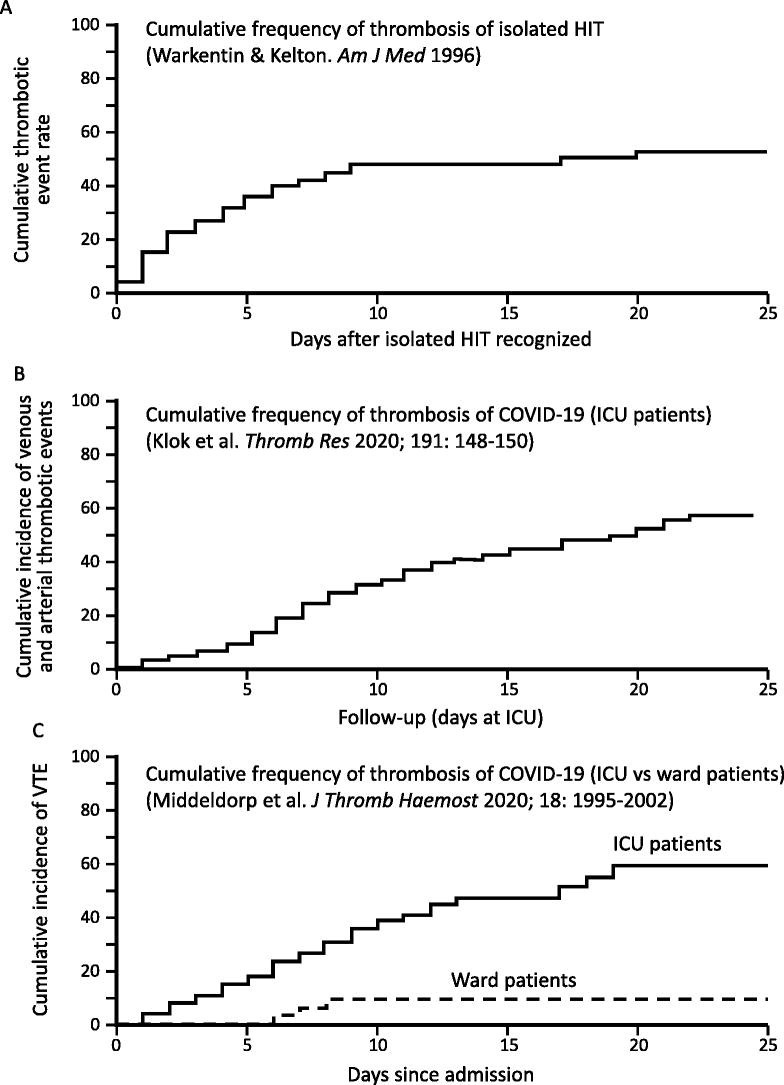
Fig. 2 suggests that the frequency of thrombosis in isolated HIT [58] could be similar to that observed in severe COVID-19 requiring ICU admission [63,64,68].
Fig. 2.
Cumulative thrombosis rates: HIT vs COVID-19 (ICU and ward patients).
A. Cumulative thrombosis frequency of isolated HIT (N = 62). Reprinted from [58], with modifications, with permission.
B. Cumulative thrombosis in ICU patients with severe COVID-19 (N = 184). Reprinted from [64], with permission.
C. Cumulative incidence of venous thromboembolism (VTE) in COVID-19: ICU patients (N = 75) versus ward patients (N = 123). Reprinted from [68], with permission.
5.1. Frequency estimates of thrombosis complicating COVID-19
Since none of the 16 studies reported rates exclusively for patients who had completed hospitalization (discharged alive or died), the true rate of in-hospital thromboembolism is unknown. The cumulative rate was estimated in 4 studies [64,66,68,72], each reporting different follow-up times, and results differ from crude rates because of competing risk (death) or longer follow-up in sicker patients than those discharged earlier. Nearly half the studies screened all patients for DVT [[59], [60], [61],65,[68], [69], [70],73] and the importance of asymptomatic DVT likely is not the same as clinically symptomatic disease. Some studies reported arterial thromboembolism in addition to VTE, with venous disease predominating. HIT also features venous thrombosis predominance, with venous:arterial thrombosis ratio of approximately 4:1 in two studies [35,58]. Similarly, venous thrombosis predominance is seen in COVID-19; however, it is less clear whether pulmonary “embolism” reflects DVT-source embolism or rather in situ pulmonary artery thrombosis (discussed subsequently).
5.2. Pulmonary embolism
Fatal COVID-19 outcomes could often reflect PE, as suggested by post-mortem studies of patients who have died of COVID-19. For example, Wichmann and colleagues [74] reported on twelve consecutive autopsies performed in Hamburg, Germany (minimizing selection bias since local law requires autopsies in patients dying with COVID-19). To their surprise, 7 of the 12 patients had previously unrecognized DVT identified at post-mortem, with 4 patients' deaths attributable to PE.
Faggiano and colleagues, in Italy, identified 7 patients with PE out of 25 COVID-19 patients admitted to their ICU over a 2-week period; 3 of the PE were already apparent at admission [75]. Poissy and colleagues [76], in France, identified 22 (21%) patients with PE among 107 consecutive patients admitted to ICU because of COVID-19. This high frequency of PE was approximately three-fold higher than that seen in the same time period one year earlier (6.1%), as well as in patients admitted during 2019 with influenza (7.5%).
Bompard and colleagues [77] found an overall 24% frequency of PE (segmental > proximal > subsegmental) in patients with COVID-19 pneumonia who underwent imaging because of clinical suspicion (including d-dimer elevation); the frequency was higher in ICU versus non-ICU patients (50% vs 18%). PE events occurred despite thromboprophylaxis in all patients including intermediate dosing in ICU patients. Similar findings were reported by Poyiadji and colleagues (Detroit, USA) [78], who found 72/328 (22%) COVID-19 patients investigated by pulmonary angiography to have confirmed PE.
Reported anecdotes have included asymptomatic or minimally symptomatic COVID-19 patients who presented abruptly with PE [79,80].
5.3. In situ pulmonary thrombosis
Diffuse alveolar disease can be complicated by pulmonary microthrombosis, irrespective of cause. For example, a 1983 study on ARDS reported a high frequency of pulmonary microthrombosis [81]. In COVID-19 ARDS, there also is evidence for in situ pulmonary artery thrombosis involving small vessels (pulmonary microthrombosis) as well as larger arteries [3,82,83].
Lax and colleagues [82] reported an 11-patient autopsy study from Switzerland which showed diffuse alveolar damage in 11 randomly-selected autopsy patients who died of COVID-19. In the authors' words, “Notably, the most striking and unexpected finding was the obstruction of pulmonary arteries by thrombotic material present at both the macroscopic and microscopic level in all cases. … The key finding of thrombosis in small to mid-sized pulmonary arteries was unexpected. On the basis of the occurrence of this finding in all patients, we assume that these thrombotic events were disease-related and were the immediate cause of death, through acute pulmonary hypertension and cessation of pulmonary circulation. … [w]e consider our findings to be caused by thrombosis rather than by thromboembolism, because most vessels were completely occluded by thrombotic material and small arteries were involved, with a diameter less than 1 mm.”
Menter and colleagues [83] reported on 21 autopsies performed on patients who died of COVID-19. They noted that “[i]n five of eleven cases where immunohistochemistry for fibrin was performed, microthrombi were detected in alveolar capillaries”; moreover, “[f]our cases presented with peripheral and prominent central pulmonary embolisms.”
Ackermann and colleagues [3] reported on the lung pathology of 7 patients who died of COVID-19. They found features of lung injury common also to influenza-associated respiratory failure, namely diffuse alveolar damage with lymphocytic infiltration, as well as precapillary vessel pathology (microthrombi in small pulmonary arteries with diameter of 1 to 2 mm); however, COVID-19 featured alveolar capillary microthrombi that were 9 times as prevalent as seen in control lungs with influenza respiratory failure.
Van Dam and colleagues [84] observed that the radiologic picture of PE features more peripheral (versus central) thrombosis, indirectly supporting a potential role for in situ thrombosis in the pathogenesis of pulmonary “emboli” in some COVID-19 patients.
5.4. Arterial thrombosis: stroke, myocardial infarction, limb artery thrombosis
Several reports on COVID-19 have focused on arterial thrombosis, either together or as separate target organ sites, such as MI/ACS, ischemic stroke, and limb artery thrombosis. Evaluation of the relative frequency of target organ involvement can provide interesting perspectives: for example, in HIT, the rank order of arterial thrombosis (limb artery > stroke > MI/ACS) [58] is inverse to usual atherothrombosis (MI/ACS > stroke > limb artery). The most common arterial thrombotic event in COVID-19 appears to be thrombotic stroke. Cantador and colleagues [85] reported on 14 patients in Spain who developed arterial thrombosis complicating COVID-19, representing about 1% of 1419 admitted patients: 8 patients had cerebrovascular events (6 strokes, 2 TIAs), 3 had MI/ACS, and 3 had limb artery thrombosis. Similar findings were made by Rey and colleagues [86]: of 38 COVID-19 patients who developed arterial thrombosis, 24 had cerebral thrombosis, 10 peripheral artery thrombosis, and 4 patients had coronary artery thrombosis. Fibrinogen and d-dimer levels were generally high, and none of the patients met criteria for DIC. Based on these studies, one can tentatively propose the hierarchy of arterial thrombosis in COVID-19 to be: stroke > limb artery > MI/ACS. Thus, in contrast to HIT, COVID-19 hypercoagulability may be associated with a greater relative risk of thrombotic stroke versus limb artery thrombosis.
Several studies have focused on strokes complicating COVID-19. In a Chinese report [87] that reviewed 214 consecutive patients hospitalized with COVID-19, the frequency of stroke was higher in patients with severe versus non-severe COVID-19 (5/88 [6%] vs 1/126 [1%], respectively). Oxley and coauthors [88] reported 5 cases of acute ischemic stroke involving large cerebral arteries in COVID-19 outpatients under the age of 50 observed during a two-week period in New York city (at most, 1 young patient would have been expected during this time period); most patients had relatively mild symptoms of COVID-19. Jain et al. [89] reviewed neuroimaging studies performed on hospitalized COVID-19 patients also in New York City; approximately 1% (35/3218) had stroke (large infarct, n = 17; lacunar infarct, n = 9; hemorrhagic stroke, n = 9). Another report from New York City by Merkler and coworkers [90] found that 31 (1.6%) of 1916 patients with COVID-19 had acute ischemic stroke, a frequency far higher than that observed (3/1486 = 0.2%) in patients with influenza; after adjustment for age, sex, and race, the likelihood of stroke was much higher with COVID-19 versus influenza (odds ratio, 7.6; 95%CI, 2.3–25.2).
Among other studies reporting on various thrombotic manifestations, the frequency of ischemic strokes was also relatively high (approximately 2–3%) in the studies of Klok [63,64] and Lodigiani [66]. The topic of cerebral venous sinus thrombosis (CVST) associated with COVID-19 is discussed later (see section 5.5 Unusual venous and arterial thromboses).
Peripheral limb artery thrombosis manifesting as limb ischemia with absent pulses is another potential complication of COVID-19, particularly in the critically ill. Bellosta and colleagues reported 20 patients who developed peripheral limb artery thrombosis after admission for COVID-19 pneumonia [91]. The frequency of acute limb ischemia was at least 5-fold greater than in the year earlier period, for which the authors proposed that COVID-19 produced a “virus-related hypercoagulable state.” Surgical revascularization was performed in 17 patients (3 were too ill), with successful outcomes infrequently seen. Mestres and colleagues, in Barcelona [92], described 4 patients who developed acute limb ischemia, 3 with infrapopliteal artery thrombosis in one or both limbs, and 1 patient with femoral-popliteal and radial-ulnar artery thromboses.
A clinical picture in keeping with symmetrical peripheral gangrene in COVID-19 ICU patients has also been reported [93]. Laboratory markers (falling platelet count, rising d-dimer levels to >20.0 mg/L, elevated PT) suggested possible overt DIC. However, the authors stated that the patients did not have elevated lactate levels and were not on vasopressors, thus somewhat unlike the typical patient with hemodynamic shock who develops DIC-associated ischemic limb injury [24]. Again, whether such patients have an unusual form of DIC that can lead to symmetrical peripheral gangrene or venous limb gangrene (as rarely occurs in severe HIT) [24] or whether these patients have superimposed bacterial sepsis is uncertain. In contrast, Helms et al. [40] noted that none of their 150 ICU patients with severe COVID-19 developed acral tissue necrosis.
Although MI/ACS in the conventional sense occur in a small minority of COVID-19 patients (discussed above), elevated cardiac biomarkers, such as troponin or creatine kinase MB, are commonly seen in COVID-19. One review of 26 studies estimated the prevalence of acute myocardial injury to be approximately 20% [94]. Plausible explanations for myocardial injury included: hyperinflammation/cytokine storm resulting in myocarditis; respiratory failure/hypoxemic cardiomyocyte injury; down-regulation of ACE2 expression with loss of protective signaling pathways in cardiac myocytes; hypercoagulability with resulting coronary microvascular thrombosis; “endotheliitis” involving cardiac vasculature; and inflammation and/or stress causing coronary plaque rupture or supply-demand mismatch with more conventional myocardial ischemia/infarction. Zhou and colleagues [26] found troponin levels >28 pg/mL in 17% of patients admitted with COVID-19, with almost half (23/50 [46%]) of patients with such elevated troponin levels dying versus patients with lower levels on admission (1/95 [1%]; P < 0.0001).
5.5. Unusual venous and arterial thromboses
Hypercoagulable states can be characterized by a propensity for unusual sites of thrombosis. This is also a feature of COVID-19, where patients have developed such unusual thrombotic events as cerebral venous sinus thrombosis [[95], [96], [97]], mesenteric artery thrombosis [[98], [99], [100]], aortic graft thrombosis [101] and mesenteric vein thrombosis [102]. However, unlike HIT, adrenal vein thrombosis with secondary adrenal infarction/hemorrhage has not been reported in COVID-19. As with HIT [103], catheter-associated upper-limb DVT can complicate COVID-19 [104].
5.6. Risk factors for thrombosis
Seven of the 16 studies in Table 2 reported adjusted analysis for laboratory thrombosis risk factors, with increased d-dimer the most consistent, identified in 5 studies. Al-Samkari and coworkers [32] found elevated d-dimer, platelet count and CRP at presentation were independent predictors of thrombosis. Adjusted odd ratios (OR) with 95% CI for d-dimer (expressed in ng/mL) were 3.04 (1.26–7.31) for d-dimer levels of 1001–2500 and 6.79 (2.39–19.30) >2500. Platelet count above 450 × 109/L had an adjusted OR of 3.56 (1.27–9.97) and CRP levels >100, OR 2.71 (1.26–5.86). Demelo-Rodríguez et al. [61] showed d-dimer levels were higher in patients with DVT found by ultrasound screening with median levels of 4527 (IQR 1925–9144) ng/mL vs 2050 (IQR 1428–3235) in patient without DVT (P < 0.001) with an adjusted OR = 9.8 (2.9–33.7). Klok identified prolongation of PTs of >3 s or PTT > 5 s as predictors of thrombotic complications, with adjusted hazard ratio 4.1 (1.9–9.1) [63].
The subdistribution hazard ratio for elevated median neutrophil-to-lymphocyte ratio was 1.7 (1.2–2.5) and 1.4 (1.1–1.9) for d-dimer in the study by Middeldorp [68]. Stoneham [71] found elevations in white blood cell count, fibrinogen and d-dimer to be associated with VTE with OR of 1.18, 1.66 and 1.39 respectively. d-dimer >1.0 at admission had an adjusted OR of 5.82 (1.42–23.81) in the study led by Zhang [73] for screen-detected DVT.
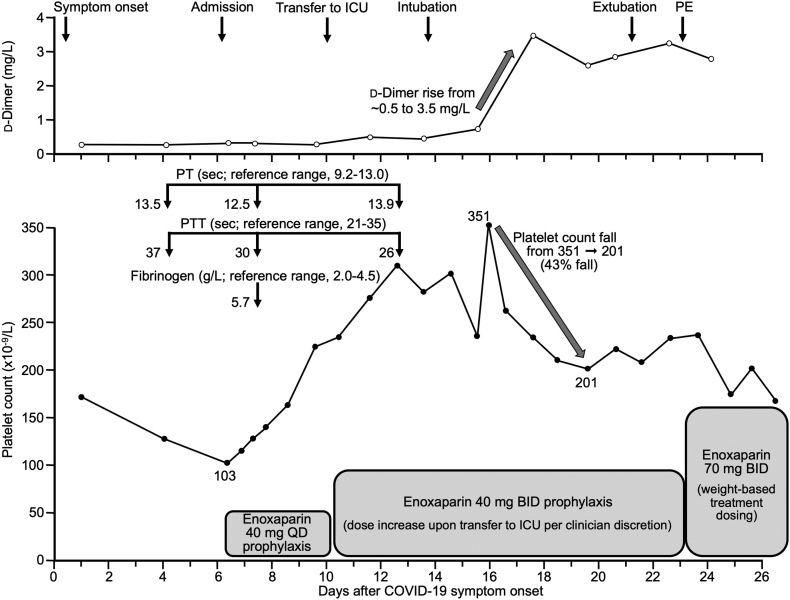
6. Illustrative case
Fig. 3 illustrates clinical events and serial laboratory values in a patient with severe COVID-19 requiring intubation/mechanical ventilation. Despite routine thromboprophylaxis with enoxaparin (40 mg/day) that was doubled (to 40 mg twice-daily) upon ICU admission, symptomatic PE (proven by CT angiography) occurred in association with right internal jugular central line removal post-extubation. The patient also received treatment with hydroxychloroquine, high-dose corticosteroids, tocilizumab, remdesivir, and COVID-19 convalescent plasma (listed in order received). There are several noteworthy features of the case. First, the patient exhibit mild thrombocytopenia (platelet count, 103 × 109/L) and mild PT elevation (13.5 s; reference range, 9.0–13.0) at hospital admission, common features of severe COVID-19. However, neither parameter showed progressive worsening as might be expected if the infection had progressed to overt DIC. Second, the patient exhibited a rapid increase in d-dimer (which was normal on admission) on day 18 despite receiving intermediate-dose enoxaparin. In hindsight, the abrupt d-dimer increase may have been a marker of development of otherwise subclinical DVT that resulted in symptomatic PE a few days later. And third, the patient also evinced a 43% decline in platelet count beginning about 10 days after starting enoxaparin thromboprophylaxis. It is possible that this platelet count fall, together with PE occurrence, reflects occurrence of HIT (not otherwise suspected nor investigated for).
Fig. 3.
Illustrative case of pulmonary embolism (PE) complicating COVID-19.
Hemostasis abnormalities were evident at onset of symptomatic COVID-19 (mild thrombocytopenia, minor increase in PT and PTT), with subsequent improvement; however, an abrupt increase in d-dimer and decrease in platelet count preceded occurrence of symptomatic PE. See text for additional clinical details.
Abbr.: BID, twice-daily; PT, prothrombin time; PTT, partial thromboplastin time; QD, once-daily.
7. HIT as a potential complication of COVID-19
HIT occurrence reflects not only exposure to heparin but also the clinical setting, heparin type, and duration of exposure. For example, HIT occurs more often with unfractionated heparin (UFH) than with low-molecular-weight heparin (LMWH) [105], more often in surgical than medical patients [106], and more often with exposure beyond 10 days than patients who receive 4 or fewer days [107]. Further, among immunized patients, higher heparin dosing can lead to greater frequency of HIT “breakthrough”, and so dosing increase from prophylactic to intermediate to therapeutic could also increase the HIT risk [108]. Inflammation is a risk factor for HIT [109], so it is possible that COVID-19 represents a high-than-usual risk for HIT than standard “medical” patients receiving thromboprophylaxis, especially as many COVID-19 patients are hospitalized for over 1 week.
There are anecdotal reports of HIT occurrence in COVID-19 patients. Riker and colleagues [110] reported 3 cases of putative HIT observed among only 16 intubated patients receiving UFH. However, although all 3 patients tested positive by PF4-dependent enzyme-immunoassay (EIA), only 1 patient tested positive by serotonin-release assay (SRA), so the true frequency of HIT in this report is uncertain.
Lingamaneni and coworkers [111] reported 1 case of confirmed HIT (DVT, thrombocytopenia after switching from prophylactic-dose LMWH to therapeutic-dose UFH, SRA-positive) among 5 COVID-19 patients investigated for HIT who tested EIA-positive (the other 4 patients with weak-positive EIA results tested SRA-negative). The authors emphasized the potential for HIT “overdiagnosis” if EIA-positive status alone is used to diagnose HIT.
Patell and coworkers [112] reported 5 patients with possible HIT—based upon suspicious platelet count decline and a positive latex immunoturbidimetric assay (LIA) for anti-PF4/heparin antibodies—among 88 COVID-19 patients who received at least 5 days of UFH (median, 11 days of UFH exposure). This corresponded to a cumulative incidence of LIA positivity of 12% at 25 days (95%CI, 4% to 26%). However, LIA reactivity was relatively weak (between 1.0 and 1.9 units/mL) in 4 of the 5 patients, which corresponds to a relatively low (20–30%) predictivity for SRA-positive status [113], and a clear positive SRA result was only observed in 1 patient (two patients had “borderline” SRA-positive results). One of the LIA-positive patients did not undergo SRA testing, but that patient's LIA result was strong-positive (>16 units/mL), which predicts for high (~90%) probability of HIT [113]. Although the true frequency of HIT in this study was unclear, the implication is that HIT is a definite potential complication of COVID-19 patients, particularly if there is prolonged exposure to UFH.
Given the importance of HIT ascertainment in this novel patient population, we recommend referral of EIA-positive or LIA-positive blood samples for testing by functional (platelet activation) assay, such as the SRA. However, given emerging data regarding occasional false-negative SRA results [114], we also suggest that SRA-negative blood samples (but with strong clinical suspicion of HIT corroborated by strong-positive EIA or LIA) be referred to a laboratory with experience in performing newer PF4-dependent platelet activation assays, such as the PF4-SRA [114], PF4/heparin-SRA [115], or the P-selectin expression assay [116].
Parzy and colleagues [117] reported 3 patients with laboratory-confirmed HIT among 13 critically-ill patients requiring venovenous extracorporeal membrane oxygenation (ECMO) with heparin anticoagulation. Since all 13 ECMO patients developed one or more venous thrombotic events, the contributory role of HIT in explaining thrombosis in this study is uncertain. Unfortunately, clinical and laboratory data supporting the HIT diagnoses were not provided.
A non-peer-reviewed paper from China found that non-surviving ICU patients frequently had progressive, severe thrombocytopenia [118]. Many of these patients were receiving LMWH. The authors found high levels of “HIT antibodies” by enzyme-immunoassay among ICU patients, and they speculated that HIT may have contributed to severe thrombocytopenia and adverse outcomes. In some patients, antibodies were detectable even before heparin treatment, raising the possibility of COVID-19-associated “spontaneous” HIT syndrome [119]. However, HIT antibody assays with higher specificity (e.g., SRA) were not performed, so the implications of these observations are uncertain.
Anticoagulant dosing in HIT offers an interesting perspective. Due to its (relative) rarity, heterogeneous clinical presentation, and (until recently) difficulty in achieving rapid real-time laboratory confirmation of a diagnosis of HIT, randomized trials evaluating different treatment approaches have not been available for HIT. Yet, the recognition of HIT as a profound hypercoagulable state, together with relevant observational studies, have led to the current treatment paradigm where—in the absence of contraindications—patients with HIT are typically treated with therapeutic-dose anticoagulation. An example of an observational study that informed this practice was one by Farner and colleagues, who reported their experience treating HIT with danaparoid [120]. Paradoxically, patients with HIT-associated thrombosis who were treated with danaparoid had a lower frequency of subsequent thrombosis than patients who had “isolated” HIT, i.e., HIT diagnosed on the basis of a platelet count fall rather than because of thrombosis occurrence that led to a diagnosis of HIT. The authors' explanation was that patients with HIT-associated thrombosis typically received therapeutic-dose danaparoid, whereas patients with isolated HIT were usually given lower (prophylactic-dose) danaparoid.
8. Prevention and treatment of thrombosis in COVID-19
There is wide variation in dosing of pharmacologic VTE prophylaxis in COVID-19 patients. Of interest is the observation that continuation of pre-hospital therapeutic-dose anticoagulation may have an important effect on reducing development of VTE. Klok and coworkers [64] noted that the risk of VTE in patients on therapeutic anticoagulation prior to ICU admission was lower in a competing risk model (HR 0.29 [95%CI, 0.091–0.92]), although no effect on mortality was seen. In another Dutch study, no patients receiving therapeutic anticoagulation prior to hospital admission developed VTE [68].
Paranjpe and colleagues studied 2773 patients hospitalized with COVID-19 within the Mount Sinai Health System (New York City), with 786 (28%) receiving therapeutic-dose anticoagulation at some point during hospitalization [121]. They used a Cox proportional hazards model to evaluate the effect of treatment-dose anticoagulation on in-hospital mortality, finding no difference. However, in patients who required mechanical ventilation (N = 395), in-hospital mortality was 29.1% (with a median survival of 21 days) for those treated with therapeutic-dose anticoagulation, as compared to 62.7% (with a median survival of 9 days) in patients who did not. In a multivariate proportional hazards model, longer duration of anticoagulant treatment was associated with reduced mortality (adjusted HR, 0.86 per day, 95%CI, 0.82–0.89, P < 0.001).
The effect of prophylactic-dose LMWH or UFH was evaluated in 449 Chinese patients with severe COVID-19, of whom 22% received an anticoagulant. There was no difference in 28-day mortality, however, in patients with a Sepsis Induced Coagulopathy (SIC) score ≥ 4 and who received prophylactic-dose heparin, mortality was lower (40.0% vs 64.2%, P = 0.029) [122]. These observations suggest anticoagulation may have a favorable effect on mortality in the sickest patients.
Several organizations have developed guidelines, guidance statements, answers to frequently asked questions, or state-of-the-art reviews (Table 3 ) [[123], [124], [125], [126], [127], [128]]. There is general agreement that all patients should receive, or be evaluated for, pharmacologic VTE prophylaxis; none recommend therapeutic-dose VTE prophylaxis unless done in the context of a clinical trial. Recommendations to use usual prophylactic- or intermediate-dose anticoagulation varies, as does dose adjustment for extremes of body weight, and some organizations differentiate approaches depending on whether the patient is in the ICU. Some statements recommend LMWH over UFH because of less patient exposure and personal protective equipment utilization. There is not strong agreement for extended prophylaxis post-discharge, but several groups recommend considering. Randomized trials are needed to address the best anticoagulant dose for VTE prevention, and we identified 9 proposed or ongoing trials comparing different doses of LMWH or UFH (Table 4 ).
Table 3.
Organizational advice and comments re: anticoagulation of COVID-19.
| Group | Anticoagulant dose recommended |
Pharmacologic and mechanical prophylaxis | Extremes of weight adjustment | Extended prophylaxis post-discharge | ||
|---|---|---|---|---|---|---|
| Prophylactic | Intermediate | Therapeutic | ||||
| Anticoagulation forum [123] | Ward | ICU | No | ICU | Yes | Case-by-case and low bleeding risk |
| International Society on Thrombosis and Haemostasis [124] | Ward, ICU | Ward (30% of respondents); ICU (50% of respondents) | No | ICU (60% of respondents) | Yes | LMWH (30% of respondents); DOAC (30% of respondents) |
| American College of Chest Physicians (ACCP) [125] | All | No | No | No | Not mentioned | No |
| Global COVID-19 Thrombosis Collaborative Group [126] | All | Insufficient data to consider | Insufficient data to consider | Not mentioned | Not mentioned | Not mentioned |
| American Society of Hematology [127] | Not specifically mentioned but implied for all | We encourage participation in clinical trials rather than empiric use of intermediate-dose heparin | We encourage participation in clinical trials rather than empiric use of therapeutic-dose heparin | Not generally recommended | May be used | It is reasonable to consider extended thromboprophylaxis after discharge using a regulatory approved regimen |
| National Institutes of Health [128] | Per standard of care for other hospitalized adults | There are currently insufficient data to recommend for or against the use of thrombolytics or increasing anticoagulant doses for VTE prophylaxis in hospitalized COVID-19 patients outside the setting of a clinical trial | Not mentioned | Not mentioned | Hospitalized patients with COVID-19 should not routinely be discharged on VTE prophylaxis. Using FDA-approved regimens, extended VTE prophylaxis can be considered in patients who are at low risk for bleeding and high risk for VTE as per protocols for patients without COVID-19. | |
Abbr: COVID-19, coronavirus disease, 2019; DOAC, direct oral anticoagulant; FDA, Food and Drug Administration; ICU, intensive care unit; LMWH, low-molecular-weight heparin; VTE, venous thromboembolism.
Table 4.
Clinical trials of anticoagulation for COVID-19.
| Trial | Identifier | Sponsor | Patients | Intervention | Comparison | Outcome | Timeframe |
|---|---|---|---|---|---|---|---|
| COVID-HEP | NCT0445848 | University Hospital, Geneva | 200 ward or ICU | Therapeutic enoxaparin or UFH | Prophylactic enoxaparin or UFH | Composite ATE, VTE, DIC and all-cause mortality | 30 days |
| X-covid 19 | NCT04366960 | Niguarda Hospital | 2712 ward | Enoxaparin 40 mg QD | Enoxaparin 40 mg BID | DVT by serial ultrasound and PE | 30 days |
| Rapid COVID COAG | NCT04362085 | St. Michael's Hospital, Toronto | 462 ward with D-dimer >2 time ULN | Therapeutic LMWH or UFH | Prophylactic LMWH, UFH or fondaparinux | Composite ICU admission, non-invasive positive pressure ventilation, mechanical ventilation or all-cause death | 28 days |
| Protect COVID 19 | NCT04359277 | NYU Langone Health | 1000 ward or ICU | Therapeutic LMWH (adjusted for obesity) or UFH | Prophylactic LMWH or UFH (adjusted for obesity) | Composite all = cause mortality, cardiac arrest, VTE, ATE, MI, stroke or shock | 30 days |
| ATTACC | NCT04372589 | University of Manitoba | 3000 or less (adaptive design) ward and ICU | Therapeutic LMWH or UFH | Usual care | Intubation and mortality | 30 days |
| CORIMMUNO-COAG | NCT04344756 | Assistance Pulique - Hopitaux de Paris | 808 ward and ICU | Therapeutic tinzaparin or UFH | Prophylactic LMWH or UFH | Survival without ventilation | 14 days |
| COVI-DOSE | NCT04373707 | Central Hospital, Nancy, France | 602 ward and ICU | Intermediate LMWH | Prophylactic LMWH | VTE and VTE related death | 28 days |
| IMPROVE | NCT04367831 | Columbia University | 100 ICU | Intermediate LMWH or UFH | Prophylactic LMWH or UFH | VTE and ATE | 30 days |
| HEP-COVID | NCT04401293 | Northwell Health | 308 ward and ICU | Therapeutic LMWH | Prophylactic or intermediate LMWH or UFH | VTE, ATE and all-cause mortality | 30 days |
Abbr.: ATE, arterial thromboembolism; BID, twice-daily; COVID-19, coronavirus disease, 2019; DIC, disseminated intravascular coagulation; DVT, deep-vein thrombosis; ICU, intensive care unit; LMWH, low-molecular-weight heparin; QD, once-daily; UFH, unfractionated heparin; VTE, venous thromboembolism.
CRediT authorship contribution statement
Theodore E. Warkentin: Contributing to the literature review, writing and editing drafts of the manuscript (primarily responsible for sections 1 through 4, inclusive, and section 7).
Scott Kaatz: Contributing to the literature review, writing and editing drafts of the manuscript (primarily responsible for sections 5, 6, and 8).
Declaration of competing interest
TEW has received royalties from Informa (Taylor & Francis) and lecture honoraria from Alexion Canada and Instrumentation Laboratory; has provided consulting services to Aspen Global, Bayer, CSL Behring, Ergomed, Instrumentation Laboratory and Octapharma; has received research funding from Instrumentation Laboratory; and has provided expert witness testimony relating to HIT and non-HIT thrombocytopenic and coagulopathic disorders.
SK, Scott Kaatz has received research funding from Janssen and Bristol-Myers Squibb, and has received consulting honoraria from Janssen, Bristol-Myers Squibb, and Portola.
References
- 1.Worldometer COVID-19. https://www.worldometers.info/coronavirus/ Available at.
- 2.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. (Epub 2020 Apr 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. (Epub 2020 May 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poncs S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CiV-2 infection. Crit. Care. 2020;24(1):353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuker A., Arepally G.M., Chong B.H., Greinacher A., Gruel Y., Linkins L.A., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood. Adv. 2018;2(22):3360–3392. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong B.H., Murray B., Berndt M.C., Dunlop L.C., Brighton T., Chesterman C.N. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood. 1994;83(6):1535–1541. [PubMed] [Google Scholar]
- 7.Rauova L., Hirsch J.D., Greene T.K., Zhai L., Hayes V.M., Kowalska M.A., et al. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116(23):5021–5031. doi: 10.1182/blood-2010-03-276964. (Epub 2010 Aug 19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gollomp K., Kim M., Johnston I., Hayes V., Welsh J., Arepally G.M., et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight. 2018;3(18):e99445. doi: 10.1172/jci.insight.99445. (eCollection 2018 Sep 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perdomo J., Leung H.H.L., Ahmadi Z., Yan F., Chong J.J.H., Passam F.H., Chong B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019;10(1):1322. doi: 10.1038/s41467-019-09160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madeeva D., Cines D.B., Poncz M., Rauova L. Role of monocytes and endothelial cells in heparin-induced thrombocytopenia. Thromb. Haemost. 2016;116(5):806–812. doi: 10.1160/TH16-02-0162. [DOI] [PubMed] [Google Scholar]
- 11.Hayes V., Johnston I., Arepally G.M., McKenzie S.E., Cines D.B., Rauova L., et al. Endothelial antigen assembly leads to thrombotic complications in heparin-induced thrombocytopenia. J. Clin. Invest. 2017;127(3):1090–1098. doi: 10.1172/JCI90958. (Epub 2017 Feb 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warkentin T.E., Hayward C.P.M., Boshkov L.K., Santos A.V., Sheppard J.I., Bode A.P., Kelton J.G. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84(11):3691–3699. [PubMed] [Google Scholar]
- 13.Kelton J.G., Sheridan D., Santos A., Smith J., Steeves K., Smith C., et al. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72(3):925–930. [PubMed] [Google Scholar]
- 14.Pouplard C., Iochmann S., Renard B., Herault O., Colombat P., Amiral J., Gruel Y. Induction of monocyte tissue factor expression by antibodies to heparin-platelet factor 4 complexes developed in heparin-induced thrombocytopenia. Blood. 2001;97(10):3300–3302. doi: 10.1182/blood.v97.10.3300. [DOI] [PubMed] [Google Scholar]
- 15.Kowalska M.A., Krishnaswamy S., Rauova L., Zhai L., Hayes V., Amirikian K., et al. Antibodies associated with heparin-induced thrombocytopenia (HIT) inhibit activated protein C generation: new insights into the prothrombotic nature of HIT. Blood. 2011;118(10):2882–2888. doi: 10.1182/blood-2011-02-335208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson I., Sarkar A., Hayes V., Koma G.T., Arepally G.M., Chen J., et al. Recognition of PF4-VWF complexes by heparin-induced thrombocytopenia antibodies contribute to thrombus propagation. Blood. 2020;135(15):1270–1280. doi: 10.1182/blood.2018881607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warkentin T.E. HIT: still stringing us along. Blood. 2020;135(15):1193–1194. doi: 10.1182/blood.2020005157.10.1182/blood.2020005157. Apr 9. [DOI] [PubMed] [Google Scholar]
- 18.Barnes B.J., Androver J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iba T T., Levy J.H., Wada H., Thachil J., Warkentin T.E., Levi M., et al. Differential diagnosis for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J. Thromb. Haemost. 2019;17(2):415–419. doi: 10.1111/jth.14354. (Epub 2019 Jan 7) [DOI] [PubMed] [Google Scholar]
- 21.Iba T., Levy J.H., Raj A., Warkentin T.E. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Clin. Med. 2019;8(5) doi: 10.3390/jcm8050728. pii: E728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.C.S. Whyte, G.B. Morrow, J.L. Mitchell, P. Chowdary, N.J. Mutch, Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19, J. Thromb. Haemost.. 18 (7) 1548–1555. doi: 10.1111/jth.14872. (Epub 2020 Jun 3). [DOI] [PMC free article] [PubMed]
- 23.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. (Epub 2020 Apr 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warkentin T.E. Ischemic limb gangrene with pulses. N. Engl. J. Med. 2015;373(7):642–655. doi: 10.1056/NEJMra1316259. [DOI] [PubMed] [Google Scholar]
- 25.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. (Epub 2020 Feb 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;95(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. ([Epub ahead of print] Correction in: JAMA Intern. Med. 2020 May 11. E201429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J., Kong J., Wang W., Wu M., Yao L., Wang Z., et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb. Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006. (Epub 2020 May 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui M., Sheppard J.I., Li N., Warkentin T.E. Neutrophil and monocyte counts in heparin-induced thrombocytopenia. Thromb. Haemost. 2019;119(6):941–951. doi: 10.1055/s-0039-1683913. [DOI] [PubMed] [Google Scholar]
- 31.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Samkari H., Leaf R.K., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warkentin T.E. Heparin-induced thrombocytopenia: pathogenesis and management. Br. J. Haematol. 2003;121(4):535–555. doi: 10.1046/j.1365-2141.2003.04334.x. [DOI] [PubMed] [Google Scholar]
- 34.Warkentin T.E. Drug-induced immune-mediated thrombocytopenia—from purpura to thrombosis. N. Engl. J. Med. 2007;356(9):891–893. doi: 10.1056/NEJMp068309. [DOI] [PubMed] [Google Scholar]
- 35.Gruel Y., Vayne C., Rollin J., Weber P., Faille D., Bauters A., et al. Comparative analysis of a French prospective series of 144 patients with heparin-induced thrombocytopenia (FRIGTIH) and the literature. Thromb. Haemost. 2020;120(7):1096–1107. doi: 10.1055/s-0040-1712957. [DOI] [PubMed] [Google Scholar]
- 36.Warkentin T.E., Roberts R.S., Hirsh J., Kelton J.G. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Arch. Intern. Med. 2003;163(20):2518–2524. doi: 10.1001/archinte.163.20.2518. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Zhao K., Wei H., Chen W., Wang W., Jia L., et al. Dynamic relationship between D-dimer and COVID-19 severity. Br. J. Haematol. 2020;190(1):e24–e27. doi: 10.1111/bjh.16811. (Epub 2020 Jun 9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients in novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. Epub 2020 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N. Engl. J. Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. (Epub 2020 May 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harzallah I., Debliquis A., Drénou B. Frequency of lupus anticoagulant in Covid-19 patients. J. Thromb. Haemost. 2020 May:29. doi: 10.1111/jth.14937. (doi: 10.1111/jth.14937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. (Epub 2020 Apr 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escher R., Breakey N., Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb. Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. (Epub 2020 May 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huisman A., Beun R., Sikma M., Westerink J., Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SRAS-CoV-2. Int. J. Lab. Hematol. 2020 May;22 doi: 10.1111/ijlh.13244. (doi: 10.1111/ijlh.13244. Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varatharajah N., Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge von Willebrand factor (eULVWF) decorated-platelet strings. Fed. Pract. 2020;37(6):e1–e2. (Epub 2020 May 15) [PMC free article] [PubMed] [Google Scholar]
- 46.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. (Epub 2020 Apr 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warkentin T.E. Heparin-induced thrombocytopenia in critically ill patients. Semin. Thromb. Hemost. 2015;41(1):49–60. doi: 10.1055/s-0034-1398381. [DOI] [PubMed] [Google Scholar]
- 48.Warkentin T.E. Anticoagulant failure in coagulopathic patients: PTT confounding and other pitfalls. Expert Opin. Drug Saf. 2014;13(1):25–43. doi: 10.1517/14740338.2013.823946. [DOI] [PubMed] [Google Scholar]
- 49.Chilver-Stainer L., Lämmle B., Alberio L. Titre of anti-heparin/PF4-antibodies and extent of in vivo activation of the coagulation and fibrinolytic systems. Thromb. Haemost. 2004;91(2):276–282. doi: 10.1160/TH03-07-0454. [DOI] [PubMed] [Google Scholar]
- 50.Warkentin T.E., Bernstein R.A. Delayed-onset heparin-induced thrombocytopenia and cerebral thrombosis after a single administration of unfractionated heparin. N. Engl. J. Med. 2003;348(11):1067–1069. doi: 10.1056/NEJM200303133481120. [DOI] [PubMed] [Google Scholar]
- 51.Hart R., Bate I., Dinh D., Elms M., Bundesen P., Hillyard C., Rylatt D.B. The detection of d-dimer in plasma by enzyme immunoassay: improved discrimination is obtained with a more specific signal antibody. Blood Coagul. Fibrinolysis. 1994;5(2):227–232. [PubMed] [Google Scholar]
- 52.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J.Thromb. Haemost. 2020 Jun 18 doi: 10.1111/jth.14975. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saba L., Sverzellati N. Is COVID evolution due to occurrence of pulmonary vascular thrombosis? J. Thorac. Imaging. 2020 Apr 28 doi: 10.1097/RTI.0000000000000530. (doi: 10.1097/RTI.0000000000000530. Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunt B.J., Levi M. Re the source of elevated plasma D-dimer levels in COVID-19 infection. Br. J. Haematol. 2020;190(3):e133–e134. doi: 10.1111/bjh.16907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) Thromb. Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 56.Takemitsu T., Wada H., Hatada T., Ohmori Y., Ishikura K., Takeda T., et al. Prospective evaluation of three different diagnostic criteria for disseminated intravascular coagulation. Thromb. Haemost. 2011;105(1):40–44. doi: 10.1160/TH10-05-0293. Jan. [DOI] [PubMed] [Google Scholar]
- 57.Warkentin T.E. Ischemic limb gangrene with pulses. 2015;373(24):2386–2388. doi: 10.1056/NEJMc1511750. [DOI] [PubMed] [Google Scholar]
- 58.Warkentin T.E., Kelton J.G. A 14-year study of heparin-induced thrombocytopenia. Am. J. Med. 1996;101(5):502–507. doi: 10.1016/s0002-9343(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 59.Criel M., Falter M., Jaeken J., Van Kerrebroeck M., Lefere I., Meylaerts L., et al. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur. Resp. J. 2020;56(1) doi: 10.1183/13993003.01201-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. (Epub 2020 May 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Marcías M., Toledo-Samaniego N., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb. Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. (Epub 2020 May 13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit. Care. 2020;24(1):275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. (Epub 2020 Apr 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. (Epub 2020 Apr 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. (Epub 2020 May 27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. (Epub 2020 Apr 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maatman T.K., Jalali F., Feizpour C., Douglas A., II, McGuire S.P., Kinnaman G., et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit. Care Med. 2020 May 27 doi: 10.1097/CCM.0000000000004466. (doi: 10.1097/CCM.0000000000004466. Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Middeldorp S., Coppens M., Van Happs T.F., Foppen M., Vlaar A.P., Müller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.M., et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren B., Yan F., Deng Z., Zhang S., Xiao L., Wu M., Cai L. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142(2):181–183. doi: 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- 71.Stoneham S.M., Milne K.M., Nuttal E., Frew G.H., Sturrock B.R., Sivaloganathan H., et al. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med (Lond) 2020;20(4):e76–e81. doi: 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K., et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. (Epub 2020 Apr 25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 74.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. A prospective cohort study. Ann. Intern. Med. 2020 May 6:M20–2003. doi: 10.7326/M20-2003. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faggiano P., Bonelli A., Paris S., Milesi G., Bisegna S., Bernardi N., et al. Acute pulmonary embolism in COVID-19 disease: preliminary report on seven patients. Int. J. Cardiol. 2020;313:129–131. doi: 10.1016/j.ijcard.2020.04.028. (Epub 2020 May 26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poissy J., Goutay J., Caplan M., Parmentier E., Duburq T., Lassalle F., et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 77.Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L., et al. Pulmonary embolism in patients with Covid-19 pneumonia. Eur. Resp. J. 2020;56(1) doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K., et al. Acute pulmonary embolism and COVID-19. Radiology. 2020 May 14:201955. doi: 10.1148/radiol.2020201955. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitali C., Minniti A., Caporali R., Del Papa N. Occurrence of pulmonary embolism in a patient with mild clinical expression of COVID-19 infection. Thromb. Res. 2020;192:21–22. doi: 10.1016/j.thromres.2020.05.002. (Epub 2020 May 5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polat V., Bostanci G.I. Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J. Thromb. Thrombolysis. 2020;50(1):239–241. doi: 10.1007/s11239-020-02132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomashefski J.F., Davies P., Boggis C., Greene R., Zapol W.M., Reid L.M. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am. J. Pathol. 1983;112(1):112–126. [PMC free article] [PubMed] [Google Scholar]
- 82.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koeblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020 May 14:M20–2566. doi: 10.7326/M20-2566. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 May 4 doi: 10.1111/his.14134. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Dam L.F., Kroft L.J.M., van der Wal L.I., Cannegieter S.C., Eikenboom J., de Jonge E., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease. Thromb. Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cantador E., Núñez A., Sobrino P., Espejo V., Fabia L., Vela L., et al. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J. Thromb. Thrombolysis. 2020 Jun 9:1–5. doi: 10.1007/s11239-020-02176-7. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rey J.R., Caro-Codón J., Pineda D.P., Merino J.L., Iniesta A.M., López-Sendón J.L., et al. Arterial thrombotic complications in hospitalized patients with COVID-19. Rev. Esp. Cardiol. (Engl Ed) 2020 May 23 doi: 10.1016/j.rec.2020.05.008. S1885-5857(20)30205-X. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. (Epub 2020 Apr 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain R., Young M., Dogra S., Kennedy H., Nguyen V., Jones S., et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J. Neurol. Sci. 2020 Jul 15;414:116923. doi: 10.1016/j.jns.2020.116923. (Epub 2020 May 19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., et al. Risk of ischemic stroke with coronavirus disease 2019 (COVID-19) vs patients with nfluenza. JAMA Neurol. 2020 Jul 2 doi: 10.1001/jamaneurol.2020.2730. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J. Vasc. Surg. 2020 Apr 29 doi: 10.1016/j.jvs.2020.04.483. S0741-5214(20)31080-6. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mestres G., Puigmacià R., Blanco C., Yugueros X., Esturrica M., Riambau V. Risk of peripheral arterial thrombosis in COVID-19. J. Vasc. Surg. 2020;72(2):756–757. doi: 10.1016/j.jvs.2020.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y., Cao W., Xiao M., Li Y.J., Yang Y., Zhao J., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28;41(0):E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [Article in Chinese] (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 94.Bavishi C., Bonow R.O., Trivedi V., Abbott J.D., Messerli F.H., Bhatt D.L. Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog. Cardiovasc. Dis. 2020 Jun 6 doi: 10.1016/j.pcad.2020.05.013. S0033-0620(20)30123-7. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur. J. Case. Rep. Intern. Med. 2020;7(5):001691. doi: 10.12890/2020_001691. (eCollection 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hemasian H., Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev. Neurol. (Paris) 2020;176(6):521–523. doi: 10.1016/j.neurol.2020.04.013. (Epub 2020 May 11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klein D.E., Libman R., Kirsch C., Arora R. Cerebral venous thrombosis: atypical presentation of COVID-19 in the young. J. Stroke Cerebrovasc. Dis. 2020;29(8):104989. doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vulliamy P., Jacob S., Davenport R.A. Acute aortic-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br. J. Haematol. 2020;189(6):1053–1054. doi: 10.1111/bjh.16760. (Epub 2020 May 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.E. Azouz, S. Yang, L. Monnier-Cholley, L. Arrivé, Systemic arterial thrombosis and acute mesenteric ischemic in a patient with COVID-19, Intensive Care Med. 46 (7) 1464–1465. doi: 10.1007/s00134-020-06079-2. (Epub 2020 May 18). [DOI] [PMC free article] [PubMed]
- 100.Beccara L.A., Pacioni C., Ponton S., Francavilla S., Cuzzoli A. Acute mesenteric thrombosis as a complication of SARS-CoV-2 infection. Eur. J. Case Rep. Intern. Med. 2020;7(5):001690. doi: 10.12890/2020_001690. (eCollection 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giacomelli E., Dorigo W., Fargion A., Calugi G., Cianchi G., Pratesi C. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19-related pneumonia. Ann. Vasc. Surg. 2020;66:8–10. doi: 10.1016/j.avsg.2020.04.040. (Epub 2020 Apr 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Barry O., Mekki A., Diffre C., Seror M., El Hajjam M., Carlier R.-Y. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol. Case Rep. 2020;15(7):1054–1057. doi: 10.1016/j.radcr.2020.04.055. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong A.P., Cook D.J., Sigouin C.S., Warkentin T.E. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049–3051. doi: 10.1182/blood-2002-05-1448. [DOI] [PubMed] [Google Scholar]
- 104.Bozzani A., Arici V., Franciscone M.M., Danesino V., Cascina A., Ticozzelli G., Ragni F. Severe acute respiratory syndrome coronavirus 2 infection and the upper limb deep vein thrombosis risk. Ann. Vasc. Surg. 2020;66:11–13. doi: 10.1016/j.avsg.2020.04.037. (Epub 2020 Apr 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martel N., Lee J., Wells P.S. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–2715. doi: 10.1182/blood-2005-04-1546. Oct 15. (Epub 2005 Jun 28) [DOI] [PubMed] [Google Scholar]
- 106.Warkentin T.E., Sheppard J.I., Sigouin C.S., Kohlmann T., Eichler P., Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937–2941. doi: 10.1182/blood-2005-11-012450. (Epub 2006 Jul 20) [DOI] [PubMed] [Google Scholar]
- 107.Warkentin T.E. In: Heparin-Induced Thrombocytopenia. 5th ed. Warkentin T.E., Greinacher A., editors. CRC Press; Boca Raton, FL: 2013. Clinical picture of heparin-induced thrombocytopenia; pp. 24–76. [Google Scholar]
- 108.Warkentin T.E., Sheppard J.I., Heels-Ansdell D., Marshall J.C., McIntyre L., Rocha M.G., et al. Heparin-induced thrombocytopenia in medical surgical critical illness. Chest. 2013;144(3):848–858. doi: 10.1378/chest.13-0057. [DOI] [PubMed] [Google Scholar]
- 109.Paparella D., Scrascia G., Galeone A., Coviello M., Cappabianca G., Venneri M.T., et al. Formation of anti-platelet factor 4/heparin antibodies following cardiac surgery: influence of perioperative platelet activation, the inflammatory response, and histocompatibility leukocyte antigen status. J. Thorac. Cardiovasc. Surg. 2008 Dec;136(6):1456–1463. doi: 10.1016/j.jtcvs.2008.06.014. (Epub 2008 Jul 24) [DOI] [PubMed] [Google Scholar]
- 110.Riker R.R., May T.L., Fraser G.L., Gagnon D.J., Bandara M., Zemrak W., et al. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res. Pract. Thromb. Haemost. 2020;4(5):936–941. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lingamaneni P., Gonakoti S., Moturi K., Vohra I., Zia M. Heparin-induced thrombocytopenia in COVID-19. J. Investig. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620944091. 2324709620944091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patel R., Khan A., Bogue T., Merrill M., Koshy A., Bindal P., et al. Heparin induced antibodies in COVID-19. Am. J. Hematol. 2020 Jul 13 doi: 10.1002/ajh.25935. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Warkentin T.E., Sheppard J.I., Linkins L.A., Arnold D.M., Nazy I. Performance characteristics of an automated latex immunoturbidimetric assay [HemosIL® HIT-Ab(PF4-H)] for the diagnosis of immune heparin-induced thrombocytopenia. Thromb. Res. 2017;153:108–117. doi: 10.1016/j.thromres.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 114.Warkentin T.E., Nazy I., Sheppard J.I., Smith J.W., Kelton J.G., Arnold D.M. Serotonin-release assay-negative heparin-induced thrombocytopenia. Am. J. Hematol. 2020;95(1):38–47. doi: 10.1002/ajh.25660. [DOI] [PubMed] [Google Scholar]
- 115.Vayne C., Guery E.A., Kizlik-Masson C., Rollin J., Bauters A., Gruel Y., Pouplard C. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin-induced thrombocytopenia antibodies. Br. J. Haematol. 2017;179(5):811–819. doi: 10.1111/bjh.14955. [DOI] [PubMed] [Google Scholar]
- 116.Padmanabhan A., Jones C.G., G C., Curtis B.R., Bougie D.W., Sullivan M.J., Peswani N., et al. A novel PF4-dependent platelet activation assay identifies patients likely to have heparin-induced thrombocytopenia/thrombosis. Chest. 2016;150(3):506–515. doi: 10.1016/j.chest.2016.02.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parzy G., Daviet F., Puech B., Sylvestre A., Guervilly C., Porto A., et al. Venous thromboembolism events following venovenous extracorporeal membrane oxygenation for severe acute respiratory syndrome coronavirus 2 based on CT scans. Crit. Care. Med. 2020 Jun 26 doi: 10.1097/CCM.0000000000004504. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu X., Zhang X., Xiao Y., Gao T., Wang G., Wang Z., et al. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-induced treatment. MedRxiv. 2020 doi: 10.1101/2020.04.23.20076851. (preprint, not peer-reviewed) [DOI] [Google Scholar]
- 119.Warkentin T.E., Basciano P.A., Knopman J., Bernstein R.A. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014;123(23):3651–3654. doi: 10.1182/blood-2014-01-549741. (Epub 2014 Mar 27) [DOI] [PubMed] [Google Scholar]
- 120.Farner B., Eichler P., Kroll H., Greinacher A. A comparison of danaparoid and lepirudin in heparin-induced thrombocytopenia. Thromb. Haemost. 2001;85(6):950–957. [PubMed] [Google Scholar]
- 121.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. (Epub 2020 May 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. (Epub 2020 Apr 27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moores L.K., Tritschler T., Brosahan S., Carrier M., Collen J.F., Doerschug K., et al. Prevention, diagnosis, and treatment of VTE in patients with COVID-19: CHEST guideline and expert panel report. Chest. 2020 Jun 2 doi: 10.1016/j.chest.2020.05.559. S0012-3692(20)31625-1. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. (Epub 2020 Apr 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
- 128.https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/