Abstract
Background & Objective:
Colonoscopy is widely recommended for colorectal (CRC) screening in the USA but evidence of effectiveness is limited. We examined if exposure to colonoscopy decreases the odds of incident CRC and death from CRC in Utah.
Methods:
We performed a case-control study of Utah residents, 54 and 90 y old, who received a CRC diagnosis from 2000 through 2010 (cases). Age and sex matched controls with no history of CRC (controls) were selected for each case. We determined receipt of colonoscopy 6 months to 10 y before the reference date for each case and control through administrative claims data. Colonoscopy exposure was compared by using conditional logistic regression.
Results:
We identified 5128 cases and 20,512 controls; 741 of cases (14%) and 5,715 of controls (28%) received a colonoscopy. Exposure to colonoscopy reduced the odds for a diagnosis of CRC: the odds ratios (OR) were 0.41 for any CRC (95% confidence interval [CI], 0.38–0.44), 0.58 for proximal colon cancer (95% CI, 0.51–0.65), and 0.29 for distal colon or rectal cancer (95% CI, 0.25–0.33). This finding was consistent among sexes, age groups, and cancer stages. Similarly, in a subgroup analysis, colonoscopy was associated with decreased odds of death from CRC (OR, 0.33; 95% CI, 0.28–0.39), in both the proximal colon (OR, 0.43; 95% CI, 0.34–0.55) and distal colon or rectum (OR, 0.23; 95% CI, 0.18–0.30).
Conclusion:
In the population of Utah, colonoscopy is associated with a large reduction in risk of new-onset CRC and death from CRC. This reduction in risk for CRC was greatest for the distal colon and rectum, with a more modest reduction for proximal colon cancer.
Keywords: colon cancer, detection, prevention, US, endoscopy, tumor, neoplasm
INTRODUCTION:
Colorectal cancer (CRC) is the fourth most common cancer in the United States and the second leading cause of cancer-related mortality in men and women1. It is considered one of the most preventable cancers, primarily through detection and removal of polyps. Adenomatous polyps are accepted as the precursor lesion for most colorectal cancer. Colonoscopy can detect and remove precursor lesions thereby preventing development of CRC or allowing diagnosis of patients at an earlier stage of cancer. Colonoscopy is one of the options for CRC screening in the United States (US) and is advocated by both lay and specialty organizations2, 3. Unlike flexible sigmoidoscopy where randomized controlled trials have shown that screening reduces the incidence and mortality from CRC4–7, comparable clinical trial data for colonoscopy are not yet available. Colonoscopy is widely endorsed largely on the basis of observational studies that have shown an association between colonoscopy and CRC incidence/mortality8–11. However, there is uncertainty about the effectiveness of colonoscopy in the proximal colon8, 12 and frequency of missed cancers in those who have recently undergone colonoscopy13–15. Most of these studies were conducted in countries with healthcare systems that differ from those of the United States. Importantly the Canadian studies may not accurately reflect practice in the USA since a substantial proportion of colonoscopies in their health system are performed by non-gastroenterologists8, 9. Studies completed solely in Veterans16, Medicare17 or health maintenance organization (HMO)11 populations may also not be generalizable to the US population and routine clinical practice – due to the older population examined in the VA or Medicare studies or nature of clinical care in an HMO.
To address some of these uncertainties regarding the protective effect of colonoscopy, we conducted a population-based case-control study to evaluate the association between colonoscopy utilization and odds for incident CRC and death from CRC in men and women, overall and separately for proximal and distal colon. The unique linkage between the Utah Population Database and Cancer Registry allowed us to confirm cancer diagnosis, ascertain nearly all colonoscopy procedures throughout the state and determine familial CRC history without ascertainment, referral or recall bias. Our secondary objectives were to determine the effectiveness of colonoscopy in different genders, age groups and cancer stages.
METHODS:
Study Design
This study was approved by the Institutional Review Boards of the University of Utah and Intermountain Healthcare (IHC) and by the Resource for Genetic and Epidemiologic Research (RGE; http://www.research.utah.edu/rge/), an administrative oversight board created to govern access to the Utah Population Database (UPDB).
We performed a population-based case-control study of the association between colonoscopy and incident CRC throughout the state of Utah. We measured the odds of exposure to colonoscopy in cases (patients diagnosed with CRC) and controls (persons without CRC) and calculated an odds ratio (OR) for exposure.
Data Sources
This study takes advantage of 3 unique linked data sources in Utah. The Utah Cancer Registry is a statewide cancer registry established in 1966 and since 1973 it has been part of the Surveillance, Epidemiology, and End Results (SEER) network of the National Cancer Institute (NCI) registries. State law requires that all cancer diagnoses be reported to the Utah Cancer Registry. The Utah Department of Health records information on all ambulatory, surgical and inpatient procedures performed in the state between 1996 and 2010, permitting identification of all colonoscopy procedures occurring in Utah. The UPDB, a genealogical database representing over 7.2 million unique individuals, was used to determine pathologically confirmed family history of CRC. The UPDB is linked to all three of these databases and also includes demographic information (from driver license data, birth/death certificates, voter registration and marriage/divorce/death certificates) and length of residence in Utah for all persons in the state. Previous demographic and genetic analyses have shown that the population recorded in the UPDB is genetically representative of US white and northern European populations with a low level of inbreeding18.
Identification of Cases
We identified cases from the Utah Cancer Registry (UCR) with a first diagnosis of colorectal adenocarcinoma between January 1, 2000 and December 31, 2010. We included only persons age 54 to 90 years so that all cases and controls would be in the screening-eligible age range during the exposure period. We excluded persons who had received a previous diagnosis of CRC, history of Crohn’s disease (ICD-9 code 555), ulcerative colitis (ICD-9 code 556) or molecular genetic diagnosis (confirmed APC mutation) of familial adenomatous polyposis (FAP) (see Supplementary Table 1 for codes utilized in the study). The diagnosis of FAP was determined by a linkage to our institutions hereditary gastrointestinal cancer registry, which cares for the vast majority of FAP families in the state (>620 FAP patients). The diagnosis of IBD was determined by linkage to our state-wide IBD registry, which encompasses all persons with an ICD-9 code for IBD in the state. By using the UPDB residence, driver’s license and voter registration records we could ensure all cases and controls lived in Utah from at least January 1, 1996 and had not emigrated during the time frame of the study. For secondary analysis, we stratified case patients by age; sex and site of primary CRC. The localization of the tumors, originally specified according to International Classification of Disease for Oncology was regrouped into proximal colon (cecum, ascending, colon, hepatic flexure, transverse colon), distal colon (splenic flexure, descending colon, sigmoid colon) and rectum (rectosigmoid junction and rectum).
For the subgroup analysis involving mortality, we limited CRC cases to those who died (both all cause and of a CRC related cause) between January 1, 2000 and December 31, 2010 using ICD-9 codes on their Utah death certificates.
Identification of Controls
From the UPDB we selected persons continually living in the state since at least January 1, 1996 without a diagnosis of CRC prior to the date of the matched case’s diagnosis of CRC (reference date). The available control population was also excluded for Crohn’s disease, ulcerative colitis or familial adenomatous polyposis as described above prior to control selection. Each case was matched to 4 controls according to age and sex. To assure comparable exposure time with cases, controls were assigned a time reference point that corresponded to the date of CRC diagnosis in their matched case subject (referred to as reference date). By matching for year of birth, case patients and controls had an equal period to be exposed to colonoscopy before the date of CRC diagnosis (reference date). The same controls were used for both the primary analysis involving incident CRC cases and the subgroup analysis involving cases who died from all cause and CRC related causes. See Flow Chart (Figure 1) for case/control selection.
Figure 1:
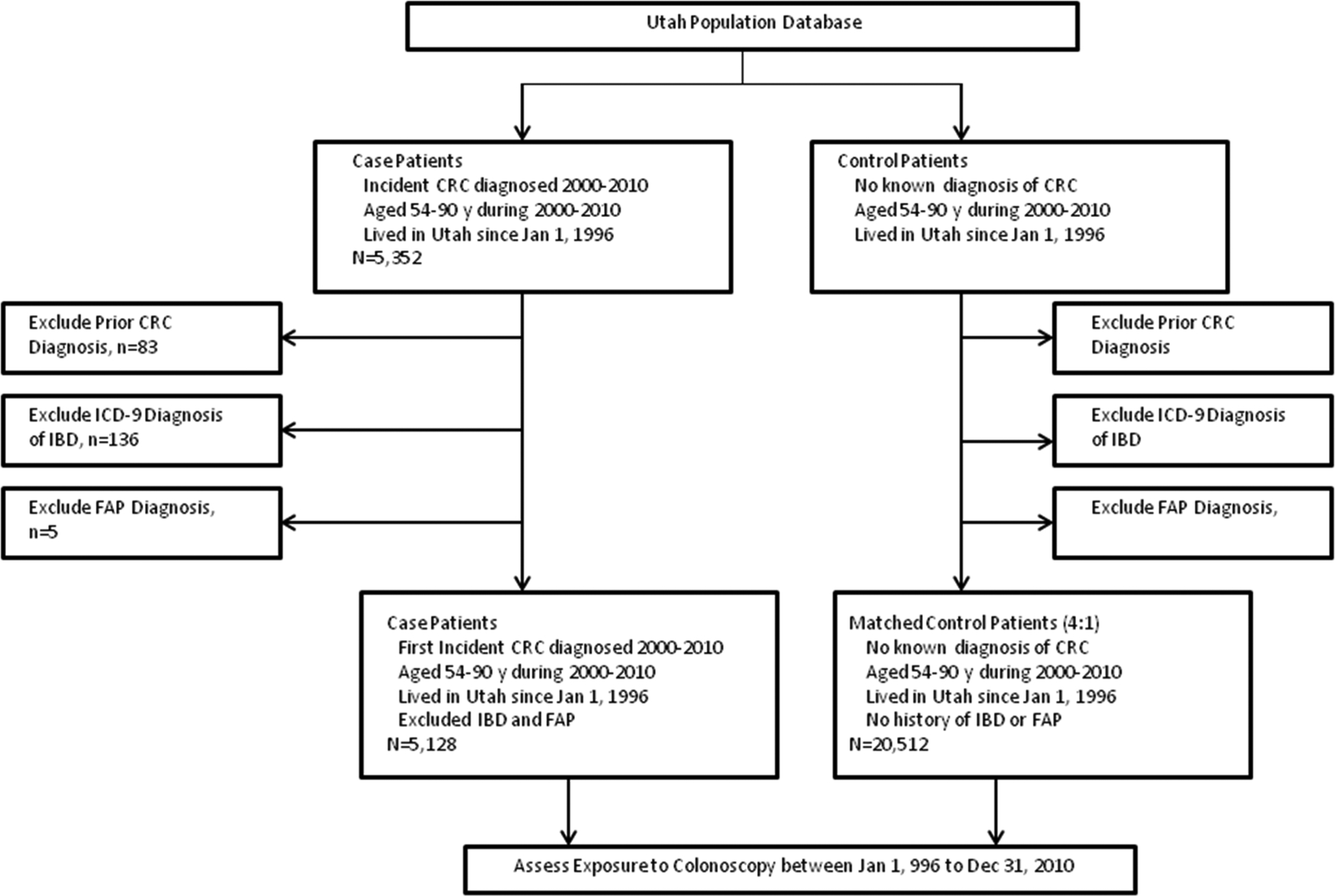
Study Flow Diagram
Determining Exposure
We identified any colonoscopy using ICD-9 and CPT codes (see Appendix Table 1 for codes utilized in the study) appearing on hospital (inpatient and outpatient), outpatient clinic and ambulatory surgical center records from the Utah Department of Health, between 10 years to 6 months before the date of CRC diagnosis/reference date. The exposure window started on January 1, 1996. To exclude colonoscopies performed to evaluate symptoms suggestive of CRC, confirm diagnosis or search for synchronous/metachronous tumors, we excluded procedures done with 6 months before the date of CRC diagnosis (reference date). Sensitivity analysis was completed by varying the time frame between CRC diagnosis date for cases (reference date for controls) and exposure to colonoscopy from 6 months to 12 months or 18 months. Every case and control patient had more than 48 months of potential exposure to colonoscopy while of screening eligible age. Exposure to colonoscopy was treated as a binary outcome: those who had at least one colonoscopy were considered exposed; for patient who had more than one colonoscopy the first colonoscopy was considered the exposure.
Covariates
We attempted to control for a family history of CRC and alcohol consumption or cigarette smoking as it relates to CRC risk. Family history of CRC in a first degree relative was determined from the unique linkage of genealogical and cancer registry records with the UPDB. Affiliation with the Church of Jesus Christ of Latter-day Saints (or Mormons) is associated with proscriptions against alcohol consumption and cigarette smoking and was used in the regression models to control for this behavior19.
Statistical Analysis
Demographic and clinical features of cases and controls were characterized by descriptive statistics. Conditional logistic regression, adjusting for family history of CRC and church affiliation status was performed to calculate the adjusted OR for the association between colonoscopy and incidence of CRC or mortality (both all cause and CRC related), with 95% CI. The study was designed with primary analysis for CRC incidence and a secondary (subgroup) analysis was performed to examine mortality. We repeated the analysis, stratified by sex, age (<65, >65), stage of cancer diagnosis and site of cancer diagnosis (proximal colon, distal colon, rectum). All analyses were done using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Patient Characteristics
We identified 5,128 cases with CRC and 20,512 controls without CRC. Table 1 shows the demographic characteristics of cases and controls; mean age was 71.6 years (standard deviation 9.7 years) and 52.2% of participants were male. Cases and controls were similar in health insurance coverage and geographical residence (urban/rural). As expected, cases (6.5%) were more likely to have had a family history of CRC in a first degree relative compared to controls (5.8%). Affiliation with the Church of Jesus Christ of Latter-day Saints was high for both cases and controls and is associated with proscription against alcohol consumption and cigarette smoking19. Colonoscopy data were available for a median of 111 months (range 48–179 months) prior to CRC diagnosis/reference date and 6,456 (25%) study participants had a colonoscopy more than 6 months prior to this reference date. The median time from colonoscopy to CRC diagnosis (reference date) was 53 months for cases and 48 months for controls. CRC was located in the proximal colon in 41.4%, distal colon in 27.9% and rectum-rectosigmoid colon in 27.6% of cases. The distribution of cancer stages is also shown in Table 1. For the subgroup analysis examining colonoscopy and death, 2,565 cases with CRC had died of any cause and 1,623 cases of CRC had died of malignancy by December 31, 2010.
Table 1:
Demographic and Clinical Characteristics of Case Patients and Control Patients
| Characteristic | Case Patients N (%) | Control Patients N (%) |
|---|---|---|
| Total, N | 5,128 (20%) | 20,512 (80%) |
| Age, n (%) | ||
| Mean (SD) | 71.6 (9.7) | 71.6 (9.7) |
| Range | 49–92 | 49–92 |
| <65 years | 1,401 (27.3) | 5,615 (27.4) |
| ≥65 years | 3,727 (72.7) | 14,897 (72.6) |
| Sex | ||
| Men | 2,676 (52.2) | 10,704 (52.2) |
| Women | 2,452 (47.8) | 9,808 (47.8) |
| Family History of CRC in First Degree Relative, n (%) | ||
| Yes | 334 (6.5) | 1,191 (5.8) |
| No | 4,794 (93.5) | 19,321 (94.2) |
| Church Affiliation1 | ||
| Non-LDS | 2,020 (39.4) | 7,595 (37.0) |
| LDS Church Affiliation | 3,108 (60.6) | 12,917 (63.0) |
| Median observation period for exposure before date of CRC/reference date (months) | 111 (48–179) | 111 (48–179) |
| >5 years of observation period for exposure before date of CRC/reference date, n (%) | 4,617 (90.1) | 18,468 (90.1) |
| Attempted colonoscopy >6 months before CRC diagnosis, n (%) | 741 (14.5) | 5,715 (27.9) |
| Median time from first observed colonoscopy to date of CRC diagnosis or reference date in months (Range), limited to those with colonoscopy | 53 (6–166) | 48 (6–171) |
| Residence Status, n (%) | ||
| Urban | 4,321 (84.3) | 17,701 (86.3) |
| Rural | 636 (12.4) | 2,587 (12.6) |
| Unknown | 171 (3.3) | 224 (1.1) |
| Health Insurance Status, n (%) | ||
| Government | 3,378 (65.9) | 12,969 (63.3) |
| Commercial | 1,633 (31.8) | 5,960 (29.1) |
| Other (self-pay, charity, workers compensation) | 48 (0.9) | 261 (1.3) |
| Unknown | 69 (1.4) | 1322 (6.3) |
| Site of Cancer at Diagnosis, n (%) | ||
| Proximal Colon | 2,124 (41.4) | NA |
| Cecum | 1,017 (19.8) | |
| Ascending colon | 625(12.2) | |
| Hepatic flexure | 176 (3.4) | |
| Transverse colon | 306 (6.0) | |
| Distal Colon | 1,432 (27.9) | |
| Splenic flexure | 99 (1.9) | |
| Descending colon | 212 (4.1) | |
| Sigmoid colon | 1121 (21.9) | |
| Rectum/Rectosigmoid | 1,414 (27.6) | |
| Unspecified | 158 (3.1) | |
| Stage | ||
| Localized (Stage 1) | 2,432 (47.4) | NA |
| Regional, direct extension only (Stage 2) | 464 (9.1) | |
| Regional with lymph nodes (Stage 3) | 1,242 (24.2) | |
| Distant metastasis (Stage 4) | 835 (16.3) | |
| Unknown stage | 155 (3.0) |
Affiliation with the Church of Jesus Christ of Latter-day Saints (LDS)
Association between Colonoscopy and Incident CRC
A total of 741 cases (14.5%) and 5715 controls (27.9%) underwent a colonoscopy between 6 months and 10 years before the CRC diagnosis/reference date. The rate of colonoscopy in cases varied by tumor site (19.3% proximal colon, 10.1% distal colon, 11.0% rectum-rectosigmoid). CRC cases were significantly less likely than controls to have undergone colonoscopy (adjusted conditional odds ratio [OR], 0.41; 95% CI: 0.38–0.44) (Table 2). The association differed by site of primary CRC and analysis stratified by anatomic location is summarized in Table 2. Colonoscopy was associated with a reduced odds of both distal and proximal CRC, but the effect was stronger for distal/rectum cancer (OR 0.29; 95% CI: 0.25–0.33) than for proximal CRC (OR 0.58; 95% CI: 0.51–0.65). In stratified analysis, the association between colonoscopy and the odds of CRC was slightly weaker in females (OR 0.46; 95% CI 0.41–0.52) compared to males (OR 0.36; 95% CI: 0.32–0.41) and individuals over age 65 years (OR 0.44; 95% CI: 0.40–0.49) compared to those younger (OR 0.33; 95% CI: 0.27–0.39). Colonoscopy was equally effective for both early stage (OR 0.43; 95% CI: 0.39–0.49) and late stage CRC (OR 0.38; 95% CI: 0.33–0.43) (Table 3).
Table 2:
Association between receipt of colonoscopy and colorectal cancer, 2000–2010
| Cancer Location | Endoscopic Test | Cases, n (%) | Controls, n (%) | OR (95% CI)* | P* |
|---|---|---|---|---|---|
| All | No Colonoscopy | 4387(85.6) | 14797 (72.1) | 1.00 | |
| Colonoscopy | 741 (14.5) | 5715 (27.9) | 0.41 (0.38–0.44) | <0.0001 | |
| Proximal Colon | No Colonoscopy | 1714 (80.7) | 6092 (71.7) | 1.00 | |
| Colonoscopy | 410 (19.3) | 2404 (28.3) | 0.58 (0.51–0.65) | <0.0001 | |
| Distal Colon/Rectum | No Colonoscopy | 2546 (89.5) | 8264 (72.6) | 1.00 | |
| Colonoscopy | 300 (10.5) | 3120 (27.4) | 0.29 (0.25–0.33) | <0.0001 | |
conditional logistic regression results
Table 3:
Results of analysis stratified by age and sex
| Odds Ratio (95% CI)* | |||
|---|---|---|---|
| Variable | All Cancer | Proximal Cancer | Distal Colon/Rectum Cancer |
| Stratified by Age | |||
| <65 | |||
| No endoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.33 (0.27–0.39) | 0.47 (0.36–0.62) | 0.25 (0.19–0.32) |
| ≥65 | |||
| No endoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.44 (0.40–0.49) | 0.61 (0.53–0.70) | 0.31 (0.26–0.36) |
| Stratified by Sex | |||
| Male | |||
| No endoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.36 (0.32–0.41) | 0.54 (0.45–0.65) | 0.27 (0.22–0.32) |
| Female | |||
| No endoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.46 (0.41–0.52) | 0.61 (0.52–0.72) | 0.32 (0.26–0.38) |
| Stratified by Stage | |||
| Early Stage (1) | |||
| No endoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.43 (0.39–0.49) | 0.62 (0.52–0.73) | 0.32 (0.27–0.37) |
| Late Stage (2–4) | |||
| No endoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.38 (0.33–0.43) | 0.54 (0.45–0.65) | 0.23 (0.18–0.29) |
conditional logistic regression results
A small number of cases (0.2%) and controls (0.9%) were exposed to flexible sigmoidoscopy between 6 months and 10 years prior to CRC diagnosis/reference date. Their exclusion from the primary analysis did not alter the primary outcomes.
Association between Colonoscopy and All-cause and CRC related Mortality
In subgroup analysis using CRC cases who died (all cause mortality and CRC related mortality) during the study window, only 12.8% of CRC cases who died of any cause and 11.5% of cases who died of CRC/malignancy underwent colonoscopy versus 25.3% and 26.2% of matched controls for each group respectively (Table 4). CRC cases who died (both all cause and CRC related) were significantly less likely than controls to have undergone colonoscopy (OR 0.40, 95% CI: 0.35–0.46 for all cause mortality; OR 0.33, 95% CI: 0.28–0.39 for CRC related mortality) (Table 4). Colonoscopy was associated with a reduced odds of death from all causes in both proximal and distal/rectum cancer, but the effect was stronger for distal/rectum (OR 0.27, 95% CI: 0.22–0.33) than for proximal CRC (OR 0.54, 95% CI: 0.45–0.65) (Table 5).
Table 4:
Association between receipt of colonoscopy and all cause and CRC related mortality, 2000–2010
| Endoscopic Test | Cases, n (%) | Controls, n (%) | OR (95% CI)* | P* | |
|---|---|---|---|---|---|
| All Cause Mortality | No Colonoscopy | 2236 (87.2) | 7667 (74.7) | 1.00 | |
| Colonoscopy | 329 (12.8) | 2593 (25.3) | 0.40 (0.35–0.46) | <0.001 | |
| CRC Related Mortality | No Colonoscopy | 1437 (88.5) | 4792 (73.8) | 1.00 | |
| Colonoscopy | 186 (11.5) | 1700 (26.2) | 0.33 (0.28–0.39) | <0.001 |
conditional logistic regression results, adjusted for church affiliation status and family history of CRC
Table 5:
Association between receipt of colonoscopy and all cause and CRC related mortality, stratified by tumor site
| All Cancer | Proximal | Distal Colon/Rectum | |
|---|---|---|---|
| All Cause Mortality | |||
| No Colonoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.40 (0.35–0.46) | 0.54 (0.45–0.65) | 0.27 (0.22–0.33) |
| CRC Related Mortality | |||
| No Colonoscopy | 1.00 | 1.00 | 1.00 |
| Colonoscopy | 0.33 (0.28–0.39) | 0.43 (0.34–0.55) | 0.23 (0.18–0.30) |
conditional logistic regression results, adjusted for church affiliation status and family history of CRC
Sensitivity Analysis
In sensitivity analyses, varying the time frame between CRC diagnosis date for cases (and the corresponding reference date for controls) and exposure to colonoscopy did not affect the main results when the preclinical period was set anywhere from 6 months to 18 months (Appendix Table 2 and Appendix Figure 1).
DISCUSSION
In this large population-based case-control study reflecting standard of care clinical practice in Utah, colonoscopy was associated with a significant reduction in incidence and death from CRC. Exposure to colonoscopy was associated with significant prevention of incident CRC in the proximal colon and distal colon/rectum. These findings were consistent for men and women, older and younger persons and for early and late cancer stage.
There have been several case-control and cohort studies evaluating the association between colonoscopy and incidence or mortality from CRC8–12, 16, 17, 20. These studies reported a reduced risk or odds of incident CRC or CRC mortality associated with colonoscopy ranging from 0.45 (95% CI: 0.20–0.98) in the Netherlands20 to 0.69 (95%CI: 0.63–0.74) in Ontario, Canada8 which are consistent with our findings. A notable finding in the early studies from Canada was that colonoscopy may not be protective against proximal bowel tumors compared to distal colon cancers8, 12. Though the magnitude of proximal CRC reduction in our study was lower than that for the distal colon and rectum, they were statistically significant. This protective effect in both the proximal and distal colon is consistent with recent studies from Germany10, the Veterans Affair Health System16, an analysis using Surveillance, Epidemiology, and End Results (SEER)-Medicare data17 and results from the Nurse’s and Health Professional’s study21.
Colonoscopy was first endorsed as a primary CRC screening modality in 1997 and it became a Medicare benefit in 2001 resulting in a considerable increase in colonoscopy screening22, 23. Prior studies conducted in populations from Canada (Ontario and Manitoba)8, 12 and Medicare beneficiaries17 were limited to a relatively short timeframe (mid 1990’s to early 2000) when colonoscopy had yet to become the preferred CRC screening procedure. Another important limitation of these prior studies is that a substantial proportion of colonoscopies are performed by non-gastroenterologists in Canada may not accurately reflect practice in the USA8, 9. Our study has the advantage of reporting on colonoscopy exposure starting in 1996 and up to 2010, which includes the period of time when colonoscopy for CRC screening has become widely endorsed, covered by most health insurance plans and widely available. Practice in Utah is also more consistent with US practice where cecal intubation is performed in >90% of procedures and gastroenterologists perform the vast majority of colonoscopies13.
Our study has several notable strengths. We took advantage of the unique linkage of the Utah Population Database to the Utah Cancer Registry to confirm cancer diagnosis and with the Utah Department of Health to ascertain nearly all colonoscopy procedures throughout the state. Linkage to the genealogical registry (UPDB) allowed assessment of familial CRC history without ascertainment, referral or recall bias. We were also able to examine the effect of colonoscopy on both incidence of CRC and death from CRC (both all cause and CRC related death), unlike other studies which have reported on only one outcome (incidence or mortality). Our study included all persons in the screening eligible age range as compared to prior Medicare and VA studies which were limited to an older population16, 17. Prior studies have had limited statistical power for subgroup analysis by primary tumor location since the number of cases with unspecified site have been high, ranging from 8% to 23%8, 17. The Utah Cancer Registry is a long-term prospective cancer registry with full coverage of the entire state population and has a very low rate of unspecified tumor site (3.1%). This is the largest population-based study, reflecting standard-of-care clinical practice in a large academic medical center, managed care organization, private hospitals and ambulatory surgical centers throughout the entire state of Utah. Health care systems outside the US have several important differences that may impact the generalizability of their findings to US practice. Inadequate performance (incomplete colonoscopy and procedures performed by non-gastroenterologists) has been suggested as a possible explanation for the weaker than expected protective effect of colonoscopy in studies from Canada8, 14, 15. Our results reflect the routine practice of colonoscopy throughout an entire US state, including academic, managed care and private practice settings. Hence, our results are more likely to be generally applicable than those from single academic centers with a referral center bias, health care systems outside the US or restricted populations such as Medicare beneficiaries (older individuals) or Veterans. Finally, the population of Utah is representative of US/European white populations with a low level of inbreeding18. Hence, the results are applicable to similar populations in the US.
The present study also has limitations. Observational studies are at risk of confounding and unmeasured bias which may threaten the validity of the findings. Although our study design matched for sex and age and assessed family risk for cancer, residual confounding by healthy behaviors may differ between those undergoing colonoscopy versus those that do not. Dietary factors, medication (NSAID use), body mass index were not available for inclusion in our analysis, nor was specific information regarding race, ethnicity or co-morbidities. The high rate of affiliation with the Church of Latter-day Saints is associated with low rates of smoking and alcohol use in this population and could also differ from other populations19. Our study design which utilized the same control group to examine both CRC incidence (primary analysis) and all-cause or CRC-related mortality (subgroup secondary analysis) may introduce bias due to the healthy nature of the controls. This design and bias is similarly present in prior studies examining Canadian8 and SEER-Medicare17 populations. Due to this potential bias our risk estimates for all cause and CRC related mortality associated with receipt of colonoscopy - which are very strong (OR 0.40 for all-cause mortality and 0.33 for CRC-related mortality) - may be potentiated in effect size slightly. Extraction of data from electronic medical records has limitations in the information that can be gathered. The indication for colonoscopy was not specifically ascertained to be a screening procedure and may also introduce bias. Many screening procedures are also coded for a diagnosis found at colonoscopy or other unrelated symptoms and thus it would be nearly impossible to establish a study population of only screening colonoscopies. This reflects gastroenterologists’ usual clinical practice of colonoscopy, with patients referred for screening as well as other indications. Similar to other studies we tried to minimize this limitation of non-screening colonoscopies completed for patient signs/symptoms by excluding procedures performed within 6 months of the CRC diagnosis. We did not have information on the documented completeness of the colonoscopy exam, quality of the bowel preparation or specialty of the physician performing the examination. We were also unable to determine exposure to fecal screening tests (such as FOBT or FIT).
In conclusion this large population-based study, encompassing care throughout the state of Utah, colonoscopy was effective in reducing the odds of developing CRC and death from CRC in men and women. Though the reduction in CRC risk was greatest for the distal colon and rectum, our results suggest that colonoscopy also has a major protective effect in the proximal colon.
Supplementary Material
Funding/Support:
Support for this project was provided by NCI grants P01-CA073992 (RWB), R01-CA040641 (RWB), an Endoscopic Research Award from the American Society for Gastrointestinal Endoscopy (NJS) and a junior faculty career development award from the American College of Gastroenterology (NJS). Partial support for the Utah Population Database and this project was provided by the Huntsman Cancer Institute Cancer Center Support Grant P30CA042014 from the National Cancer institute and the Huntsman Cancer Foundation. Support for the Utah Cancer Registry is provided by Contract #HHSN 261201000026C from the National Cancer Institute with additional support from the Utah Department of Health and the University of Utah.
Primary Funding Source: National Cancer Institute, American Society for Gastrointestinal Endoscopy, American College of Gastroenterology and Huntsman Cancer Foundation.
Role of the Funding Source: The study was funded by the National Cancer Institute, American Society for Gastrointestinal Endoscopy, American College of Gastroenterology and the Huntsman Cancer Foundation. The funding sources did not play a role in the design, conduct or reporting of the study or in the decision to submit the manuscript for publication.
Appendix Table 1:
Procedure and Diagnostic Codes
| Diagnosis | Codes |
|---|---|
| Colorectal Cancer | SEER primary site codes 180–189 (excluding appendiceal 180.1), 199, 209 |
| IBD | ICD-9: 555.x, 556.x |
| Cancer Site | |
| Proximal | SEER primary site codes 180–184 (excluding appendiceal) |
| Distal | SEER primary site codes 185–187 |
| Rectum | SEER primary site codes 199, 209 |
| Unknown Site | SEER primary site codes 188, 189 |
| Procedure | |
| Colonoscopy | ICD-9: 45.23, 45.25, 45.42, 45.43 CPT: 45378–45385 |
Appendix Table 2:
Influence of the exclusion window on the conditional odds ratio for the association between colonoscopy and incident CRC
| Exclusion Window (months) | % Exposed (control/case) | OR | 95% CI |
|---|---|---|---|
| 6 | 27.9/14.5 | 0.41 | 0.38–0.45 |
| 9 | 27.0/14.0 | 0.41 | 0.38–0.45 |
| 12 | 26.0/13.7 | 0.42 | 0.39–0.46 |
| 15 | 25.0/13.2 | 0.43 | 0.39–0.47 |
| 18 | 24.1/12.9 | 0.44 | 0.40–0.48 |
conditional logistic regression results, adjusted for church affiliation status and family history of CRC
Appendix Figure 1:

Influence of the exclusion window on the conditional odds ratio for the association between colonoscopy and incident CRC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: RWB is a consultant for Myriad Genetics and NJS is a consultant for Cook Medical Inc. No other authors have a conflict of interest to disclose.
Presentation: This study was presented in part at the GI Oncology Distinguished Abstracts Plenary session at Digestive Diseases Week (Chicago, IL) in May 2014.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104:739–50. [DOI] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–95. [DOI] [PubMed] [Google Scholar]
- 4.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. [DOI] [PubMed] [Google Scholar]
- 6.Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst 2011;103:1310–22. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Turner D, Xue L, et al. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA 2006;295:2366–73. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 11.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med 2013;158:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh H, Nugent Z, Demers AA, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010;139:1128–37. [DOI] [PubMed] [Google Scholar]
- 13.Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of Missed or Interval Colorectal Cancer and Patient Survival: A Population-Based Study. Gastroenterology 2014;146:950–60. [DOI] [PubMed] [Google Scholar]
- 14.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 2007;132:96–102. [DOI] [PubMed] [Google Scholar]
- 15.Singh H, Nugent Z, Demers AA, et al. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol 2010;105:2588–96. [DOI] [PubMed] [Google Scholar]
- 16.Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology 2014;146:718–725 e3. [DOI] [PubMed] [Google Scholar]
- 17.Baxter NN, Warren JL, Barrett MJ, et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol 2012;30:2664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorde LB. Inbreeding in the Utah Mormons: an evaluation of estimates based on pedigrees, isonymy, and migration matrices. Ann Hum Genet 1989;53:339–55. [DOI] [PubMed] [Google Scholar]
- 19.West DW, Lyon JL, Gardner JW. Cancer risk factors: an analysis of Utah Mormons and non-Mormons. J Natl Cancer Inst 1980;65:1083–95. [PubMed] [Google Scholar]
- 20.Mulder SA, van Soest EM, Dieleman JP, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a case-control study. Eur J Gastroenterol Hepatol 2010;22:437–43. [DOI] [PubMed] [Google Scholar]
- 21.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenck AP, Klabunde CN, Warren JL, et al. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev 2007;16:2118–27. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GS, Doug Kou T. Underuse of colorectal cancer screening in a cohort of Medicare beneficiaries. Cancer 2008;112:293–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


