Abstract
The rapid spread of the Coronavirus pandemic and its significant health and social impact urges the search for effective and readily available solutions to mitigate the damages. Thus, evaluating the effectiveness of existing vaccines like Bacillus Calmette–Guérin (BCG) has attracted attention. The aim of this review was evidence synthesis on the effect of BCG vaccine in preventing severe infectious respiratory disease including COVD-19, but not tuberculosis. We considered studies conducted on human participants of any study design from any country setting that were published in Enlgish. We did a systematic literature search in MEDLINE, Scopus and Google scholar databases and a free search on Google. The identified studies were appraised and relevant data were extracted using Joanna Briggs Institute tools. The extracted findings were synthesized with tables and narrative summary. Nine studies met the inclusion criteria. The findings indicated that BCG vaccine has a strong protective effect against both upper and lower acute respiratory tract infections. For instance in countries with universal BCG vaccination policy, the incidence of COVID-19 was lower compared to the counterparts. Addtionally, BCG vaccine was found to protect against infections like lethal influenza A virus, pandemic influenza (H1N1), and other acute respiratory tract infections. BCG improved the human body’s immune response involving antigen-specific T cells and memory cells. It also induced adaptive functional reprogramming of mononuclear phagocytes that induce protective effects against different respiratory infections other than tuberculosis. In countries with universal BCG vaccination, the incidence and death from acute respiratory viral infection including COVID – 19 is significantly low. However, there is an urgent need for further evidence from well-designed studies to understand the possible role of BCG vaccination over time and across age groups, its possible benefits in special populations such as health workers and cost-savings related to a policy of universal BCG vaccination.
Keywords: Bacillus Calmette–Guérin, Respiratory tract infections, Novel coronavirus (COVID 19)
1. Introduction
In 1924, Albert Calmette and Camille Guérin developed Bacillus Calmette-Guérin (BCG) [1], which serves as the only available vaccine against tuberculosis (TB) since then. BCG has a protection duration from 10 to 25 years [2], [3]. The world health organization (WHO) recommends a single birth dose of the vaccine in a setting with high risk of TB [4]. The introduction of the vaccine has resulted in a vastly positive effect in eliminating severe disease following TB infections especially among children [5].
Respiratory tract infections (RTIs) are infections that attack either the upper or the lower respiratory organs of an individual. Upper respiratory tract infections (URTIs) include laryngitis, common cold, acute rhinitis, pharyngitis/tonsillitis, acute rhinosinusitis and acute otitis media. Lower respiratory tract infections (LRTIs), on the other hand, include bronchiolitis, acute bronchitis, pneumonia and tracheitis [6]. Other infections attack both the upper and lower respiratory tract. Infections like different forms of Coronavirus attack the upper and lower airways [7]. These infections range from mild to severe, some are even fatal. Most of them are highly contagious and affect many people who are at risk [8], [9].
Various studies have implied that the BCG vaccine reduces severe diseases related to other infections other than TB [10], [11]. For instance, a review by Moorlag and his colleagues indicated that BCG vaccination improved antibody production against viral infections [11]. Furthermore, BCG reduces non-tuberculosis mycobacterial infections like leprosy and Buruli ulcer [12]. There is also evidence that shows BCG has a preventive effect on bladder cancer and atopic disorders including asthma [13], [14]. BCG vaccination minimized and eliminated morbidities and mortalities from various upper and lower respirator viral ifections [15], [16], [17]. We aimed to evaluate the effect BCG vaccination in preventing severe infectious respiratory diseases other than TB as evidence input to guide the strategy of COVID 19 prevention. Therefore the review was ‘What is the effect of BCG vaccination in preventing severe infectious respiratory diseases other than TB?’.
2. Methods
We developed the review protocol and get it registred on PROSPERO with registration number: CRD42020177274. Two reviewers conducted the literature search and selection of eligible publications. As a means of monitoring quality and consistency, the identified papers were appraised with the Joanna Briggs Institute (JBI) critical appraisal tool for each of the study design.
2.1. Inclusion criteria
-
•
Participants
Human with infectious respiratory disease. There was no exclusion based on age, geographic limit and type of respiratory infection.
-
•
Intervention
Vaccination with BCG.
-
•
Comparator
Not vaccinated with BCG.
-
•
Outcomes
Severity of infectious respiratory disease of URTIs or LRTIs, viral or any other form, except TB. There was no time limit to measure outcome of injection with BCG. Outcome of BCG on the participants was considered at any time from one injected with BCG vaccine strains.
2.2. Types of studies
In this review we included both experimental and quasi-experimental study designs including randomized controlled trials, non-randomized controlled trials, and before and after studies. Furthermore, analytical observational studies including cohort studies, case-control studies and analytical cross-sectional studies were inclued. Only studies published in English were included. We did not set a time limit for studies.
2.3. Search strategies
An iterative process was used to search the literature aimed to find both published and unpublished studies. To find potentially relevant articles, a comprehensive search with no date limits was performed in MEDLINE, and PubMed platforms followed by an analysis of the text words contained in the title and abstract and the index terms used to describe the article. A second search using all the identified keywords and index terms was undertaken across the same databases with no time limit. The search strategies used for the databases searched are detailed in Annex I. The search for unpublished studies/grey literature was performed in Google scholar and through the review of reference lists and input of content experts. Finally, the reference lists of all reports and articles selected for critical appraisal were searched for additional studies.
2.4. Study selection
The final selection was informed by the agreed PICO question. Studies identified by our search strategy were collated and uploaded into Mendeley [18] software and duplicates were removed. Titles and abstracts were screened by two independent reviewers for assessment against the inclusion criteria for the review. Full texts of potentially eligible studies were retrieved and assessed in detail against the inclusion criteria by two independent reviewers. Full-text studies that didn’t meet the inclusion criteria were excluded, and reasons for exclusion are provided in Annex II. Any disagreements that arose between the reviewers were resolved through discussion. The search of the literature was conducted between April and July 2020. All papers published until 9 July 2020 were considered. The search used the following keywords: “Effect”, “BCG vaccine”, “prevention”, “infectious”, “severe”, “respiratory disease”, “other than”, “tuberculosis”, “non-specific effect”. The search terms were used separately and in combination using Boolean operators like “AND”, “OR” and “NOT” (Annex I). The results of the search were reported in full in the final systematic review and presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.
2.5. Assessment of methodological quality
Studies meeting the inclusion criteria were assessed by two independent reviewers for methodological validity before inclusion in the review using standardized critical appraisal instruments from JBI for all types of studies [19], [20], [21], [22], [23]. Any disagreements that arose between the reviewers were resolved through discussion. All studies regardless of their methodological quality underwent data extraction and synthesis. The findings of the quality assessment were presented under Annex III.
2.6. Data extraction
Data from included articles were extracted using a standardized data extraction format from the Joanna Briggs Institute (JBI) [24], by two independent reviewers. The data extracted included specific details about the intervention, populations, study methods and outcomes of significance to the review question. For the outcome (prevalence), the data extraction format included primary author, publication year, country where the study was conducted, study area, study design, sample size, and prevalence with 95% CI. Any disagreements during the data extraction were resolved through discussion and consensus. Authors of papers were contacted to request missing or additional data where required.
2.7. Data synthesis
Pieces of evidences were synthesized descriptively meta-analysis was not feable because of the methodological and study subject heterogeneity of the included studies. Thus, variables were synthesized narratively and summarized as frequencies and proportions in tables.
3. Results
3.1. Study inclusion
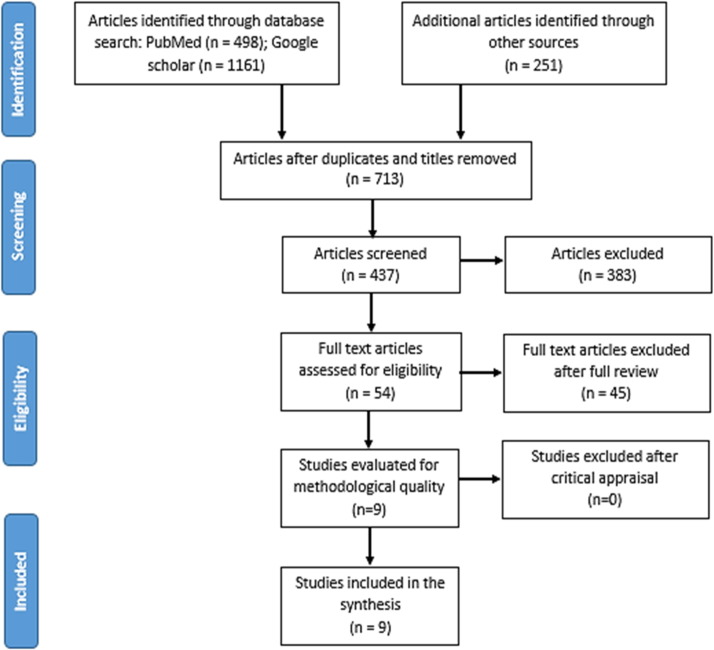
The intial comprehensive literature search yielded 19,010 articles which reported on the non-specific effect of BCG vaccination in preventing severe infectious respiratory diseases (Fig. 1 ). Of these, 1197 articles were excluded due to duplication and the unrelated topic of interest. From the remaining 713 articles, 276 articles were excluded after review of their titles and abstracts confirmed non-relevance to this review. Then, 437 articles were screened and among these, 383 were excluded from the reading of the abstracts. Therefore, 54 articles were retrieved for full-text review. Forty-five articles were excluded after full text review. Annex II lists the excluded articles with the rationale for exclusion.
Fig. 1.
PRISMA flow diagram of study selection and inclusion process.
3.2. Characteristics of included studies
The nine studies reviewed aimed to show effect of BCG vaccine on either URTIs or LRTIs, represented both in low and high income settings (Table 1 ). The study period ranged from 2005 to 2020.
Table 1.
Characteristics of reviewed studies.
| Author(s)/year | Objective | Study design | Country or setting |
|---|---|---|---|
| Escobar et al. 2020 | To examine BCG vaccine protection from severe coronavirus disease | Multinational data analysis (prevalence data) | 22 countries |
| Miller A et al, 2020 | To examine the correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19 | Multinational data analysis | Low income, middle-high income and high income countries |
| Leentjens et al., 2015 | To test that BCG vaccination could enhance immune responses to influenza vaccination | Double blinded randomized controlled trial | The Netherlands |
| De Castro, Pardo-Seco and Martinón-Torres, 2015 | To assess difference in hospitalization rate due to selected pathologies between BCG-vaccinated and non-BCG-vaccinated children < 15 years | Retrospective epidemiological and hospital-based surveillance (prevalence data) | Spain |
| Hollm-Delgado, Stuart and Black, 2014 | To determine whether Bacille Calmette-Guerin (BCG) vaccination is linked to the risk of acute lower respiratory infection (ALRI) among children < 5 years of age | Multinational data analysis based of DHS data (prevalence data) | 37 countries |
| Kleinnijenhuis et al., 2012 | To explore the mechanisms of the enhanced immune function induced by BCG both in vitro and in vivo | Experimental | Netherlands |
| Wardhana et al., 2011 | To assess the efficacy of BCG vaccinations for the prevention of acute respiratory tract infection. | Experimental prospective study | Indonesia |
| Roth et al., 2005 | To identify possible differences in the major causes of death for children with and without a BCG scar | Cohort | Guinea-Bissau |
| Stensballe et al., 2005 | To assess whether BCG vaccination is associated with a reduced risk of developing ALRI, caused by RSV or not | Matched cases-control | Guinea-Bissau |
3.3. Review findings
Respiratory tract infections attack one or more parts of the respiratory organs of an individual. Most of them are caused by either virus or bacteria. Some of them attack mostly the lower part of the respiratory tract and the others may affect the upper part and some both [9], [25]. The infection can vary from mild to severe. Although there are infections that can be treated or prevented effectively, there are not effective vaccine or treatment for some [26], [27], [28]. Although it has been used to develop antibody against mycobacterium Tuberculosis, according to studies BCG vaccine has a non-specific preventive effect on respiratory tract infections other than TB [11], [29].
3.3.1. Effect of BCG vaccine beyond TB
Bacillus Calmette–Guérin (BCG) vaccination was found to have a strong relationship with on COVID-19 disease burden and mortality: 60 individuals per 1 million people were infected in countries with universal BCG vaccination compared with 265 per 1 million people in countries with no BCG vaccination policy. Miller et al. [30] showed that countries without universal BCG vaccination like Italy, Netherland and the USA have been more severely affected with COVID-19 compared to countries with universal and long-lasting BCG vaccination. Most of the low-income countries with universal BCG vaccination had no reproted COVID-19 related deaths. In 55 countries with universal BCG policy, mortality from COVID-19 was less than one individual per 1 million people compared to 16 per 1 million in countries where BCG vaccination is not universal. The starting time of universal BCG policy was also found to affect death caused by COVID- 19. Those late starter countries were highly affected in this regard. For instance Iran has declared universal BCG policy in 1984. The death in this country is among the highest (Table 2 ).
Table 2.
Summary of studies on the effect of BCG vaccination on infectious respiratory tract infections.
| Author, year | Target population | Outcome | Key findings |
|---|---|---|---|
| Escobar et al. 2020) [31] | All age group human | Death with COVID – 19 | Every 10% increase in the BCG index was associated with a 10.4% reduction in COVID-19 realted mortality |
| Miller A et al, 2020 [30] | All age group human | Infection and death with COVID – 19 | Countries without universal policies of BCG vaccination have been more severely affected with COVID – 19 compared to countries with universal and long-lasting BCG policies. |
| Leentjens et al., 2015 [32] | Human population | Pandemic influenza A (H1N1) | Combined vaccination of BCG and influenza improved immunity against pandemic influenza A (H1N1) |
| Hollm-Delgado MG. et al., 2014 [16] | Children < 5 years of age | Acute lower respiratory infection (ALRI) | Children vaccinated with BCG had a significantly lower risk of suspected ALRI. BCG vaccination was associated with a 17% to 37% risk reduction for suspected ALRI. |
| Stensballe LG. et al., 2005 [33] | Infants | Acute lower respiratory tract infection | BCG vaccination has a non-targeted protective effect against ALRI, the effect being most marked in girls. |
| Wardhana et al., 2011 [29] | Humans, 60 and 75 years | Acute URTIs respiratory tract infection | BCG vaccine has a protective effect on AURTI |
Another global study in 22 countries by Escobar et al. [31] has strangthend this argument with an imperical analysis. The study categorize countries in to three groups including countries with current BCG policy, interrupted BCG vaccination and never have BCG policy. The findings indicated that countries with current BCG vaccination have lower mortality of COVID – 19 as compared to those with interapted vaccine and never had BCG vaccine. Moreover, they found a negative association between mean BCG coverage and death per one million population. The study generally shown there is a 10.4% reduction in COVID – 19 related deaths for every 10% increase in the BCG index (Table 2).
An experimental study by Leentjens et al. [32] has shown that BCG vaccination significantly enhanced antibody which protects the human body from the 2009 pandemic influenza A (H1N1). In BCG-vaccinated subjects, hemagglutination inhibiting (HI) antibody responses against the 2009 pandemic influenza A (H1N1) vaccine strain were significantly enhanced, compared with the placebo group, and there was a trend toward more-rapid seroconversion. At the baseline, there was no significant difference between BCG vaccinated and unvaccinated participants. Afterward, those in the experimental group developed a significantly higher HI antibody to protect against H1N1 influenza (Table 2).
Another experimental study by Hollm-Delgado et al. [16] revealed that BCG vaccination has a protective effect against acute lower respiratory infection (ALRI). They have tested that BCG vaccination reduced the risk of suspected ALRI by 17% to 37%. Age at vaccination was a major factor that created variation in BCG’s protective effect on ALRI. If the vaccination is given for those less than or equal to three months at the time of vaccination, it has a better protective effect. When the BCG vaccine was given before diphtheria-tetanus-pertussis (DTP) it was found to have a better protective effect than with or after DTP (Table 2).
Stensballe et al. [33] have shown that BCG vaccination has a non-targeted protective effect on minimizing the risk of ALRI among children. The effect was found to be even better among girls. Among BCG unvaccinated children, 9.3% were caught by ALRI while only 4.4% were BCG unvaccinated from healthy infants. ALRI case infants were about three times more likely to be unvaccinated as compared to ALRI negative infants. Stensballe et al. [33] specifically looked at the effect of BCG vaccination on URTIs. The study has showed that BCG vaccination has a protective effect against acute upper respiratory tract infection (AURTI). In comparison between two groups in the period of the study, it was uncovered that there was a significant reduction in the prevalence of AURTI in the BCG group. There was also a significant increase in IFN-γ and IL-10 levels in the BCG group compared to the non-BCG (placebo) group. However, the change in pre- and post- BCG on IFN-γ and IL-10 levels was insignificant. The increase of the IFN-γ level was positively and significantly correlated with the increase of the infiltrate and scar diameters in the BCG group (Table 2).
De Castro MJ et al.[34] found out that BCG vaccination at birth may decrease hospitalization due to respiratory infections not related to TB through heterologous protection. They showed that 90% of admissions due to respiratory infection not attributable to TB occurred in children under 4 years old. Only 11 028 (2.5%) of the cases were in BCG-vaccinated children which was significantly lower compared with in non-BCG-vaccinated children giving total prevention fraction of 41.4% (Table 3 ).
Table 3.
The effect of BCG on respiratory tract infection related consequences.
| Author, year | Target population | Outcome | Result |
|---|---|---|---|
| De Castro MJ et al, 2015 [34] | Children < 15 years of age | Hospitalization Due to Respiratory Infection other than TB | BCG vaccinated children, hospitalization rates related to respiratory infection were significantly lower than in non-vaccinated children |
| Kleinnijenhuis et al., 2012 [36] | People between 20 and 36 years of age | Induction of trained immunity and non-specific protection from infections | Among BCG vaccinated volunteers, there was an increment in non-specific production of proinflammatory cytokines than volunteers injected saline |
| Roth A. et al., 2005 [35] | Children between 3 months and 5 years of age | Death on children | The study found that lower mortality for children with a BCG scar than without. The number of deaths per 100 person-year caused by pneumonia among BCG vaccinated children was 0.31 as compared to 0.44 among the unvaccinated ones. |
Roth et al. [35] have identfied variability in the overall mortality ratio by BCG vaccination status. They found that five major causes of mortality in the study were malaria, pneumonia, acute diarrhea, chronic diarrhea, and meningitis/encephalitis. The number of deaths per 100 person-year caused by pneumonia among BCG vaccinated children was 0.31 as compared to 0.44 among the unvaccinated ones (Table 3).
3.3.2. Mechanism of BCG’s effect beyond TB
A study by Kleinnijenhuis et al. [36] demonstrated that monocytes can be functionally reprogrammed or ‘trained’ to exhibit an enhanced and lasting phenotype after vaccination with BCG. Additionally, it was implied that vaccination with BCG induces two types of immune responses. First, it induces a classic specific immune response involving antigen-specific T cells and memory leading to protection against TB. Second, BCG induces adaptive trained immunity based on functional reprogramming of mononuclear phagocytes that induce protective effects not only against TB but also against other infections. In a combination of in vivo and in vitro experiments, the researchers demonstrate that a NOD2-mediated epigenetic change at the level of histone methylation (H3K4me3) is the mechanism through which BCG enhances innate immune responses. In conclusion, the study showed that innate immunity in humans has adaptive features and that it has the capacity to display an enhanced response upon re-infection. The immune response of BCG vaccine has a further effect on hospitalization and death.
Improvements in a body’s clearance of apoptotic or dead cells by alveolar phagocytes could be facilitated by BCG vaccines. It is also evident in an experimental study that [37] BCG vaccine brings about a TH1/TH17 T cell repertoire. This process then facilitates an efficient virus clearance and also it protects the lung from inflammation. It was also seen that BCG is a strong inducer of T-helper type 1 immune responses [15].
4. Discussion
Though BCG vaccine was developed targeting childhood TB, its non-specific immune effects have been figured out [38]. For instance, it has a protective effect against those respiratory tract infections that have no specific vaccine-like RSV [37], [39]. Generally, BCG vaccine reduced morbidity and mortality among children from severe infectious diseases such as pneumonia. There is also evidence that BCG vaccine halve the deaths caused by non-TB infections [40], [41], [17].
Although BCG protected against both the upper and lower respiratory system infections [29], [33], [34], the majority of the studies identified in this review focused on LRTI of viral origin. A study by Miller et al. [30] for instance, revealed that BCG vaccination has a strong protective effect on the current pandemic of COVID 19. This argument was strongly supported by Escobar et al. [31]. A significant reduction in COVID – 19 realted mortality was achived in countries with BCG vaccine policy. It was argued that in countries with universal BCG vaccination like Japan, the incidence and the spread of the virus is lower than countries with no universal BCG vaccination like the USA, Italy, Spain and the Netherlands. The spread rate and number of deaths in these countries supported the authors’ argument [42]. A significant amount of risk of acute LRTIs has been reduced with BCG vaccine among children under-five years of age [16]. Similarly, Stensballe et al have uncovered the protective effect of BCG vaccine against ALRTIs in Guinea-Bissau, with girls better protected than boys [33] .
The 2009 pandemic influenza A virus (H1N1) was known for its fast spread [43], [44]. A clinical trial conducted in 2015 has shown that a combined vaccination of BCG and influenza vaccine strains had a better effect on preventing the virus [32]. The vaccine plays a significant role in the clearance of apoptotic or dead cells. This enables the body to prevent lethal influenza A virus pneumonia. Human RSV, another major cause for hospitalization mostly among immune-compromised individuals, can be curbed with BCG vaccine [37]. A commentary by Rey-Jurado E. et al. suggested that BCG vector vaccine is a safe and efficient mechanism to prevent hRSV [39].
Respiratory tract infections also attack the upper respiratory tract in humans [45], [46]. These infections are common among old age population [47]. The immune system changes with age. Though the number of T cells does not decrease with aging, their function decreases significantly. Therefore, individuals with old age are susceptible for infectious diseases [48], [49]. BCG vaccine was found to protect against AURTIs among old age population [29]. The vaccine increased the Th1 response, shown by the increase of IFN-γ level, reflecting through the formation of infiltrates and scars at the site of the BGC vaccinations.
The effect of BCG vaccine included reduction in mortality and hospitalization. For example, Roth A. et al. [35]. showed that BCG vaccine can significantly reduce mortality from pneumonia among children. Similar findings were also reported by others [50], [51]. Furthermore, hospitalization related to respiratory tract infection among BCG vaccinated children was significantly lower than in non-vaccinated children [34].
We recognize the difference in susceptibility to infectious respiratory infections among BCG vaccinated and unvaccinated individuals. This disparity is perhaps because of BCG’s effect on the immune system. The immune response to BCG vaccine is twofold. It is evident that the vaccine strengthens the human T cell immunity against respiratory tract infections [36], [37]. BCG facilitates development of immune memory leading to protection against TB via classic specific immune response involving antigen-specific T cells. Secondly, it induces adaptive trained immunity based on functional reprogramming of mononuclear phagocytes that induce protective effects not only against tuberculosis but also against other infections. There is also an evidence immunization with BCG significantly improved efferocytosis (the clearance of apoptotic or dead cells) by alveolar phagocytes and rendered 100% protection against lethal influenza A virus pneumonia [52].
There are certain limitations of this review. The studies lacked homogeneity due to the differences in the study populations and outcome measures. Hence, the dissimilarity between the study design and populations of the studies limited the ability to synthesize results for a meta-analysis. Additionally, the search strategy limited the results to English and therefore limited the number of studies available for review.
5. Conclusion and implications
BCG vaccine has been shown a tremendous effect in averting severe cases of childhood TB since its introduction. Its protective effect, however, goes beyond TB to include other bacterial and viral respiratory infections through immune modluations. This reviewe showed that both URTIs and LRTIs were lower among BCG vaccinated individuals. The incidence of and mortality from COVID 19 was significantly lower in countries with universal BCG vaccination as compared to countries without such a policy.
As synthesized in this review BCG vaccine has the potential to offer nonspecific immune protection against many respiratory tract infections including COVID 19. We recommend countries with universal BCG vaccination to enhance effective vaccination coverage. Countries with no universal policy of BCG vaccination should reintroduce the vaccine targeting those groups highly exposed to COVID 19 indlcusing frontlone ehalth workers and individuals with high risk of dealth such as those above 60 years of age.
Given the potential benefits of BCG vaccination in preventing severe infectious respiratory infections, it is crucial to deterime the mechanism. Further, research is needed to continue to develop the evidence base on the effect of BCG vaccination in preventing severe infectious respiratory diseases like COVID-19. Well-designed studies such as randomized control trials with appropriate sample size and power are recommended to establish whether the vaccine could be beneficial in special populations such as the health workforce. Evidence that reflects on the effect of BCG vaccination overtime, in different context and across age groups in the prevention of severe infectious respiratory diseases are also lacking. Additionally, new research will help to determine cost savings related to BCG vaccination in preventing severe infectious respiratory diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to acknowledge the Scientific Advisory Panel for the Oromia Regional Health Bureau, for forwarding the review question.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.08.018.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Seki M., Honda I., Fujita I., Yano I., Yamamoto S., Koyama A. Whole genome sequence analysis of Mycobacterium bovis bacillus Calmette-Guérin (BCG) Tokyo 172: a comparative study of BCG vaccine substrains. Vaccine. 2009;27(11):1710–1716. doi: 10.1016/j.vaccine.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Cernuschi T., Malvolti S., Nickels E., Friede M. Bacillus Calmette-Guérin (BCG) vaccine: a global assessment of demand and supply balance. Vaccine. 2018;36(4):498–506. doi: 10.1016/j.vaccine.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abubakar I., et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013 doi: 10.3310/hta17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal A., Dutta A.K. Timing and dose of BCG vaccination in infants as assessed by postvaccination tuberculin sensitivity. Indian Pediatr. 1995;32(6):635–639. [PubMed] [Google Scholar]
- 5.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 6.NHS and Centre for Clinical Practice at NICE. Respiratory tract infections – antibiotic prescribing; 2018.
- 7.Oxford University press. “Oxford Medicine Online: Infectious diseases”; 2020. [Online]. Available: https://oxfordmedicine.com/.
- 8.Chang A.B., Chang C.C., O’Grady K., Torzillo P.J. Lower respiratory tract infections. Pediatr Clin North Am. 2009 doi: 10.1016/j.pcl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Heikkinen T, Ruuskanen O. Upper respiratory tract infection. In: Encyclopedia of respiratory medicine, four-volume set; 2006. p. 385–8.
- 10.Gomes R.R., Antunes D.E., dos Santos D.F., Sabino E.F.P., Oliveira D.B., Goulart I.M.B. BCG vaccine and leprosy household contacts: protective effect and probability to becoming sick during follow-up. Vaccine. 2019;37(43):6510–6517. doi: 10.1016/j.vaccine.2019.08.067. [DOI] [PubMed] [Google Scholar]
- 11.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Tanghe A., Content J., Van Vooren J.P., Portaels F., Huygen K. Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer. Infect Immun. 2001;69(9):5403–5411. doi: 10.1128/IAI.69.9.5403-5411.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Zein M., Conus F., Benedetti A., Menzies D., Parent M.E., Rousseau M.C. Association between bacillus Calmette-Guérin vaccination and childhood asthma in the Quebec birth cohort on immunity and health. Am J Epidemiol. 2017;186(3):344–355. doi: 10.1093/aje/kwx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thøstesen L.M., et al. Neonatal BCG vaccination and atopic dermatitis before 13 months of age: a randomized clinical trial. Allergy Eur J Allergy Clin Immunol. 2018;73(2):498–504. doi: 10.1111/all.13314. [DOI] [PubMed] [Google Scholar]
- 15.Gen-sheng Z., Ping-li W., Hua-qiong H., Hua-hao S. Human immunodeficiency virus and hepatitis C virus co-infection: epidemiology, natural history and the situation in China. Chin Med J (Engl) 2009;122(1):93–97. [PubMed] [Google Scholar]
- 16.Hollm-Delgado M.-G., Stuart E.A., Black R.E. Acute lower respiratory infection among Bacille Calmette-Guérin (BCG)–vaccinated children. Pediatrics. 2014;133(1):e73–e81. doi: 10.1542/peds.2013-2218. [DOI] [PubMed] [Google Scholar]
- 17.Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther. 2013;35(2):109–114. doi: 10.1016/j.clinthera.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 18.The Mendeley Support Team. “Getting Started with Mendeley.” London: Mendeley Desktop. Mendeley Ltd.; 2011. p. 1–16.
- 19.Checklist for Cohort Studies: The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews; 2017.
- 20.Checklist for Case Control Studies: The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews; 2017.
- 21.Checklist for Randomized Controlled Trials: The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews; 2017.
- 22.Checklist for Prevalence Studies: The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews; 2017.
- 23.Pearson A, Field J, Field J. Critical appraisal tools JBI critical appraisal checklist for experimental studies.
- 24.J.B. Institute. JBI Reviewer Mannual: Data extercation; 2019.
- 25.Meers P.D., et al. Respiratory tract infections. J Hosp Infect. 1981;2(SUPPL. 1):19–22. [Google Scholar]
- 26.Piedimonte G., Perez M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35(12):519–530. doi: 10.1542/pir.35-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43(3):185–190. [PubMed] [Google Scholar]
- 30.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. J Chem Inf Model. 2020;53(9):1689–1699. [Google Scholar]
- 31.Escobar L.E., Molina-cruz A., Barillas-mury C. BCG vaccine protection from severe coronavirus. PNAS Appl Biol Sci. 2020:1–7. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leentjens J., et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 33.Stensballe L.G., et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls Community based case – control study. Vaccine. 2005;23:1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 34.De Castro M.J., Pardo-Seco J., Martinón-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60(11):1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 35.Roth A., et al. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol. 2005;34(3):540–547. doi: 10.1093/ije/dyh392. [DOI] [PubMed] [Google Scholar]
- 36.Kleinnijenhuis J., et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Céspedes P.F., et al. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35(5):757–766. doi: 10.1016/j.vaccine.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 38.Luca S., Mihaescu T. History of BCG Vaccine. Maedica (Buchar) 2013;8(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- 39.Rey-jurado E., Soto J. A safe and ef fi cient BCG vectored vaccine to prevent the disease caused by the human Respiratory Syncytial Virus. Hum Vaccin Immunother. 2017;13(9):2092–2097. doi: 10.1080/21645515.2017.1334026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shann F. The heterologous (non-specific) effects of vaccines: implications for policy in high-mortality countries. Trans R Soc Trop Med Hyg. 2015;109:5–8. doi: 10.1093/trstmh/tru161. [DOI] [PubMed] [Google Scholar]
- 41.Nissen T.N., et al. Bacillus Calmette-Guérin vaccination at birth and in vitro cytokine responses to non-specific stimulation. A randomized clinical trial. Eur J Clin Microbiol Infect Dis. 2018;37(1):29–41. doi: 10.1007/s10096-017-3097-2. [DOI] [PubMed] [Google Scholar]
- 42.worldometers.info. Coronavirus Update (Live): Worldometer; 2020. [Online]. Available: https://www.worldometers.info/coronavirus/ [accessed: 04-Jun-2020].
- 43.Itoh Y., et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009 doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chowell G., et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009 doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 45.Grief SN. Upper Respiratory Infections. Elsevier Inc; 2013. p. 757–70. [DOI] [PMC free article] [PubMed]
- 46.Teng C.L., et al. The management of upper respiratory tract infections. Contin Med Educ. 2001;56(2):260–267. [PubMed] [Google Scholar]
- 47.Childs A., et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19(210):1–10. doi: 10.1186/s12877-019-1236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montecino-rodriguez E., Berent-maoz B., Dorshkind K. Review series Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyand C.M., Goronzy J.J. Aging of the immune system mechanisms and therapeutic targets. Transatl Airw Conf. 2016;13(Suppl. 5):S422–S428. doi: 10.1513/AnnalsATS.201602-095AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butkeviciute E., Jones C.E., Smith S.G. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13(10):1193–1208. doi: 10.2217/fmb-2018-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kjærgaard J., et al. Nonspecific effect of BCG vaccination at birth on early childhood infections: a randomized, clinical multicenter trial. Pediatr Res. 2016 doi: 10.1038/pr.2016.142. [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee S., Subramaniam R., Chen H., Smith A., Keshava S., Shams H. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS ONE. 2017;12(7):1–19. doi: 10.1371/journal.pone.0180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.