Abstract
Primary malignant CNS tumors are the leading cause of childhood cancer-related death and morbidity. While advances in surgery, radiation and chemotherapy have improved the survival rates in children with malignant brain tumors, mortality persists in certain subpopulations and current therapies are associated with extreme morbidity. This is especially true for children with malignant infratentorial tumors. Accordingly, G207, a genetically engineered herpes simplex virus (HSV-1) capable of selectively targeting cancer cells has emerged as a promising therapeutic option for this patient population. Herein, we demonstrate that cerebellar inoculation of G207 was systemically non-toxic in an immunocompetent, HSV-1 sensitive mouse strain (CBA/J). Mice had neither abnormal brain/organ pathology nor evidence of G207 replication by immunohistochemistry at days 7 and 30 after cerebellar G207 inoculation. While a minute amount viral DNA was recovered in the cerebellum and brainstem of mice at day 7, no viral DNA persisted at day 30. Critically, G207 delivered to the cerebellum was able to target/treat the highly aggressive MYC-overexpressed group 3 murine medulloblastoma increasing survival vs controls. These results provide critical safety and efficacy data to support the translation of G207 for pediatric clinical trials in intractable cerebellar malignancies.
Keywords: HSV, virotherapy, oncolytic, G207, cerebellum, pediatric, brain tumors
INTRODUCTION:
Immunovirotherapy with engineered, oncolytic HSV-1 (oHSV) is an emerging therapy capable of targeting both extracranial solid and brain tumors in children.(1, 2) Several viruses that contain deletions in the diploid neurovirulence gene γ134.5, a gene that prevents productive infection in normal post-mitotic cells while maintaining the virus’ ability to infect, replicate in, and ultimately kill dividing cancer cells, have advanced to Phase I clinical trials in pediatric patients.(3) The first completed study in children and young adults utilized HSV1716, a γ134.5-deleted virus derived from the wild-type strain 17+, in patients with recurrent, advanced non-central nervous system (CNS) solid tumors.(4) The study involved computed tomography-guided intratumoral inoculation of a single dose of the virus at 105-107 plaque-forming units (PFU).(4) Virus inoculation was safe; further, evidence of an immune response and viral replication was detected in some study patients. A second Phase I study centered on the intravenous (IV) delivery of HSV1716 is currently enrolling children and young adults with extracranial solid tumors (clinicaltrials.gov identifier NCT00931931).
The first pediatric trial of an oHSV in brain tumors is currently ongoing and continues to recruit patients (NCT02457845). The study is testing the safety and tolerability of HSV G207 alone or combined with a single dose of radiation in children 3-18 years old with recurrent or progressive supratentorial malignant brain tumors.(5) G207 contains a deletion of both copies of the γ134.5 gene and a lacZ insertion into the UL39 gene which encodes the heavy chain of viral ribonucleotide reductase thereby adding an additional layer of protection from viral infection in non-cancer cells.(6) The decision to treat supratentorial tumors in this first study was based on previous safety data whereby G207 was proven safe when inoculated supratentorially in the cerebral cortex of both mice and non-human primates.(7, 8) Three Phase I studies in adults with recurrent supratentorial high-grade gliomas (HGG) also demonstrated the safety of virus delivered intratumorally, in surrounding brain tissue, and combined with a single dose of radiation.(9-11)
While most adult malignant brain tumors occur supratentorially, the majority of pediatric brain tumors arise in an infratentorial location, most frequently within the cerebellum.(12) It is also prudent to note pediatric malignant cerebellar brain tumors are often aggressive and require intensive combinatorial therapies including surgery, radiation and chemotherapy, which frequently cause lifelong disability in survivors. As such, novel therapeutic approaches are urgently warranted in an effort to treat malignant pediatric infratentorial tumors. Recent preclinical data from our group and others indicate that malignant pediatric brain tumors including cerebellar tumors are highly sensitive to oHSV.(13-16) Concordant with such thinking is the putative repositioning of G207 (i.e. from trials centered on supratentorial tumors to those centered on infratentorial brain malignancies). Herein, we have demonstrated the safety and efficacy of cerebellar inoculation of oHSV G207 in pertinent preclinical mouse models and in so doing establish the groundwork for the development of oHSV G207-centered clinical trials in children diagnosed with intractable cerebellar malignancies.
MATERIALS AND METHODS:
Herpes simplex viruses
G207 contains a deletion of both copies of the γ134.5 gene and a disrupting lacZ insertion within the UL39 gene which encodes the heavy chain of viral ribonucleotide reductase, as has been previously described.(6) G207 virus was purified on OptiPrep gradients (AXIS-SHIELD PoC AS, Oslo, Norway) as described by Shah et al.(17) Briefly, when Vero cells reached ~100% CPE following infection, culture supernates and cells were collected and separately harvested. Culture supernates underwent three centrifugation steps and viral pellets were resuspended in 1X phosphate-buffered saline (PBS) containing 10% glycerol. Cells were scraped, lysed and subjected to the same centrifugation steps as supernates prior to suspension in PBS-10% glycerol. Virus pellets were then purified on OptiPrep gradients; following purification, pellets from supernates and lysates were combined in order to determine the titer on Vero cells as described elsewhere.(18) For a control, wild-type HSV-1 (F) was used.
Animal studies
Animal experiments were approved by the University of Alabama at Birmingham (UAB) Animal Care and Use Committee (ACUC) (protocol # IACUC-0873) and were performed in accordance with all relevant experimental guidelines. Four-week-old CBA/J and C57BL/6 mice were purchased from ENVIGO (Prattville, AL).
In vivo studies / cell line (9728)
Ten CBA/J mice were stereotactically injected with 1x107 PFU of G207 in the right cerebellum (coordinates: 1mm lateral from the midline and 2 mm posterior to the lambdoid suture) as previously described.(13) Mice were assessed daily for signs of toxicity. Two mice were randomly chosen and sacrificed at day 7 and the organs harvested and prepared for studies as described below. At day 30, the remaining eight mice were sacrificed and organs were harvested and prepared for studies described below. The brain tissue used for slide preparation were sectioned from hemispheres that were injected with virus. For evaluation of the side effect profiles of injecting wild-type HSV verses the oHSV G207, 10 total CBA/J mice were stereotactically injected with 1x103 PFU wild-type HSV and 1x107 G207. All mice injected with wild-type HSV were sacrificed by day 7, whereas those randomized to receive G207 survived to the 30-day time point, where they were euthanized for evaluation of tissue.
To evaluate the efficacy of G207 in targeting 9728 MYC-overexpressed group 3 (non SHH/NonWNT) murine medulloblastoma cells, 4-week old C57BL/6 mice received an intracranial injection of 5x104 9728 MYC-overexpressed group 3 (non-SHH/non-WNT) medulloblastoma cells. 3 days and 9 days later 10 mice per group received a single intratumoral injections of 5μL of saline or 1x107 PFU of G207. Briefly, the 9728 line [courtesy of Martine F. Roussel, St. Jude Children’s Research Hospital] grows as neurospheres which give rise to group 3 (as characterized by Affymetrix arrays) medulloblastoma after implantation in the cerebellum or cortex of recipient mice. Line 9728 was originally derived from tumors harvested from mice implanted with granule neuron progenitors purified from the cerebellum of 5-7 day old Trp53−/− C57BL/6 pups, infected with high titer retroviruses expressing C-MYC and the cyan fluorescent protein (CFP); the vector employed was MSCV-C-MYC-IRES-CFP.
Tissue processing
Organs were immediately fixed in a 10% formalin solution overnight (Fisher Scientific, Pittsburgh, PA) and subsequently stored in 70% ethanol. Organs and tumors were cut into small tissue pieces and placed in tissue cassettes for processing at the UAB immunohistochemistry core facility. After processing, tissues were embedded in surgipath paraplast embedding medium (Fisher Scientific, Pittsburgh, PA). Tissue blocks were placed on ice for approximately 30 minutes until they became solid. Tissue sections were cut with a microtome (6 μm) and placed on glass slides (Fisher Scientific) for subsequent staining/imaging.
Immunohistochemistry (IHC) and immunofluorescence (IF)
Slides from organs and controls were dewaxed with xylene and then rehydrated by sequential incubation in PBS with various concentrations of ethanol. Antigen retrieval was conducted by incubating slides at 80°C in 10 mM Citrate buffer solution at pH=6 (EMD Millipore, Temecula, CA) for 15 minutes. Slides were then cooled for 60 minutes at room temperature (RT) and then washed three times with phosphate buffered saline (PBS) (5 minutes per wash). For IHC staining, endogenous peroxidases were quenched by incubation of slides with 3% hydrogen peroxide (Fisher Scientific) for 15 minutes. Slides were blocked with 5% BSA in PBS for 60 minutes at RT. Slides were incubated overnight with an anti-HSV1 antibody 1:400 (Abcam Cambridge, MA) at 4°C. The following day, slides were washed 3 times with PBS and then incubated with Polymar HRP anti-rabbit (GeneTex, Irvine, CA) for 60 minutes at RT. Slides were again washed 3 times with PBS, incubated with substrate DAB (BioGenex, Fremont, CA) for 5-10 minutes, washed with PBS, and counterstained with hematoxylin and eosin (Fisher Scientific). Slides were then washed with tap water, dehydrated by sequential treatment with various concentrations of ethanol and xylene, and covered with a coverslip (Fischer Scientific). DBT murine tumors grafted into BALB/c mice [courtesy of Dr. Michael R. Chicoine, Washington University School of Medicine in St. Louis] were utilized as positive IHC controls having been injected with (107 PFU/5μl) of γ134.5-deleted virus.
For IF, sections were blocked with 10% normal goat serum (NGS) in 0.1% Triton X-100 in PBS for 60 minutes at RT. Slides were then incubated overnight with anti-CD4 antibody 1:200 (Abcam Cambridge, MA) or anti-CD8 antibody 1:100 (Thermo Scientific, CA) at 4°C. The following day, slides were washed 3 times with PBS and incubated with appropriate secondary antibody 488- or 546-conjugated 1:1000 (Alexafluor, CA) for 60 minutes at RT. Slides were again washed 3 times with PBS. Nuclei were counterstained with Dapi 1:5000 in PBS for 5 minutes at RT. Slides were mounted with aqueous mounting medium (Abcam Cambridge, MA). Nonspecific staining was observed in control incubations in which primary antibodies were omitted. Digital images were acquired using a Nikon Aperio’s Slide scanner ScanScope and a Leica DM IRB fluorescence microscope.
Hematoxylin and eosin staining
Slides were deparaffinized with incubation in xylene for 15 minutes three times with change of fresh xylene every time. The slides were then incubated for 2 minutes, three times in 100% and three times in 95% ethanol with change of fresh solution for each incubation. After being washed with water, slides were incubated in hematoxylin at room temperature for 10 minutes and rinsed thoroughly in tap water. Slides were dipped three times in clarifier and bluing agent, rinsed in tap water for 3 minutes, and incubated with 95% ethanol for 2 minutes. Slides were then stained with eosin for 1 minute and rinsed thoroughly in tap water. Slides were then incubated for 2 minutes, three times in 95% and three times in 100% ethanol with change of fresh solution for each incubation. Slides were covered with coverslips.
DNA purification
Mouse organs and blood samples were harvested and immediately snap frozen in liquid nitrogen. ~ 20mg of organ and 80μl of blood were ultimately utilized for the preparation of DNA. DNA was purified with the DNeasy Blood & Tissue Kit according to the manufacturer’s instruction (Qiagen, Gaithersburg, MD). Briefly, organ tissues were incubated in lysis buffer with proteinase K at 56°C for ~18-20 hours and blood samples were incubated at 56°C for 10 minutes. DNA in lysates was purified using DNeasy columns. The purity (UV 260/280 ratio) of DNA was ~ 2. 10μl of G207 (2.85 x 107 PFU) was used for preparation of control DNA. DNA was extracted with the QIAamp Ultrasens Virus (Qiagen) according to the manufacturer’s instruction and was purified using DNeasy columns.
Real-time quantitative polymerase chain reaction (RT-qPCR)
The RT-qPCR primers (HSV.2 RT For: CATCACCGACCCGGAGAGGGAC, HSV.2 RT Rev: GGGCCAGGCGCTTGTTGGTGTA) and probe (HSV.2 RTProbe: [6FAM]CCGCCGAACTGAGCAGACACCCGCGC[TAM]) were purchased from Sigma-Aldrich (St. Louis, MO). The Taqman Genotyping Master Mix was purchased from Applied Biosystems (Waltham, MA). RT-qPCR was carried out using an Applied Biosystems 7900HT Fast Real-Time PCR system according to the manufacturer’s instructions. Each RT-qPCR reaction tube contained 70ng of total DNA (from each organ or blood sample), primers (250nM of each primer), and probe (900nM) in 15ul reaction volumes; each sample was run in triplicate. The RT-qPCR thermal cycle conditions employed were as follows: 50°C-2min, 96°C-10min, and then 40 cycles of 95°C −15 sec and 60°C-1min. G207 was used as a positive control and to develop a standard curve, as described below, with the following serial dilutions: 1:50, 1:100, 1:500, 1:1000, 1:20000.
Statistical analyses
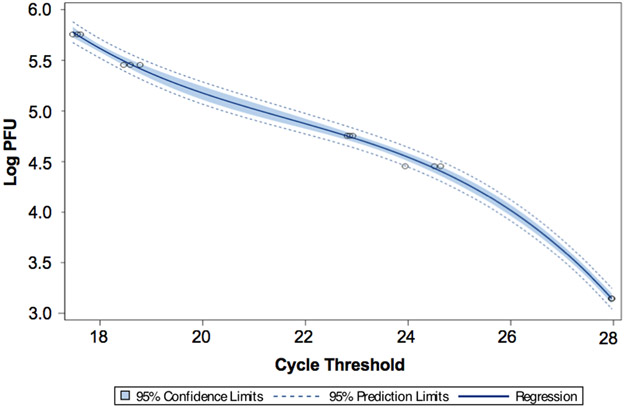
To examine the association between cycle threshold (Ct) and PFU, we utilized a general linear regression model using log PFU as the outcome and Ct as the independent variable via the application of a 3rd order polynomial. Utility of the model was tested using 5% level of significance. Using the fitted model, we obtained the estimated curve with confidence bounds based on individual 95% confidence intervals; SAS version 9.4 (Cary, NC) was used to perform the analyses. Survival curves were generated with SigmaPlot v12.0 (Systat Software) using Kaplan–Meier analysis and median survival time; the log-rank test was applied to compare survival between groups.
RESULTS:
Histopathology of mouse organs
To determine the safety and toxicity of oHSV G207 in the cerebellum, CBA/J mice were employed based on their documented sensitivity to HSV-1, manifested as herpes encephalitis, as compared with other strains such as C57/BL6 or BALB/c.(19-21) Mice received a single injection of 107 PFU of G207 in the right cerebellum and were monitored for signs of toxicity for 30 days; control animals received wild-type HSV-1 (F) and were similarly monitored. As expected and concordant with the literature, all wild-type HSV-1 control animals became moribund and required euthanization by day 7. (19-21)
For the G207-treated animals, there were neither alterations in physical presentation nor behavioral abnormalities. All mice had normal grooming and avoidance behavior with normal posture and gait, and normal feeding. Two randomly chosen G207-treated mice were sacrificed at day 7 and sections of the brain (i.e. cerebellum, brainstem, and cerebrum), other critical organs (i.e. heart, kidneys, liver, lung, spleen) and blood were harvested to evaluate for pathologic changes and/or evidence of HSV-1 infection. Organs were stained with hematoxylin and eosin and examined by a clinically trained pathologist (R.L.) with experience examining mouse organs (Figure 1). No abnormalities were identified other than the expected surgical tract within the cerebellum (Figure 2A-B). Proximal to the sites of injection, we also noted a decreased presence of immune effector cells (CD4 and CD8 cells) in the G207 animals as compared to that of the wild-type HSV controls (Figure 2C-F). At day 30, the remaining eight G207-treated mice were euthanized and the aforementioned tissues were again harvested. Similar to day 7, there was no abnormal histopathology noted in any part of the brain or organ at day 30 (Figure 3).
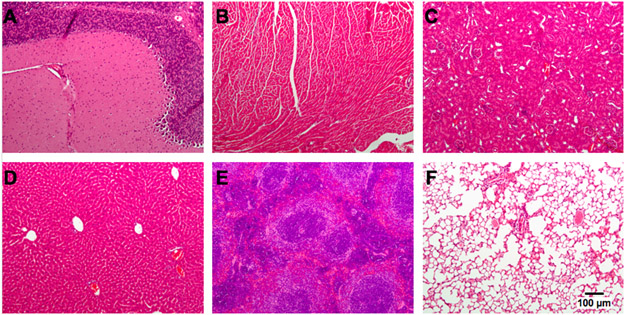
Figure 1. Organ pathology 7 days after cerebellar inoculation of HSV G207.
CBA/J mice were inoculated with 1x107 plaque-forming units of G207 in the right cerebellum, sacrificed at day 7 post-virus injection, and organs were harvested and stained with hematoxylin and eosin. There were no pathologic abnormalities identified in multiple organs. (A) cerebellum; (B) heart; (C), kidney; (D) liver; (E) spleen; (F) lung.
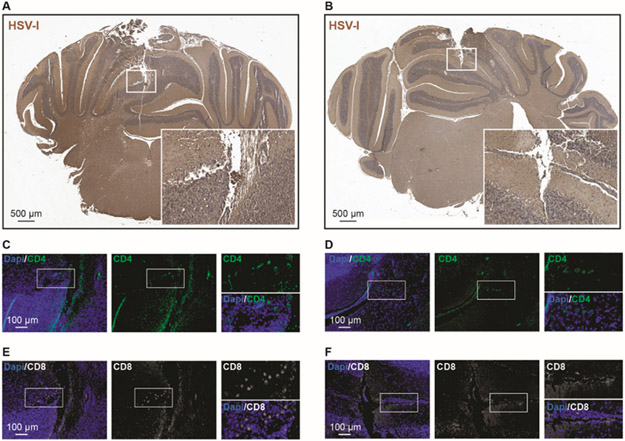
Figure 2. Cerebellar analysis for HSV-I in mice inoculated with 1x107 plaque-forming units of WT HSV-I or G207.
(A-B) At day 7 post-viral injection we found HSV-I immuno-positive area in the needle tract of WT HSV-I injected cerebellum (A) and very little staining was observed in G207 injected cerebellum (B). (C-F) We evaluated the CD4 (C-D) and CD8 (E-F) positive cells in the WT HSV-I versus G207 injected cerebella. We found a small number of CD4 and CD8 positive cells in G207 cerebella as compared with WT HSV-I injected-cerebella.
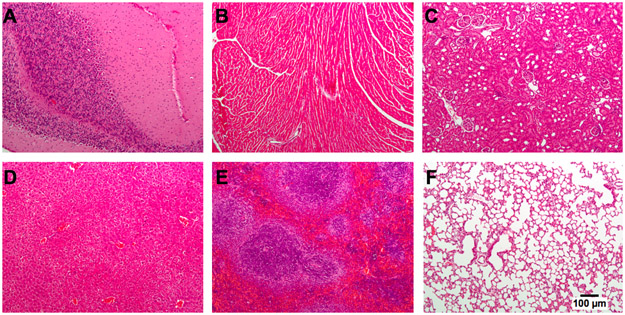
Figure 3. Organ pathology 30 days after cerebellar inoculation of HSV G207.
CBA/J mice were inoculated with 1x107 plaque-forming units of G207 in the right cerebellum, sacrificed at day 30 post-virus injection, and organs were harvested and stained with hematoxylin and eosin. There were no pathologic abnormalities identified in multiple organs. (A) cerebellum; (B) heart; (C), kidney; (D) liver; (E) spleen; (F) lung.
HSV-1 immunostaining
To determine if HSV-1 was present within any subsections of brain (i.e. cerebellum, brainstem, cerebrum) or any of the other organs (i.e. heart, kidneys, liver, lung, spleen) harvested from CBA/J mice treated with G207, immunostaining for HSV-1 was performed. Of note, the immunostain of the positive control, murine DBT astrocytoma cells infected with 34.5-deleted oHSV, reacted appropriately. Critically, all organs were negative for HSV-1 immunostaining at day 7 (Figure 4) and day 30 (data not shown) indicating a lack of HSV-1 replication after cerebellar inoculation of G207; HSV-1 staining was noted in wild-type HSV-1 controls proximal to the needle track (Figure 2A).
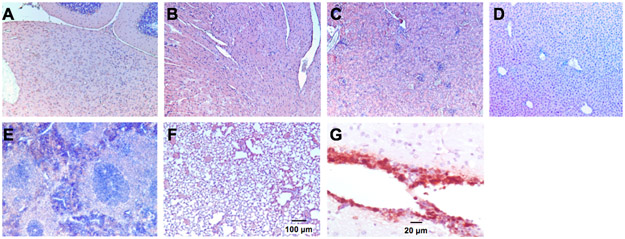
Figure 4. Immunostaining for HSV-I.
Mice were inoculated with 1x107 plaque-forming units of G207 in the right cerebellum. Mice were sacrificed at day 7 (shown) and 30 (not shown) post-viral injection and organs were harvested and evaluated for HSV-1 via immunohistochemistry. No positive staining for HSV-1 was seen in multiple organs. (A) cerebellum; (B) heart; (C), kidney; (D) liver; (E) spleen; (F) lung; (G) positive control.
Real-time quantitative polymerase chain reaction
To confirm the absence of HSV-1 via viral DNA, real-time quantitative polymerase chain reaction (RT-qPCR) was performed on the brain/organs and blood at days 7 and day 30. The mean cycle threshold (Ct) was determined using triplicate samples. A known concentration of G207 (2.85 x 107 PFU) and serial dilutions of that concentration (1:50, 1:100, 1:500, 1:1000, 1:20000) were used both as a positive control and to develop a standard curve from which the PFU in target organs/blood was ultimately extrapolated (Figure 5). The estimated mean log PFU as a function of Ct is given by the equation 99.78546 – (11.59322 x Ct) + (0.52010 x Ct2) - (0.00801 x Ct3). This provided an r-squared value of 0.9986. A Ct mean greater than 31.61442, which was the predicted value of 1 PFU, was considered below the limit of detection. Table 1 shows the Ct mean and predicted PFU for brain regions/organs and blood at day 7. Small (<46 PFU) amounts of viral DNA were detected in the cerebellum and brainstem but not in the cerebrum. Furthermore, viral DNA was below the limit of detection in the blood and all non-CNS organs at day 7, and viral DNA was below the limit of detection in the blood and all organs or brain regions including the cerebellum and brainstem at day 30 suggesting a lack of viral DNA persistence after cerebellar inoculation of G207.
Figure 5. Association between real-time quantitative polymerase chain reaction (RT-qPCR) cycle threshold (Ct) and plaque-forming units (PFU) of G207.
Ct was determined by RT-qPCR using five dilutions (1:50, 1:100, 1:500, 1:1000, 1:20000) of a known concentration of G207 (2.85x107 PFU). Utilizing a linear regression model that employed log PFU as the outcome and Ct as the independent variable, an estimated curve was obtained with a 5% level of significance and confidence bounds based on individual 95% confidence intervals.
Table 1.
Mean cycle threshold (Ct) and estimated plaque-forming units (PFU) in organs and blood 7 days after inoculation of 1x107 PFU of G207 in the cerebellum.
| Area of Brain/Organ |
Mean Ct | Estimated PFU |
|---|---|---|
| Brainstem 1 | 31.59 ± 0.15 | 1 |
| Brainstem 2 | 30.64 ± 0.28 | 11 |
| Cerebellum 1 | 30.00 ± 0.32 | 45 |
| Cerebellum 2 | 31.35 ± 0.29 | 2 |
| Cerebrum 1 | (-) | BLD |
| Cerebrum 2 | (-) | BLD |
| Blood 1 | (-) | BLD |
| Blood 2 | (-) | BLD |
| Heart 1 | (-) | BLD |
| Heart 2 | (-) | BLD |
| Kidney 1 | (-) | BLD |
| Kidney 2 | (-) | BLD |
| Liver 1 | (-) | BLD |
| Liver 2 | (-) | BLD |
| Lung 1 | (-) | BLD |
| Lung 2 | (-) | BLD |
| Spleen 1 | (-) | BLD |
| Spleen 2 | (-) | BLD |
(-), undetermined; BLD, below limit of detection
In vivo efficacy
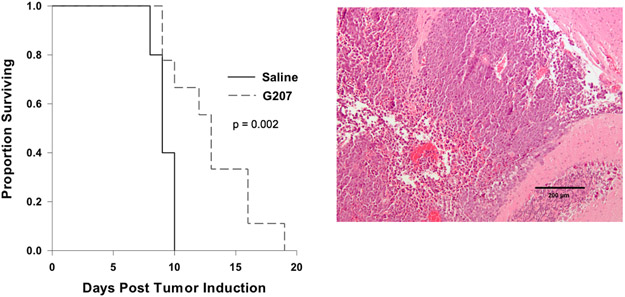
To evaluate the efficacy of G207 in targeting highly aggressive MYC-overexpressed group 3 (non SHH/Non-WNT) murine medulloblastoma cells, C57BL/6 mice underwent intracranial injection of 5x104 9728 MYC-overexpressed group 3 medulloblastoma cells. Three and 9 days later mice received intratumoral injections of 5μL of saline or 1x107 PFU of G207. The median survival for mice that received G207 was 13 ± 0.775 days compared to 9 ± 0.387 days for saline mice (Figure 6) demonstrating that G207 increased survival of treated animals by 44%.
Figure 6. Treatment of MYC-overexpressed group 3 (non-SHH/non-WNT) medulloblastoma in immunocompetent mice with G207 prolonged survival.
(A) Kaplan-Meier survival plot of C57BL/6 mice after intracranial injection of 5x104 9728 medulloblastoma cells. 3 days and 9 days later mice received a intratumoral injections of 5μL of saline or 1x107 PFU of G207. G207 significantly prolonged survival. (B) H&E stained sections of cerebellum demonstrate a hypercellular malignant neoplasm composed of sheets of densely packed primitive tumor cells with a high ratio of nucleus to cytoplasm; apoptotic cells are frequently seen.
DISCUSSION:
The safety of direct cerebrum inoculation of G207 has been confirmed in mice, non-human primates, and in three Phase I clinical trials (37 adults with recurrent HGG).(7-11) In the murine study, BALB/c mice were inoculated with 107 PFU of G207 which was delivered into the cerebral cortex.(7) These animals showed no symptomatic evidence of disease during a year of follow-up observation, whereas 50% of mice died within 3-14 days after intracerebral inoculation of wild-type HSV-1 strain F (parent strain of G207) at a dose of only 103 PFU.(7) Similarly, Aotus nancymai owl monkeys had no CNS or non CNS-organ abnormalities after receiving an intracerebral dose of up to 109 PFU (maximum dose tested), while 103 PFU of wild-type HSV-1 strain F resulted in HSV encephalitis and rapid mortality; these data indicate that G207 has at least a 10-million fold safety margin compared to the wild-type virus.8 The three human trials conclusively established the safety of inoculating G207 in the cerebral cortex in one (up to 3 x 109 PFU) or two doses (total of 1.15 x 109 PFU) directly into the tumor or the surrounding brain tissue alone or when combined with a single dose of radiation.(9-11) Currently the safety and tolerability of G207 alone and combined with a single dose of radiation is being tested in children with progressive or recurrent supratentorial malignant brain tumors at our institution (NCT02457845).(5, 22)
While approximately 80-85% of adult malignant brain tumors are supratentorial, only 30-40% of pediatric malignant brain tumors are supratentorial with the majority of tumors located infratentorially.(12, 23) The most common location for pediatric malignant brain tumors is the cerebellum.(12) Tumor types found in the cerebellum include medulloblastoma, the most common malignant brain tumor of childhood, and atypical teratoid/rhabdoid (AT/RT) tumors and ependymoma, which both may arise in infratentorial or supratentorial locations.(12, 24) Outcomes for these tumors depend on the molecular subgroups and each contain subgroups with very poor outcomes ranging from 20%−50% survival.(24, 25) Furthermore, the burden of cure is very high for medulloblastoma, which requires surgery, craniospinal irradiation (CSI) and multi-agent chemotherapy; and AT/RT which typically requires surgery, induction chemotherapy, focal or CSI, and triple autologous stem cell transplantation.(24) Patients often experience permanent disabilities such as hormone dysfunction, neurosensory damage, and neurocognitive impairments that are attributed to such treatments.(26-28) Therefore, there is a great need for targeted approaches like oHSV for these aggressive cerebellar malignancies that can kill tumor cells without injuring normal cells and that result in less local and systemic toxicities.
Preclinical data indicates that pediatric medulloblastoma and AT/RT are highly sensitive to oHSV. We established that pediatric patient-derived medulloblastoma xenografts, including subgroup 3 tumors, which portend a poor prognosis (i.e. survival ≤ 50%), and the pediatric cancer stem-like cells (CD133+ or CD15+ cells), were highly sensitive to killing by G207 and interleukin-12 producing, 34.5-deleted oHSV M002 in vitro and in vivo.(13) Similarly, using rRp450, an attenuated oHSV with intact 34.5 genes but deficient in the viral-encoded ribonucleotide reductase (ICP6), Studebaker, et al. demonstrated that a single intratumoral injection of the virus resulted in significantly prolonged survival in mice bearing medulloblastoma and AT/RT orthotopic xenografts.(15) Collectively, the high propensity of pediatric tumors occurring in the cerebellum, the heavy burden of current therapies for cerebellar malignancies, and the promising preclinical data in tumor types that occur in the cerebellum suggest that oHSV trials targeting pediatric cerebellar tumors are warranted. Such a rationale informed the current study which was designed to confirm the safety and efficacy of inoculating oHSV G207 in the cerebellum of immunocompetent mice.
Our results establish the safety of cerebellar inoculation of oHSV in an immunocompetent HSV-1 sensitive murine strain and suggest that the virus may harbor potential as a focused treatment for medulloblastoma. CBA/J mice have previously been shown to be highly sensitive to HSV-1 and HSV encephalitis as compared to other less sensitive strains such as C57/BL6.(19-21) Here we have demonstrated that mice appear physically well after cerebellar injection of a high dose of oHSV and have no abnormal brain/organ pathology or evidence of HSV-1 replication by immunohistochemistry at days 7 and 30 after the inoculation. While a small amount of viral DNA was recovered in the cerebellum and brainstem of mice at day 7, no viral DNA persisted at day 30. In addition to preventing the virus from overcoming a normal cell’s host defense mechanism against infection, the deletion of the γ134.5 genes removes the opposite strand which encodes a portion of the latency-activated transcripts (LAT) making the γ134.5-deleted HSVs like G207 less capable of establishing latency or reactivating from latency.(29-31) Furthermore, we demonstrated safety and efficacy of targeting a highly aggressive MYC-overexpressed group 3 murine medulloblastoma model with G207. These results along with our previous data demonstrating sensitivity of patient-derived medulloblastoma xenograft models to G207 strongly support the utility of the virus in treating these malignant cerebellar tumors.(13, 16)
Taken together, our findings indicate that similar to cerebral inoculation, cerebellar inoculation of G207 is safe in an immunocompetent, wild-type HSV-1 sensitive murine model, and effective at targeting cerebellar malignancies. This preclinical safety data along with the clinical safety reported to date in pediatric and adult humans and the promising preclinical efficacy data in pediatric cerebellar tumor models strongly supports the translation of G207 and the development of a pediatric Phase I clinical trial in children with recurrent or progressive cerebellar malignancies.
ACKNOWLEDGEMENTS:
This research was supported in part by a grant from the Rally Foundation for Childhood Cancer Research, the Truth 365, the National Institutes of Health (R01FD005379), and the Department of Defense (W81XWH-15-1-0108) to GKF, and from the National Institutes of Health (P20CA151129 to GYG and R01CA217179 to JMM and GYG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense. We thank the UAB Neuroscience Molecular Detection and Stereology Core for assistance with IHC.
Footnotes
CONFLICTS OF INTEREST:
Drs. Markert and Gillespie are founders of and own stock and stock options (<8% interest) in Aettis, Inc., a biotech company that has licensed M032 HSV from The Board of Trustees of the University of Alabama for the University of Alabama at Birmingham and is developing other oHSVs that are not the subject of this current investigation. Dr. Gillespie currently serves as one of five unpaid members of the Board of Directors for Aettis, Inc. Dr. Gillespie is a founder of and owns stock and stock options (<10%) in Maji Therapeutics, which is developing other HSVs that are not the subject of the current investigation. Drs. Markert and Gillespie were also founders of and owned stock and stock options (<8%) in Catherex Inc., a biotechnology company that had licensed additional intellectual property related to oHSV. Catherex, Inc., was sold to Amgen, Inc., on December 18, 2015, and they no longer participate in any decision making or have any control of any aspect of Catherex or Amgen, although they did receive proceeds from the sale of the company. Dr. Gillespie has served as a paid advisor to the Program Project at the Ohio State University that seeks to find improved methods for application of distinct oHSV to treat localized and metastatic cancers. This is generally, but not specifically, related to the subject matter of this investigation. Dr. Bernstock has equity (≤ 5°%) in both CITC Ltd and Avidea Technologies.
REFERENCES:
- 1.Cripe TP, Chen C-Y, Denton NL, Haworth KB, Hutzen B, Leddon JL, et al. Pediatric cancer gone viral part I: the potential of oncolytic HSV virotherapy in children. Mol Ther Oncolytics. 2015;2:15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman GK, Beierle EA, Gillespie GY, Markert JM, Waters AM, Chen C-Y, et al. Pediatric cancer gone viral part II: potential clinical application of oncolytic herpes simplex virus-1 in children. Mol Ther Oncolytics. 2015;2:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters AM, Friedman GK, Ring EK, Beierle EA. Oncolytic virotherapy for pediatric malignancies: future prospects. Oncolytic Virother. 2016;5:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streby KA, Geller JI, Currier MA, Warren PS, Racadio JM, Towbin AJ, et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin Cancer Res. 2017;23(14):3566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters AM, Johnston JM, Reddy AT, Fiveash J, Madan-Swain A, Kachurak K, et al. Rationale and Design of a Phase 1 Clinical Trial to Evaluate HSV G207 Alone or with a Single Radiation Dose in Children with Progressive or Recurrent Malignant Supratentorial Brain Tumors. Hum Gene Ther Clin Dev. 2017;28(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mineta T, Rabkin S, Yazaki T, Hunter W, Martuza R. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–43. [DOI] [PubMed] [Google Scholar]
- 7.Sundaresan P, Hunter WD, Martuza RL, Rabkin SD. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation in mice. J Virol. 2000;74(8):3832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter WD, Martuza RL, Feigenbaum F, Todo T, Mineta T, Yazaki T, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73(8):6319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(1):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markert J, Medlock M, Rabkin S, Gillespie G, Todo T, Hunter W, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. [DOI] [PubMed] [Google Scholar]
- 11.Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014;22:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, et al. Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol. 2015;16 Suppl 10:x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman GK, Moore BP, Nan L, Kelly VM, Etminan T, Langford CP, et al. Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol. 2016;18(2):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman GK, Langford CP, Coleman JM, Cassady KA, Parker JN, Markert JM, et al. Engineered herpes simplex viruses efficiently infect and kill CD133+human glioma xenograft cells that express CD111. J Neuro-Oncol. 2009;95(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Studebaker AW, Hutzen BJ, Pierson CR, Haworth KB, Cripe TP, Jackson EM, et al. Oncolytic Herpes Virus rRp450 Shows Efficacy in Orthotopic Xenograft Group 3/4 Medulloblastomas and Atypical Teratoid/Rhabdoid Tumors. Mol Ther Oncolytics. 2017;6:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman GK, Bernstock JD, Chen D, Nan L, Moore BP, Kelly VM, et al. Enhanced Sensitivity of Patient-Derived Pediatric High-Grade Brain Tumor Xenografts to Oncolytic HSV-1 Virotherapy Correlates with Nectin-1 Expression. Scientific reports. 2018;8(1):13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah AC, Price KH, Parker JN, Samuel SL, Meleth S, Cassady KA, et al. Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors. Journal of virology. 2006;80(15):7308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreansky S, He B, van Cott J, McGhee J, Markert JM, Gillespie GY, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5(1):121–30. [DOI] [PubMed] [Google Scholar]
- 19.Kastrukoff LF, Lau AS, Thomas EE. The effect of mouse strain on herpes simplex virus type 1 (HSV-1) infection of the central nervous system (CNS). Herpesviridae. 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halford WP, Balliet JW, Gebhardt BM. Re-evaluating natural resistance to herpes simplex virus type 1. J Virol. 2004;78(18):10086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassady KA, Bauer DF, Roth J, Chambers MR, Shoeb T, Coleman J, et al. Pre-clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-human Primates. Mol Ther Oncolytics. 2017;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstock JD, Wright Z, Bag AK, Gessler F, Gillespie GY, Markert JM, et al. Stereotactic Placement of Intratumoral Catheters for Continuous Infusion Delivery of HSV-1 G207 in Pediatric Malignant Supratentorial Brain Tumors. World Neurosurg. 2019;122:e1592–e1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol. 2015;33(27):2986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramaswamy V, Taylor MD. Medulloblastoma: From Myth to Molecular. J Clin Oncol. 2017;35(21):2355–63. [DOI] [PubMed] [Google Scholar]
- 26.Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packer RJ, Sutton LN, Atkins TE, Radcliffe J, Bunin GR, D'Angio G, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70(5):707–13. [DOI] [PubMed] [Google Scholar]
- 28.Ribi K, Relly C, Landolt MA, Alber FD, Boltshauser E, Grotzer MA. Outcome of medulloblastoma in children: long-term complications and quality of life. Neuropediatrics. 2005;36(6):357–65. [DOI] [PubMed] [Google Scholar]
- 29.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science (New York, NY). 1990;250:1262–6. [DOI] [PubMed] [Google Scholar]
- 30.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(3):843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993;91(6):2837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]