Abstract
The severe form of coronavirus disease 19 (COVID-19) is characterized by cytokine storm syndrome (CSS) and disseminated intravascular coagulation (DIC). Diabetes, obesity, and hypertension have, as minor common denominators, chronic low-grade inflammation and high plasma myeloperoxidase levels, which could be linked to pulmonary phagocytic hyperactivation and CSS. The hyperactivation of M1 macrophages with a proinflammatory phenotype, which is linked to aerobic glycolysis, leads to the recruitment of monocytes, neutrophils, and platelets from circulating blood and plays a crucial role in thrombo-inflammation (as recently demonstrated in COVID-19) through the formation of neutrophil extracellular traps and monocyte-platelet aggregates, which could be responsible for DIC. The modulation of glucose availability for activated M1 macrophages by means of a eucaloric ketogenic diet (EKD) could represent a possible metabolic tool for reducing adenosine triphosphate production from aerobic glycolysis in the M1 macrophage phenotype during the exudative phase. This approach could reduce the overproduction of cytokines and, consequently, the accumulation of neutrophils, monocytes, and platelets from the blood. Second, an EKD could be advantageous for the metabolism of anti-inflammatory M2 macrophages because these cells predominantly express oxidative phosphorylation enzymes and are best fed by the oxidation of fatty acids in the mitochondria. An EKD could guarantee the availability of free fatty acids, which are an optimal fuel supply for these cells. Third, an EKD, which could reduce high lactate formation by macrophages due to glycolysis, could favor the production of interferon type I, which are inhibited by excessive lactate production. From a practical point of view, the hypothesis, in addition to being proven in clinical studies, must obviously take into account the contraindications of an EKD, particularly type 1 or 2 diabetes treated with drugs that can cause hypoglycemia, to avoid the risk for side effects of the diet.
Keywords: Ketogenic diet, Cytokine storm syndrome, COVID-19, Macrophage, Disseminated intravascular coagulation, Warburg effect, Aerobic glycolysis, Interferon type I, Hyperglycemia, Alveolar cell type II, Obesity, Diabetes
Highlights
-
•
Macrophage hyperactivation in COVID-19 is linked to cytokine storm syndrome.
-
•
Macrophage phenotype M1 in the exudative phase metabolically depend on aerobic glycolysis (Warburg-like effect).
-
•
M1 recruitment of neutrophil and platelets plays a crucial thrombo-inflammatory role.
-
•
Eucaloric ketogenic diet (EKD) could immunomodulate macrophage M1 limiting cytokine storm syndrome.
-
•
EKD could guarantee optimal fuel supply for phenotype M2 macrophages.
-
•
EKD, limiting lactate production, could stimulate type I interferon synthesis.
-
•
Viral replication could be inhibited by the antiglycolytic action of EKD.
Introduction
Coronavirus disease 19 (COVID-19) is a predominantly respiratory viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Recent evidence suggests that the subgroup of patients who have the most severe clinical manifestation of COVID-19 and require hospitalization in intensive care units may have a cytokine storm syndrome (CSS) [1].
CSS is characterized by acute respiratory distress syndrome (ARDS) and septic shock, followed by multiorgan failure and disseminated intravascular coagulation (DIC), which represent the main causes of mortality [1], [2], [3].
CSS seems to be due to the hyperactivation of the innate immune system by SARS-CoV-2 and the hyperproduction of proinflammatory cytokines and chemokines in patients with more severe disease [1], [2], [3], [4].
In particular, CSS is more frequently observed in patients with diabetes, hypertension, cardiovascular disease, or obesity [4,5]. This last clinical condition is characterized by systemic chronic inflammation, increased complement system activation and interleukin (IL)-6 secretion [5].
In this regard, it has been suggested that mild immunosuppression should be achieved to avoid progression toward CSS [1].
In COVID-19, interstitial pneumonia causes significant hypoxemia, which significantly reduces the energy input from cellular metabolism in alveolar epithelial cell type II (ATII) and macrophage cells and increases the uptake and utilization of glucose via glycolysis to obtain energy [6], [7], [8]. Oxidative phosphorylation (OXPHOS) in the mitochondria and gluconeogenesis are reduced; however, reactive oxygen species (ROS) and cytokine release are increased, as previously observed in severe acute respiratory syndrome (SARS) [6], [7], [8].
In autopsy findings of patients with SARS, coronavirus RNA was detected in ATII and macrophage cells [8]. Indeed, coronavirus, as observed in SARS, binds to the SARS receptor angiotensin-converting enzyme 2 to infect ATII cells, which represent the first line of innate alveolar immunity [8,9].
ATII cells release cytokines and chemokines that activate alveolar macrophages and lead to the migration of neutrophils [7], [8], [9], [10]. Due to genetic and epigenetic factors [10], the intensity of cytokine production by macrophages is variable and, if excessive, could be deleterious, leading to ARDS [9,10].
In the course of ARDS, the presence of neutrophils in the lungs is described, and it has been proposed that ROS derived from leukocyte myeloperoxidase (MPO; present in neutrophils) are responsible for the development of lung tissue injury [3,10].
MPO can modulate immune responses by activating macrophages [7,11]. The exposure of macrophages to MPO in vitro results in the release of tumor necrosis factor (TNF)-α, low levels of interferon (IFN)-γ, and increased macrophage-dependent cytotoxicity [11].
Hyperglycemia, which may be caused by stress and infection and has been reported in 51% of patients with COVID-19 [12], is poorly compensated in patients with diabetes and might be a contributing factor to COVID-19 mortality.
It is well known that diabetes and obesity often are associated with an altered inflammatory response, which leads to a greater vulnerability of the host to new inflammatory situations, such as infections and fever. In hyperglycemia, macrophage numbers increase in tissues due to increased monocyte infiltration and in situ macrophage proliferation [13].
Diabetes, obesity, and hypertension have, as minor common denominators, chronic low-grade inflammation and high plasma MPO levels, which could be linked to pulmonary phagocytic hyperactivation and CSS. MPO could amplify oxidative stress in hypertension in the presence of hyperglycemia [11,13,14].
Currently, there is increasing evidence to suggest that macrophages, including resident alveolar macrophages and macrophages recruited from the blood, are crucial in the pathogenesis of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) [10].
In the alveoli, AM are located in the interface between the air and cellular tissue as a uniform and dormant population. During the exudative phase of ALI/ARDS, resident AMs are activated and differentiate into the M1 phenotype. Proinflammatory cytokines (IFN-γ, TNF-α, and IL-1 β) are secreted by M1 macrophages into the site of inflammation, recruiting monocytes from the blood by means of monocyte chemoattractant protein and driving them toward the M1 phenotype [10]. At the end of the process, the M1 phenotype shifts to the M2 phenotype, which eliminates apoptotic cells, debris, and pathogens. These resident macrophages represent the frontline against viruses and release powerful inflammatory mediators, such as IL-1, IL-6, and IL-18 [10].
M1 chemokines (i.e., macrophage inflammatory protein-2 and IL-8) attract neutrophils from the circulating blood to the alveolar space. As previously reported, the infiltration of neutrophils and the excessive accumulation of inflammatory cytokines are important factors that cause tissue damage due to proinflammatory cytotoxic mediators in ARDS [10,15].
Additionally, in acute pulmonary lesions of ARDS, platelets and neutrophils are observed and play crucial roles in thrombo-inflammation (as recently suggested in COVID-19), forming neutrophil extracellular traps and monocyte–platelet aggregates that could be responsible for DIC [2,15].
Therefore, in the exudative phase, a possible depletion of activated M1 macrophages could reduce neutrophil-induced alveolitis by reducing inflammatory stimulation; however, this is difficult to achieve outside of experimental models [10,15].
A possible clinical solution that reduces the activity of M1 macrophages in the early stages of inflammation could be to target their metabolic specificity [9,10,15].
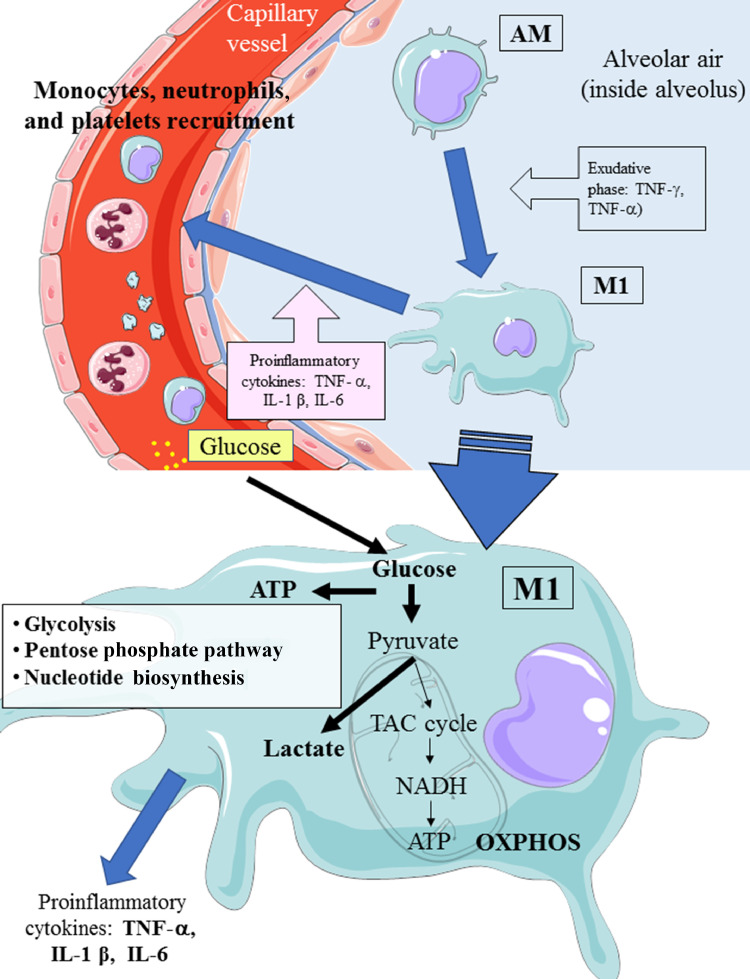
From a metabolic point of view, activation of the M1 phenotype induces a metabolic shift in adenosine triphosphate (ATP) production from OXPHOS to aerobic glycolysis (Warburg-like effect; Fig. 1 ) [9,10]. The activity of the tricarboxylic acid (TCA) cycle is reduced; whereas lactate production is increased in the presence of hypoxia, glucose overload, or both [6,16].
Fig. 1.
Possible metabolic pathways during the activation of M1 from AM during COVID-19 infection. AM, alveolar quiescent macrophage; ATP, adenosine triphosphate; IFN, interferon; IL, interleukin; M1, activated macrophage; MCP, monocyte chemoattractant protein; OXPHOS, oxidative phosphorylation; TAC cycle, tricacrboxylic acid cycle; TNF, tumor necrosis factor. This figure was drawn adapting the vector image form the Servier Medical Art bank (http://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
After the activation of hypoxia-inducible factors, ATII cells exclusively employ glycolysis throughout changes in epigenomic and metabolomic signaling pathways [6,16].
Zhang et al. demonstrated that under conditions of glucose overload and high lactate production, the binding of mitochondrial antiviral signaling (MAVS) to lactate in ATII cells interrupts MAVS/mitochondria localization and consequently reduces innate immune type I interferon (IFN I) production; these phenomena play vital roles in host defense against viruses [17]. This fact could explain why a weaker IFN response was generated by SARS than by traditional IFN-inducing viruses [18].
Hypothesis
We hypothesize that a eucaloric ketogenic diet (EKD), reducing the dietetic oral supply of glucose, could favor the anti-inflammatory process through the modulation of immune metabolism.
The metabolic modulation induced by a ketogenic diet (KD) can affect the following four targets: inhibition of M1 macrophages, activation of M2 macrophages, disinhibition of the IFN-I production induced by the overproduction of lactate, and decreased virus synthesis in cells.
The reduction of glucose uptake by M1 macrophages represents the main target of the metabolic treatment of CSS because aerobic glycolysis is the main way by which ATP is produced in activated M1 macrophages and enables M1 macrophages to carry out effector functions, such as inflammatory cytokine production [19] (Fig. 2 ).
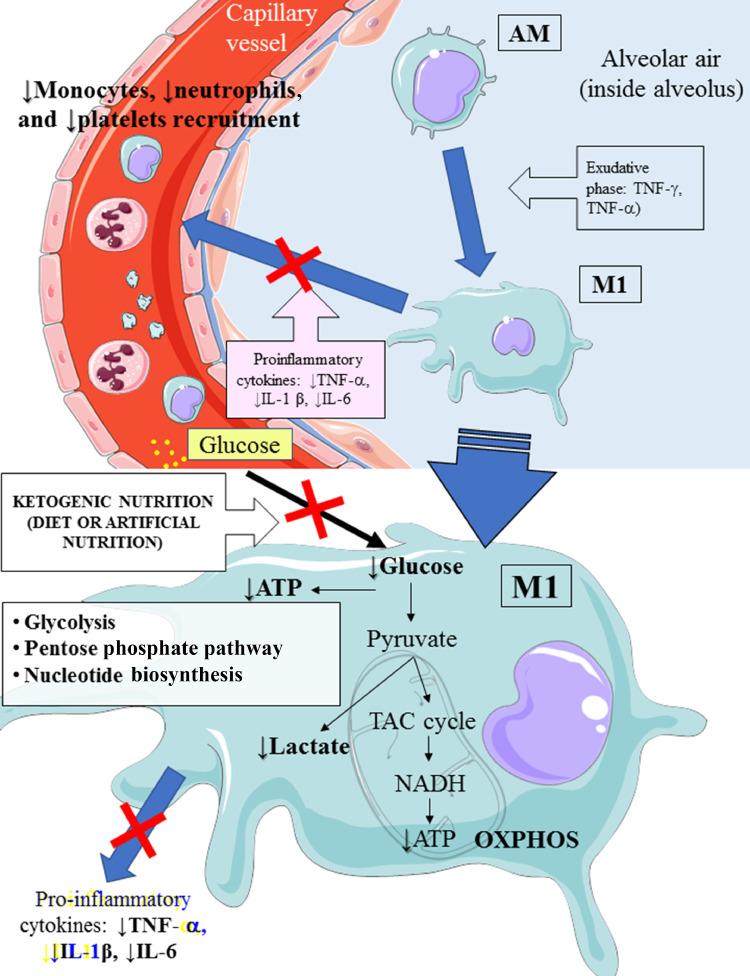
Fig. 2.
Hypothetical attenuation of phagocyte hyperactivation by means of an EKD. An EKD could reduce glucose availability for aerobic glycolysis (Warburg-like effect) in M1 macrophages. The main target of this approach is to inhibit M1 phagocyte hyperactivation, which provokes the overproduction of proinflammatory cytokines (IFN-γ, TNF-α, and IL-1 β), leading to excessive accrual of monocytes, neutrophils, and platelets from the blood. AM, alveolar quiescent macrophage; ATP, adenosine triphosphate; EKD, eucaloric ketogenic diet; IFN, interferon; IL, interleukin; M1, activated macrophage; OXPHOS, oxidative phosphorylation; TAC cycle,tricarboxylic acid cycle ; TNF, tumor necrosis factor. This figure was drawn adapting the vector image form the Servier Medical Art bank (http://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Second, an EKD could be advantageous for the metabolism of anti-inflammatory M2 macrophage metabolism because these cells are best fed by the oxidation of fatty acids in the mitochondria [9,10]. These anti-inflammatory cells, which are derived from M1 cells, appear in the rehabilitation phase of ALI/ARDS and limit proinflammatory cytokines in the alveolar space through the production of anti-inflammatory cytokines (IL-10 and IL-1) [9,15].
M2 macrophages predominantly expresses OXPHOS enzymes, and for this reason, an EKD could guarantee the availability of free fatty acids, which are an optimal fuel supply for these cells.
Third, because the modulation of the glucose load could reduce the production of lactate, there would be a better production of IFN-I, which is inhibited by the excessive production of lactate [17].
A final metabolic hypothesis in the treatment of COVID-19 emerges from a recent in vitro study of the proteomics of host cells infected with SARS-CoV-2, which aimed to detect potential therapeutic targets. Recently, Bojkova et al. observed that by targeting glycolysis with the deoxy-d-glucose glycolysis inhibitor, an exokinase inhibitor that is effective against other cultivated viruses, the replication of COVID-19 in Caco-2 cells was inhibited [20]. Because agents that reduce activity of glycolysis could be potential therapeutic agents for the treatment of COVID-19 [20], a similar antiglycolytic effect could be obtained by means of KDs. A summary of the hypothesis is provided in Table 1 .
Table 1.
Hypothetical effects of eucaloric ketogenic diets in COVID-19
| Inhibition of Warburg-like effect |
| • Modulation of CSS by reducing metabolism of M1 macrophage phenotype by reducing glucose availability |
| • Metabolic promotion of the anti-inflammatory M2 macrophage phenotype fueled by fatty acids |
| • Reduced production of lactate associated with disinhibition of IFN-I production |
| Antiglycolytic effects in infected cells leading to inhibition of virus replication |
CSS, cytokine storm syndrome; IFN, interferon
In obese patients affected by COVID-19, a low-calorie KD could also be used, considering the various dietary therapeutic models described in the literature [21].
The term ketogenic diet describes a variety of diets of varying composition that are rich in fat, very low in carbohydrates, and are classically composed of a 4:1 macronutrient ratio of fat to protein (which varies according to the desired caloric intake) and carbohydrates <30 g/d [21].
Although low-calorie KDs and very low-calorie KDs (VLCKDs) are used mainly in the treatment of obesity, EKDs are clinically used in the treatment of refractory epilepsy but may also have therapeutic effects in the treatment of gliomas and other diseases [21], [22], [23]. Many of these diseases present underlying metabolic diseases and chronic inflammation that have been linked to a state of hyperglycemia [24], [25], [26], [27], [28], [29], [30].
Evidence that supports hypothesis
There is a consensus that ketosis protects healthy tissues against oxidative stress by simultaneously decreasing ROS production and increasing endogenous antioxidant capacity, even if hypoxia has metabolic effects similar to those of hyperglycemia such that reducing hyperglycemia might not cause substantial metabolic shifts [27], [28], [29].
A KD minimizes spikes in blood glucose, reduces oxidative stress in mice [29], and reduces circulating inflammatory markers in humans [30]. A KD, increasing the levels of hydroxybutyrate, is capable of activating hydroxycarboxylic acid receptor 2, which is a G protein-coupled receptor that inhibits nuclear factor-κB in macrophages, dendritic cellsn and microglia and reduces neuroinflammation [31], [32], [33].
In preclinical studies in mice, a KD provoked an expansion of γδ T cells in the lung, improving barrier functions and antiviral resistance against influenza A virus [34].
KDs are associated with improvement in respiratory function in obese patients [35,36] After 10 d of a VLCKD, statistically significant improvements in the functional residual capacity and expiratory reserve volume were observed [35]. Additionally, a 20-d VLCKD shows a significant decrease in end-tidal carbon dioxide tension [36].
Carbohydrate restriction represents the most important tool in diabetes management [37], and KDs offer further benefits [23]. In intensive care units (ICUs), there is a close relationship between glycemia upon admission and mortality, not only in patients with type 2 diabetes but also in those without a history of diabetes mellitus (in which the form is U-shaped) [38].
According to the literature, hyperlipidic diets, even if not ketogenic, could be beneficial in ICU patients undergoing artificial ventilation [39,40], and they could even improve respiratory failure [41].
Limitations of the hypothesis
Before ketosis occurs, glucose may be released during the first 12 h from glycogen reserves and from 24 to 72 h by gluconeogenesis (from gluconeogenetic amino acids); thus, the effect of the diet can only be observed starting after 72 h, when glycerol becomes the main substrate for gluconeogenesis. According to this consideration, it might be more beneficial to start the treatment at the onset of symptoms, which could represent a limitation to the efficacy of the treatment, even if a low intake of glucose could also be beneficial.
A second limitation is that hypoxia has effects on redox potential that could be increased by ketogenic diet. The lack of glucose could lead to an insufficient NADPH/NADP+ ratio necessary to counteract the acute oxidative challenge (rapid enzymatic reduction of glutathione and other oxidized thiols), biosynthesis, and/or superoxide generation during immune responses or as physiologic redox signaling [42].
Oxidative pentose phosphate pathway (PPP), however, remains active under conditions of limited glucose intake because it is a thermodynamically more favorable process than glycolysis [42].
The reactions of the TCA cycle can be effectively maintained in the limited glucose supply by the oxidation of fats and amino acids and the minimum glucose content is redirected to the PPP to maintain the redox balance in the cells [42].
Additionally, the KDs restore the physiologic redox signaling by means of the β-hydroxybutyrate ketone contributing to the protection against oxidative stress by decreasing the production of reactive mitochondrial oxygen species [43].
Another limitation could be the possible difficulty in the use of EKD due to contraindications, in particular in some patients with diabetes in whom a KD is not indicated (Table 2 ). EKD can be implemented in patients with diabetes with great care, and many hypothetical contraindications have been discredited. In this regard, Bruci et al. recently demonstrated that KDs have no detrimental effects on the kidney in mild renal failure [44], and good evidence is available regarding liver safety as well [20].
Table 2.
Main contraindications for ketogenic diets
| Type 1 diabetes mellitus |
| Type 2 diabetes treated with: |
| Insulin |
| • Derivatives of sulfonylurea |
| • Secretagogues non sulfonylurea (i.e., repaglinide) |
| • Analogs of GLP-1 (i.e., exenatide, liraglutide, and lixisenatide) |
| • SGLT2 inhibitors (risk for euglycemic diabetic ketoacidosis; i.e., dapagliflozin, canagliflozin, and empagliflozin) |
| Acute cardiovascular event within 1 mo |
| Food allergies to diet components |
| Any metabolic disorder that may affect gluconeogenesis or the ability to adapt to periods of hypoglycemic diet (i.e., pyruvate kinase deficiency) |
| Pregnancy and breastfeeding |
| Pancreatitis |
| Liver failure |
| Disorders of fat metabolism (primary carnitine deficiency, carnitine palmitoyltransferase, carnitine deficiency, translocase deficiency, etc.) |
| Porphyrias |
A recent report by Li et al. suggests that COVID‐19 infection may cause ketosis and ketoacidosis, which are correlated with a longer median hospital length of stay and a higher mortality rate [45].
Ketosis is a physiologic process that is observed after prolonged fasting, and ketonuria and ketonemia are detectable after ≥1 d of fasting [46,47]. Ketone bodies increase under conditions of fasting, starvation, and low-carbohydrate diets (i.e., KD); intense exercise; and uncompensated diabetes mellitus [48].
The study does not report any information regarding nutritional status, spontaneous oral food and water intake, or artificial nutritional support (parenteral or enteral nutrition), which are fundamental for verifying any relationship among ketosis, COVID-19 infection, and clinical outcomes.
Patients with ketosis were probably in worse clinical condition and were probably fasting because 21.4% of patients with ketosis versus 6.7% of patients without ketosis (P < 0.002) were receiving invasive mechanical ventilation; 31% of patients with ketosis versus 12% of patients without ketosis (P < 0.001) had digestive disorders; and 28.6% of patients with ketosis versus 13.5% of those without ketosis (P < 0.007) had ARDS.
Finally, the development of ketoacidosis in three patients with diabetes could be caused by poor control of their glycemic status, which is common during starvation or hypercatabolic conditions [49], or by insulin deficiency and dehydration, particularly if the patients were treated with hypoglycemic drugs [50]. However, in a paper by Li et al. there is no information about the treatment of diabetes in these patients [45]. In the absence of nutritional and diabetologic data, it is quite difficult to hypothesize a cause–effect relationship between ketosis and COVID-19 infection. The only plausible reason why patients were in ketosis at admission was the prolongation of physiologic fasting conditions due to starvation, which is frequently observed in patients with ARDS with COVID-19, especially if subintensive or intensive care is required [51].
To our knowledge, apart from this report, no other study actually suggests a possible exacerbation of the clinical conditions of ARDS and COVID-19 patients due to a KD, and a trial concerning the use of KDs in critical care patients is ongoing [52].
How this hypothesis may be tested
For this purpose, a randomized controlled trial has been developed at IRCCS San Martino, Genoa, Italy, and submitted to the Regional Ethical Committee (KETOCOV-1 protocol 10517). The trial involves the treatment of patients with moderate severity COVID-19 in inpatient centers by using an EKD with natural Mediterranean food. The main endpoint is to try to prevent the progression toward CSS, ARDS, and mortality/transfer to subintensive/ICUs or to prevent the need for continuous positive airway pressure or intubation.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
SGS was responsible for the conceptualization and design of the study and writing the manuscript. MB was responsible for the study design.
References
- 1.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thachil J. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/JTH.14810. Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS‐CoV‐2: a population to safeguard [Epub ahead of print] Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 6.Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome–coronavirus. Am J Respir Cell Mol Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshikawa T., Hill T., Li K., Clarence J.P., Chien-Te K.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly B., O'Neill L. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X., Xiu H., Zhang S., Zhang G. The Role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate R.M., Repine J.E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu S., Bian Z., Tremblay A., Luo Y., Kidder K., Mansour A. Broad infiltration of macrophages leads to a proinflammatory state in streptozotocin-induced hyperglycemic mice. J Immunol. 2016;197:3293–3301. doi: 10.4049/jimmunol.1502494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Zwan L.P., Scheffer P.G., Dekker J.M., Stehouwer C.D., Heine R.J., Teerlink T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxidase and blood pressure. Hypertension. 2010;55:1366–1372. doi: 10.1161/HYPERTENSIONAHA.109.147231. [DOI] [PubMed] [Google Scholar]
- 15.Matthay M.A., Ware L.B., Zimmerman G A. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lottes R.G., Newton D.A., Syropoulos D.D., Baatz J.E. Lactate as substrate for mitochondrial respiration in alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Phys. 2015;309:L953–L961. doi: 10.1152/ajplung.00335.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., Wang G., Xu Z.G., Tu H., Hu F., Dai J. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell. 2019;178:176–189. doi: 10.1016/j.cell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scagnolari C., Trombetti S., Cicetti S., Antonelli S., Selvaggi C., Perrone L. Severe acute respiratory syndrome coronavirus elicits a weak interferon response compared to traditional interferon-inducing viruses. Intervirology. 2008;51:217–223. doi: 10.1159/000154258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everts B., Amiel E., Huang S.C.C., Smith A., Chang C.H., Lam W.Y. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe M., Tozzi R., Risi R., Tuccinardi D., Mariani S., Basciani S. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes Rev. 2020;21:e13024. doi: 10.1111/obr.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poff A., Koutnik A.P., Egan K.M., Sahebjam S., D'Agostino D., Kumar N.B. Targeting the Warburg effect for cancer treatment: ketogenic diets for management of glioma. Semin Cancer Biol. 2019;56:135–148. doi: 10.1016/j.semcancer.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hite A.H., Berkowitz V.G., Berkowitz K. Low-carbohydrate diet review: shifting the paradigm. Nutr Clin Pract. 2011;26:300–308. doi: 10.1177/0884533611405791. [DOI] [PubMed] [Google Scholar]
- 24.Paoli A., Rubini A., Volek J.S., Grimaldi K.A. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative Diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 26.Picard M., Turnbull D.M. Linking the metabolic state and mitochondrial DNA inchronic disease, health and aging. Diabetes. 2013;63:672–678. doi: 10.2337/db12-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito K., Marfella R., Giugliano D. Stress hyperglycemia, inflammation, and cardiovascular events. Diabetes Care. 2003;26:1650–1651. doi: 10.2337/diacare.26.5.1650-a. [DOI] [PubMed] [Google Scholar]
- 28.Gyurko R., Siqueira C.C., Caldon N., Gao L., Kantarci A., Van Dyke T.E. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nandivada P., Fell G.L., Pan A.H., Nose V., Ling P.R., Bistrian B.R. Eucaloric ketogenic diet reduces hypoglycemia and inflammation in mice with endotoxemia. Lipids. 2016;51:703–714. doi: 10.1007/s11745-016-4156-7. [DOI] [PubMed] [Google Scholar]
- 30.Veech R.L. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Maalouf M., Rho J.M., Mattson M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman M., Muhammad S., Khan M.A., Chen H., Ridder D.A., Muller-Fielitz H. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 33.Zandi-Nejad K., Takakura A., Jurewicz M., Chadraker A.K., Offermanns S., Mount D. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. 2013;27:4366–4374. doi: 10.1096/fj.12-223933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg E.L., Molony R.D., Kudo E., Sidorov S., Kong Y., Dixit V.D. Ketogenic diet activates protective γδ T cell responses against influenza virus infection. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aav2026. eaav2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sukkar S.G., Signori A., Borrini C., Barisione G., Ivaldi C., Romeo C. Feasibility of protein-sparing modified fast by tube (ProMoFasT) in obesity treatment: a phase II pilot trial on clinical safety and efficacy (appetite control, body composition, muscular strength, metabolic pattern, pulmonary function test) Med J Nutrition Metab. 2013;6:165–176. doi: 10.1007/s12349-013-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alessandro R., Gerardo B., Alessandra L., Cenci L., Parmagnani A., Grimaldi K. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung. 2015;193:939–945. doi: 10.1007/s00408-015-9806-7. [DOI] [PubMed] [Google Scholar]
- 37.Feinman R.D., Pogozelski W.K., Astrup A., Bernstein R.K., Fine E.J., Westman E.C. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Gunst J., De Bruyn A., Van den Berghe G. Glucose control in the ICU. Curr Opin Anaesthesiol. 2019;32:156–162. doi: 10.1097/ACO.0000000000000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Saady N.M., Blackmore C.M., Bennett E.D. High fat, low carbohydrate, enteral feeding lowers PaCO2 and reduces the period of ventilation in artificially ventilated patients. Intensive Care Med. 1989;15:290–295. doi: 10.1007/BF00263863. [DOI] [PubMed] [Google Scholar]
- 40.Van den Berg B., Bogaard J.M., Hop W.C.J. High fat, low carbohydrate, enteral feeding in patients weaning from the ventilator. Intensive Care Med. 1994;20:470–475. doi: 10.1007/BF01711897. [DOI] [PubMed] [Google Scholar]
- 41.Venkat G., Tirlapur M., Afzal M. Effect of low calorie intake on abnormal pulmonary physiology in patients with chronic hypercapneic respiratory failure. Am J Med. 1984;77:987–994. doi: 10.1016/0002-9343(84)90177-3. [DOI] [PubMed] [Google Scholar]
- 42.Cherkas A., Holota S., Mdzinarashvili T., Gabbianelli R., Zarkovic N. Glucose as a major antioxidant: when, what for and why it fails? Antioxidants. 2020;9:140. doi: 10.3390/antiox9020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller V.J., Villamena F.A., Volek J.S. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018 doi: 10.1155/2018/5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruci A., Tuccinardi D., Tozzi R., Balena A., Santucci S., Frontani R. Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. 2020;12:333. doi: 10.3390/nu12020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis [Epub ahead of print] Diabetes Obes Metab. 2020 doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alemany M. Adjustment to dietary energy availability: from starvation to overnutrition. RSC Adv. 2013;3:1636–1651. [Google Scholar]
- 47.Elia M., Wood S., Khan K., Pullicino E. Ketone body metabolism in lean male adults during short-term starvation, with particular reference to forearm muscle metabolism. Clin Sci. 1990;78:579–584. doi: 10.1042/cs0780579. [DOI] [PubMed] [Google Scholar]
- 48.Gershuni V.M., Yan S.L., Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. 2018;7:97–106. doi: 10.1007/s13668-018-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soeters M.R., Soeters P.B., Schooneman M.G., Houten S.M., Romijn J.A. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am J Physiol Endocrinol Metab. 2012;303:E1397–E1407. doi: 10.1152/ajpendo.00397.2012. [DOI] [PubMed] [Google Scholar]
- 50.Misra S., Oliver N.S. Diabetic ketoacidosis in adults. BMJ. 2015;351:h5660. doi: 10.1136/bmj.h5660. [DOI] [PubMed] [Google Scholar]
- 51.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keto-diet for Intubated Critical Care COVID-19 (KICC-COVID-19). ClinicalTrials.gov Identifier: NCT04358835. 2020.48.