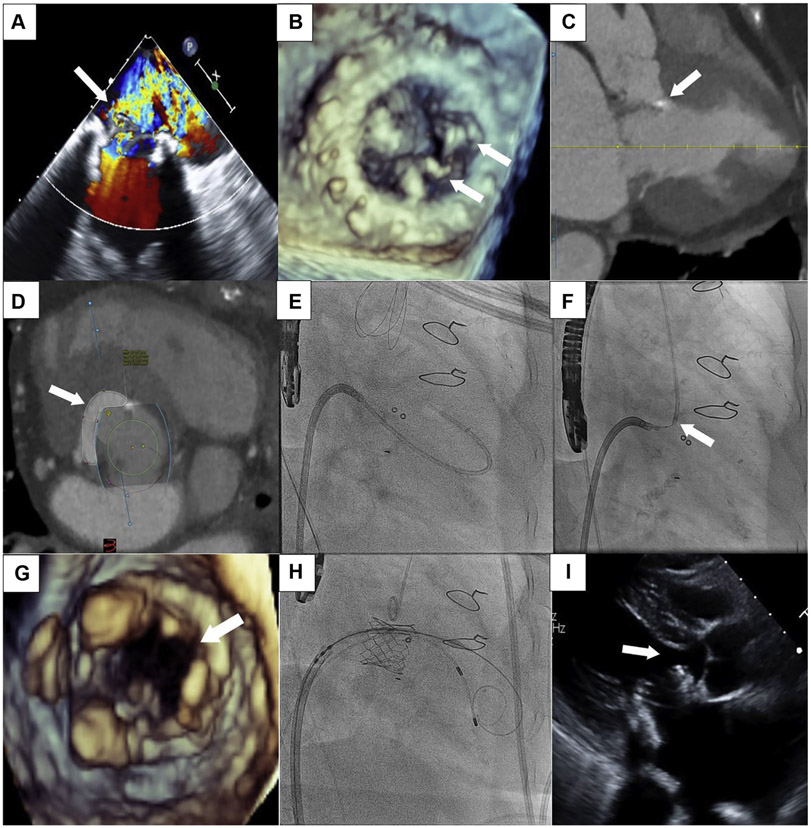
An 84-year-old woman with a history of a 29-mm bioprosthetic Mosaic (Medtronic, Minneapolis, Minnesota) mitral valve replacement presented with heart failure. Transesophageal echocardiography (TEE) demonstrated severe regurgitation (Figure 1A) from torn prosthetic leaflets (Figure 1B). Computed tomography revealed bioprosthetic leaflet near the septum in systole (Figure 1C). Predicted neo-left ventricular outflow tract (LVOT) using virtual 26-mm Sapien S3 (Edwards Lifesciences, Irvine, California) was 177.5 mm2 (Figure 1D). We performed a modification of laceration of the anterior mitral leaflet to prevent LVOT obstruction (LAMPOON) to achieve tip-to-base laceration of the bioprosthetic to prevent LVOT obstruction from valve-in-valve (ViV) transcatheter mitral valve replacement (TMVR).
FIGURE 1. Tip-to-Base LAMPOON to Facilitate ViV TMVR.
(A) Transesophageal echocardiography (TEE) showing severe bioprosthetic mitral regurgitation (arrow). (B) 3-Dimensional TEE demonstrating 2 torn prosthetic leaflets (arrows). (C) Computed tomography demonstrating bioprosthetic leaflet near the septum in systole (arrow). (D) CT predicting neo-left ventricular outflow tract (arrow) of 177.5 mm2. (E) Fluoroscopy showing catheter advanced into the aorta. (F) Fluoroscopy of the tip-to-base LAMPOON electrification of the bioprosthetic leaflet using the “flying-V” (arrow). (G) 3-Dimensional TEE of the split bioprosthetic leaflet (arrow) after tip-to-base LAMPOON. (H) Fluoroscopy of the valve-in-valve (ViV) transmitral valve replacement (TMVR). (I) Post-procedure echocardiogram demonstrating valve close to the septum (arrow).
After transseptal puncture, a balloon-wedge endhole catheter was floated through the mitral prosthesis, aortic valve, into the aorta (Figure 1E). A 0.014-inch Astato XS20 wire (Asahi-Intecc, Tokyo, Japan), focally denuded and kinked mid-shaft to form a “flying-V,” was positioned at the offending bioprosthetic leaflet’s tip using TEE and fluoroscopy (Figure 1F). The guidewire was pulled toward the bioprosthetic valve ring and electrified at 70 W with continuous 5% dextrose flush until it reached the base; TEE confirmed tip-to-base leaflet split (Figure 1G). ViV TMVR was then performed using a 26-mm Sapien S3 valve (Figure 1H). Final assessment demonstrated no evidence of LVOT obstruction (Figure 1I).
This is the first report of tip-to-base (sometimes referred to as “reverse”) LAMPOON to prevent bioprosthetic leaflet LVOT obstruction before ViV TMVR. In native valves, LAMPOON is performed from base to tip to avoid inadvertent laceration of the aortomitral continuity, requiring traversal of the base of the A2 scallop, which can be technically challenging and time-consuming. By contrast, tip-to-base LAMPOON involves lacerating the leaflet from tip to base using a simple venoarterial rail, with the surgical ring of the bioprosthesis preventing extension into the aortomitral continuity. In summary, for select patients with high-risk anatomy undergoing ViV TMVR, tip-to-base LAMPOON appears to be an effective solution to prevent LVOT obstruction.