ABSTRACT
Recent years have witnessed evolution of lung allocation strategies to prioritize sicker recipients. In the pre-transplant period, this has translated into increased utilization of invasive extracorporeal or mechanical ventilatory support as a bridge to lung transplantation. The morbidity associated with these strategies warrants consideration to less invasive respiratory support modalities. Herein, we present a case highlighting successful bridge to lung transplantation with a relatively non-invasive negative pressure ventilator.
INTRODUCTION
Over the last three decades, lung transplantation has evolved as an effective therapy for end- stage lung disease of diverse etiologies. However, paucity of suitable donor organs frequently translates into prolonged waitlist times for patients. Not infrequently, patients experience clinical and potentially terminal decline, while awaiting a transplant. These situations warrant consideration to invasive strategies including mechanical ventilator support as well as extracorporeal membrane oxygenation support (ECMO) as a bridge to lung transplant and a chance at survival. The morbidity associated with these interventions can result in a narrower window of transplant as well as poorer outcomes after transplant compared to patients who do not require bridging. We report a relatively non-invasive negative pressure biphasic cuirass ventilator (BPCV) to successfully bridge a patient in respiratory failure to lung transplantation.
CASE REPORT
A 48-year-old woman with end-stage lung disease secondary to immotile cilia syndrome with bronchiectasis was evaluated for suitability for lung transplantation. She was deemed a satisfactory candidate and placed on the lung transplant waitlist. Baseline arterial blood gas analysis at time of lung transplant listing demonstrated a compensated respiratory acidosis with a partial pressure of carbon dioxide (PaCO2) of 54 mm Hg with hypoxemia with PaO2/FiO2 ratio of 318. Over the ensuing months, while awaiting a suitable donor, she developed worsening respiratory insufficiency. She was diagnosed with bronchiectasis exacerbation and hospitalized for management of respiratory failure. Her arterial blood gas analysis revealed worsening hypercapnia and hypoxemia with a PaCO2 of 68 and PaO2/FiO2 ratio of 153. Therapy for medical management of bronchiectasis exacerbation was initiated with intravenous antibiotics as well as bronchodilators. Attempts at optimization of respiratory failure with non-invasive positive pressure ventilation with BIPAP (Philips Respironics) were complicated by intolerance of mask for prolonged periods of time due to claustrophobia and extreme anxiety. Concerns about the potential adverse impact of prolonged invasive mechanical ventilatory support on patient’s transplant candidacy prompted the medical team to explore the option of negative pressure ventilation (NPV) with BPCV. She was initiated on a control mode with an initial setting of −15 cm H2O synchronized to patient’s inspiration and + 5 cm H2O synchronized for expiration. Frequency as well as inspiratory-to-expiratory ratio was adjusted to synchronize patient efforts. Patient demonstrated easy tolerance for BPCV. Follow-up arterial blood gas after the first 60 min session of BPCV revealed significant improvement in hypercapnia (Fig. 1). Over the next few days, patient was able to maintain acceptable PaCO2 levels with BPCV. She was discharged home with the device with recommendations to use BPCV with sleep as well as on an as needed basis in the day. The patient reported excellent compliance with the device. She also utilized vibration and cough modes of this device on a routine basis to assist with clearance of secretions. Patient underwent successful bilateral sequential lung transplantation approximately 4 months after initiation of BPCV. Patient is currently more than 6 months post lung transplant, and continues to do well with excellent functional and respiratory status.
Figure 1.
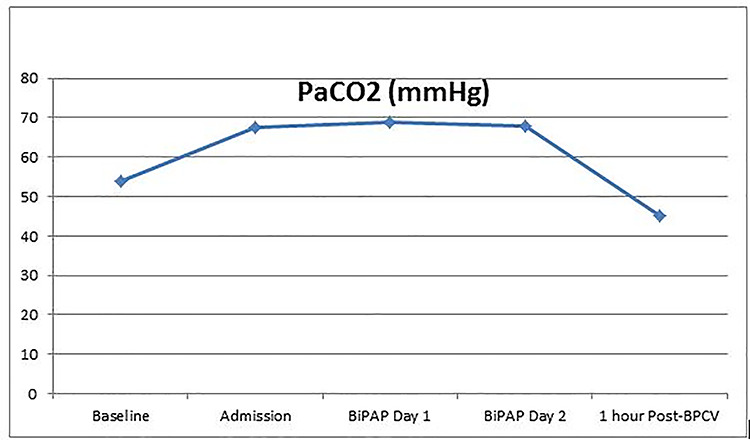
Changes in PaCO2.
DISCUSSION
A change in lung allocation strategy with the introduction of lung allocation score in 2005 has allowed prioritization of donor organs to sicker patients with the most urgent transplant needs. This has necessitated increased reliance on invasive respiratory support strategies to assist in bridging these critically ill patients to transplant. Even though invasive mechanical ventilatory support is still frequently utilized, the last decade has witnessed a growing number of lung transplant programs embrace ECMO support as a bridge to lung transplant. With technical advancements in ECMO circuits as well as improved ECMO care with multidisciplinary teams, outcomes with ECMO bridging are now comparable to invasive mechanical ventilation [1, 2]. Despite these improvements, there is significant morbidity associated with these invasive support strategies. Mechanically ventilated patients remain at risk of rapid deconditioning, hemodynamic compromise, ventilator-induced lung injury and pneumonia. Even though ECMO bridging may allow avoidance of sedation, early mobilization and participation in physical therapy, these patients also remain at significant risk of complications including hemodynamic compromise, bleeding, thromboembolism and limb ischemia. Post-transplant outcomes in patients who require bridging with invasive strategies continue to lag behind those for recipients who do not require bridging [3]. For a select group of patients, NPV offers a less invasive respiratory support strategy as a bridge to lung transplant. NPV applies alternate sub-atmospheric (negative) and atmospheric (zero) pressures around the thorax and the abdomen facilitating airflow into the lung [4]. NPV is suitable for patients with abnormal facial morphologies, excessive oropharyngeal secretions as well as patients who experience anxiety and claustrophobia from non-invasive positive pressure ventilation. NPV has been shown to assist with ventilatory support with effects comparable to non-invasive positive pressure ventilation for avoiding tracheal intubation in COPD patients [5].
BPCV, a newer generation version of the historic iron lung, is a non-invasive negative pressure ventilator. It consists of an extra-thoracic cuirass and a pressure source capable of providing both positive and negative pressures across the chest wall (Fig. 2). Beyond the benefits with ventilation, BPCV has been shown to improve the hemodynamic profile in patients with pulmonary hypertension secondary to underlying lung disease. This benefit has been hypothesized to reflect effects of decreased intrathoracic pressure on eliminating auto-positive end-expiratory pressure with resultant decrease in pulmonary artery occlusion pressure as well as right atrial pressure [6]. In 2005, Shoseyov et al. [7] reported a patient with cystic fibrosis who was successfully weaned off invasive mechanical ventilatory support, decannulated from his tracheostomy and then bridged to lung transplantation with BPCV.
Figure 2.
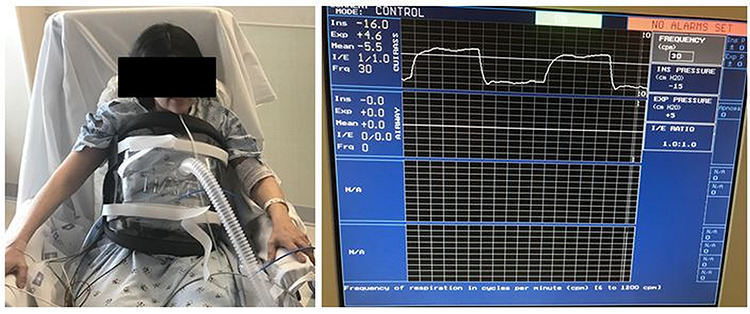
Patient using BPCV.
The physiologic benefits coupled with avoidance of sedation allow active participation in physical rehabilitation, a critical component of pre-lung transplant care. In addition, the ability to talk and eat without the invasion of medical devices promises value beyond the objective measures of outcomes and survival.
Our report adds to the only other prior report highlighting the feasibility of using NPV with BPCV in carefully selected critically ill patients awaiting lung transplant. With an increasing reliance on bridging strategies, time is ripe for prospective investigations to better define the role of NPV in pre-lung transplant patients.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
FUNDING
None.
ETHICAL APPROVAL
No approval is required.
CONSENT
Patient consent was obtained for production of this manuscript.
GUARANTOR
Tathagat Narula, MD.
ACKNOWLEDGEMENTS
None.
REFERENCES
- 1. Hayanga JWA, Hayanga HK, Holmes SD, Ren Y, Shigemura N, Badhwar V et al. Mechanical ventilation and extracorporeal membrane oxygenation as a bridge to lung transplantation: closing the gap. J Heart Lung Transplant 2019;38:1104–11. doi: 10.1016/j.healun.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 2. Mattar A, Chatterjee S, Loor G. Bridging to lung transplantation. Crit Care Clin 2019;35:11–25. doi: 10.1016/j.ccc.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 3. Chiumello D, Coppola S, Froio S, Colombo A, Del Sorbo L. Extracorporeal life support as bridge to lung transplantation: a systematic review. Crit Care 2015;19:19. doi: 10.1186/s13054-014-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med 2001;163:540–77. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 5. Corrado A, Gorini M, Melej R, Baglioni S, Mollica C, Villella G et al. Iron lung versus mask ventilation in acute exacerbation of COPD: a randomised crossover study. Intensive Care Med 2009;35:648–55. doi: 10.1007/s00134-008-1352-9. [DOI] [PubMed] [Google Scholar]
- 6. Sato Y, Saeki N, Asakura T, Aoshiba K, Kotani T. Effects of extrathoracic mechanical ventilation on pulmonary hypertension secondary to lung disease. J Anesth 2016;30:663–70. doi: 10.1007/s00540-016-2172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shoseyov D, Linton D, Kramer MR, Kerem E. Non invasive negative biphasic cuirass ventilator as a bridge to lung transplant in cystic fibrosis. J Cyst Fibros 2005;4:S59–73. [Google Scholar]


