ABSTRACT
The USA is witnessing an outbreak of vaping-induced lung injuries associated with the drastic rise in e-cigarette use, especially among teenagers and young adults. Our understanding of the harmful effects of these products is expanding as an increasing amount of consumers seek medical care for lung-related illnesses. The knowledge of the long-term sequelae of e-cigarette use is limited due to their novelty, but a growing association exists between use and acute lung injury. We describe a case vignette of vaping-induced lung injury to increase physician awareness and discuss the applicability of preliminary diagnostic criteria.
INTRODUCTION
The use of e-cigarettes, colloquially known as vaping, has seen a drastic rise in popularity since its introduction to the USA in 2006, especially among youth. According to the National Youth Tobacco Survey in 2019, the prevalence of self-reported current e-cigarette use was 27.5% among high school students and 10.5% among middle school students [1]. This portable device delivers aerosolized compounds for inhalation to the user [2]. Several risks related to these products have already been documented. Injuries related to vaping have varied in type and severity according to many factors such as the components of the compound used, the amount inhaled and its solubility [3]. The liquid compounds used in e-cigarettes are brand or flavor dependent. They usually contain nicotine, propylene glycol/glycerol, heavy metals (lead, tin, nickel, cobalt, manganese, chromium and arsenic), tetrahydrocannabinol (THC), cannabidiol flavorings in variable concentrations and preservatives like Vitamin-E [4–7]. Pathology associated with use of e-cigarettes has demonstrated a wide range of injury severity, from minor respiratory tract discomfort to pneumonitis, adult respiratory distress syndrome (ARDS), respiratory failure and even cases of death. The pathophysiology of injury involves alveolar inflammation, edema of airways leading to epithelial sloughing and ultimately hypoxemia [8].
CLINICAL CASE
A 29-year-old man with a history of exercise-induced asthma (not requiring treatment for 10 years) and obesity (body mass index 39) presented to the emergency room in respiratory distress with evidence of systemic inflammatory response syndrome. His history was pertinent for heavy e-cigarette use for the previous 2 years. Of note, he admitted that he previously smoked ~1–2 packs of cigarettes and 0.5 g of cannabis per day from ages 19 to 27 but quit both of those when he started vaping. He admitted to owning 12 different vaping pens, 8 of which were used for nicotine and 4 for THC. He used a variety of flavors and estimated that he went through a 30-ml bottle every 7–10 days. He also reported that 5 days prior to the onset of symptoms, he tried a new branded vaping product that contained THC. Two days prior to presentation, he experienced rigors with associated nausea and several episodes of emesis. Despite cessation from all vaping activities, he became increasingly dyspneic with shortness of breath with minor activity prompting him to seek emergent evaluation. Upon presentation, he demonstrated evidence of hypoxemic respiratory distress with severe tachypnea with increased work of breathing and mild accessory muscle use. He reported coughing bouts with deep inhalation and associated production of white frothy sputum. He denied hemoptysis, chest pain, recent illness, travel or sick contacts. His examination was significant for tachycardia, tachypnea and distant breathing sounds with fine crackles in bilateral lung fields. An electrocardiogram showed sinus tachycardia (heart rate 115) with no ST-segment changes. Chest radiograph showed diffuse pulmonary infiltrates bilaterally (Fig. 1). Laboratory evaluation demonstrated an elevated white blood cell count of 19.5 cells/μl (the cell differentials by percentage were all within normal range and there was no eosinophilia present). Troponin was negative, and both brain natriuretic peptide and D-dimer were not significantly elevated (82 pg/ml and 195 ng/ml, respectively).
Figure 1.
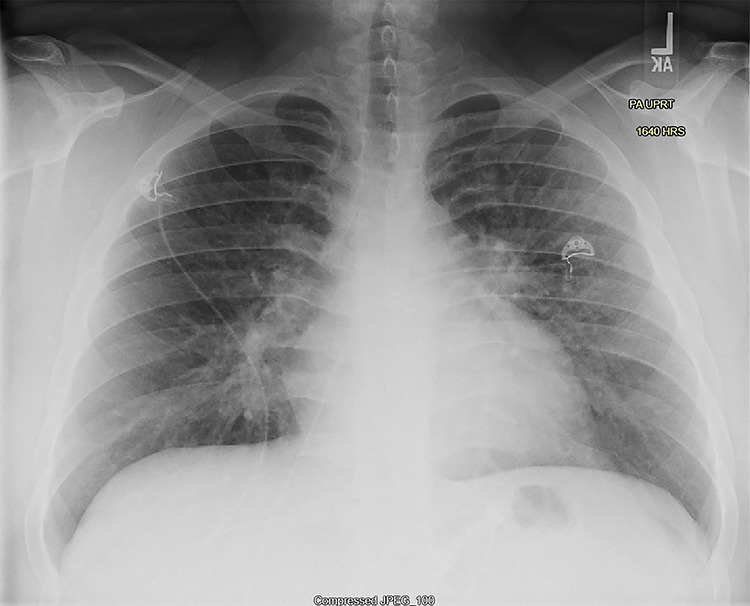
Posterior-anterior chest X-ray on day of presentation shows mild underinflation with diffuse interstitial opacities in a perihilar distribution, slightly greater on the left, without lobar consolidation.
Therapy consisted of scheduled nebulized albuterol, methylprednisolone (30 mg intravenous [i.v.] given twice over 8 hours, and then changed to prednisone 40 mg b.i.d.) and empirical antibiotic treatment with azithromycin and ceftriaxone. The patient continued to have fevers (Tmax 101.6), worsening hypoxemia (requiring up to 12 l via high-flow nasal cannula) and leukocytosis.
On Day 2, the patient’s respiratory status worsened and an arterial blood gas (ABG) analysis revealed a pH of 7.48, pCO2 of 33 and a pO2 of only 48 while on 8 l O2 high-flow nasal cannula. He did meet the Berlin criteria for severe ARDS and therefore was transferred to the intensive care unit (ICU) for closer observation but never required invasive mechanical ventilation. A computerized tomography (CT) chest angiogram with i.v. contrast revealed findings significant for pneumonitis (Fig. 2A–B).
Figure 2.
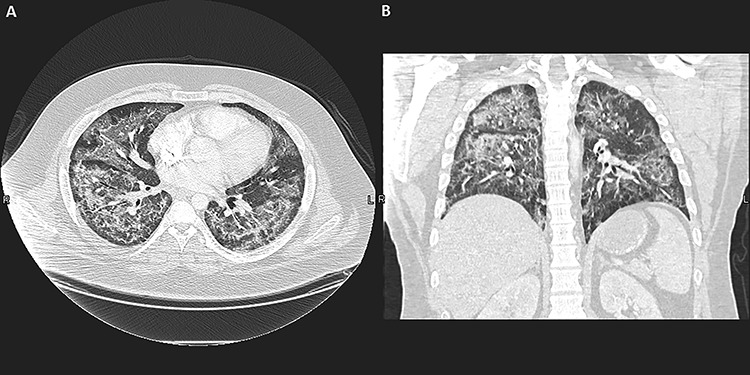
(A) Chest CT scan, axial view, lung window, shows diffuse bilateral ground-glass opacities throughout with subpleural sparing, including the fissures. (B) Chest CT scan, coronal view and lung window, shows disease was more confluent in the superior segment of the right lower lobe with alveolar consolidation. The findings are consistent with pneumonitis, especially with a history of heavy vaping.
At this point, a workup for an infectious source including blood cultures, urine legionella, antigen, urine antigen for Streptococcus pneumoniae, antibody screen for coccidiomycosis, human immunodeficiency virus and respiratory polymerase chain reaction panel was negative. Antinuclear antibody (ANA), antineutrophil cytoplasmic antibodies, cytoplasmic & perinuclear and anti–double-stranded DNA antibody were also negative. An ABG showed respiratory alkalosis with hypoxemia. Acute phase reactants were elevated including: C-reactive protein of 243.93 mg/l, procalcitonin of 0.17 ng/ml and sedimentation rate of 35 mm/h. Antimicrobials were discontinued after 3 days of duration and supportive care continued including supplemental oxygen, steroids and albuterol nebulizer treatments. By the sixth day of his hospital stay, the patient’s symptoms had resolved and was able to ambulate without supplemental oxygen. He was discharged home with an inhaler and to complete a course of prednisone 40 mg daily for five more days. His vaping paraphernalia was submitted to the county health department and was subsequently found to indeed contain THC. His case was also reported to the Centers for Disease Control and Prevention. The patient followed up with his primary care provider within 2 weeks of discharge and no further treatment was indicated. Additionally, upon contacting the patient 8 months after his hospitalization, we were pleased to discover that he has quit using e-cigarettes and has not had any further episodes of respiratory distress or need for an inhaler.
DISCUSSION
E-cigarette or vaping-associated lung injury is a novel disease with recent recognition and correlation to e-cigarettes use. Disease and awareness of this uptrending disease presentation and diagnostic criteria is pivotal for practicing clinicians due to the increasing e-cigarette use and reports of variable-related disease. The Department of Public Health of Illinois and Wisconsin have reported the following surveillance criteria: use of e-cigarettes in the last 90 days and pulmonary infiltrates such as opacities on plain chest radiograph or ground-glass opacities on CT scan and complete negative workup for infection and finally no alternative diagnosis (cardiac, autoimmune, neoplasm, etc.) to explain presenting symptoms.
Layden et al. [9], in one of the most comprehensive descriptive reviews of 98 case patients, reported that patients presented with a combination of respiratory, gastrointestinal and constitutional symptoms (>90%) had abnormal imaging during their evaluation. For these patients, the median duration of hospitalization was 6 days. More than half were admitted to the ICU and one-third of them required mechanical ventilation for respiratory failure. They noted that 45% of cases received antibiotics in the outpatient and 92% received them during hospitalization. Significantly, all the patients who received antibiotics on an outpatient basis had reported progression of respiratory symptoms. Among the 84% of patients who received systemic steroids, 51% had documented clinical improvement attributed to their use. All but five cases received a course of systemic steroid for at least 7 days.
As previously described, this disease process represents a heterogeneous display of lung pathology. In a review article published in the New England Journal of Medicine by Henry et al [10], they classified vaping pathological findings into four categories: (i) diffuse alveolar damage, (ii) lipoid pneumonia, (iii) organizing pneumonia, (iv) acute eosinophilic pneumonia. Of the described cases, subpleural sparing on CT was a common image finding and may be a clue to practitioners. In two cases, pathology was defined as diffuse alveolar hemorrhage and giant cell interstitial pneumonia [10].
In this case presentation, crucial diagnostic indicators include: history of heavy e-cigarette use and use of new product, presentation of acute respiratory distress with negative infectious, autoimmune and cardiac evaluation and imaging demonstrating evidence of pneumonitis. Although VILI is a diagnosis of exclusion with extensive evaluation, treatment with steroids can begin early if the indicators above are present in addition to other supportive measures such as nebulizer treatment and supplemental oxygen. Early recognition may remain key to avoid complications, prolonged hospitalization, unnecessary use of antimicrobials or even death. While more research is being conducted to delineate diagnostic guidelines and treatment recommendations, the key is clinician awareness and recognition of clinical presentation and imaging with exclusion of infectious etiologies for symptoms. Until more information is elucidated or safe products identified, physicians should recommend against the use of vaping products and should provide education to teenagers and young adults about the health risks associated with vaping.
CONFLICT OF INTEREST STATEMENT
None declared. This patient has consented for this publication.
REFERENCES
- 1. Cullen KA, Gentzke AS, Sawdey MD, et al. . E-cigarette use among youth in the United States. JAMA 2019;322:2095–103. doi: 10.1001/jama.2019.18387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic cigarettes—a narrative review for clinicians. Am J Med 2015;128:674–81Epub 27 Feb 27 2015. [DOI] [PubMed] [Google Scholar]
- 3. Kales SN, Christiani DC. Acute chemical emergencies. N Engl J Med 2004;350:800–8. [DOI] [PubMed] [Google Scholar]
- 4. Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, et al. . American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation 2014;130:1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. . Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med 2019. doi: 10.1056/NEJMoa1916433. [DOI] [Google Scholar]
- 6. Pellegrino RM, Tinghino B, Mangiaracina G, Marani A, Vitali M, Protano C, et al. . Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig 2012;24:279–88. [PubMed] [Google Scholar]
- 7. Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. . Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014 Jul;23:iii3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthay MA, Zemans RL, Zimmerman GA, et al. . Acute respiratory distress syndrome. Nat Rev Dis Primers 2019;5:18–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Layden JE, Ghinai I, Pray I, et al. . Pulmonary illness related to e-cigarette use in Illinois and Wisconsin — preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 10. Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]


