Abstract
OBJECTIVES:
A timely, accurate assessment and decision-making process is essential for the diagnosis and treatment of the acute stroke, which is the world's third leading cause of death. This process is often performed using the traditional method that increases the complexity, duration, and medical errors. The present study aimed to design and evaluate an intelligent system for improving adherence to the guidelines on the assessment and treatment of acute stroke patients.
METHODS:
Decision-making rules and data elements were used to predict the severity and to treat patients according to the specialists' opinions and guidelines. A system was then developed based on the intelligent decision-making algorithms. The system was finally evaluated by measuring the accuracy, sensitivity, specificity, applicability, performance, esthetics, information quality, and completeness and rates of medical errors. The segmented regression model was used to evaluate the effect of systems on the level and the trend of guideline adherence for the assessment and treatment of acute stroke.
RESULTS:
Fifty-three data elements were identified and used in the data collection and comprehensive decision-making rules. The rules were organized in a decision tree. In our analysis, 150 patients were included. The system accuracy was 98.30%. Evaluation results indicated an error rate of 1.69% by traditional methods. Documentation quality (completeness) increased from 78.66% to 100%. The average score of system quality was 4.60 indicating an acceptable range. After the system intervention, the mean of the adherence to the guideline significantly increased from 65% to 99.5% (P < 0.0008).
CONCLUSION:
The designed system was accurate and can improve adherence to the guideline for the severity assessment and the determination of a therapeutic trend for acute stroke patients. It leads to physicians' empowerment, significantly reduces medical errors, and improves the documentation quality.
Keywords: Acute stroke, clinical decision-making, guideline adherence, intelligent system
Introduction
Acute stroke is rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting for more than 24 h or leading to death without any apparent cause, unlike vascular origins.[1] This disease affects all ages, but its incidence rate increases by age.[2] According to the statistics, acute stroke affects more than 15 million people in the world every year. It is also known as the third cause of death.[3,4] It is vital to utilize a quick, timely and an accurate method for the assessment and treatment of acute stroke as the wrong and time-consuming decision-making leads to irreparable complications for patients.[5] Assessment and treatment guidelines for acute stroke diseases aim to improve the outcomes and cost-effectiveness. However, it remains a challenge to effectively integrate these guidelines into clinical practice because medical providers do not have adequate sources, time, and mental focus for the treatment to provide accurate healthcare services, and also, they are often faced with incomplete data, leading to increasingly misdiagnosis and medical errors.[6] Using modern technologies, such as intelligent systems, can be an effective way to solve this challenge.[7]
Box-ED.
Acute stroke is rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 h or leading to death with no apparent cause other than that of vascular origin
A quick, timely and accurate assessment and decision-making for the diagnosis and treatment of acute stroke, which is the third leading cause of death in the world, is necessary
This process is often performed using a traditional method that increases the complexity, duration, and medical errors
An intelligent system is an effective tool to implement and apply clinical guidelines
In our study, a rule-based reasoning approach was used to assess the severity and to determine the therapeutic trend of acute stroke patient
This mobile-based intelligent system reduces the error rate and increases the guideline adherence and diagnostic accuracy in the emergency situations.
Intelligent systems were defined as the system that incorporates intelligence into applications being handled by machines. Intelligent systems also perform complex automated tasks that are not possible by the traditional computing paradigm. Rule-based reasoning systems are the form of artificial intelligence system.[8] This system uses rules as the knowledge representation for knowledge coded into the system. The definitions of the rule-based systems depend almost entirely on expert systems, which are a system that mimics the reasoning of a human expert in solving a knowledge-intensive problem. Instead of representing knowledge in a declarative, static way as a collection of facts that are valid, rule-based system represents knowledge in terms of a set of rules that tells what to do in various circumstances or what to infer.[9]
These systems, if properly designed, play an important role in collecting useful information such as initial data, documents, personal knowledge, and business models to solve problems and subsequently allow decision-makers to quickly administrate a huge volume of information computing and processing. They can thus be used as a solution for complex issues and emergency situations that often require quick and accurate responses.[10] Our study was conducted with the aim to design and evaluate an intelligent system for increasing adherence to the guidelines on assessment and treatment of acute stroke to reduce error rates and increase the diagnostic accuracy in emergency situations.
Methods
Information requirement engineering
This process is considered one of the most critical aspects of constructing an intelligent system because during this process is determined what is to be designed.[11] We first observed the acute stroke assessment and treatment workflow in some emergency departments (EDs). Then, we conducted unstructured interviews with four clinicians (emergency medicine specialist and neurologist) to examine the current efficiency of the existing system and determined the major weak and strong points. The clinicians were asked about their perceptions, opinions, beliefs, and attitudes toward the shortcomings of the paper-based workflow. The potential strong points of implementing an intelligent system were explained to them. The Ethics Committee of Tabriz University of Medical Sciences approved the procedures of the study.
Intelligent system was developed in three phases consisting of descriptive, developmental, and evaluation data [Figure 1]. To identify and determine the data elements for patient assessment and determination of acute stroke therapeutic trends in the descriptive phase, guidelines and published resources were first searched and collected on active global websites in the field of acute stroke. Collected resources were then provided for four emergency medicine physicians and neurologists as a checklist. They reviewed the checklist and selected and approved important resources. In the next step, data elements were extracted from verified resources. To determine a decision-making process (decision model), a focus group meeting was held with four physicians (emergency medicine specialist and neurologist) and three technical specialists as the members of the intelligent system team who were responsible for system implementation in a clinical environment. During this meeting, team experts carried out a focus group discussion that was facilitated by a health information technology expert and used the verified data elements to create decision-making rules.
Figure 1.

Overview of methodology
System design and development
In the design and development phase, the system was developed in an agile methodology based on the extracted rules and knowledge in the previous phase.
In the design phase, the system architecture was proposed based on the existing technologies for developing diagnostic and treatment systems. System features and axes were then determined for requirements analysis to integrate intelligent tools and visualize knowledge. The system was finally designed in a comprehensive decision tree. In the present study, the rule-based reasoning determined related decision-making rules based on the representation of the domain knowledge that was available in guidelines, studies, and expert' opinions using the “if-then” format. Other decision-making points were modeled as qualitative or quantitative rules. Figure 2 shows the knowledge representation process. Besides, some requirements were explained and modeled by selected unified modeling language diagrams such as the use case.
Figure 2.
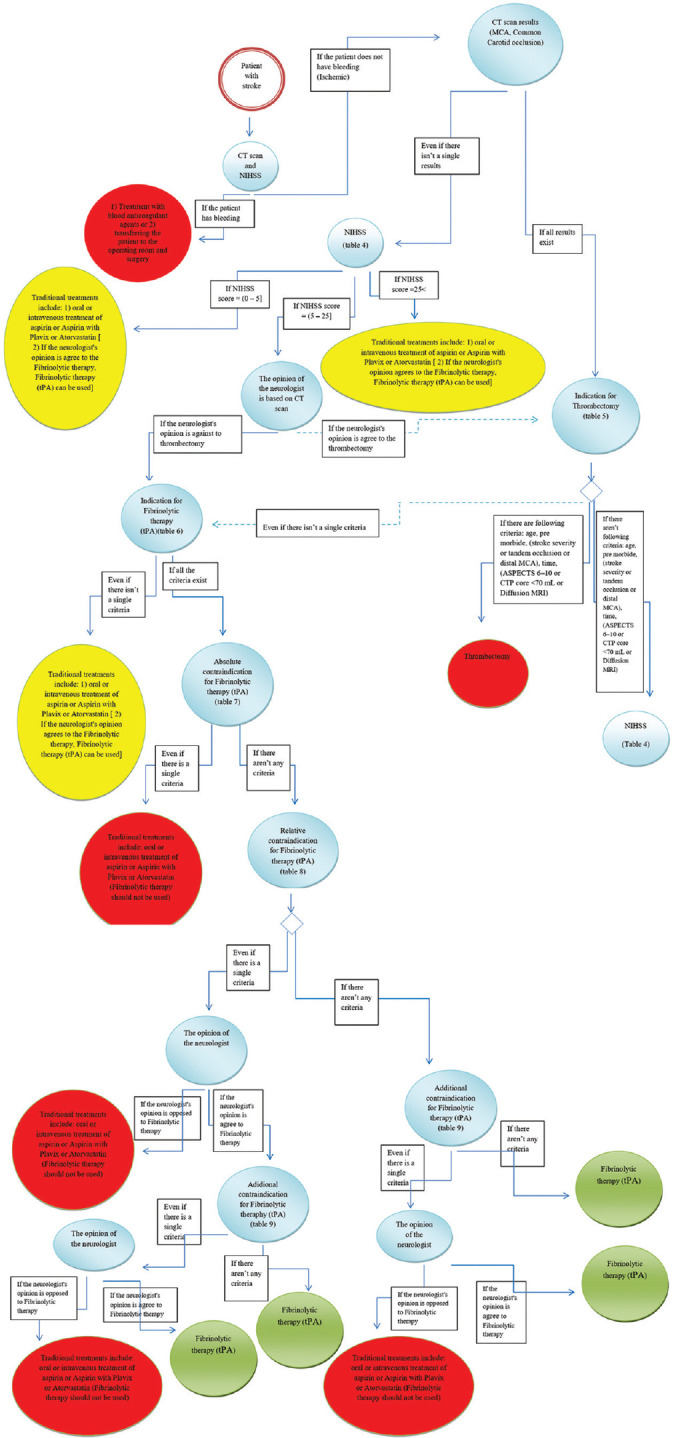
A comprehensive scheme of decision-making rules
In the development phase, the user interface and database layers of the system were designed using Android Studio, Android SQLLite, and Java programming language in a monolingual (English) format. The database contained four tables including physician demographic data, patient information, acute stroke severity, and therapeutic trend as well as reasons for proposed therapeutic procedures. The intelligent system was an android mobile application.
System function
When the system was running, it asked a specialist physician to create a user profile. The system then asked the specialist physician to enter the patient's code. In the next step, the system evaluated and determined the severity of the patient's acute stroke based on the items selected by the specialist physician. After the severity of the patient's acute stroke is determined, the system suggested treatment methods based on the decision-making rules (guidelines) in its rule base and the conditions selected by the specialist physician. After the process was completed, the system showed a form containing the details of the relevant specialist, the patient's code, the severity of the acute stroke, the chosen treatment, and the reason for choosing that treatment and stored in the database. The system had features such as profile editing, reset, search, settings, ability to go back to the previous step, status bar, and help and explain how to use in each step.
Data collection and system evaluation
In the evaluation phase, the application was installed on the emergency medicine specialist (resident of emergency medicine) smartphone or tablet. The emergency medicine specialist (resident of emergency medicine) was trained on using the application. Then, the designed system was exposed to users and evaluated using the mobile application rating scale (MARS) tool, which provided comprehensive coverage of user experience,[12] as well as accuracy, sensitivity, specificity, physicians' errors rates, guideline adherence, and documentation quality (completeness).
All data were collected from the patients who had visited the ED of Imam Reza Hospital (Tabriz-Iran) from March 2018 to August 2018. Data were also simultaneously collected by physicians (residents of emergency medicine) on paper forms and through system users on an intelligent system. During the data collection period, data from patients whose primary diagnosis was an acute stroke were included in the study, and data from other patients were excluded. Further, we included residents of emergency medicine with more than 3 years' experience in the ED and interested to collaborate in the study, and others were excluded from the study. The system was evaluated based on the collected dataset, which included 150 items, as well as the MARS questionnaire that was completed by relevant experts.
A confusion matrix was used to measure the accurate prediction of the classification model and physicians' (residents of emergency medicine) error rates using a paper-based method. The measurement was aimed to determine the accuracy, sensitivity, specificity, and error rates as presented in the following equations:

Where TP refers to a true-positive rate; TN is a true-negative rate; FP is a false-positive rate, and FN is a false-negative rate.
The adherence percentages were assessed with the time series data. The segmented regression model was used to evaluate the effect of the system on the level and trend of guideline adherence in the severity assessment and determination of a therapeutic trend of acute stroke. Guideline adherence was determined by the concordance between guideline-recommended therapy and prescribed treatment. The time-series data are the most efficient, quasi-experimental method for evaluating the longitudinal effects of such time-limited interventions. Segmented regression analysis of time series data helps us to measure, in statistical terms, how much an intervention changed an outcome of interest, immediately and over time; instantly or with delay; transiently or long-term; and whether variables other than the intervention could explain the change.[13]
Two assessors reviewed paper forms and evaluated the number and distribution of completeness or incompleteness of records to evaluate the completeness of documentation. A technical assessor (query) did the same for registered cases in the system. Incompleteness means that a record has one or more missing data items in every form. The following equations were used to calculate the record completeness measure:
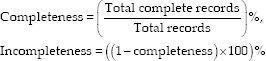
Descriptive statistics were used to describe patient and expert attributes. A segmented regression analysis was used for determining the change in level and trend of adherence after using the system. The Durbin–Watson test was used to test the autocorrelation in the regression model. The statistical analysis was performed in IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. A P < 0.05 was considered statistically significant. Figure 1 shows the overview of the study methodology.
Results
Descriptive data analysis
Two members of the focus group were emergency medicine specialist; two members were neurologist, and three members were technical expert. Four members of the participants worked in the hospital, and three members were employed at the university. Four members of the participants also had more than 5 years of job experience. The mean age of the visited patients was 56 years. 98 people (65.33%) out of 150 patients with acute stroke were male and 52 (34.67%) were female. In addition, most patients were illiterate and married. Table 1 presents the characteristics of focus group members and patients.
Table 1.
Characteristics of the participants
| Items | Frequency (%) |
|---|---|
| Characteristics of focus group members | |
| Gender | |
| Male | 7 (100) |
| Female | 0 |
| Age | |
| 20-30 | 1 (14.28) |
| 30-40 | 3 (42.86) |
| 40-50 | 3 (42.86) |
| More than 50 | 0 |
| Academic background | |
| Bachelor | 0 |
| Master | 1 (14.28) |
| Doctor | 6 (85.72) |
| Organization | |
| Hospital | 4 (57.14) |
| Company | 0 |
| University | 3 (42.86) |
| Others | 0 |
| Job experience | |
| <5 (years) | 3 (42.86) |
| 6-10 | 2 (28.57) |
| 11-15 | 2 (28.57) |
| More than 15 | 0 |
| Characteristics of the patients | |
| Gender | |
| Male | 98 (65.33) |
| Female | 52 (34.67) |
| Age | |
| 10-20 | 3 (2) |
| 20-30 | 14 (9.33) |
| 30-40 | 23 (15.33) |
| 40-50 | 41 (27.34) |
| More than 50 | 69 (46) |
| Academic background | |
| Illiterate | 48 (32) |
| High school | 35 (23.33) |
| Diploma | 29 (19.33) |
| Bachelor | 23 (15.33) |
| Master | 11 (7.33) |
| PhD | 4 (2.68) |
| Marital status | |
| Single | 33 (22) |
| Married | 117 (78) |
Fifty-three data elements were identified for acute stroke severity assessment and therapeutic trend determination. To assess the severity of the acute stroke, the National Institutes of Health Stroke Scale (NIHSS) scores were selected and confirmed from all the items. NIHSS is a reliable, accurate, and sensitive instrument for measuring the severity of the stroke. NIHSS needs less equipment for a stroke patient to be provided quickly. It is also free to support doctors and conveniently available. Some evidence indicates that the NIHSS is sensitive over time toward recognizing important clinical changes.[14] Therapeutic trends include traditional therapies, fibrinolytic therapy, and thrombectomy. Considering criteria such as indications and contraindications for fibrinolytic therapy, and indications and contraindications for thrombectomy were also selected and approved for the treatment of acute stroke. Results of the used resources and data elements for creating the decision-making rules and decision tree were presented in Appendices 1-12.
Rule-based reasoning results
The extracted rules, including 43 rule-based reasoning, were created in the rule-based engine and were used for the assessment and treatment of acute stroke patients. Figure 2 shows the knowledge representation process as a decision tree.
The present study recommended an appropriate architecture. The architecture consists of seven main components: user- and case-specific data, database, rule base, inference engine, explanation system, rule base editor, and the user interface. The provided system architecture is presented in Appendices 1-12.
Figure 3 (Part A) shows a section of the designed graphic user interface for the NIHSS calculation and an acute stroke severity assessment module. In this module, data elements related to the acute stroke severity assessment were entered in 15 categories, including the level of consciousness which itself has three parts, A, B, and C, best Gaze, visual, facial palsy, the motor arm which has two parts, right and left, motor leg which has two parts, right and left, limb ataxia, sensory, best language, dysarthria, extinction, and inattention.
Figure 3.
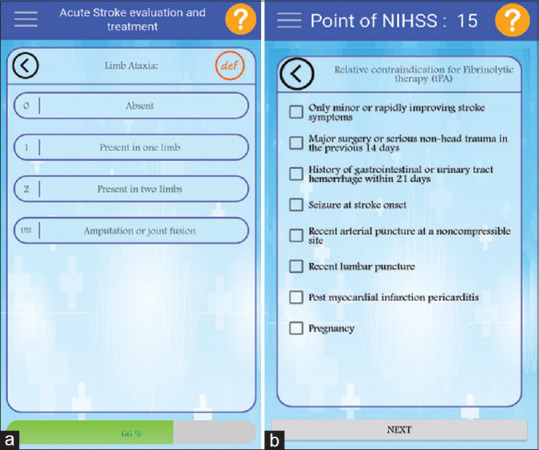
(a) The user interface of severity assessment of systems. (b) The user interface of therapeutic trend determination in systems
In the acute stroke therapeutic trend determination module [Figure 3 (Part B)], the therapeutic trend was selected and recommended according to each patient's conditions based on the data elements (patient characteristics) that were chosen by specialists and system decision-making rules. The existing paper layout and content were mirrored to facilitate the required training, but the optimization was implemented for device sizes.
Evaluation results
A comparison of the system-based and paper-based documentation showed that the documentation of 150 cases (100%) was completed in 150 collected records in the system-based method, while 118 cases (78.66%) of all cases were completely documented in the paper-based method. One or more items of missing data were identified in 32 records. The missing data included NIHSS (5), therapeutic trend (16), and data related to date, time, and documentary identity (11). The system accuracy (in the test data) was 98.30%. The system sensitivity and specificity were also 98.29% and 100%, respectively [Table 2]. Evaluation results indicated that the severity of acute stroke and therapeutic trend of 1.69% of the patients were mistakenly determined, and in the traditional method, it was statistically significant (P < 0.05).
Table 2.
Confusion matrix for acute stroke assessment and treatment model using rule-based reasoning
| Selected condition by physician | Predicted condition by system | |
|---|---|---|
| True | False | |
| True | 115 | 0 |
| False | 2 | 1 |
The Durbin–Watson statistic was 1.85 when the dependent variable was adherence to the guideline. This shows that there was no autocorrelation. Before the beginning of the data collection period, the mean of the adherence to the guideline was 65%. Before the intervention, there was no significant change in the trend of the adherence to the guideline (P < 0.27). After the system intervention, the mean of the adherence to the guideline significantly increased from 65% to 99.5% (P < 0.0001). Since the decision-making rules of the system are based on guidelines, and the system based on these guidelines guides the specialist physician at each stage, as a result when a specialist physician uses this system, the adherence to the guidelines increases. It is obvious when using a traditional method (paper-based). Guidelines may not be followed at some stage.
According to the results of the system evaluation using MARS questioners, the average score of system applicability was 4.6; system performance was 4.75; system esthetics was 4.66; and system information quality was 4.42. In general, the mean quality score of the system was 4.60 and the subjective quality score was 3.75, indicating the excellent and acceptable system quality [Table 3].
Table 3.
System quality score
| Sections | Items | Item names | Mean | SD | Quality |
|---|---|---|---|---|---|
| Engagement | 5 | Entertainment, interest, customization, interactivity, target group | 4.6 | 0.73 | Acceptable - very good |
| Functionality | 4 | Performance, ease of use, navigation, gestural design | 4.75 | 0.68 | Acceptable - very good |
| Esthetics | 3 | Layout, graphics, visual appeal | 4.66 | 0.78 | Acceptable - very good |
| Information | 7 | Accuracy of app, goals, quality of information, quantity of information, visual information, credibility, evidence base | 4.42 | 0.85 | Acceptable - very good |
| App quality score | 19 | All items in above sections | 4.60 | 0.64 | Acceptable - very good |
| App subjective score | 4 | Recommendations, usage, pay, rating | 3.75 | 0.76 | Acceptable - good |
SD=Standard deviation
Discussion
The present study consisted of an intelligent information system for the severity assessment and determination of a therapeutic trend for the acute stroke. In this study, 53 data elements were identified for the severity assessment and determination of a therapeutic trend for the acute stroke. Identifying and determining data elements are the main tasks of data collection to achieve the appropriate functionality of intelligent systems.[15] A review showed that there was no standard and uniform format for data collection in this field.[16] Therefore, it is essential to determine the data elements for mobile-based intelligent systems for severity assessment and determination of a therapeutic trend for acute stroke patients at early stages.
Various methods have been suggested for assessing acute stroke severity in recent studies. Among them, the NIHSS is the most common method. A system was developed to diagnose the stroke severity based on the NIHSS in a study by Rajan et al.[17] This study was consistent with the present study for assessing the stroke severity, but it does not analyze the severity and does not provide any treatment, while the present research included all of these advantages. The mentioned study did not report the accuracy. Therefore, it can be claimed that the present system improved the compliance with acute stroke management tools and guidelines with a high accuracy.
A smartphone platform was created for the triage of patients with stroke in a study by Nogueira et al.[18] The above-mentioned system was compatible with the designed system of the present study in terms of their platforms, but they vary in terms of their application. The smartphone is a complex tool connecting people to a world of information. In recent years, the use of smartphones has been significantly growing. Smartphone features such as interactive screens, fast and easy access, data transfer and tracking, and pervasive influence have led to the more common use than other equipment for access to the internet and use in health applications.[19]
Results indicated that the documentation quality increased from 78.66% to 100%. A review of studies shows that completeness was the most commonly assessed dimension of data quality as an area of focus in 64% of papers.[20] Potential benefits of an intelligent system in the healthcare documentation include the improved quality of documentation, increased communication between users, reduced paperwork, and cost-saving. Electronic records allow for the real-time access that leads to faster data searches and increased physician efficiency.[21]
Our results show that the system improved adherence to the guideline for the severity assessment and determination of a therapeutic trend for acute stroke patients. This improvement may have occurred because the system reduced guideline complexity by simplifying calculation and interpreted the risk scores.[22] This result was similar to those of some other studies investigating the effect of intelligent systems on adherence to the guidelines. In one study, computer-assisted, nurse-driven, and guideline-based decision support system (DSS) was developed as an intervention. In this trial, the adherence to the guidelines was 96% in the intervention group compared to 70% in the control group (P < 0.001).[23] Contrary to these results, in another study, a DSS was developed to improve guideline adherence in the treatment of atrial fibrillation. There was no significant difference between the intervention groups and the control groups. Lack of clinical DSS use (5%), alert fatigue, and need to click to access information was stated as reasons for lack of effect.[24]
Most studies, which focused on information systems in the field of stroke, were developed with aims such as detecting the risk of stroke, helping to rehabilitate patients, and triage of stroke patients and compared the performance of different clinical methods. For instance, a study by Mehdipour et al. proposed a model to prediction of cerebrovascular accident. The comparison of results with neurologists' opinions indicated an acceptable result.[25] Other studies have been also conducted using nonlinear learning models such as the neural network, Nave Bayesian, and support vector machine.[26,27]
According to the compared results of the present study with studies above, the proposed system had higher accuracy than other systems. All of these studies predicted the stroke risk using machine learning (ML) algorithms, while the proposed system used rule-based reasoning methods instead of ML algorithms, due to the nature of acute stroke diagnosis and treatment process. A rule-based reasoning approach is a well-known method that is applicable to design evidence-based expert systems. Results of some studies indicate that a rule-based reasoning approach has high accuracy (nearly 100%) compared with other ML methods.[28] The current study indicated that the domain knowledge should be available to developers in the evidence-based diagnosis and guideline-based medicine, because rule-based reasoning methods are more efficient in designing and they are easy-to-understanding for stakeholders. Some ML algorithms such as the artificial neural network behave as a black box, and the inference process is unclear for being interpretation by experts. However, a rule-based reasoning algorithm performs like a white box. It is important to analyze the problem-solving path in many health challenges. Owing to the uncertainty of medicine, rule-based reasoning methods help to analyze relations of rules and outcomes.[29]
Limitation
Coordinating the time of meeting with specialized physicians to extract and approve decision-making rules due to their busy schedule was one of the most important limitations of the present study, which was best managed with careful planning by the research team.
Conclusion
A system based on guidelines and clinicians' opinions was designed and evaluated in the present study. It was found that designed system effectively assessed the severity of acute strokes and determined its therapeutic trends. This system reduced medical errors and improved the quality of documentation and adherence to the guideline. Therefore, due to the high prevalence of acute stroke in the world, the use of intelligent systems in the field of acute stroke diseases empowers physicians to assess and treat acute stroke and improve the quality of emergency services.
Acknowledgements
This study as an MSc dissertation with Thesis number 59584/323 was done in School of Health Management and Medical Informatics of Tabriz University of Medical Sciences. Authors express their gratitude to emergency specialists at Imam Reza Hospital for their assistance and cooperation.
Appendix 1
The extracted library resources and pivots related to the severity assessment and therapeutic trend determination of acute stroke patient
| Information requirement | library resources |
|---|---|
| Indication and contraindication for fibrinolytic therapy in studies | ECASS |
| Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II) | |
| Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials | |
| Thrombolysis with alteplase 3-4.5 h after acute ischemic stroke | |
| Guidelines for the early management of adults with ischemic stroke | |
| Qualification requirements for performing neuro-interventional procedures: A report of the practice guidelines committee of the American Society of Neuroimaging and the Society of Vascular and Interventional Neurology | |
| Safety and efficacy of intra-arterial thrombolysis for perioperative stroke after cardiac operation | |
| STAIR recommendations for extended window acute stroke therapy trials | |
| Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: Meta-analysis of randomized controlled trials | |
| MELT Japan | |
| Thrombolysis with alteplase 3-4.5 h after acute ischemic stroke (SITS-ISTR) | |
| Indication and contraindication for thrombectomy in studies | The emergency medicine physicians can manage all emergent procedures in the emergency department |
| Stent-retriever thrombectomy after intravenous t-PA versus t-PA alone in stroke | |
| Endovascular therapy following imaging evaluation for ischemic stroke 3 (DEFUSE 3) | |
| A guideline for healthcare professionals from the AHA/ASA | |
| Rapidly improving symptoms and neurologic deficits (PRISMS) | |
| HERMES collaborators. Endovascular thrombectomy after large-vessel ischemic stroke | |
| Endothelial trauma from mechanical thrombectomy in acute stroke | |
| A guideline for healthcare professionals from the AHA/ASA | |
| Thrombolysis with alteplase for acute ischemic stroke in the SITS-MOST | |
| Safety of mechanical thrombectomy and intravenous tissue PA in acute ischemic stroke. Results of the multi-MERCI trial, part I | |
| Stroke severity assessment factors | Scandinavian stroke scale |
| NIHSS | |
| Scaling neurological impairment after middle cerebral artery infarction | |
| Canadian neurological | |
| Down with stroke scale |
ECASS=The European Cooperative Acute Stroke Study, STAIR=Stroke therapy academic industry roundtable, PA=Plasminogen activator, MELT=Middle cerebral artery embolism local fibrinolytic intervention trial, SITS-ISTR=Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register, AHA/ASA=American Heart Association/American Stroke Association, SITS-MOST=Safe implementation of thrombolysis in stroke-monitoring study, MERCI=Mechanical embolus removal in cerebral ischemia, NIHSS=National Institutes of Health Stroke Scale, PRISMS, HERMES
Appendix 2
The confirmed library resources and pivots related to the severity assessment and therapeutic trend determination of acute stroke patient
| Information requirement | library resources |
|---|---|
| Indication and contraindication for fibrinolytic therapy in studies | The ECASS |
| Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II) | |
| Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials | |
| Guidelines for the early management of adults with ischemic stroke | |
| STAIR recommendations for extended window acute stroke therapy trials | |
| The MELT Japan | |
| Thrombolysis with alteplase 3-4.5 h after acute ischemic stroke (SITS-ISTR) | |
| Indication and contraindication for thrombectomy in studies | Stent-retriever thrombectomy after intravenous t-PA versus t-PA alone in stroke |
| Endovascular therapy following imaging evaluation for ischemic stroke 3 (DEFUSE 3) | |
| A guideline for healthcare professionals from the AHA/ASA | |
| HERMES collaborators. Endovascular thrombectomy after large-vessel ischemic stroke | |
| A guideline for healthcare professionals from the AHA/ASA | |
| Thrombolysis with alteplase for acute ischemic stroke in the SITS-MOST | |
| Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi-MERCI trial, Part I | |
| Stroke severity assessment factors | Scandinavian stroke scale |
| NIH stroke scale | |
| Canadian neurological |
ECASS=European cooperative acute stroke study, MERCI=Mechanical embolus removal in cerebral ischemia, NIH=National Institutes of Health, SITS-MOST=Safe implementation of thrombolysis in stroke- monitoring study, STAIR=Stroke therapy academic industry roundtable, MELT=Middle cerebral artery embolism local fibrinolytic intervention trial, AHA/ASA=American Heart Association/American Stroke Association
Appendix 3
Acute stroke severity assessment criteria’s (NIHSS)
| Instructions | Scale definition | Score |
|---|---|---|
| LOC The investigator must choose response, even if a full evaluation is prevented by such obstacles as an endotracheal tube, language barrier, orotracheal trauma/bandages. A “3” is scored only if the patient makes no movement (other than reflexive posturing) in response to noxious stimulation. |
0: Alert; keenly responsive 1: Not alert; but arousable by minor stimulation to obey, answer, or respond 2: Not alert; requires repeated stimulation to attend, or is obtunded and requires strong or painful stimulation to make movements (not stereotyped) 3: Responds only with reflex motor or autonomic effects, or totally unresponsive, flaccid, and areflexic |
|
| LOC questions The patient is asked the month and his/her age. The answer must be correct - there is no partial credit for being close. Aphasic and stuporous patients who do not comprehend the questions will score 2. Patients unable to speak because of endotracheal intubation, orotracheal trauma, severe dysarthria from any cause, language barrier, or any other problem not secondary to aphasia are given a 1. It is important that only the initial answer be graded and that the examiner not “help” the patient with verbal or non-verbal cues |
0: Answers both questions correctly 1: Answers one question correctly 2: Answers neither question correctly |
|
| LOC questions The patient is asked to open and close the eyes and then to grip and release the non-paretic hand. Substitute another one-step command if the hands cannot be used. Credit is given if an unequivocal attempt is made but not completed due to weakness. If the patient does not respond to command, the task should be demonstrated to him or her (pantomime), and the result scored (i.e., follows none, one, or two commands). Patients with trauma, amputation, or other physical impediments should be given suitable one-step commands. Only the first attempt is scored |
0: Performs both tasks correctly 1: Performs one task correctly 2: Performs neither task correctly |
|
| Best gaze Only horizontal eye movements will be tested. Voluntary or reflexive (oculocephalic) eye movements will be scored, but caloric testing is not done. If the patient has a conjugate deviation of the eyes that can be overcome by voluntary or reflexive activity, the score will be 1. If a patient has an isolated peripheral nerve paresis (CN III, IV, or VI), score a 1. Gaze is testable in all aphasic patients. Patients with ocular trauma, bandages, preexisting blindness, or other disorder of visual acuity or fields should be tested with reflexive movements, and a choice made by the investigator. Establishing eye contact and then moving about the patient from side to side will occasionally clarify the presence of a partial gaze palsy |
0: Normal 1: Partial gaze palsy; gaze is abnormal in one or both eyes, but forced deviation and total gaze paresis is not present 2: Forced deviation, or total gaze paresis is not overcome by the oculocephalic maneuver |
|
| Visual Visual fields (upper and lower quadrants) are tested by confrontation, using finger counting or visual threat, as appropriate. Patients may be encouraged, but if they look at the side of the moving fingers appropriately, this can be scored as normal. If there is unilateral blindness or enucleation, visual fields in the remaining eye are scored. Score 1 only if a clear-cut asymmetry, including quadrantanopia, is found. If patient is blind from any cause, score 3. Double simultaneous stimulation is performed at this point. If there is extinction, patient receives a 1, and the results are used to respond to item 1 |
0: No visual loss 1: Partial hemianopia 2: Complete hemianopia 3: Bilateral hemianopia (blind including cortical blindness) |
|
| Facial palsy Ask or use pantomime to encourage the patient to show teeth or raise eyebrows and close eyes. Score symmetry of grimace in response to noxious stimuli in the poorly responsive or noncomprehending patient. If facial trauma/bandages, orotracheal tube, tape, or other physical barriers obscure the face, these should be removed to the extent possible |
0: Normal symmetrical movements 1: Minor paralysis (flattened nasolabial fold, asymmetry on smiling) 2: Partial paralysis (total or near-total paralysis of lower face) 3: Complete paralysis of one or both sides (absence of facial movement in the upper and lower face) |
|
| Motor arm The limb is placed in the appropriate position: extend the arms (palms down) 90° (if sitting) or 45° (if supine). Drift is scored if the arm falls before 10 s. The aphasic patient is encouraged using urgency in the voice and pantomime, but not noxious stimulation. Each limb is tested in turn, beginning with the nonparetic arm. Only in the case of amputation or joint fusion at the shoulder, the examiner should record the score as UN and clearly write the explanation for this choice. |
0: No drift; limb holds 90° (or 45°) for full 10 s 1: Drift; limb holds 90° (or 45°), but drifts down before full 10 s; does not hit bed or other support 2: Some effort against gravity; limb cannot get to or maintain (if cued) 90° (or 45°), drifts down to bed, but has some effort against gravity 3: No effort against gravity; limb falls 4: No movement UN: Amputation or joint fusion, explain |
|
| Motor leg The limb is placed in the appropriate position: hold the leg at 30° degrees (always tested supine). Drift is scored if the leg falls before 5 s. The aphasic patient is encouraged using urgency in the voice and pantomime but not noxious stimulation. Each limb is tested in turn, beginning with the nonparetic leg. Only in the case of amputation or joint fusion at the hip, the examiner should record the score as UN and clearly write the explanation for this choice |
0: No drift; leg holds 30° position for full 5 s 1: Drift; leg falls by the end of the 5-s period but does not hit the bed 2: Some effort against gravity; leg falls to bed by 5 s but has some effort against gravity 3: No effort against gravity; leg falls to bed immediately 4: No movement UN: Amputation or joint fusion, explain |
|
| Limb ataxia This item is aimed at finding evidence of a unilateral cerebellar lesion. Test with eyes open. In case of visual defect, ensure testing is done in intact visual field. The finger nose-finger and heel-shin tests are performed on both sides, and ataxia is scored only if present out of proportion to weakness. Ataxia is absent in the patient who cannot understand or is paralyzed. Only in the case of amputation or joint fusion, the examiner should record the score as UN and clearly write the explanation for this choice. In case of blindness, test by having the patient touch nose from extended arm position. |
0: Absent 1: Present in one limb 2: Present in two limbs UN: Amputation or joint fusion, explain |
|
| Sensory Sensation or grimace to pinprick when tested, or withdrawal from noxious stimulus in the obtunded or aphasic patient. Only sensory loss attributed to stroke is scored as abnormal and the examiner should test as many body areas (arms [not hands, legs, trunk, face) as needed to accurately check for hemi sensory loss. A score of 2, “severe or total sensory loss,” should only be given when a severe or total loss of sensation can be clearly demonstrated. Stuporous and aphasic patients will, therefore, probably score 1 or 0. The patient with brainstem stroke who has bilateral loss of sensation is scored 2. If the patient does not respond and is quadriplegic, score 2. Patients in a coma (item 1a=3) are automatically given a 2 on this item |
0: Normal; no sensory loss 1: Mild-to-moderate sensory loss; patient feels pinprick is less sharp or is dull on the affected side; or there is a loss of superficial pain with pinprick, but patient is aware of being touched 2: Severe or total sensory loss; patient is not aware of being touched in the face, arm, and leg |
|
| Best language A great deal of information about comprehension will be obtained during the preceding sections of the examination. For this scale item, the patient is asked to describe what is happening in the attached picture, to name the items on the attached naming sheet, and to read from the attached list of sentences. Comprehension is judged from responses here, as well as to all of the commands in the preceding general neurological exam. If visual loss interferes with the tests, ask the patient to identify objects placed in the hand, repeat, and produce speech. The intubated patient should be asked to write. The patient in a coma (item 1a=3) will automatically score 3 on this item. The examiner must choose a score for the patient with stupor or limited cooperation, but a score of 3 should be used only if the patient is mute and follows no one-step commands |
0: No aphasia; normal 1: Mild-to-moderate aphasia; some obvious loss of fluency or facility of comprehension, without significant limitation on ideas expressed or form of expression. Reduction of speech and/or comprehension, however, makes conversation about provided materials difficult or impossible. For example, in conversation about provided materials, examiner can identify picture or naming card content from patient’s response 2: Severe aphasia; all communication is through fragmentary expression; great need for inference, questioning, and guessing by the listener. Range of information that can be exchanged is limited; listener carries burden of communication. Examiner cannot identify materials provided from patient response 3: Mute, global aphasia; no usable speech or auditory comprehension |
|
| Dysarthria If patient is thought to be normal, an adequate sample of speech must be obtained by asking patient to read or repeat words from the NIH Stroke Scale document pages 6 and 8 (pdf, 495 kb). If the patient has severe aphasia, the clarity of articulation of spontaneous speech can be rated. Only if the patient is intubated or has other physical barriers to producing speech, the examiner should record the score as UN and clearly write the explanation for this choice. Do not tell the patient why he/she is being tested. |
0: Normal 1: Mild-to-moderate dysarthria; patient slurs at least some words and, at worst, can be understood with some difficulty 2: Severe dysarthria; patient’s speech is so slurred as to be unintelligible in the absence of or out of proportion to any dysphasia, or is mute/anorthic UN: Intubated or another physical barrier, explain |
|
| Extinction and inattention (formerly neglect) Sufficient information to identify neglect may be obtained during the prior testing. If the patient has a severe visual loss preventing visual double simultaneous stimulation, and the cutaneous stimuli are normal, the score is normal. If the patient has aphasia but does appear to attend to both sides, the score is normal. The presence of visual spatial neglect or anosagnosia may also be taken as evidence of abnormality. Since the abnormality is scored only if present, the item is never untestable |
0: No abnormality 1: Visual, tactile, auditory, spatial, or personal inattention, or extinction to bilateral simultaneous stimulation in one of the sensory modalities 2: Profound hemi-inattention or extinction to more than one modality; does not recognize own hand or orients to only one side of space |
LOC=Level of consciousness, UN=Untestable
Appendix 4
Thrombectomy indication
| Criteria | Yes | No |
|---|---|---|
| Patients should receive mechanical thrombectomy with a stent retriever if they meet all the following criteria: (1) Prestroke mRS score of 0 to 1 (2) Causative occlusion of the internal carotid artery or MCA segment 1 (M1) (3) Age ≥18 years (4) NIHSS score of ≥6 (5) ASPECTS of ≥6 (6) Treatment can be initiated (groin puncture) within 6 h of symptom onset |
Yes | No |
| Age: No justification for arbitrary upper age limits. Older age is associated with worse prognosis but treatment effect is consistent across the age spectrum Pre-morbid function: Important to consider current quality of life and probability of maintaining an acceptable quality of life. This can be challenging to accurately assess in the emergency department and, if in doubt, it is best to err on the side of treating Stroke severity: Patients with NIHSS ≥6 definitely benefit. No upper limit for severity has been demonstrated. Milder patients still have approximately 10% incidence of large vessel occlusion and so CTA should be routine. This group has a high risk of later deterioration. If symptoms are uncharacteristically mild, consider whether the occlusion could be chronic, particularly relevant in Asian countries with high prevalence of intracranial atherosclerosis Tandem occlusion of the internal carotid artery: Very strong benefit in this subpopulation. Stenting before versus after thrombectomy is controversial. In general, removal of the intracranial occlusion to allow collateral flow via circle of Willis is logical, followed by treatment of the cervical ICA, unless this is impassable Distal MCA (M2) occlusion: Uncertain benefit in trials. Consider territory at risk (clinical signs and/or perfusion lesion and accessibility of the clot to thrombectomy) Time: Thrombectomy has clear-cut benefit 0-6 h after stroke onset. Beyond 6 h, or if onset time is uncertain, RCT participation is encouraged, but data suggest benefit in the presence of favorable imaging in ESCAPE and DEFUSE-2. Core volume Patients with ASPECTS 6-10 definitely benefit. If ASPECTS 0-5 benefit is uncertain-consider core location and acknowledge potential imprecision in NCCT assessment of subtle signs (CTA collaterals and/or CTP may improve reliability) Patients with CTP core <70 mL definitely benefits. If core volume >70 mL benefit is uncertain-consider core location and overall patient resilience (comorbidities, tolerance for extended rehabilitation) and preferences (tolerance of disability, remembering that perceptions change poststroke). Note that SICH may be considerably more frequent in patients with large core (e.g., 20% in a series of patients with core >70 mL) Diffusion MRI-more precise than CTP but generally associated with greater treatment delay, which offsets that benefit. Large core volume appears prognostic but may not modify treatment effect as greatly as previously thought Other imaging parameters Collateral grade-patients with moderate-to-good collaterals definitely benefit. Benefit in patients with poor/absent collaterals is uncertain. Assessing collaterals on standard static CTA risks underestimating collateral flow, which is, by its nature, delayed and therefore may not have arrived in the arterial phase imaging. Multiphase acquisition or CTP source images avoid this problem Clot length-there is currently no evidence that short clots reanalyze sufficiently often to justify waiting to see if thrombolysis works or omitting thrombectomy. Rates of preangiogram recanalization were <10% in the trials (none excluded short clots except THERAPY, which was neutral) Residual anterograde flow-requires dynamic angiography (e.g., raw CTP data). There is evidence that thrombolysis works much better, but it is not sufficient to justify waiting to see if thrombolysis works or for omitting thrombectomy |
NIHSS=National Institutes of Health Stroke Scale, CTA=CT angiography, CTP=CT perfusion, SICH=Symptomatic intracerebral hemorrhage, RCT=Randomized controlled trial, NCCT=Noncontrast computed tomography, CT=Computed tomography
Appendix 5
Fibrinolytic therapy (tissue plasminogen activator) indication
| Criteria | Yes | No |
|---|---|---|
| Eligibility for tPA | ||
| 65> age ≥18 years | ||
| Clinical diagnosis of acute stroke causing neurological deficit | ||
| Time of symptom onset <4.5 h (See Additional Warnings to tPA at 3-4.5 h below) | ||
| CT scan does not indicate bleeding |
CT=Computed tomography, tPA=Tissue plasminogen activator
Appendix 6
Fibrinolytic therapy (tissue plasminogen activator) absolute contraindication
| Criteria | Yes | No |
|---|---|---|
| Absolute contraindications to tPA | ||
| 1. Intracranial hemorrhage on CT | ||
| 2. Clinical presentation suggests subarachnoid hemorrhage | ||
| 3. Neurosurgery, head trauma, or stroke in past 3 months | ||
| 4. Uncontrolled hypertension (>185 mmHg SBP or >110 mmHg DBP) | ||
| 5. History of intracranial hemorrhage | ||
| 6. Known intracranial arteriovenous malformation, neoplasm, or aneurysm | ||
| 7. Active internal bleeding | ||
| 8. Suspected/confirmed endocarditis | ||
| 9. Known bleeding diathesis ((1) Platelet count <100,000; (2) Patient has received heparin within 48 h and has an elevated aPTT (greater than upper limit of normal for laboratory); (3) Current use of oral anticoagulants (ex: warfarin) and INR >1.7; (4) Current use of direct thrombin inhibitors or direct factor Xa inhibitors) | ||
| 10. Abnormal blood glucose (<50 or >400 mg/dL) |
aPTT=Activated partial thromboplastin time, INR=International normalized ratio, CT=Computed tomography, SBP=Systolic blood pressure, DBP=Diastolic blood pressure, tPA=Tissue plasminogen activator
Appendix 7
Fibrinolytic therapy (tissue plasminogen activator) relative contraindication
| Relative contraindications/warnings to tPA |
| Only minor or rapidly improving stroke symptoms |
| Major surgery or serious nonhead trauma in the previous 14 days |
| History of gastrointestinal or urinary tract hemorrhage within 21 days |
| Seizure at stroke onset |
| Recent arterial puncture at a noncompressible site |
| Recent lumbar puncture |
| Postmyocardial infarction pericarditis |
| Pregnancy |
tPA=Tissue plasminogen activator
Appendix 8
Fibrinolytic therapy (tissue plasminogen activator) additional contraindication
| Additional warnings to tPA >3 h onset |
| Age >80 years |
| History of prior stroke and diabetes |
| Any active anticoagulant use (even with INR <1.7) |
| NIHSS >25 |
| CT shows multi lobar infarction (hypo density >1/3 cerebral hemisphere |
tPA=Tissue plasminogen activator, NIHSS=National Institutes of Health Stroke Scale, CT=Computed tomography
Appendix 9
Severity assessment and therapeutic trend determination data element
| Variable | Item |
|---|---|
| NIHSS | 15 |
| CT scan results | 2 |
| Thrombectomy indication | 12 |
| Opinion of the neurologist | 2 |
| Fibrinolytic therapy indication | 4 |
| Fibrinolytic therapy absolute contraindication | 10 |
| Fibrinolytic therapy relative contraindication | 8 |
| Fibrinolytic therapy additional contraindication | 5 |
| Traditional treatments include: Oral or intravenous treatment of aspirin or aspirin with Plavix or atorvastatin or other drugs | 4 |
| Thrombectomy | 1 |
| Fibrinolytic therapy | 1 |
| Treatments with blood anticoagulant agents or surgery | 1 |
NIHSS=National Institutes of Health Stroke Scale, CT=Computed tomography
Appendix 10
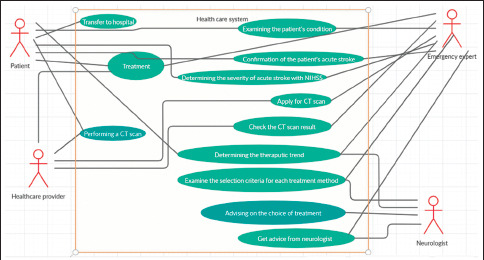
A use case diagram for the diagnosis and treatment of acute stroke patients
Appendix 11

System architecture
Appendix 12

Graphical abstract of the study
Footnotes
Conflicts of interest
None declared.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The Ethics Committee of Tabriz University of Medical Sciences has confirmed this research.
Consent to participate
Human subjects were not included in the project.
Funding
None declared.
References
- 1.Birtane M, Taştekin N. Quality of life after stroke. Med J Trakya Univer. 2010;27:63–8. [Google Scholar]
- 2.Brust JC. Current Diagnosis and Treatment Neurology. New York: McGraw Hill Professional; 2011. [Google Scholar]
- 3.Roudbary SA, Saadat F, Forghanparast K, Sohrabnejad R. Serum C-reactive protein level as a biomarker for differentiation of ischemic from hemorrhagic stroke. Acta Medica Iranica. 2011:149–52. [PubMed] [Google Scholar]
- 4.MEMBERS WG. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125:e2. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saposnik G, Johnston SC. Decision making in acute stroke care: Learning from neuroeconomics, neuromarketing, and poker players. Stroke. 2014;45:2144–50. doi: 10.1161/STROKEAHA.114.005462. [DOI] [PubMed] [Google Scholar]
- 6.Rothschild JM, Hurley AC, Landrigan CP, Cronin JW, Martell-Waldrop K, Foskett C, et al. Recovery from medical errors: The critical care nursing safety net. Jt Comm J Qual Patient Saf. 2006;32:63–72. doi: 10.1016/s1553-7250(06)32009-0. [DOI] [PubMed] [Google Scholar]
- 7.Handel D, Epstein S, Khare R, Abernethy D, Klauer K, Pilgrim R, et al. Interventions to improve the timeliness of emergency care. Acad Emerg Med. 2011;18:1295–302. doi: 10.1111/j.1553-2712.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- 8.Mankad KB. An intelligent process development using fusion of genetic algorithm with fuzzy logic. Artificial Intelligence: Concepts, Methodologies, Tools, and Applications. IGI Global. 2017:245–81. [Google Scholar]
- 9.Abu-Nasser BS, Abu Naser SS. Rule-based system for watermelon diseases and treatment. Int J Acad Inf Syst Res. 2018;2:1–7. [Google Scholar]
- 10.Lehmann CU. Medical information systems in pediatrics. Pediatrics. 2003;111:679. doi: 10.1542/peds.111.3.679. [DOI] [PubMed] [Google Scholar]
- 11.Arayici Y, Ahmed V, Aouad GF. A requirements engineering framework for integrated systems development for the construction industry. J Inf Technol Constr. 2006;11:35–55. [Google Scholar]
- 12.Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, Mani M. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR mHealth and uHealth. 2015;3:e27. doi: 10.2196/mhealth.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nistal-Nuño B. Segmented regression analysis of interrupted time series data to assess outcomes of a South American road traffic alcohol policy change. Public Health. 2017;150:51–9. doi: 10.1016/j.puhe.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS) J Physiother. 2014;60:61. doi: 10.1016/j.jphys.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Kalankesh LR, Dastgiri S, Rafeey M, Rasouli N, Vahedi L. Minimum data set for cystic fibrosis registry: A case study in iran. Acta Inform Med. 2015;23:18–21. doi: 10.5455/aim.2015.23.18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins SC, Martins MC, Carbonera LA, Souza AC, Portal M, Martin K, et al. Abstract TP312: Assessment of the data collection strategies for stroke patient-centered outcomes: Implementation of the International Consortium for Health Outcomes in Brazil. Stroke. 2018;49(Suppl 1):ATP312. [Google Scholar]
- 17.Rajan V, Bhattacharya S, Shetty R, Sitaram A, Vivek G. Pacific-Asia Conference on Knowledge Discovery and Data Mining. Springer, Cham; 2016. Clinical Decision Support for Stroke Using Multi–view Learning Based Models for NIHSS Scores; pp. 190–9. [Google Scholar]
- 18.Nogueira RG, Silva GS, Lima FO, Yeh YC, Fleming C, Branco D, et al. The FAST-ED App: A Smartphone Platform for the Field Triage of Patients With Stroke. Stroke. 2017;48:1278–84. doi: 10.1161/STROKEAHA.116.016026. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka Y, Kamitani E, Dracup K, Jong SS. New insights into compliance with a mobile phone diary and pedometer use in sedentary women. J Phys Act Health. 2011;8:398–403. doi: 10.1123/jpah.8.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiskopf NG, Hripcsak G, Swaminathan S, Weng C. Defining and measuring completeness of electronic health records for secondary use. J Biomed Inform. 2013;46:830–6. doi: 10.1016/j.jbi.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai J, Bond G. A comparison of electronic records to paper records in mental health centers. Int J Qual Health Care. 2008;20:136–43. doi: 10.1093/intqhc/mzm064. [DOI] [PubMed] [Google Scholar]
- 22.Goud R, van Engen-Verheul M, de Keizer NF, Bal R, Hasman A, Hellemans IM, et al. The effect of computerized decision support on barriers to guideline implementation: A qualitative study in outpatient cardiac rehabilitation. Int J Med Inform. 2010;79:430–7. doi: 10.1016/j.ijmedinf.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Hendriks JL, Nieuwlaat R, Vrijhoef HJ, de Wit R, Crijns HJ, Tieleman RG. Improving guideline adherence in the treatment of atrial fibrillation by implementing an integrated chronic care program. Neth Heart J. 2010;18:471–7. doi: 10.1007/BF03091818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arts DL, Abu-Hanna A, Medlock SK, van Weert HC. Effectiveness and usage of a decision support system to improve stroke prevention in general practice: A cluster randomized controlled trial. PLoS One. 2017;12:e0170974. doi: 10.1371/journal.pone.0170974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehdipour Y, Ebrahimi S, Karimi A, Alipour J, Khammarnia M, Siasar F. Presentation a model for prediction of cerebrovascular accident using data mining algorithm. Sadra Med Sci J. 2016;4:255–66. [Google Scholar]
- 26.Letham B, Rudin C, McCormick TH, Madigan D. An interpretable model for stroke prediction using rules and Bayesian analysis InProceedings of 2014. KDD Workshop on Data Science for Social Good. 2014 [Google Scholar]
- 27.Pardamean B, Christian R, Abbas BS. Expert-system based medical stroke prevention. J Comput Sci. 2013;9:1099–105. [Google Scholar]
- 28.Nabaei A, Hamian M, Parsaei MR, Safdari R, Samad-Soltani T, Zarrabi H, et al. Topologies and performance of intelligent algorithms: a comprehensive review. Artificial Intelligence Review. 2018;49:79–103. [Google Scholar]
- 29.Sumarlinda S, Rahmat A, Long ZA. Clinical decision support system in computational methods: A review study. Proc ICOHETECH. 2019;1:242–5. [Google Scholar]


