Abstract
Purpose:
This study aimed to investigate the influence of cleaned-up knowledge-based treatment planning (KBP) models on the plan quality for volumetric-modulated arc therapy (VMAT) of prostate cancer.
Materials and Methods:
Thirty prostate cancer VMAT plans were enrolled and evaluated according to four KBP modeling methods as follows: (1) model not cleaned – trained by fifty other clinical plans (KBPORIG); (2) cases cleaned by removing plans that did not meet all clinical goals of the dosimetric parameters, derived from dose–volume histogram (DVH) (KBPC-DVH); (3) cases cleaned outside the range of ±1 standard deviation through the principal component analysis regression plots (KBPC-REG); and (4) cases cleaned using both methods (2) and (3) (KBPC-ALL). Rectal and bladder structures in the training models numbered 34 and 48 for KBPC-DVH, 37 and 33 for KBPC-REG, and 26 and 33 for KBPC-ALL, respectively. The dosimetric parameters for each model with one-time auto-optimization were compared.
Results:
All KBP models improved target dose coverage and conformity and provided comparable sparing of organs at risks (rectal and bladder walls). There were no significant differences in plan quality among the KBP models. Nevertheless, only the KBPC-ALL model generated no cases of >1% V78 Gy (prescribed dose) to the rectal wall, whereas the KBPORIG, KBPC-DVH, and KBPC-REG models included two, four, and three cases, respectively, which were difficult to overcome with KBP because the planning target volume (PTV) and rectum regions overlapped.
Conclusions:
The cleaned-up KBP model based on DVH and regression plots improved plan quality in the PTV–rectum overlap region.
Keywords: Cleaned-up model, knowledge-based treatment planning, plan quality, prostate cancer
INTRODUCTION
Knowledge-based treatment planning (KBP) with a machine-learning technique is an approach used to reduce variations in plan quality in high-precision radiotherapy, thereby improving planning consistency.[1] A commercial KBP module, RapidPlan® (Varian Medical Systems, Palo Alto, CA, USA), has been released for use with the Eclipse (Varian) treatment planning system. The KBP uses a statistical model generated from a library of clinically accepted, high-quality plans to train dose–volume histograms (DVHs).[1,2] This model predicts an achievable DVH range and generates dose–volume objectives based on the relationships between geometric and dosimetric features, to optimize intensity-modulated radiation therapy and volumetric-modulated arc therapy (VMAT) plans.[1,2]
Many studies have reported that KBP can generate better (or at least comparable) dosimetric results at some anatomical sites.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] Our previous study showed that KBP with one-time auto-optimization could create an acceptable VMAT plan for prostate cancer that could be used in clinical practice with no major problems concerning dosimetric accuracy or mechanical performance.[10,16] Ueda et al. suggested that sharing the KBP model could enable other institutions to reproduce the dose distributions, although whether the registered DVH curves match the plan design of the institution required verification.[17] However, the volumes over which high doses were delivered to organs at risks (OARs) in the KBP were inferior to those of clinical plans (CPs)[10,16] because the doses applied to any overlapping regions of the target and OARs were not considered in the KBP system.[18] Some reports described the effects of outliers in the KBP model on the plan quality, or investigated whether a “cleaned-up” KBP model created by removing the outlier plans or structures which have potential of the negative effect on the model could improve the plan's quality.[1,19,20,21] Aviles et al. showed that the DVHs estimated using cleaned-up KBP had greater accuracy.[20] In contrast, Hussein et al.[1] and Delaney et al.[21] reported that statistical outliers had no significant impact on plan quality. Therefore, it remains unclear whether a cleaned-up KBP model can improve plan quality. Additionally, the modeling process itself is not completely understood.
The purpose of this study was to investigate how a cleaned-up KBP model affects the plan quality of VMAT for new prostate cancer patients. For this study, we created a cleaned-up KBP model by excluding outlying items according to the DVH and/ or regression plots, and investigated whether the cleaned-up KBP model could improve the plan quality involving the target–OAR overlap region with one-time auto-optimization application, which is easy to install in a clinical situation. The one-time auto-optimization can eliminate the subjectivity and heuristics, which results in the standardization of high VMAT plan quality at many institutions.
MATERIALS AND METHODS
Volumetric-modulated arc therapy planning for prostate cancer
Thirty prostate cancer patients (T1–T2c) who underwent VMAT with CPs during 2016–2017 were selected for the KBP model validation. All VMAT plans for prostate cancer were created using 10-MV photon beams, two full arcs (gantry angles rotating clockwise from 181° to 179° and counterclockwise from 179° to 181°), and collimator angles of 30° and 330°, calculated using the Varian analytic anisotropic algorithm[22] and the Eclipse treatment planning system (version 13.6; Varian Medical Systems, Palo Alto, CA, USA) of a TrueBeam® radiotherapy system (Varian).[16] The clinical target volume (CTV) in the present study was defined as the prostate and seminal vesicle. It was delineated by experienced radiation oncologists. The planning target volume (PTV) was defined as a 6-mm posterior margin and a 10-mm margin in all other directions added to the CTV, to reduce the dose at the prostate–rectal interface. The OARs were the rectal and bladder walls. The rectum was delineated as a region up to 1.0 cm above and below the PTV. The rectal and bladder walls were delineated as regions 4.0 mm inside the outer surface of the rectum and bladder. The prescribed dose was 78 Gy in 39 fractions to 95% of the volume of the PTV minus the rectum (PTV − R).[16] All patients underwent urine collection for 1–2 h before computed tomography simulation and treatment.
The clinical goals and acceptable criteria for treatment plans in our institution are shown in Table 1.[16,23] The overlap region between the PTV and rectal wall was covered with a 90% isodose line.
Table 1.
Clinical goal and acceptable criteria for each structure in our institution
| Parameter | Clinical goal (%) | Acceptable criteria (%) | |
|---|---|---|---|
| PTV-R | Dmax | <110 | |
| D95 | 100 | ||
| Dmean | >99, <103 | ||
| Rectal wall | V40 Gy | <60 | <65 |
| V60 Gy | <30 | <35 | |
| V70 Gy | <20 | <25 | |
| V78 Gy | <1 | ||
| Bladder wall | V40 Gy | <60 | <65 |
| V70 Gy | <35 | ||
PTV−R: Planning target volume minus the rectum
Original knowledge-based treatment planning model library
The KBP model was trained with fifty cases of T1–T2c prostate cancer treated during 2015–2016. This model was defined as the original KBP model (KBPORIG). Informed consent was obtained from all patients, and our institutional ethics committee approved this study (institutional review board number: 29–133).
The KBP model configuration and training process are well explained in the literature.[5,7,10] The fifty structures of the PTV − R, rectum, and bladder were registered in the original KBP library. The geometric and dosimetric outliers were not excluded from this KBPORIG model.
Methods for cleaning-up the knowledge-based treatment planning model
Three cleaned-up KBP models were derived from the KBPORIG model:
Cleaned cases by removing the plans that did not meet the clinical goal of the dosimetric parameters; derived from DVH plots (KBPC-DVH model)
Cleaned cases by removing the plans that were outside ±1 standard deviation (SD); derived from principal component analysis (PCA) regression plots (KBPC-REG model)
Cleaned cases by removing the plans using the filters (1 and 2) (KBPC-ALL model).
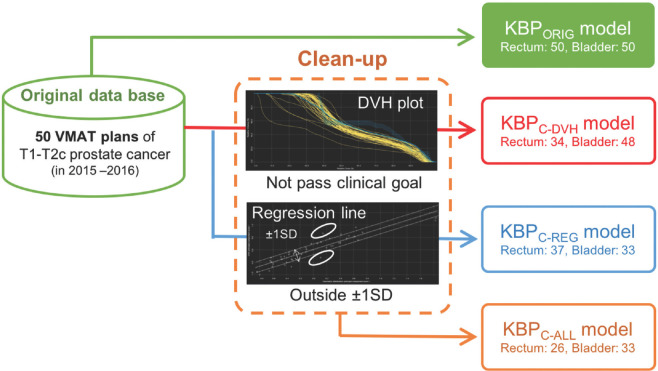
The schema of the cleaned-up KBP modeling methods are shown in Figure 1. The clean-up processes were performed using the above-mentioned methods and a Varian model analytical tool.[21] The number of rectal and bladder structures used to train the model was 34 and 48 for the KBPC-DVH model, 37 and 33 for the KBPC-REG model, and 26 and 33 for the KBPC-ALL model, respectively. The volume of the PTV-R in all KBP models was within the range of 56.28–202.94 cm3. The rectal volume ranges for the KBPORIG, KBPC-DVH, KBPC-REG, and KBPC-ALL models were 28.39–117.26 cm3, 28.39–116.46 cm3, 33.39–117.26 cm3, and 33.39–116.46 cm3, respectively. The bladder volume ranges for the KBPORIG, KBPC-DVH, KBPC-REG, and KBPC-ALL models were 49.18–486.52 cm3, 58.08–486.52 cm3, 58.08–486.52 cm3, and 58.08–486.52 cm3, respectively. All objectives for the KBP models were generated automatically.
Figure 1.
Schema of the cleaned-up KBP modeling methods. The KBPC-DVH model was created by excluding cases that did not meet the clinical goal based on DVH plots. The KBPC-REG model was created by excluding cases outside ±1 standard deviation from PCA regression plots. Finally, the KBPC-ALL model was created by excluding all cleaned-up cases as in both the KBPC-DVH and KBPC-REG models. KBP: Knowledge-based treatment planning, DVH: Dose–volume histogram, PCA: Principal component analysis
Plan evaluation
The thirty plans used for the KBP validation were compared across the CPs and four KBPs using the following dosimetric parameters.[16]
Maximum (Dmax), minimum (Dmin), and mean (Dmean) doses of the PTV–R volume (D95 = 100%)
Homogeneity index = 100 × (D2% − D98%)/D50%, where D98%, D2%, and D50% are doses received by 98%, 2%, and 50% of the PTV − R, respectively[24]
The 95% isodose conformity index (CI95) = V 95%/ VPTV − R, where V95% is the volume covered by 95% of the prescribed dose (74.1 Gy), and VPTV − R is the PTV–R volume[1]
Dose–volume parameters of the rectal wall: V40 Gy, V60 Gy, V70 Gy, V78 Gy
Dose–volume parameters of the bladder wall: V40 Gy, V70 Gy
Modulation complexity scores (MCSs) and monitor unit (MU) values.[10] The MCS assesses the variability between multi-leaf collimator positions and the aperture opening, and has values ranging from 0 to 1, with lower values indicating greater modulation.[1]
Statistical analysis
Data were expressed as means and SDs, unless otherwise indicated. The Wilcoxon signed-rank test was used to compare continuous variables and trends between the each KBP model and the CP. All statistical analyses were performed using R version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered to indicate statistical significance.
RESULTS
Table 2 summarizes the results of the dosimetric parameters and plan complexity between the each KBP model and the CP. Figures 2 and 3 compare the dose parameters between each KBP model and its CP for PTV − R and OARs.
Table 2.
Dosimetric parameters and plan complexity for each knowledge-based treatment planning model and clinical plan.
| Parameter | CP | KBPORIG | KBPC-DVH | KBPC-REG | KBPC-ALL | |
|---|---|---|---|---|---|---|
| PTV-R | Dmax (%) | 106.63±1.31 | 105.61±0.50 | 105.77±0.93 | 105.63±0.54 | 105.66±0.69 |
| Dmin (%) | 91.90±2.74 | 91.54±1.67 | 91.58±1.87 | 91.76±1.64 | 91.53±1.60 | |
| Dmean (%) | 102.30±0.66 | 102.01±0.31 | 102.09±0.36 | 102.08±0.27 | 102.06±0.29 | |
| HI | 0.053±0.013 | 0.047±0.003 | 0.048±0.006 | 0.047±0.004 | 0.047±0.004 | |
| CI95 | 1.28±0.065 | 1.19±0.025 | 1.18±0.024 | 1.19±0.025 | 1.19±0.023 | |
| Rectal wall | V40 Gy (%) | 48.95±6.38 | 49.24±4.94 | 49.35±3.96 | 50.51±4.94 | 50.05±5.28 |
| V60 Gy (%) | 26.90±4.32 | 27.62±4.32 | 27.58±3.94 | 27.85±4.69 | 27.52±4.54 | |
| V70 Gy (%) | 15.00±3.49 | 15.94±2.25 | 15.86±2.15 | 15.69±2.80 | 15.61±2.33 | |
| V78 Gy (%) | 0.07±0.17 | 0.35±0.29 | 0.44±0.47 | 0.33±0.36 | 0.29±0.24 | |
| Bladder wall | V40 Gy (%) | 38.56±10.80 | 38.34±14.38 | 37.91±13.38 | 38.36±13.75 | 38.23±13.71 |
| V70 Gy (%) | 21.00±6.40 | 20.47±6.19 | 20.29±7.23 | 20.39±7.29 | 20.31±7.38 | |
| MU | 619.20±60.88 | 621.26±24.77 | 625.26±37.90 | 619.41±30.28 | 625.11±26.92 | |
| MCS | 0.27±0.022 | 0.27±0.015 | 0.27±0.020 | 0.27±0.017 | 0.27±0.018 |
Results are expressed as means±1 SD. KBP: Knowledge-based treatment planning, CP: Clinical plan, MCSs: Modulation complexity scores, MU: Monitor unit, PTV-R: Planning target volume minus the rectum, SD: Standard deviation
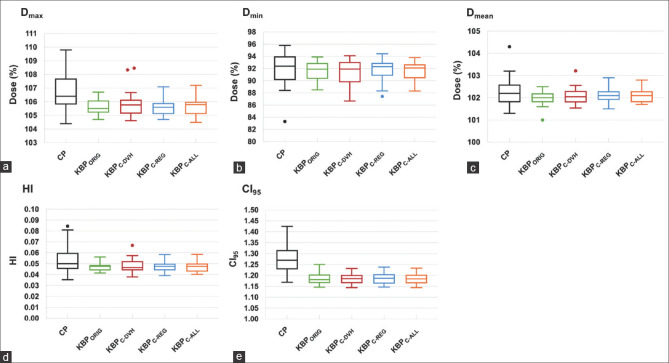
Figure 2.
(a) Dmax, (b) Dmin, (c) Dmean, (d) HI, and (e) CI95. Comparison of dose parameters for the PTV − R among the KBPs and the clinical plans. Middle, lower, and upper lines in each box are the median value, first quartile, and third quartile, respectively. Whisker values do not contain the outliers, which are plotted as individual points. PTV − R: Planning target volume minus the rectum, KBP: Knowledge-based treatment planning
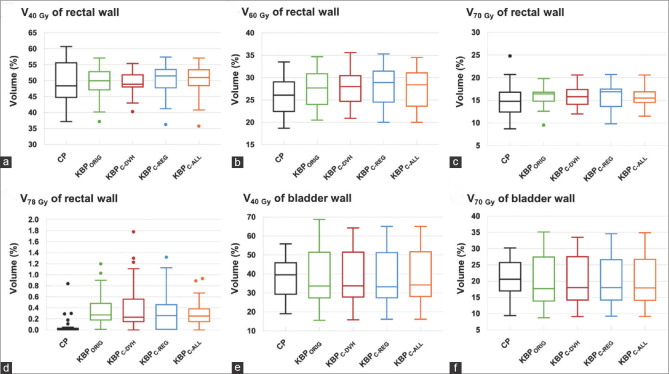
Figure 3.
(a) V40Gy of rectal wall, (b) V60Gy of rectal wall, (c) V70Gy of rectal wall, (d) V78Gy of rectal wall, (e) V40Gy of bladder wall, and (f) V70Gy of bladder wall. Comparison of dose parameters for the organs at risks for all knowledge-based treatment plannings and clinical plans. Middle, lower, and upper lines in each box are the median value, first quartile, and third quartile, respectively. Whisker values do not contain the outliers, which are plotted as individual points.
The values of Dmin and Dmean for PTV–R were comparable between the CP and all KBPs. Regarding Dmax for PTV − R, all KBPs were statistically significantly lower than those with the CP (P < 0.001). For the homogeneity of PTV − R, all KBPs were better than that found with the CP, although only KBPORIG showed a statistically significant difference from the CP (P = 0.04). Additionally, the PTV − R coverage of all KBPs was more conformal than that of the CP (P < 0.001). For the OARs, the dose parameters of all KBPs were comparable to those of the CP, except for the V78 Gy of the rectal wall. The V78 Gy of the rectal wall was significantly higher for all KBPs than for the CPs. However, the KBPC-ALL was the only planning whose V78 Gy of the rectal wall was <1% for all cases, whereas KBPORIG, KBPC-DVH, and KBPC-REG resulted in two, four, and three cases, respectively, with a V78 Gy of the rectal wall >1%.
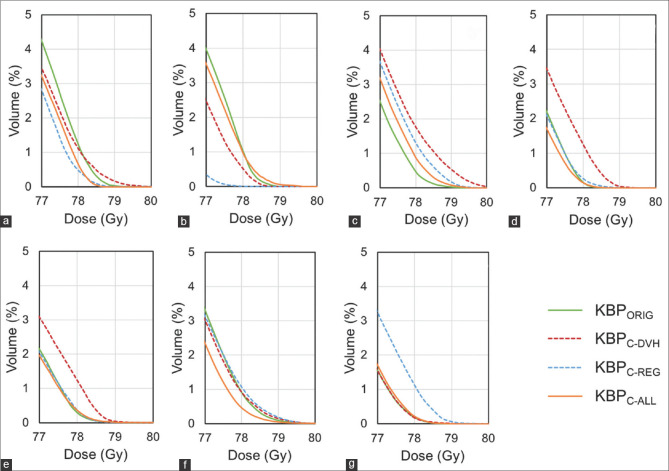
Figure 4 shows the DVH curves of the cases that did not pass the criterion of V78 Gy <1% for any of the four KBP models. The DVH curves for the KBPORIG, KBPC-DVH, and KBPC-REG models display a long tail close to the maximum dose in some cases with V78 Gy >1%, whereas none of the KBPC-ALL curves show such a tail. KBPC-DVH and KBPC-REG had one case each with V60 Gy of the rectal wall >35%. KBPORIG had one case with V40 Gy of the bladder wall >65% and one case with V70 Gy of the bladder wall >35%, whereas KBPC-REG had one case with V40 Gy of the bladder wall >65%. Hence, only KBPC-ALL fulfilled all the criteria for all cases. For the MU and MCS, there were no significant differences in CP and each KBP model.
Figure 4.
DVH-based curves of the rectal wall for cases that did not pass the criterion of V78 Gy <1% for any of the four KBP models. The KBPORIG, KBPC-DVH, and KBPC-REG models had two cases (a and b), four cases (a and c-e), and three cases (c, f and g), respectively, that did not meet the V78 Gy <1% criterion. The DVH curves for KBPORIG, KBPC-DVH, and KBPC-REG show a long tail close to the maximum dose in some cases, whereas that for KBPC-ALL had no tail in any case. KBP: Knowledge-based treatment planning, DVH: Dose–volume histogram
DISCUSSION
In this study, we investigated how the cleanup of KBP models affected the quality of a VMAT plan for treating prostate cancer with one-time auto-optimization. The cleaned-up KBPs, based on DVH and regression plots, may overcome one of the characteristics of KBPs that the high-dose delivered volumes of the OARs are inferior to those of the CPs.
Other studies have also investigated the effect of a cleaned-up KBP model on plan quality.[1,19,20,21] Delaney et al. showed the effect of dosimetric outliers for head-and-neck cancer and concluded that the cleaned-up KBP did not improve the plan quality, although the presence of many outliers deteriorated the plan quality.[21] Hussein et al. established a model in the pelvic region and also noted that the cleaned-up KBP model had no significant impact.[1] Conversely, Aviles et al. concluded that a cleaned-up KBP could improve the accuracy of the estimated DVHs,[20] although its usefulness in a new patient was unclear. Our study showed that in new patients, a cleaned-up KBP model could address weak points where the PTV overlapped with an organ. The KBPC-ALL model was the only one that did not generate cases with V78 Gy >1%, as shown in Figures 3d and 4, although KBPORIG, KBPC-DVH, and KBPC-REG had two, four, and three such cases, respectively, among the thirty evaluated clinical cases. Additionally, KBPC-ALL was the only model that could meet all the criteria of the dosimetric parameters, as shown in Table 2 and Figures 2 and 3, although only a few structures were used. Thus, cleaned-up KBPs could improve the accuracy of estimated DVHs, especially in regions of high-dose delivery where a long tail appears following a close-to-maximum dose to the OARs (estimated using DVHs), as shown by Aviles et al.[20]
KBPs were shown to be inferior to clinically accepted plans for the high-dose volumes delivered to OARs[10,16] because the only priority in the KBP system was the PTV, although the line objectives for the OARs were used during the optimization process. These line objectives for the OARs were placed horizontally in the overlap region between the PTV and OARs to prevent underdosing with the PTV.[10,18] The KBP model has heuristic factors, one of which may be manual clean-up modeling derived from both DVH and PCA regression plots, although the upgraded version of the KBP software may solve this problem. This KBP modeling method may be helpful for updating the KBP model and for creating a KBP model that could be used in treatment sites with many overlap regions, such as in head-and-neck cancer. The performance of the cleaned-up database has been described; few got some advantages, whereas few did not get any.[21] Cleaning up the database may be an obvious fact; however, no previous reports described step-wise outlier cleanup of the database. In this study, we showed that the KBPC-ALL model could improve plan quality in the overlap region, while the KBPC-DVH and KBPC-REG models might be inferior to the CP and the plans generated with the original model. Cleaning up of a KBP model must be performed carefully with adequate model validations.
CONCLUSIONS
The cleaned-up KBP model created using both DVH and PCA regression plots could improve plan quality, especially for overlap regions, without causing any deterioration in the coverage of the target with one-time auto-optimization for prostate VMAT.
Financial support and sponsorship
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant number: 16K10406) and the Japanese Society of Radiological Technology (JSRT) Research Grant (2019, 2020).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Nancy Schatken, BS, MT (ASCP), from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
REFERENCES
- 1.Hussein M, South CP, Barry MA, Adams EJ, Jordan TJ, Stewart AJ, et al. Clinical validation and benchmarking of knowledge-based IMRT and VMAT treatment planning in pelvic anatomy. Radiother Oncol. 2016;120:473–9. doi: 10.1016/j.radonc.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, Jiang F, Yue H, Li S, Zhang Y. A dosimetric evaluation of knowledge-based VMAT planning with simultaneous integrated boosting for rectal cancer patients. J Appl Clin Med Phys. 2016;17:78–85. doi: 10.1120/jacmp.v17i6.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tol JP, Delaney AR, Dahele M, Slotman BJ, Verbakel WF. Evaluation of a knowledge-based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:612–20. doi: 10.1016/j.ijrobp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Fogliata A, Wang PM, Belosi F, Clivio A, Nicolini G, Vanetti E, et al. Assessment of a model based optimization engine for volumetric modulated arc therapy for patients with advanced hepatocellular cancer. Radiat Oncol. 2014;9:236. doi: 10.1186/s13014-014-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogliata A, Belosi F, Clivio A, Navarria P, Nicolini G, Scorsetti M, et al. On the pre-clinical validation of a commercial model-based optimisation engine: Application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother Oncol. 2014;113:385–91. doi: 10.1016/j.radonc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Fogliata A, Nicolini G, Bourgier C, Clivio A, de Rose F, Fenoglietto P, et al. Performance of a Knowledge-Based Model for Optimization of Volumetric Modulated Arc Therapy Plans for Single and Bilateral Breast Irradiation. PLoS One. 2015;10:e0145137. doi: 10.1371/journal.pone.0145137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogliata A, Nicolini G, Clivio A, Vanetti E, Laksar S, Tozzi A, et al. A broad scope knowledge based model for optimization of VMAT in esophageal cancer: Validation and assessment of plan quality among different treatment centers. Radiat Oncol. 2015;10:220. doi: 10.1186/s13014-015-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin Snyder K, Kim J, Reding A, Fraser C, Gordon J, Ajlouni M, et al. Development and evaluation of a clinical model for lung cancer patients using stereotactic body radiotherapy (SBRT) within a knowledge-based algorithm for treatment planning. J Appl Clin Med Phys. 2016;17:263–75. doi: 10.1120/jacmp.v17i6.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang AT, Hung AW, Cheung FW, Lee MC, Chan OS, Philips H, et al. Comparison of planning quality and efficiency between conventional and knowledge-based algorithms in nasopharyngeal cancer patients using intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:981–90. doi: 10.1016/j.ijrobp.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Kubo K, Monzen H, Ishii K, Tamura M, Kawamorita R, Sumida I, et al. Dosimetric comparison of rapidplan and manually optimized plans in volumetric modulated arc therapy for prostate cancer. Phys Med. 2017;44:199–204. doi: 10.1016/j.ejmp.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Kamima T, Ueda Y, Fukunaga JI, Shimizu Y, Tamura M, Ishikawa K, et al. Multi-institutional evaluation of knowledge-based planning performance of volumetric modulated arc therapy (VMAT) for head and neck cancer. Phys Med. 2019;64:174–81. doi: 10.1016/j.ejmp.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kubo K, Monzen H, Ishii K, Tamura M, Nakasaka Y, Kusawake M, et al. Inter-planner variation in treatment-plan quality of plans created with a knowledge-based treatment planning system. Phys Med. 2019;67:132–40. doi: 10.1016/j.ejmp.2019.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Ueda Y, Miyazaki M, Sumida I, Ohira S, Tamura M, Monzen H, et al. Knowledge-based planning for oesophageal cancers using a model trained with plans from a different treatment planning system. Acta Oncol. 2020;59:274–83. doi: 10.1080/0284186X.2019.1691257. [DOI] [PubMed] [Google Scholar]
- 14.Inoue E, Doi H, Monzen H, Tamura M, Inada M, Ishikawa K, et al. Dose-volume histogram analysis of knowledge-based volumetric-modulated arc therapy planning in postoperative breast cancer irradiation. In Vivo. 2020;34:1095–101. doi: 10.21873/invivo.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uehara T, Monzen H, Tamura M, Ishikawa K, Doi H, Nishimura Y. Dose-volume histogram analysis and clinical evaluation of knowledge-based plan with manual objective constraints for pharyngeal cancer. J Radiat Res. 2020;61:499–505. doi: 10.1093/jrr/rraa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura M, Monzen H, Matsumoto K, Kubo K, Otsuka M, Inada M, et al. Mechanical performance of a commercial knowledge-based VMAT planning for prostate cancer. Radiat Oncol. 2018;13:163. doi: 10.1186/s13014-018-1114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueda Y, Fukunaga JI, Kamima T, Adachi Y, Nakamatsu K, Monzen H. Evaluation of multiple institutions’ models for knowledge-based planning of volumetric modulated arc therapy (VMAT) for prostate cancer. Radiat Oncol. 2018;13:46. doi: 10.1186/s13014-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tol JP, Dahele M, Delaney AR, Slotman BJ, Verbakel WF. Can knowledge-based DVH predictions be used for automated, individualized quality assurance of radiotherapy treatment plans? Radiat Oncol. 2015;10:234. doi: 10.1186/s13014-015-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogliata A, Reggiori G, Stravato A, Lobefalo F, Franzese C, Franceschini D, et al. RapidPlan head and neck model: The objectives and possible clinical benefit. Radiat Oncol. 2017;12:73. doi: 10.1186/s13014-017-0808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviles JE, Marcos MI, Sasaki D, Sutherland K, Kane B, Kuusela E. Creation of knowledge-based planning models intended for large scale distribution: Minimizing the effect of outlier plans. J Appl Clin Med Phys. 2018;19:215–26. doi: 10.1002/acm2.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaney AR, Tol JP, Dahele M, Cuijpers J, Slotman BJ, Verbakel WF. Effect of dosimetric outliers on the performance of a commercial knowledge-based planning solution. Int J Radiat Oncol Biol Phys. 2016;94:469–77. doi: 10.1016/j.ijrobp.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Esch A Van, Tillikainen L, Huyskens DP. Testing of the analytical anisotropic algorithm for photon dose calculation. Med Phys. 2006;33:4130–48. doi: 10.1118/1.2358333. [DOI] [PubMed] [Google Scholar]
- 23.Norihisa Y, Mizowaki T, Takayama K, Miyabe Y, Matsugi K, Matsuo Y, et al. Detailed dosimetric evaluation of intensity-modulated radiation therapy plans created for stage C prostate cancer based on a planning protocol. Int J Clin Oncol. 2012;17:505–11. doi: 10.1007/s10147-011-0324-1. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Jiang F, Yue H, Zhang H, Wang K, Zhang Y. Applying a RapidPlan model trained on a technique and orientation to another: A feasibility and dosimetric evaluation. Radiat Oncol. 2016;11:108. doi: 10.1186/s13014-016-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]