Abstract
Background
In animal studies, zinc supplementation inhibited phosphate-induced arterial calcification. We tested the hypothesis that higher intake of dietary zinc was associated with lower abdominal aortic calcification (AAC) among adults in the USA. We also explored the associations of AAC with supplemental zinc intake, total zinc intake and serum zinc level.
Methods
We performed cross-sectional analyses of 2535 participants from the National Health and Nutrition Examination Survey 2013–14. Dietary and supplemental zinc intakes were obtained from two 24-h dietary recall interviews. Total zinc intake was the sum of dietary and supplemental zinc. AAC was measured using dual-energy X-ray absorptiometry in adults ≥40 years of age and quantified using the Kauppila score system. AAC scores were categorized into three groups: no AAC (AAC = 0, reference group), mild–moderate (AAC >0–≤6) and severe AAC (AAC >6).
Results
Dietary zinc intake (mean ± SE) was 10.5 ± 0.1 mg/day; 28% had AAC (20% mild–moderate and 8% severe), 17% had diabetes mellitus and 51% had hypertension. Higher intake of dietary zinc was associated with lower odds of having severe AAC. Per 1 mg/day higher intake of dietary zinc, the odds of having severe AAC were 8% lower [adjusted odds ratio 0.92 (95% confidence interval 0.86–0.98), P = 0.01] compared with those without AAC, after adjusting for demographics, comorbidities and laboratory measurements. Supplemental zinc intake, total zinc intake and serum zinc level were not associated with AAC.
Conclusions
Higher intake of dietary zinc was independently associated with lower odds of having severe AAC among noninstitutionalized US adults.
Keywords: cardiovascular disease, mineral metabolism, nutrition, vascular calcification, zinc
INTRODUCTION
Patients with chronic kidney disease (CKD) often develop arterial calcification, an important predictor of cardiovascular mortality [1–3]. The development of arterial calcification in CKD is due in part to disordered mineral metabolism such as hyperphosphatemia, which induces phenotypic transformation of vascular smooth muscle cells into collagen-secreting osteoblasts [4]. Recently animal studies suggest that zinc may protect against phosphate-induced arterial calcification by inducing the production of a zinc-finger protein, tumor necrosis factor (TNF)-α-induced protein 3, and suppressing the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [5]. Importantly, CKD patients may develop a deficiency in zinc due to decreased dietary intake and intestinal absorption of zinc [6, 7].
Zinc is essential for the production and function of many structural proteins, enzymes and transcription factors [7]. Dietary sources of zinc include red meat, poultry, whole grains and dairy products [8]. Approximately 20% of the world and 10% of the US population are estimated to be at risk of inadequate zinc intake [9]. Zinc deficiency can lead to growth retardation, impaired immunity and dysregulation of blood pressure [10, 11]. Several observational studies show that low intake of dietary zinc is associated with cardiovascular mortality [12, 13], but it is unknown whether this association with cardiovascular disease is due to arterial calcification.
No study has examined the direct relationship between dietary zinc intake and arterial calcification in humans. Using the National Health and Nutrition Examination Survey (NHANES) cohort 2013–14, we tested the hypothesis that higher dietary zinc intake is associated with lower abdominal aortic calcification (AAC) among US adults. AAC is common in CKD and is an independent predictor of cardiovascular mortality in patients with and without CKD [3, 14]. As exploratory analyses, we tested the associations of AAC with supplemental zinc intake, total zinc intake and serum zinc level.
MATERIALS AND METHODS
Study population
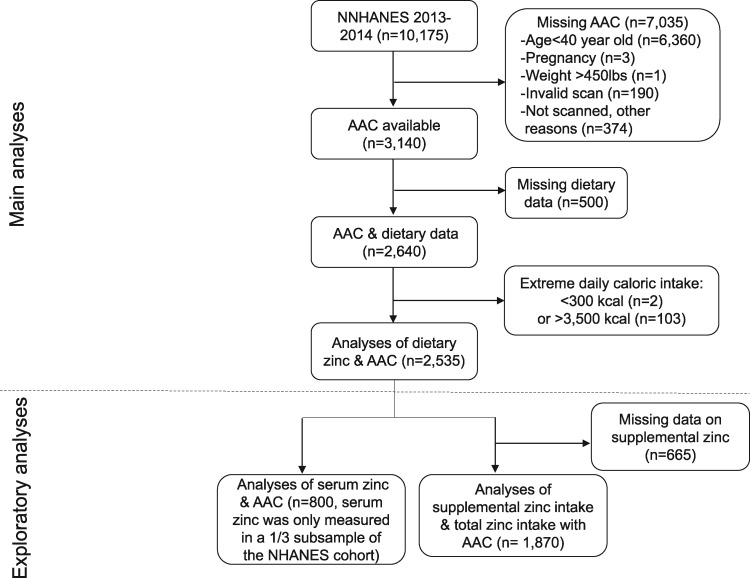
The NHANES is a nationally representative survey of a noninstitutionalized civilian population in the US using a stratified, multistage and probability sampling design [15]. The NHANES protocol was approved by the National Center for Health Statistics Ethics Review Board and written informed consent was obtained from all participants. AAC was obtained in participants ≥40 years of age in the 2013–14 cohort. In this cross-sectional study we included participants with available information on both AAC and dietary zinc intake (n = 2640; Figure 1). After excluding those with extreme caloric intake (n = 105), which was defined as >2 standard deviations greater or less than the mean caloric intake of the cohort (i.e. <300 kcal/day or >3500 kcal/day), we included 2535 participants in the main analyses, who represented ∼125 million Americans.
FIGURE 1.
Participant flowchart.
Independent variables
The main independent variable was dietary zinc intake. Dietary data were collected using the Automated Multiple-Pass Method, which provides an efficient and accurate means of collecting dietary data for a large-scale national survey such as NHANES [16]. Two 24-h dietary recalls were performed by trained dietary interviewers. The first was conducted in-person and the second was done via telephone 3–10 days later. Dietary zinc intake was calculated based on the nutrient values using the US Department of Agriculture’s Food and Nutrient Database for Dietary Studies and was averaged from the two recalls [17].
For exploratory analyses, the independent variables were supplemental zinc intake, total zinc intake and serum zinc level. Similar to dietary zinc, supplemental zinc intake was the average of two recalls for dietary supplement use. Total zinc intake was the sum of dietary and supplemental intake. After excluding 665 participants with missing data on supplements, 1870 participants were included in the analyses of supplemental and total zinc intake (Figure 1). Serum zinc was measured in a one-third subsample of the NHANES cohort. For the analyses of serum zinc, 800 participants were included.
Outcome variable
The outcome variable was AAC, which is a recognized predictor of cardiovascular mortality [1–3]. AAC was obtained from a lateral scan of the lumbar spine (vertebrae L1–L4) using dual-energy X-ray absorptiometry (DXA; Densitometer Discovery A, Hologic, Marlborough, MA, USA) and quantified using the Kauppila score system [2, 18]. Lateral abdominal radiograph is suggested by the Kidney Disease: Improving Global Outcomes 2017 clinical practice guidelines to detect arterial calcification in patients with CKD [19]. Participants were ineligible for DXA scan in this study if they were <40 years old, pregnant, weighed >450 pounds or used barium in the past 7 days (Figure 1). The Kauppila score ranges from 0 to 24 [2]. We categorized AAC scores into three groups: no calcification (AAC = 0), mild–moderate calcification (AAC >0–≤6) and severe calcification (AAC >6). A Kauppila score >6 is considered as significant calcification and has been used as a cutoff point in prior studies [1, 2].
Covariates
Potential covariates included participant demographics, comorbidities, smoking history, albumin, lipid profile, kidney function, serum markers of bone mineral metabolism, medication use and caloric intake. We categorized age in groups of 10 years, because ages ≥80 years were captured only as ‘80’ in NHANES. Poverty was defined as having a family income:poverty ratio ≤1.3 [20]. Smoker was defined as ever smoked ≥100 cigarettes in a lifetime. Diabetes mellitus (DM) was defined as taking hypoglycemic medications or having a diagnosis of DM, a hemoglobin A1c level ≥6.5%, a fasting plasma glucose ≥126 mg/dL or a 2-h plasma glucose ≥200 mg/dL [21]. Hypertension was defined as taking antihypertensive medications, having a diagnosis of hypertension or having three consecutive systolic blood pressure readings ≥140 mmHg or diastolic blood pressure ≥90 mmHg [22]. Serum albumin was included because it is a marker of inflammation and zinc is bound to albumin [23]. The lipid profile was presented as a ratio of total cholesterol to high-density lipoprotein (HDL) cholesterol [24].
Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [25]. Albuminuria was defined as a urine albumin:creatinine ratio >30 mg/g. CKD groups were categorized as CKD Stages 1–2 (presence of albuminuria and eGFR ≥60 mL/min/1.73 m2), Stage 3 (eGFR ≥30 and <60 mL/min/1.73 m2) and Stages 4–5 (eGFR <30 mL/min/1.73 m2) [26]. Serum markers of bone mineral metabolism included total 25-hydroxyvitamin D, serum calcium and phosphorus [27–29]. Diuretics included loop diuretics and thiazides since these diuretics are commonly used and may increase urinary excretion of zinc [30]. Steroids included glucocorticoid, androgen and anabolic steroids.
Statistical analyses
All analyses were performed using appropriate sampling weights and accounted for complex multistage cluster survey design using SAS 9.4 (SAS Institute, Cary, NC, USA). The significance level was set as P < 0.05. Participant demographic and clinical characteristics were tabulated by AAC group. Associations of covariates with AAC were tested using survey linear regression or Rao–Scott chi-squared tests. Associations of covariates with dietary zinc intake were examined using survey linear regression with dietary zinc intake as the dependent variable (data are presented in the Supplementary data). Multinomial logistic regression was used to test the association between dietary zinc intake (per 1 mg/day) and AAC group. Participants without AAC were used as the reference group. We tested the interactions of dietary zinc intake with sex, DM status and CKD. The associations of dietary zinc with AAC may differ between men and women [12]. DM and CKD are risk factors for arterial calcification and may mask the association between dietary zinc and AAC [1, 31]. Covariates for the adjusted models were selected based on a combination of clinical and statistical significance. Covariates were considered statistically significant if they were associated with both dietary zinc and AAC group at the level of P < 0.05. Model 1 was adjusted for age, sex and race/ethnicity. Model 2 was adjusted for the covariates in Model 1 plus caloric intake, DM, hypertension, smoking history, eGFR, albuminuria, albumin, serum phosphorus, proton pump inhibitor and diuretic use. Separately we also explored the associations of supplemental zinc intake, total zinc intake and serum zinc level with AAC group. These models were adjusted for the same covariates as in the analyses of dietary zinc.
For sensitivity analyses, we repeated the main analyses after dichotomizing dietary zinc intake, treating the AAC score as a continuous variable and choosing a different cutoff point for the AAC score. Dietary zinc was dichotomized using 11 mg as the cutoff point for men and 8 mg for women since these values are the recommended dietary allowance of zinc according to the Institute of Medicine [32]. To analyze the AAC score as a continuous variable, we log-transformed the AAC score plus 1 [i.e. log (AAC + 1)] and calculated the percentage change in the AAC score by transforming the β coefficient [i.e. percentage change in AAC = 100 × (eβ − 1)]. The AAC score was dichotomized at 0 and we compared dietary zinc intake between participants with AAC (AAC > 0) and without AAC (AAC = 0). In addition, we repeated the main analyses in the subgroups of participants with CKD Stages 1–5 (n = 526) and with CKD Stages 3–5 (n = 294).
RESULTS
Participant characteristics
Among all participants, 28% had AAC (20% mild–moderate and 8% severe; Table 1), 16.9% had DM, 51% had hypertension and 18.1% had CKD (7.1% Stages 1–2, 10.3% Stage 3 and 0.7% Stages 4–5). Compared with those without AAC, participants with AAC were older and more likely to be smokers and to have DM, hypertension and CKD. They had lower eGFR and higher hemoglobin A1c. More participants with AAC were taking proton pump inhibitors, diuretics, steroids and antihyperlipidemic medications. Mean serum phosphorous (mean ± SE) was 3.83 ± 0.02 mg/dL and it did not differ by AAC group.
Table 1.
Participant characteristics by AAC group
| Characteristics | Total (n = 2535) | No AAC (n = 1764) | Mild–moderate AAC (n = 532) | Severe AAC (n = 239) | P-value |
|---|---|---|---|---|---|
| Age group (years), % (SE) | <0.001 | ||||
| 40–49 | 27.3 (1.9) | 32.8 (1.8) | 17.9 (4.0) | 1.8 (0.4) | |
| 50–59 | 31.1 (1.8) | 33.5 (2.4) | 30.4 (5.4) | 12.1 (4.1) | |
| 60–69 | 23.5 (1.1) | 23.4 (1.4) | 24.5 (2.9) | 21.7 (5.7) | |
| >69 | 18.1 (1.4) | 10.3 (1.3) | 27.2 (4.1) | 64.4 (5.1) | |
| Female, % (SE) | 53.9 (1.6) | 54.1 (1.6) | 52.3 (3.0) | 56.5 (5.7) | 0.75 |
| Race/ethnicity, % (SE) | 0.06 | ||||
| Non-Hispanic White | 70.5 (3.4) | 68.9 (3.6) | 73.7 (4.5) | 76.1 (4.8) | |
| Non-Hispanic Black | 10.3 (1.5) | 10.8 (1.7) | 10.0 (2.1) | 6.4 (2.0) | |
| Hispanic | 11.5 (2.2) | 12.9 (2.4) | 8.5 (2.2) | 6.6 (1.8) | |
| Multiracial/other | 7.7 (1.0) | 7.3 (0.9) | 7.8 (1.7) | 10.9 (4.3) | |
| Less than high school diploma, % (SE) | 13.6 (1.9) | 13.3 (2.0) | 12.9 (2.4) | 17.4 (3.6) | 0.39 |
| Poverty, % (SE) | 17.6 (2.2) | 16.7 (2.4) | 20.1 (4.0) | 19.1 (3.6) | 0.55 |
| Body mass index (kg/m2) | 28.6 ± 0.1 | 28.8 ± 0.2 | 28.3 ± 0.3 | 27.4 ± 0.7 | 0.09 |
| Smoker, % (SE) | 43.2 (1.7) | 39.9 (1.7) | 49.6 (4.6) | 56.3 (5.6) | 0.01 |
| DM, % (SE) | 16.9 (1.3) | 12.9 (1.3) | 22.4 (2.0) | 38.0 (5.6) | <0.001 |
| Hypertension, % (SE) | 51.0 (1.5) | 44.9 (1.7) | 60.4 (3.0) | 81.0 (3.8) | <0.001 |
| CKD group, % (SE) | <0.001 | ||||
| No CKD | 81.9 (1.0) | 86.4 (1.0) | 79.2 (2.3) | 49.6 (4.5) | |
| Stages 1–2 | 7.1 (0.8) | 7.1 (0.9) | 5.7 (1.1) | 10.0 (2.6) | |
| Stage 3 | 10.3 (0.8) | 5.9 (0.7) | 14.2 (2.2) | 38.4 (5.9) | |
| Stages 4–5 | 0.7 (0.2) | 0.5 (0.2) | 0.9 (0.5) | 2.1 (0.9) | |
| eGFR (mL/min/1.73 m2) | 83.3 ± 0.5 | 86.0 ± 0.7 | 80.7 ± 1.1 | 65.6 ± 1.6 | <0.001 |
| Albumin (g/dL) | 4.26 ± 0.01 | 4.27 ± 0.02 | 4.26 ± 0.02 | 4.17 ± 0.03 | 0.04 |
| Albuminuria, % (SE) | 10.0 (0.9) | 8.8 (1.0) | 10.7 (1.7) | 18.4 (4.0) | 0.004 |
| Total 25-hydroxyvitamin D (ng/mL) | 30.5 ± 0.5 | 30.2 ± 0.5 | 30.2 ± 1.0 | 33.9 ± 1.2 | 0.007 |
| Serum total calcium (mg/dL) | 9.46 ± 0.02 | 9.45 ± 0.02 | 9.47 ± 0.03 | 9.46 ± 0.05 | 0.95 |
| Serum phosphorus (mg/dL) | 3.83 ± 0.02 | 3.84 ± 0.03 | 3.78 ± 0.02 | 3.94 ± 0.09 | 0.06 |
| Total cholesterol/HDL | 3.87 ± 0.04 | 3.85 ± 0.05 | 4.03 ± 0.07 | 3.69 ± 0.09 | 0.001 |
| Hemoglobin A1c (%) | 5.75 ± 0.03 | 5.69 ± 0.03 | 5.84 ± 0.05 | 6.05 ± 0.07 | 0.001 |
| Proton pump inhibitor, % (SE) | 14.4 (1.2) | 11.3 (1.0) | 19.6 (2.6) | 29.1 (6.0) | <0.001 |
| Diuretic use, % (SE) | 14.9 (1.2) | 11.3 (1.1) | 20.8 (2.5) | 31.6 (5.1) | <0.001 |
| Antihyperlipidemic medications, % (SE) | 28.9 (1.2) | 23.6 (1.5) | 36.0 (2.3) | 56.9 (5.6) | <0.001 |
| Steroid use, % (SE) | 3.4 (0.6) | 3.1 (0.6) | 2.0 (0.4) | 9.6 (5.6) | 0.03 |
| Caloric intake (kcal/day) | 1924 ± 21 | 1940 ± 25 | 1916 ± 31 | 1805 ± 57 | 0.07 |
Values are presented as mean ± SE unless stated otherwise.
Mean dietary zinc intake was 10.5 ± 0.1 mg/day. Women had a lower intake of dietary zinc compared with men (Supplementary data, Table S1). Compared with those without CKD, participants with CKD Stages 4–5 had a lower intake of dietary zinc (7.7 ± 0.7 versus 10.5 ± 0.1 mg/day, P = 0.001). Participants with less than a high school diploma, hypertension and those taking proton pump inhibitors and diuretics had a lower intake of dietary zinc.
Association between dietary zinc intake and AAC
Dietary zinc intake of participants without AAC (10.6 ± 0.1 mg/day) was similar to those with mild–moderate AAC (10.4 ± 0.3 mg/day, P = 0.33), whereas those with severe AAC had a lower intake of dietary zinc (9.5 ± 0.3 mg/day, P = 0.003). Sex, DM and CKD did not modify the relationship between dietary zinc and AAC (P for interaction terms: 0.48, 0.44 and 0.33, respectively). A higher intake of dietary zinc was not associated with the odds of having mild–moderate AAC, but was associated with lower odds of having severe AAC (Table 2). Per 1 mg/day higher intake of dietary zinc, the odds of having severe AAC were 5% lower in the unadjusted model (P = 0.01) and were 8% lower in the fully adjusted model {odds ratio [OR] 0.92 [95% confidence interval (CI) 0.86–0.98], P = 0.01}.
Table 2.
Multinomial logistic regression models of AAC group and dietary zinc intake (per 1 mg/day), N = 2535
| Models | Mild–moderate AAC versus no AAC |
Severe AAC versus no AAC |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Unadjusted | 0.99 (0.96–1.01) | 0.34 | 0.95 (0.91–0.99) | 0.01 |
| Model 1 | 0.98 (0.96–1.00) | 0.10 | 0.94 (0.89–0.99) | 0.02 |
| Model 2 | 0.97 (0.94–1.00) | 0.05 | 0.92 (0.86–0.98) | 0.01 |
Model 1: Adjusted for age, sex and race/ethnicity.
Model 2: Adjusted for variables in Model 1 plus caloric intake, diabetes, hypertension, smoking history, eGFR, albuminuria, albumin, serum phosphorus, proton pump inhibitor and diuretic use.
Age, race/ethnicity, diabetes, hypertension, smoking history and eGFR remained significantly associated with the odds of having severe AAC in the fully adjusted model (Table 3). Compared with those ages 40–49 years, participants >69 years had ∼50 times higher odds of having severe AAC (P < 0.001). Compared with non-Hispanic Whites, non-Hispanic Blacks had 62% lower odds of having severe AAC (P = 0.002). The odds of having severe AAC approximately doubled with the presence of DM, hypertension or smoking history (P < 0.01 for all). Per 1 mL/min/1.73 m2 higher eGFR, the odds of severe AAC were lowered by 2% (P < 0.001).
Table 3.
Multinomial logistic regression model of AAC group (n = 2535)
| Variables | Mild–moderate AAC versus no AAC |
Severe AAC versus no AAC |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Dietary zinc (mg/day) | 0.97 (0.94–1.00) | 0.05 | 0.92 (0.86–0.98) | 0.01 |
| Age group (versus 40–49 years) | ||||
| 50–59 years | 1.42 (0.74–2.70) | 0.29 | 4.39 (1.35–14.26) | 0.01 |
| 60–69 years | 1.45 (0.74–2.83) | 0.28 | 6.98 (3.45–14.15) | <0.001 |
| >69 years | 4.41 (2.19–8.89) | <0.001 | 50.59 (21.14–121.05)a | <0.001 |
| Female (versus male) | 1.00 (0.71–1.42) | 0.98 | 0.93 (0.61–1.43) | 0.74 |
| Race/ethnicity (versus non-Hispanic White) | ||||
| Non-Hispanic Black | 0.85 (0.51–1.43) | 0.54 | 0.38 (0.21–0.70) | 0.002 |
| Hispanic | 0.80 (0.54–1.17) | 0.24 | 0.76 (0.34–1.71) | 0.51 |
| Multiracial/other | 1.22 (0.71–2.09) | 0.47 | 3.09 (1.00–9.53) | 0.05 |
| Caloric intake (kcal/day) | 1.00 (1.00–1.00) | 0.22 | 1.00 (1.00–1.00) | 0.06 |
| Diabetes (yes versus no) | 1.64 (1.24–2.17) | <0.001 | 2.33 (1.32–4.08) | 0.003 |
| Hypertension (yes versus no) | 1.28 (0.98–1.68) | 0.07 | 2.13 (1.20–3.76) | 0.009 |
| Smoker (yes versus no) | 1.47 (0.97–2.22) | 0.07 | 2.34 (1.41–3.89) | 0.001 |
| eGFR (mL/min/1.73 m2) | 1.00 (0.99–1.01) | 0.70 | 0.98 (0.97–0.99) | <0.001 |
| Albuminuria (yes versus no) | 0.84 (0.53–1.34) | 0.47 | 0.81 (0.38–1.74) | 0.59 |
| Albumin (g/dL) | 1.48 (0.82–2.68) | 0.19 | 1.07 (0.51–2.26) | 0.85 |
| Serum phosphorus (mg/dL) | 0.86 (0.68–1.08) | 0.20 | 1.57 (0.87–2.83) | 0.13 |
| Proton pump inhibitor (yes versus no) | 1.37 (0.93–2.03) | 0.11 | 1.44 (0.71–2.95) | 0.31 |
| Diuretic use (yes versus no) | 1.42 (0.86–2.35) | 0.17 | 1.46 (0.79–2.69) | 0.23 |
The unit for continuous variables and the reference group for categorical variables are provided next to the variables. The OR of having mild–moderate or severe AAC was per unit increase of continuous variables and compared with the reference group for categorical variables.
Wide CI is due to the small sample size for this comparison.
Associations of AAC with supplemental zinc intake, total zinc intake and serum zinc level
The mean intake of supplemental zinc was 6.6 ± 0.4 mg/day and of total zinc was 17.3 ± 0.4 mg/day. The mean serum zinc level was 81.8 ± 1.2 μg/dL. A higher serum zinc level was associated with higher supplemental zinc intake (Pearson correlation coefficient = 0.09, P = 0.02) but not with dietary or total zinc intake. Compared with the participants without AAC, those with severe AAC had a higher intake of supplemental and total zinc (supplemental: 10.3 ± 1.2 versus 6.1 ± 0.5 mg/day, P = 0.01; total: 19.9 ± 1.2 versus 16.9 ± 0.5 mg/day, P = 0.04). Per 1 mg/day higher intake of supplemental zinc, the odds of having severe AAC versus no AAC were 3% higher [OR 1.03 (95% CI 1.01–1.04), P = 0.002; Table 4]. The result was similar for total zinc intake, with an OR of 1.02 (95% CI 1.00–1.03, P = 0.01). However, these associations became nonsignificant after adjusting for demographics in Model 1. Serum zinc level was not associated with AAC groups in either unadjusted or adjusted models.
Table 4.
Multinomial logistic regression models of AAC group with supplemental zinc, total zinc intake and serum zinc level
| Variables | Mild–moderate AAC versus no AAC |
Severe AAC versus no AAC |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Supplemental zinc intake (per 1 mg/day; n = 1870) | ||||
| Unadjusted | 1.01 (0.99–1.03) | 0.20 | 1.03 (1.01–1.04) | 0.002 |
| Model 1 | 1.00 (0.98–1.01) | 0.86 | 1.00 (0.98–1.02) | 0.95 |
| Model 2 | 1.00 (0.98–1.01) | 0.91 | 1.00 (0.97–1.02) | 0.74 |
| Total zinc intake (per 1 mg/day; n = 1870) | ||||
| Unadjusted | 1.01 (0.99–1.02) | 0.36 | 1.02 (1.00–1.03) | 0.01 |
| Model 1 | 1.00 (0.98–1.01) | 0.53 | 0.99 (0.97–1.01) | 0.42 |
| Model 2 | 1.00 (0.98–1.01) | 0.55 | 0.99 (0.97–1.01) | 0.42 |
| Serum zinc level (per 1 μg/dL; n = 800) | ||||
| Unadjusted | 1.01 (1.00–1.03) | 0.07 | 1.00 (0.97–1.04) | 0.76 |
| Model 1 | 1.02 (1.00–1.03) | 0.06 | 1.01 (0.98–1.04) | 0.50 |
| Model 2 | 1.01 (1.00–1.03) | 0.11 | 1.01 (0.98–1.03) | 0.71 |
Model 1: Adjusted for age, sex and race/ethnicity.
Model 2: Adjusted for variables in Model 1 plus caloric intake, diabetes, hypertension, smoking history, eGFR, albuminuria, albumin, serum phosphorus, proton pump inhibitor and diuretic use.
Sensitivity analyses
Approximately 55% of participants had a high intake of dietary zinc, defined as zinc intake ≥11 mg for men and ≥8 mg for women [32]. Compared with those with low intake, participants with high intake of dietary zinc had 56% lower odds of having severe AAC [adjusted OR 0.44 (95% CI 0.25–0.76), P = 0.003]. Per 1 mg/day higher intake of dietary zinc, the AAC score was lowered by 1.43% [percent change: −1.43 (95% CI −2.22 to −0.63), P = 0.002] and the odds of having AAC > 0 versus AAC = 0 were 4% lower [OR 0.96 (95% CI 0.93–0.99), P = 0.007]. In participants with CKD Stages 1–5 or CKD Stages 3–5, the odds of having severe AAC were lower with higher intake of dietary zinc after adjusting for demographics in Model 1. Per 1 mg/day higher intake of dietary zinc, the OR was 0.93 (95% CI 0.88–0.99, P = 0.02) for those with CKD Stages 1–5 and 0.94 (95% CI 0.89–0.99, P = 0.01) for those with CKD Stages 3–5 (Supplementary data, Table S2). However, these associations were not significant in the fully adjusted models.
DISCUSSION
To our knowledge, we are the first to examine the association between dietary zinc intake and arterial calcification in humans. Using a nationally representative cohort, we found that AAC was prevalent among noninstitutionalized US adults and that higher intake of dietary zinc was independently associated with lower odds of having severe AAC. After adjusting for covariates including demographics, comorbidities, kidney function and serum phosphorous level, the odds of having severe AAC were 8% lower per 1 mg/day higher intake of dietary zinc. Examining AAC score as a continuous variable and using a different cutoff point of the score yielded similar results.
As elegantly demonstrated by Voelkl et al. [5] in a series of in vitro and animal experiments, zinc supplementation protected against phosphate-induced arterial calcification. Using cultured human aortic vascular smooth muscle cells, zinc sulfate blunted phosphate-induced calcification and decreased the messenger RNA expression of osteogenic markers by inducing zinc-finger protein TNF-α-induced protein 3 and suppressing the activation of NF-κB. Activation of the NF-κB pathway is critical for phosphate-induced arterial calcification, and the inhibition of NF-κB reduces phosphate-induced arterial medial calcification in mice with CKD [33, 34].
In the same research article, Voelkl et al. demonstrated that the addition of zinc sulfate in drinking water ameliorated arterial calcification in ‘klotho’ knockout mice as well as mice with CKD and cholecalciferol overload, which were murine models of arterial calcification [5]. In our study, higher intake of dietary zinc was associated with lower odds of having severe AAC after adjusting for serum phosphorous, which is associated with arterial calcification even within a normal range [28, 29]. Our findings are consistent with the results from animal studies and support the potential effect of dietary zinc intake on arterial calcification.
Although no prior studies have examined the association between dietary zinc intake and arterial calcification in humans, several observational studies have examined the relationship between dietary zinc intake and cardiovascular morbidity/mortality, where cardiovascular disease was mostly defined as having coronary heart disease or stroke [35]. We identified six of these publications, which included cohorts from multiple countries [12, 13, 36–39]. With a higher intake of dietary zinc, three studies showed a decreased risk [12, 13, 37], two showed an increased risk [36, 39] and one showed no association with cardiovascular morbidity or mortality [38]. The discrepancy in the conclusions of these studies could be partly explained by the difference in the sources of dietary zinc. In Japan, the major source of dietary zinc is rice [40], and higher dietary zinc intake was associated with lower mortality due to coronary heart disease [12]. In the USA, the major source of dietary zinc is red meat [8]. In a study using the US Multi-Ethnic Study of Atherosclerosis cohort, 14% of dietary zinc intake was from red meat, and higher zinc intake from red meat, but not other sources, was associated with a higher risk of cardiovascular disease [39]. High consumption of red meat is associated with cardiovascular morbidity [41], and other constituents of red meat might have confounded the association between dietary zinc and cardiovascular morbidity. For future studies, examining the relationship between different sources of dietary zinc and arterial calcification may provide further insight into the pathophysiology of zinc and arterial calcification.
We found that the serum zinc level was positively associated with supplemental zinc intake but not associated with dietary zinc intake. This is consistent with prior studies that showed the serum zinc level was elevated by zinc supplementation [42], but serum zinc level was poorly correlated with dietary zinc intake due to the differences in bioavailability and metabolism from dietary sources [43, 44]. In our study, AAC was not associated with supplemental zinc intake, total zinc intake or serum zinc level after adjusting for demographics. Prior studies also found no association between supplemental zinc intake and cardiovascular morbidity [45, 46]. For the relationship between blood zinc level and cardiovascular morbidity/mortality, we identified six publications [13, 47–51]. Among these studies, two of them showed a decreased risk with high zinc level, whereas the rest showed no association [48, 49]. Compared to our study and the studies that showed no association [13, 47, 50, 51], the study population from the studies that showed a decreased risk with high zinc level had higher cardiovascular risk at baseline (e.g. the need for coronary angiography) [48, 49].
Our study has limitations. First, given the cross-sectional nature of the study, we cannot infer causation between dietary zinc and arterial calcification; however, together with the findings from the animal studies and biological plausibility [5], our findings provide support for conducting further studies in humans to examine the relationship between dietary zinc intake and arterial calcification. Second, only a small portion of the study population had advanced CKD. In the subgroup analyses of participants with CKD, dietary zinc intake was not associated with AAC in the fully adjusted model. The lack of association could be due to low statistical power from the small sample size (n = 526 for participants with CKD Stages 1–5 and n = 294 for those with CKD Stages 3–5). The low prevalence of CKD in our study population limits the generalizability of our findings to people with CKD, who have a high prevalence of arterial calcification [1]. Consistent with the published literature [6, 7], we found that participants with advanced CKD (i.e. Stages 4–5) had lower dietary zinc intake compared with those without CKD, suggesting that participants with advanced CKD could be a practical population for future interventional studies involving the modification of dietary zinc intake.
Our study has several strengths. First, this is the first study in humans to examine the relationship between dietary zinc and arterial calcification. Second, dietary data in NHANES were obtained by trained interviewers in two 24-h dietary recalls using a validated method [16]. By using the average of two recalls, we had a relatively accurate assessment of participants’ usual dietary intake. Third, while our primary independent variable was dietary zinc intake, we also studied supplemental zinc intake and serum zinc level. Few cohorts have all the parameters of zinc status [35]. We found that AAC was associated with dietary zinc intake, but not with supplemental zinc intake or serum zinc level. These findings suggest that not all sources of zinc intake are equivalent with regard to their association with arterial calcification. These relationships are comparable to that of calcium and cardiovascular risk, in which dietary calcium may reduce cardiovascular risk while supplemental calcium may increase it [52].
In conclusion, we found that with every 1 mg/day higher intake of dietary zinc, the odds of having severe AAC versus having no AAC were 8% lower among noninstitutionalized US adults. Together with the findings from animal studies [5], our findings suggest potential beneficial effects of dietary zinc on arterial calcification. Further studies are needed to confirm this relationship.
FUNDING
W.C. is supported by K23 DK114476 from the National Institutes of Health (NIH) and American Society of Nephrology Carl W. Gottschalk Research Scholar Grant. M.K.A. is supported by K23 DK099438 and R03 DK116023 from the NIH. D.A.B. is supported by R01 DK075462 from the NIH. J.W.-R. is supported by the New York Regional Center for Diabetes Translational Research (DK111022) from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
AUTHORS’ CONTRIBUTIONS
W.C., R.E., W.B.M., J.W.-R., D.A.B. and M.L.M. contributed to the research idea and study design. W.C., R.E., W.B.M., J.W.-R., M.K.A., D.A.B. and M.L.M. contributed to the data processing, analysis and interpretation. W.C., R.E. and W.B.M. were responsible for the statistical analysis. D.A.B. and M.L.M. provided mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
W.C., R.E., W.B.M., J.W.-R., M.K.A., D.A.B. and M.L.M. declare that they have no relevant financial interests. D.A.B. is a consultant for Relyspa/Vifor/Fresenius, Amgen, Sanofi/Genzyme and Tricida and has an equity interest in Amgen and Tricida. Results presented in this article have not been published previously in whole or part.
(See related article by Cardozo and Mafra. Don’t forget the zinc. Nephrol Dial Transplant 2020; 35: 1094--1098)
Supplementary Material
REFERENCES
- 1. Gorriz JL, Molina P, Cerveron MJ. et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol 2015; 10: 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kauppila LI, Polak JF, Cupples LA. et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997; 132: 245–250 [DOI] [PubMed] [Google Scholar]
- 3. Wilson PWF, Kauppila LI, O’Donnell CJ. et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001; 103: 1529–1534 [DOI] [PubMed] [Google Scholar]
- 4. Paloian NJ, Giachelli CM.. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 2014; 307: F891–F900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voelkl J, Tuffaha R, Luong TTD. et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J Am Soc Nephrol 2018; 29: 1636–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foote JW, Hinks LJ.. Zinc absorption in haemodialysis patients. Ann Clin Biochem 1988; 25: 398–402 [DOI] [PubMed] [Google Scholar]
- 7. Mahajan SK. Zinc in kidney disease. J Am Coll Nutr 1989; 8: 296–304 [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health Office of Dietary Supplements. Zinc: fact sheet for health professionals. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/#en11 (24 January 2019, date last accessed)
- 9. Wuehler SE, Peerson JM, Brown KH.. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutr 2005; 8: 812–819 [DOI] [PubMed] [Google Scholar]
- 10. Little PJ, Bhattacharya R, Moreyra AE. et al. Zinc and cardiovascular disease. Nutrition 2010; 26: 1050–1057 [DOI] [PubMed] [Google Scholar]
- 11. Williams CR, Mistry M, Cheriyan AM. et al. Zinc deficiency induces hypertension by promoting renal sodium reabsorption. Am J Physiol Renal Physiol 2019; 316: F646–F653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eshak ES, Iso H, Yamagishi K. et al. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J Nutr Biochem 2018; 56: 126–132 [DOI] [PubMed] [Google Scholar]
- 13. Bates CJ, Hamer M, Mishra GD.. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr 2011; 105: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verbeke F, Van Biesen W, Honkanen E. et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the Calcification Outcome in Renal Disease (CORD) study. Clin J Am Soc Nephrol 2011; 6: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. National Health and Nutrition Examination Survey, 2013-2014 https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013-14_overview_brochure.pdf (14 January 2019, date last accessed)
- 16. Moshfegh AJ, Rhodes DG, Baer DJ. et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008; 88: 324–332 [DOI] [PubMed] [Google Scholar]
- 17.Food Surveys Research Group. Food and Nutrient Database for Dietary Studies https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/ (14 January 2019, date last accessed)
- 18. Schousboe JT, Debold CR.. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int 2006; 17: 281–289 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cowan AE, Jun S, Gahche JJ. et al. Dietary supplement use differs by socioeconomic and health-related characteristics among US adults, NHANES 2011–2014. Nutrients 2018; 10: 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menke A, Casagrande S, Geiss L, Cowie CC.. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314: 1021–1029 [DOI] [PubMed] [Google Scholar]
- 22. Yoon SS, Carroll MD, Fryar CD.. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief 2015; 220: 1–8 [PubMed] [Google Scholar]
- 23. Rucker D, Thadhani R, Tonelli M.. Trace element status in hemodialysis patients. Semin Dial 2010; 23: 389–395 [DOI] [PubMed] [Google Scholar]
- 24. Lemieux I, Lamarche B, Couillard C. et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med 2001; 161: 2685–2692 [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Summary of recommendation statements. Kidney Int Suppl 2013; 3: 263–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park KS, Park J, Choi SH. et al. Serum phosphorus concentration and coronary artery calcification in subjects without renal dysfunction. PLoS One 2016; 11: e0151007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheridan K, Logomarsino JV.. Effects of serum phosphorus on vascular calcification in a healthy, adult population: a systematic review. J Vasc Nurs 2017; 35: 157–169 [DOI] [PubMed] [Google Scholar]
- 29. Hruska KA, Mathew S, Lund R. et al. Hyperphosphatemia of chronic kidney disease. Kidney Int 2008; 74: 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wester PO. Urinary zinc excretion during treatment with different diuretics. Acta Med Scand 1980; 208: 209–212 [DOI] [PubMed] [Google Scholar]
- 31. Katz R, Budoff MJ, O'Brien KD. et al. The metabolic syndrome and diabetes mellitus as predictors of thoracic aortic calcification as detected by non-contrast computed tomography in the Multi-Ethnic Study of Atherosclerosis. Diabet Med 2016; 33: 912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of Medicine Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press, 2001 [PubMed]
- 33. Yoshida T, Yamashita M, Horimai C. et al. Smooth muscle-selective nuclear factor-κB inhibition reduces phosphate-induced arterial medial calcification in mice with chronic kidney disease. J Am Heart Assoc 2017; 6: e007248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voelkl J, Lang F, Eckardt KU. et al. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci 2019; 76: 2077–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chu A, Foster M, Samman S.. Zinc status and risk of cardiovascular diseases and type 2 diabetes mellitus-a systematic review of prospective cohort studies. Nutrients 2016; 8: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Milton AH, Vashum KP, McEvoy M. et al. Prospective study of dietary zinc intake and risk of cardiovascular disease in women. Nutrients 2018; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee DH, Folsom AR, Jacobs DR Jr.. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women’s Health Study. Am J Clin Nutr 2005; 81: 787–791 [DOI] [PubMed] [Google Scholar]
- 38. Shi Z, Chu A, Zhen S. et al. Association between dietary zinc intake and mortality among Chinese adults: findings from 10-year follow-up in the Jiangsu Nutrition Study. Eur J Nutr 2018; 57: 2839–2846 [DOI] [PubMed] [Google Scholar]
- 39. de Oliveira Otto MC, Alonso A, Lee DH. et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr 2012; 142: 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarukura N, Kogirima M, Takai S. et al. Dietary zinc intake and its effects on zinc nutrition in healthy Japanese living in the central area of Japan. J Med Invest 2011; 58: 203–209 [DOI] [PubMed] [Google Scholar]
- 41. Quintana Pacheco DA, Sookthai D, Wittenbecher C. et al. Red meat consumption and risk of cardiovascular diseases-is increased iron load a possible link? Am J Clin Nutr 2018; 107: 113–119 [DOI] [PubMed] [Google Scholar]
- 42.Age-Related Eye Disease Study Research Group. The effect of five-year zinc supplementation on serum zinc, serum cholesterol and hematocrit in persons randomly assigned to treatment group in the age-related eye disease study: AREDS Report No. 7. J Nutr 2002; 132: 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonoodi K, Moslem A, Darroudi S. et al. Serum and dietary zinc and copper in Iranian girls. Clin Biochem 2018; 54: 25–31 [DOI] [PubMed] [Google Scholar]
- 44. Chu A, Holdaway C, Varma T. et al. Lower serum zinc concentration despite higher dietary zinc intake in athletes: a systematic review and meta-analysis. Sports Med 2018; 48: 327–336 [DOI] [PubMed] [Google Scholar]
- 45. Mursu J, Robien K, Harnack LJ. et al. Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med 2011; 171: 1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al-Delaimy WK, Rimm EB, Willett WC. et al. Magnesium intake and risk of coronary heart disease among men. J Am Coll Nutr 2004; 23: 63–70 [DOI] [PubMed] [Google Scholar]
- 47. Tonelli M, Wiebe N, Bello A. et al. Concentrations of trace elements and clinical outcomes in hemodialysis patients: a prospective cohort study. Clin J Am Soc Nephrol 2018; 13: 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pilz S, Dobnig H, Winklhofer-Roob BM. et al. Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br J Nutr 2009; 101: 1534–1540 [DOI] [PubMed] [Google Scholar]
- 49. Soinio M, Marniemi J, Laakso M. et al. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007; 30: 523–528 [DOI] [PubMed] [Google Scholar]
- 50. Leone N, Courbon D, Ducimetiere P. et al. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006; 17: 308–314 [DOI] [PubMed] [Google Scholar]
- 51. Marniemi J, Jarvisalo J, Toikka T. et al. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol 1998; 27: 799–807 [DOI] [PubMed] [Google Scholar]
- 52. Li K, Kaaks R, Linseisen J. et al. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg). Heart 2012; 98: 920–925 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.