Abstract
Immunoglobulins (Ig) are essential components in the colostrum of bovine species that enable passive immunization of newborn calves. Concentrations of fat and protein are greater in colostrum compared with mature milk and represent a vital source of energy and nutrients. Colostral IgG was shown to vary between individual dairy cows, but comparative data on different breeds and performance levels are scarce. The objective of the present field study was to investigate the contents of total IgG, fat, protein, and lactose in colostrum in different Swiss and German dairy and dual-purpose breeds. We collected colostrum samples of 458 cows of 13 different breeds (dairy breeds: Brown Swiss, Swiss and German Holstein Friesian, and New Zealand Holstein; dual-purpose breeds: German Fleckvieh, Holstein Friesian × Montbéliarde, Montbéliarde, Murnau-Werdenfels, Original Braunvieh, Pinzgauer, Rhetic Gray, and Simmental; and beef-type crossbred: Charolais × Holstein Friesian). Colostrum samples were obtained between 5 and 900 min after calving and analyzed for total IgG, fat protein, and lactose contents. Immunoglobulin G concentrations varied between 12.7 and 204.0 mg/mL. No effect of breeding purpose (i.e., dairy or dual-purpose) nor of previous lactation yield on IgG content was observed. However, milking of cows for the first time later than 12 h after parturition resulted in lower colostrum IgG concentrations compared with colostrum harvest within 9 h after calving (P < 0.05). Multiparous cows had a higher colostral IgG concentration than primiparous cows (P < 0.0001). Overall, concentrations of IgG and other constituents in colostrum varied widely in the different cattle breeds. High-yielding dairy cows did not have poorer colostrum quality compared with lower-yielding animals or beef and dual-purpose breeds, which suggests an individually different transfer of circulating IgG into colostrum.
Keywords: breed, cattle, colostrum, dairy cow, immunoglobulin G
Introduction
Colostrum contains characteristically high concentrations of immunoglobulin (Ig) G, protein, fat, and other bioactive compounds compared with mature milk (Blum and Hammon, 2000). To ensure a sufficient passive transfer of colostral IgG, calves depend on a timely supply with high-quality colostrum (Besser et al., 1985). In this context, the colostrum of dairy cows is assumed to be of poorer quality compared with colostrum in beef cows, most likely due to a greater milk production and consequently dilution of IgG. One study of Guy et al. (1994) showed that dairy cows have lower IgG concentrations in colostrum compared with beef cows, but secreted a higher mass of IgG into colostrum. However, dairy cows in the study of Guy et al. (1994) showed on average a rather poor colostrum quality. In contrast, other studies observed much greater IgG contents in the colostrum of dairy cows (Morin et al., 2001; Moore et al., 2005). In addition, Guy et al. (1994) compared only a few animals (13 Holstein dairy vs. 15 Charolais and Hereford beef cows). Meanwhile, more than 25 yr have passed and milk production per cow increased significantly. Estimation of colostrum quality was mostly conducted in the major dairy breeds such as Holstein or Jersey cows (Kehoe et al., 2007; Bielmann et al., 2010; Morrill et al., 2012), whereas only a few studies included beef cattle or local breeds (Gulliksen et al., 2008; Vandeputte et al., 2014). The objective of the present descriptive study was to compare the colostrum composition of different cattle breeds that are considered dairy and dual-purpose types, and are partly used for crossbreeding worldwide. In addition, we evaluated the impact of parity number, milk yield of the previous lactation, gestation and dry period length, and time of first milking relative to parturition on the concentration of selected colostrum components.
Materials and Methods
Animals and colostrum sampling
The European Convention for the Protection of Animals kept for Farming Purposes (treaty ETS No.087) was followed by all participating farmers. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes.
For the present descriptive study, colostrum samples of 458 cows from 13 different cattle breeds were collected by farmers in Switzerland and Germany. At least five colostrum samples of one breed were provided by one farm. In total, 28 dairy farms contributed to this field study, with one farm per breed (Rhetic Gray, 5 cows) up to 10 farms (German Fleckvieh, 177 cows). Details on the enrolled number of cows, parity, gestation and dry period length, and the previous 305-d lactation yield are shown in Table 1. All cows enrolled calved within half a year. Colostrum samples (approximately 50 mL) were obtained from the first milking after parturition (4.1 ± 3.7 h after parturition; range from 0 to 15 h) and immediately frozen at −20 °C until analysis. In addition, participating farmers filled in a form with data about the individual calving cows (e.g., parity number, date of insemination date and dry-off, and previous lactation yield), time of parturition, and first milking.
Table 1.
Cattle breeds, purpose, number of animals, and individual data (parity, gestation and dry period length, and previous lactation yield) of cows included in the present study1
| Breed | Purpose | Cows, n | Parity | Gestation length, d | Dry period length, d | Previous 305-d lactation yield, kg |
|---|---|---|---|---|---|---|
| Brown Swiss | Dairy | 34 | 2.4 ± 1.5 | 288.3 ± 6.0 | 71.3 ± 14.1 | 8,510 ± 2,330 |
| Charolais × Holstein Friesian | Beef-crossbred | 23 | 2.0 ± 0.0 | 282.0 ± 3.7 | 304.7 ± 110.1 | 1,361 ± 1,420 |
| German Fleckvieh | Dual | 177 | 2.8 ± 1.8 | 287.8 ± 5.5 | 64.0 ± 18.1 | 6,791 ± 1,874 |
| Holstein Friesian (German) | Dairy | 21 | 3.9 ± 0.7 | 280.9 ± 4.8 | 53.0 ± 15.9 | 10,483 ± 451 |
| Holstein Friesian (Swiss) | Dairy | 76 | 3.3 ± 1.9 | 283.7 ± 6.2 | 62.4 ± 30.8 | 8,839 ± 1,720 |
| Holstein Friesian × Montbéliarde | Dual | 20 | 2.4 ± 1.5 | 286.8 ± 5.9 | 57.9 ± 10.3 | 6,364 ± 1,027 |
| Montbéliarde | Dual | 11 | 3.9 ± 2.2 | 288.3 ± 5.7 | 59.6 ± 9.6 | 8,139 ± 1,548 |
| Murnau-Werdenfels | Dual | 13 | 2.6 ± 0.8 | 290.7 ± 5.5 | 62.3 ± 7.9 | 4,770 ± 615 |
| New Zealand Holstein | Dairy | 17 | 2.9 ± 2.1 | 278.6 ± 3.3 | 59.8 ± 7.2 | 6,062 ± 1,108 |
| Original Braunvieh | Dual | 9 | 3.9 ± 2.5 | 289.3 ± 7.9 | 66.4 ± 21.2 | 6,614 ± 338 |
| Pinzgauer | Dual | 37 | 2.6 ± 1.2 | 288.4 ± 5.8 | 60.3 ± 29.2 | 5,709 ± 1,297 |
| Rhetic Gray | Dual | 5 | 2.6 ± 0.9 | 291.0 ± 5.7 | Not available | Not available |
| Simmental | Dual | 15 | 3.5 ± 2.0 | 286.9 ± 5.2 | 46.2 ± 6.8 | 5,887 ± 998 |
1Data on parity, gestation and dry period length, and previous lactation yield are presented as mean values ± SD.
Measurement of IgG and colostrum constituents
Total IgG concentration in colostrum was analyzed with a modified commercial enzyme-linked immunosorbent assay (ELISA) kit (Bovine IgG ELISA Quantitation Set; Cat. No. E10-118; Bethyl Laboratories Inc., Montgomery, TX) following the manufacturer’s protocol and with slight modifications as described by Lehmann et al. (2013). In brief, after thawing at room temperature, colostrum samples were serially diluted in ELISA wash buffer (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, adjusted to pH 8.0) to final dilutions of 1:800,000 and 1:1,600,000. The inter-assay coefficient of variation (CV) was 6.1% and the intra-assay CV was 9.5%. Colostral IgG concentrations are expressed in mg/mL. Due to their high viscosity, colostrum samples were diluted 1:2 with distilled water before the analysis of fat, protein, and lactose concentrations by a milk infrared analyzer (MilkoScan 7 RM, Foss Analytical A/S, Hillerød, Denmark; Milchprüfring Bayern e.V., Wolnzach, Germany).
Statistical analysis
Statistical analysis was carried out with the software SAS, version 9.4 (SAS Institute, Cary, NC). For the evaluation of associations of various parameters on IgG, fat, protein, and lactose concentrations in colostrum, a generalized linear model (GLM) procedure with breed and parity number as class variables and additionally either gestation and dry period length or previous lactation yield as an individual covariate was used. Significant effects of breed and other variables, respectively, were detected by the Tukey–Kramer post-hoc test at P < 0.05. In terms of the effect of dry period length and milk yield of the previous 305-d lactation period on colostrum components, data of the Charolais × German Holstein Friesian breed were excluded from the statistical analysis due to the very low milk yield and extraordinary long dry period length compared with the other breeds (Table 1). The impact of the interval length between parturition and time of first milking on colostral IgG concentration was evaluated with a GLM procedure with time relative to parturition as a fixed effect. Data presented are mean values ± SD.
Results and Discussion
IgG concentration in colostrum is usually studied in popular dairy breeds such as Holstein, Jersey, and Brown Swiss (Morin et al., 2001; Kehoe et al., 2007; Morrill et al., 2012) or local dairy breeds (e.g., Norwegian Red cattle; Gulliksen et al., 2008), whereas only few literature are available on dual-purpose and beef breeds (Vandeputte et al., 2014). In view of the widespread assumption that beef and low-yielding cows produce colostrum of much better quality (i.e., with a higher IgG content) compared with dairy cows, we examined the colostrum composition of dairy and dual-purpose breeds popular in Central Europe such as Brown Swiss, Montbéliarde, and Simmental. Due to limitations of this field study (different farms and feeding systems, dry cow, and calving management), the effect of the individual farm on colostrum characteristics within breed cannot be excluded. However, we intended to give a descriptive presentation of colostrum data that reflect the variation among breeds under practical conditions.
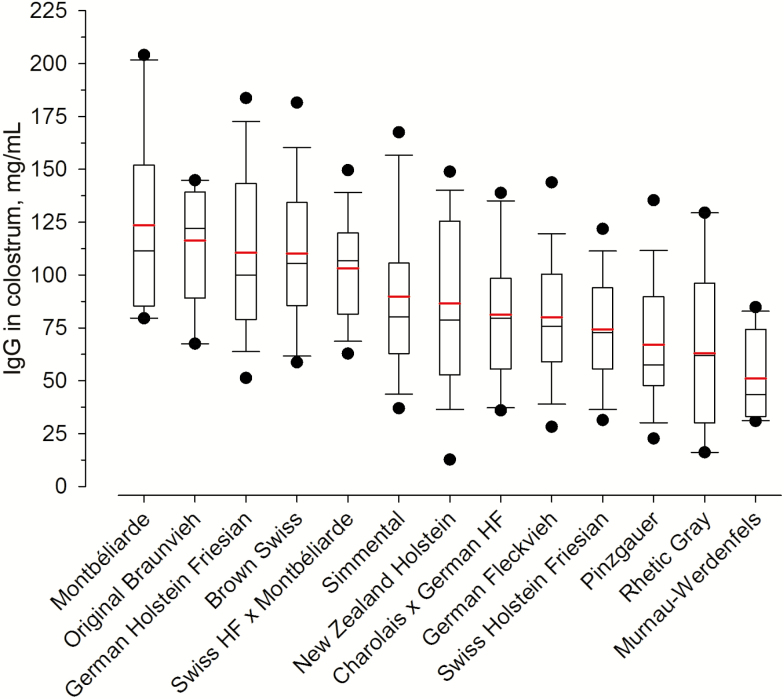
In the present study, the IgG concentration varied broadly between and also within breeds, ranging from 12.7 to 204.1 mg/mL (84.2 ± 35.2 g/mL; Figure 1). This wide spectrum of values is consistent with data from diverse individual studies conducted during the last decade (e.g., Morrill et al., 2012; Conneely et al., 2013; Dunn et al., 2017). We observed the highest average IgG concentrations in two dual-purpose breeds, Montbéliarde (123.6 ± 43.6 mg/mL; Figure 1) and Original Braunvieh (116.4 ± 28.6 mg/mL; Figure 1), followed by two high-yielding dairy breeds, German Holstein Friesian (110.5 ± 39.0 mg/mL; Figure 1) and Brown Swiss (110.2 ± 34.0 mg/mL; Figure 1). Lowest colostral IgG were measured in Murnau-Werdenfels dual-purpose cows (51.0 ± 20.3 mg/mL; Figure 1). Based on these observations, we conclude that cows that are not exclusively selected for high milk production and thus showing a lower lactation performance do not necessarily produce better quality colostrum than high-yielding dairy cows. This result is further supported by the fact that in the present study previous lactation yield was not related to IgG concentration in colostrum (P > 0.05). Vandeputte et al. (2014) analyzed the colostral IgG content in four different beef cattle breeds and observed average IgG concentrations of 95.9 ± 36.2 mg/mL that are comparable to our measurements in dairy cow colostrum. When looking at IgG concentrations in dairy cows of other reports (Bielmann et al., 2010; Rivero et al., 2012), traditional dairy breeds such as Holstein or Brown Swiss in the present study produced colostrum with similar or even higher IgG concentrations. Accordingly, the assumption that colostrum quality of (high-yielding) dairy cows is basically poorer compared with beef or low-yield cows must be clearly rejected. However, the respective variation among individuals must be considered.
Figure 1.
IgG concentrations in colostrum (mg/mL) of different cattle breeds. The box gives the 25th to 75th quartile, whereas the whiskers show the 5th to 95th percentile distribution of the data. The solid red line within the box represents the mean, and the solid black line shows the median of the data.
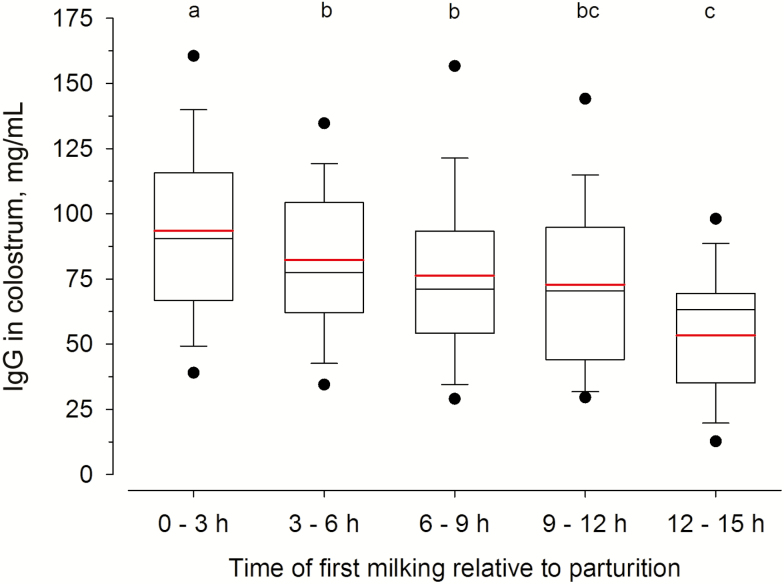
We further observed the highest IgG concentrations in colostrum milked within 3 h post partum (p.p.; Figure 2), which supports the claim that cows should be milked as soon as possible after calving to obtain high-quality colostrum. Up to approximately 9 to 12 h p.p., colostral IgG content remained rather constant (Figure 2), confirming the findings of previous studies (Morin et al., 2010; Conneely et al., 2013; Cabral et al., 2016). After approximately 12 h, IgG concentration in colostrum declined concomitantly with the start of copious milk production, reflected by a significant rise in lactose concentration (data not shown). Therefore, the first milking to obtain colostrum should not be carried out too long after calving. Otherwise, colostral Ig content might be diluted due to the onset of lactogenesis.
Figure 2.
IgG concentration (mg/mL) in colostrum grouped by intervals relative to calving. The box gives the 25th to 75th quartile, whereas the whiskers show the 5th to 95th percentile distribution of the data. The solid red line within the box represents the mean, and the solid black line shows the median of the data. Differences in colostral IgG concentrations over time after parturition are indicated by different letters (P < 0.05).
A widely recognized criterion to evaluate colostrum quality is the threshold of 50 mg IgG/mL colostrum. The average colostral IgG content of cows in the present study was 84.2 ± 35.2 mg/mL, where 15.3% were below the cutoff of 50 mg IgG/mL. Respective data in the literature on the proportion of cows with adequate colostrum quality vary between less than 50% (Gulliksen et al., 2008) or even 30% (Morrill et al., 2012) and values above 90% (Bielmann et al., 2010; Conneely et al., 2013). Only a few primiparous cows in the present study had an IgG concentration < 50 mg/mL, demonstrating that the colostrum of primiparous cows can also be of sufficient quality and should not be discarded without estimation of its IgG content on-farm (e.g., via a Brix refractometer).
Similar to IgG, the contents of fat, protein, and lactose in colostrum also showed wide ranges within and among breeds, from 1.10% to 20.88%, 3.34% to 26.50%, and 1.61% to 4.60%, respectively (Table 2). On average over all breeds, we observed results of 5.93 ± 3.09% for fat, 14.04 ± 3.70% for protein, and 2.95 ± 0.56% for lactose that are in line with earlier studies (Kehoe et al., 2007; Morrill et al., 2012; Dunn et al., 2017). The protein content in colostrum largely mirrored the colostral IgG concentration since cows with a greater IgG content also showed higher protein concentrations and vice-versa (Table 2).
Table 2.
Fat, protein, and lactose content (%) and IgG concentrations (mg/mL) in colostrum of different cow breeds
| Breed | Fat, % | Protein, % | Lactose, % | IgG, mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | |
| Brown Swiss | 7.88 | 4.11 | 1.56 | 20.88 | 14.56 | 3.65 | 6.41 | 23.22 | 2.80 | 0.53 | 1.76 | 3.89 | 110.2 | 34.0 | 52.3 | 185.3 |
| Charolais × Holstein Friesian | 4.56 | 1.85 | 1.10 | 8.66 | 13.59 | 3.41 | 8.96 | 21.62 | 3.29 | 0.47 | 2.10 | 4.10 | 81.2 | 30.6 | 35.9 | 139.3 |
| German Fleckvieh | 6.15 | 3.21 | 1.53 | 19.02 | 14.20 | 3.7 | 4.92 | 26.50 | 2.87 | 0.57 | 1.70 | 4.50 | 80.0 | 32.3 | 17.3 | 171.5 |
| Holstein Friesian (German) | 4.44 | 1.75 | 2.16 | 8.78 | 18.20 | 3.94 | 11.28 | 24.63 | 2.85 | 0.59 | 1.82 | 3.81 | 110.5 | 39.0 | 49.9 | 184.6 |
| Holstein Friesian (Swiss) | 5.46 | 2.80 | 1.32 | 14.21 | 12.61 | 2.90 | 3.34 | 17.12 | 3.19 | 0.53 | 1.94 | 4.60 | 74.3 | 27.3 | 12.7 | 135.6 |
| Holstein Friesian × Montbéliarde | 6.08 | 2.97 | 2.48 | 13.68 | 15.65 | 2.24 | 12.20 | 19.13 | 2.67 | 0.27 | 2.27 | 3.11 | 103.3 | 23.9 | 62.5 | 150.1 |
| Montbéliarde | 5.88 | 2.48 | 2.78 | 9.89 | 16.17 | 3.47 | 11.04 | 22.07 | 2.44 | 0.61 | 1.70 | 3.50 | 123.6 | 43.6 | 79.5 | 204.1 |
| Murnau-Werdenfels | 6.33 | 2.11 | 1.37 | 9.59 | 12.00 | 4.71 | 8.07 | 25.29 | 3.16 | 0.63 | 1.61 | 4.05 | 51.0 | 20.3 | 30.9 | 84.7 |
| New Zealand Holstein | 5.35 | 2.76 | 1.22 | 10.40 | 14.30 | 4.21 | 6.26 | 21.89 | 3.06 | 0.55 | 1.89 | 4.17 | 86.5 | 39.7 | 12.7 | 148.9 |
| Original Braunvieh | 6.45 | 2.32 | 4.11 | 11.24 | 15.54 | 4.26 | 8.19 | 20.87 | 2.70 | 0.79 | 1.83 | 4.22 | 116.4 | 28.6 | 67.5 | 144.8 |
| Pinzgauer | 5.72 | 3.21 | 1.85 | 13.56 | 12.27 | 2.68 | 6.51 | 17.99 | 3.17 | 0.41 | 2.22 | 4.22 | 67.0 | 30.0 | 22.2 | 143.6 |
| Rhetic Gray | 8.23 | 3.17 | 5.46 | 12.02 | 11.32 | 2.86 | 9.09 | 15.51 | 3.09 | 0.64 | 2.13 | 3.47 | 62.9 | 41.8 | 16.1 | 129.5 |
| Simmental | 4.50 | 2.31 | 1.91 | 10.35 | 14.65 | 3.36 | 11.67 | 22.95 | 2.82 | 0.44 | 1.95 | 3.54 | 89.8 | 37.6 | 36.9 | 167.5 |
We further analyzed the effect of parity, previous lactation yield, dry period, and gestation length on colostral IgG, fat, protein, and lactose concentrations. As expected, IgG and also protein concentrations were higher in multiparous compared with primiparous cows (P < 0.0001). In contrast, fat and lactose contents were greater in first lactating cows (P < 0.0001) confirming the results from the study of Dunn et al. (2017) and Gross et al. (2017). Colostral fat represents an important energy source for newborn calves. However, the underlying physiological mechanisms for greater fat contents in the first lactating cows are still unclear. A possible explanation might be an upconcentration of colostral fat, and also lactose, since younger cows produce lower amounts of colostrum than older cows (Devery-Pocius and Larson, 1983; Kessler et al., 2014; Gross et al., 2017).
The relationship between IgG concentration in colostrum and dry period length is controversially discussed. Some studies did not find any association between the length of the dry period and colostral IgG content (Shoshani et al., 2014; Mayasari et al., 2015; Kessler et al., 2020), whereas in agreement with our present results other authors described a positive effect on IgG concentration in cows with a dry period lasting for at least 8 wk (Watters et al., 2008; Dunn et al., 2017). However, as long as the dry period is not entirely omitted, its length seems to be only of minor relevance in regard to colostrum quality. Interestingly, our findings indicate an increase in colostral fat content in cows with a longer dry period (P < 0.0001). Van Hoeij et al. (2017) showed that cows without a dry period have a lower fat yield during the first 3 wk p.p. compared with cows with a preceding dry period of 30 d. Another study observed a higher milk fat percentage in cows dry for 60 d than 40 d during the first month of lactation (Shoshani et al., 2014).
Recently, we described an inverse relationship between gestation length and IgG concentration in colostrum suggesting that a prolonged gestation might not necessarily increase the colostrum quality (Kessler et al., 2020). In the present study, however, no effect of gestation length on colostrum composition was identified (P > 0.05). Similarly, no association was found between milk yield in the previous lactation and colostrum constituents (P > 0.05).
Conclusions
The present field study confirmed the significant variation in IgG and other colostrum constituents within and among breeds. Although differences between cattle breeds were detected, high-yielding dairy cows did not have poorer colostrum quality compared with lower-yielding animals such as beef and dual-purpose breeds. Our findings strongly suggest that colostrogenesis, that is, the transfer of IgG into colostrum, occurs at individually different rates. Overall, colostrum quality among all breeds could be mainly classified as sufficient to meet calf requirements. From a long-term perspective, selection criteria might be defined as a breeding objective to improve the colostrum quality.
Acknowledgments
We thank the participating farmers for providing colostrum samples. We also thank the Milchprüfring Bayern e.V. (Wolnzach, Germany) for their support and analysis of bovine colostrum samples.
Glossary
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- GLM
generalized linear model
- Ig
immunoglobulin
- p.p.
post partum
Conflict of interest statement
The authors declare that they have no conflict of interest.
Literature Cited
- Besser T. E., Garmedia A. E., McGuire T. C., and Gay C. C.. . 1985. Effect of colostral immunoglobulin G1 and immunoglobulin M concentrations on immunoglobulin absorption in calves. J. Dairy Sci. 68:2033–2037. doi: 10.3168/jds.S0022-0302(85)81065-1 [DOI] [PubMed] [Google Scholar]
- Bielmann V., Gillan J., Perkins N. R., Skidmore A. L., Godden S., and Leslie K. E.. . 2010. An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle. J. Dairy Sci. 93:3713–3721. doi: 10.3168/jds.2009-2943 [DOI] [PubMed] [Google Scholar]
- Blum J. W., and Hammon H. M.. . 2000. [Bovine colostrum: more than just an immunoglobulin supplier]. Schweiz. Arch. Tierheilkd. 142:221–228. [PubMed] [Google Scholar]
- Cabral R. G., Chapman C. E., Aragona K. M., Clark E., Lunak M., and Erickson P. S.. . 2016. Predicting colostrum quality from performance in the previous lactation and environmental changes. J. Dairy Sci. 99:4048–4055. doi: 10.3168/jds.2015-9868 [DOI] [PubMed] [Google Scholar]
- Conneely M., Berry D. P., Sayers R., Murphy J. P., Lorenz I., Doherty M. L., and Kennedy E.. . 2013. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Animal 7:1824–1832. doi: 10.1017/S1751731113001444 [DOI] [PubMed] [Google Scholar]
- Devery-Pocius J. E., and Larson B. L.. . 1983. Age and previous lactations as factors in the amount of bovine colostral immunoglobulins. J. Dairy Sci. 66:221–226. doi: 10.3168/jds.S0022-0302(83)81780-9 [DOI] [PubMed] [Google Scholar]
- Dunn A., Ashfield A., Earley B., Welsh M., Gordon A., and Morrison S. J.. . 2017. Evaluation of factors associated with immunoglobulin G, fat, protein, and lactose concentrations in bovine colostrum and colostrum management practices in grassland-based dairy systems in Northern Ireland. J. Dairy Sci. 100:2068–2079. doi: 10.3168/jds.2016-11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. J., Kessler E. C., and Bruckmaier R. M.. . 2017. Quarter vs. composite colostrum composition assessed by Brix refractometry, specific gravity and visual color appearance in primiparous and multiparous dairy cows. Transl. Anim. Sci. 1:26–35. 10.2527/tas2016.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliksen S. M., Lie K. I., Sølverød L., and Østerås O.. . 2008. Risk factors associated with colostrum quality in Norwegian dairy cows. J. Dairy Sci. 91:704–712. doi: 10.3168/jds.2007-0450 [DOI] [PubMed] [Google Scholar]
- Guy M. A., McFadden T. B., Cockrell D. C., and Besser T. E.. . 1994. Regulation of colostrum formation in beef and dairy cows. J. Dairy Sci. 77:3002–3007. doi:10.3168/jds.S0022-0302(94)77241-6 [DOI] [PubMed] [Google Scholar]
- van Hoeij R. J., Dijkstra J., Bruckmaier R. M., Gross J. J., Lam T. J. G. M., Remmelink G. J., Kemp B., and van Knegsel A. T. M.. . 2017. The effect of dry period length and postpartum level of concentrate on milk production, energy balance, and plasma metabolites of dairy cows across the dry period and in early lactation. J. Dairy Sci. 100:5863–5879. 10.3168/jds.2016-11703 [DOI] [PubMed] [Google Scholar]
- Kehoe S. I., Jayarao B. M., and Heinrichs A. J.. . 2007. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci. 90:4108–4116. doi: 10.3168/jds.2007-0040 [DOI] [PubMed] [Google Scholar]
- Kessler E. C., Bruckmaier R. M. and Gross J. J.. . 2014. Milk production during the colostral period is not related to the later lactational performance in dairy cows. J. Dairy Sci. 97:2186–2192. 10.3168/jds.2013-7573 [DOI] [PubMed] [Google Scholar]
- Kessler E. C., Pistol G. C., Bruckmaier R. M., and Gross J. J.. . 2020. Pattern of milk yield and immunoglobulin concentration and factors associated with colostrum quality at the quarter level in dairy cows after parturition. J. Dairy Sci. 103:965–971. doi: 10.3168/jds.2019-17283 [DOI] [PubMed] [Google Scholar]
- Lehmann M., Wellnitz O., and Bruckmaier R. M.. . 2013. Concomitant lipopolysaccharide-induced transfer of blood-derived components including immunoglobulins into milk. J. Dairy Sci. 96:889–896. doi: 10.3168/jds.2012-5410 [DOI] [PubMed] [Google Scholar]
- Mayasari N., de Vries Reilingh G., Nieuwland M. G., Remmelink G. J., Parmentier H. K., Kemp B., and van Knegsel A. T.. . 2015. Effect of maternal dry period length on colostrum immunoglobulin content and on natural and specific antibody titers in calves. J. Dairy Sci. 98:3969–3979. doi: 10.3168/jds.2014-8753 [DOI] [PubMed] [Google Scholar]
- Moore M., Tyler J. W., Chigerwe M., Dawes M. E., and Middleton J. R.. . 2005. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 226:1375–1377. doi: 10.2460/javma.2005.226.1375 [DOI] [PubMed] [Google Scholar]
- Morin D. E., Constable P. D., Maunsell F. P., and McCoy G. C.. . 2001. Factors associated with colostral specific gravity in dairy cows. J. Dairy Sci. 84:937–943. doi: 10.3168/jds.S0022-0302(01)74551-1 [DOI] [PubMed] [Google Scholar]
- Morin D. E., Nelson S. V., Reid E. D., Nagy D. W., Dahl G. E., and Constable P. D.. . 2010. Effect of colostral volume, interval between calving and first milking, and photoperiod on colostral IgG concentrations in dairy cows. J. Am. Vet. Med. Assoc. 237:420–428. 10.2460/javma.237.4.420 [DOI] [PubMed] [Google Scholar]
- Morrill K. M., Conrad E., Lago A., Campbell J., Quigley J., and Tyler H.. . 2012. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J. Dairy Sci. 95:3997–4005. doi: 10.3168/jds.2011-5174 [DOI] [PubMed] [Google Scholar]
- Rivero M. J., Valderrama X., Haines D., and Alomar D.. . 2012. Prediction of immunoglobulin G content in bovine colostrum by near-infrared spectroscopy. J. Dairy Sci. 95:1410–1418. doi: 10.3168/jds.2011-4532 [DOI] [PubMed] [Google Scholar]
- Shoshani E., Rozen S., and Doekes J. J.. . 2014. Effect of a short dry period on milk yield and content, colostrum quality, fertility, and metabolic status of Holstein cows. J. Dairy Sci. 97:2909–2922. doi: 10.3168/jds.2013-7733 [DOI] [PubMed] [Google Scholar]
- Vandeputte S., Detilleux J., and Rollin F.. . 2014. Investigation of colostrum quality in beef cattle by radial immunodiffusion and Brix refractometry. Vet. Rec. 175:353. doi: 10.1136/vr.101590 [DOI] [PubMed] [Google Scholar]
- Watters R. D., Guenther J. N., Brickner A. E., Rastani R. R., Crump P. M., Clark P. W., and Grummer R. R.. . 2008. Effects of dry period length on milk production and health of dairy cattle. J. Dairy Sci. 91:2595–2603. doi: 10.3168/jds.2007-0615 [DOI] [PubMed] [Google Scholar]