Abstract
Context
Urinary aldosterone levels (Uald) are widely measured in the oral sodium-loading test to confirm primary aldosteronism (PA), but reliable studies on their diagnostic value are limited. This may be due to the difficulty in collecting urine with reliable accuracy, keeping oral sodium intake constant between patients. Therefore, we focused on 24-hour Uald after intravenous saline infusion in a hospitalized setting, which provides a reliable sodium load in consistent amounts.
Objective
Comparing plasma aldosterone concentrations (PAC) and Uald after saline infusion in the sitting position, to evaluate the accuracy in determining PA subtypes and the correlation of both measurements.
Design and Setting
This was a retrospective cross-sectional study in a single referral center.
Patients
Of 53 patients without renal dysfunction who were diagnosed with PA and underwent adrenal venous sampling, 16 and 37 were diagnosed with unilateral and bilateral PA, respectively.
Main Outcome Measures
Uald collected for 24 hours and PAC after saline infusion.
Results
The area under the receiver operating characteristic curve for diagnosing unilateral PA was not significantly different between Uald and PAC after saline infusion (0.921 and 0.958, respectively; P = 0.370). The predicted optimal cutoff value of Uald was 16.5 μg/day (sensitivity, 87.5%; specificity, 100%), and that of PAC after saline infusion was 19.3 ng/dL (sensitivity, 87.5%; specificity, 97.3%). In studied patients with PA, Uald was positively correlated with PAC after saline infusion (r = 0.617; P < 0.001).
Conclusions
We reassessed Uald in PA patients under sufficient sodium loading and demonstrated the correlation between Uald and PAC after saline infusion.
Keywords: adrenal venous sampling, aldosterone, primary aldosteronism, saline infusion test, urinary aldosterone levels
Primary aldosteronism (PA) is the most common cause of secondary hypertension [1, 2]. Patients with PA exhibit various cardiovascular morbidities associated with aldosterone excess [3, 4]. Early diagnosis of PA is crucial, so that the affected patients can be given specific medical or surgical treatment. If hypertensive patients are found to have PA and desire surgery, adrenal venous sampling (AVS) is performed to determine which of 2 major subtypes they have: unilateral PA, mainly due to aldosterone-producing adenoma, or bilateral, mainly due to idiopathic hyperaldosteronism. Subtype diagnosis of PA is important because patients with unilateral PA are at higher risk of cardiovascular disease than those with bilateral PA and need more specific treatment [5]. Although AVS is the standard procedure for diagnosing the subtype of PA, it is invasive, expensive, and technically demanding [1]. Therefore, other methods of predicting subtype diagnosis of PA before AVS are desired.
We and others previously showed that plasma aldosterone concentrations (PAC) after seated saline infusion test (SSIT) are useful in predicting subtype diagnosis of PA [6, 7]. However, aldosterone secretion is not constant; aldosterone is secreted in a pulsatile manner throughout the day [8]. This secretion pattern is also observed in PA patients [9]. The value of accumulated aldosterone in a 24-hour urine sample has the advantage as it does not depend on circadian variation in its secretion [10]. The 24-hour urinary aldosterone level (Uald) measured in the oral sodium-loading test is an established and widely used technique for confirmatory test of PA [11]. The oral sodium-loading test is noninvasive and the cheapest confirmatory test, but reliable studies on its diagnostic value are limited [12]. This may be due to the difficulty in collecting urine with reliable accuracy, keeping oral sodium intake constant between patients. Therefore, we focused on 24-hour Uald after intravenous saline infusion in a hospitalized setting, which provides a reliable sodium load in consistent amounts.
The present study was planned to investigate Uald after saline infusion equivalent to the total amount of secretion on the day among patients with suspected PA in order to establish the evidence of utility of Uald including the oral sodium-loading test, similar to PAC after saline infusion equivalent to the level of hormone at the spot. To evaluate this, we compared the accuracy of subtype diagnosis between Uald and PAC after saline infusion in the sitting position and investigated the correlation of both measurements.
Materials and Methods
Patients and PA confirmation and subtype criteria
In this retrospective study, we consecutively included patients with PA who were referred to the Department of Endocrinology and Metabolism, National Hospital Organization Kyoto Medical Center, and underwent AVS with adrenocorticotropic hormone (ACTH) stimulation between June 2015 and July 2019. All patients were diagnosed with PA according to the guidelines of the Japan Endocrine Society [13] and Japanese Society of Hypertension [14], including case detection and confirmatory tests. Case detection and confirmatory tests of PA and AVS were performed and evaluated as previously described [6]. Before the diagnosis, antihypertensive drugs were generally changed to calcium channel blockers and/or α-adrenergic blockers. Patients with hypokalemia were generally given oral potassium supplementation. Thin-slice (3-mm thick) adrenal computed tomography (CT) was performed in all patients to evaluate adrenal nodules, which were defined as those with a diameter ≥10 mm on CT [15]. In AVS with ACTH stimulation, if the ratio of cortisol concentration in the adrenal vein to that in the inferior vena cava (selectivity index) was >5, adrenal vein cannulation was considered successful [11]. We considered unilateral lateralization when the aldosterone-to-cortisol ratio on the dominant adrenal side was at least 4 times that on the nondominant adrenal side (lateralization index) [11]. This retrospective study was approved by the institutional ethics committee of the National Hospital Organization Kyoto Medical Center.
Uald and PAC after saline infusion studies
SSIT was performed as previously described in a hospitalized setting [6]. Briefly, patients remained in the sitting position during the transvenous infusion of 2 L of 0.9% saline, which contains 18 g sodium chloride, over 4 hours starting at 0900 am (Day 2). PAC was measured after 4 hours, with blood pressure and heart rate monitored. A 24-hour urine sample was collected in a hospitalized setting for 24 hours from the evening before SSIT (Day 1) and was kept refrigerated until analysis. The 24-hour Uald was calculated by multiplying the urinary aldosterone value by the 24-hour urine amount. In a hospitalized setting, the amount of daily oral sodium chloride intake for each patient was almost the same at 6.6 to 6.9 g before Day 1. Patients with severe hypertension, severe hypokalemia, renal insufficiency, and/or cardiovascular complications did not undergo SSIT.
Assay methods
PAC, Uald, and plasma renin activity (PRA) were measured using commercial kits. PAC was measured by radioimmunoassay (RIA) (SPAC-S Aldosterone Kit [16]; Fuji Rebio, Tokyo, Japan) or chemiluminescent enzyme immunoassay (CLEIA) (Accuraseed Aldosterone [17]; FUJIFILM Wako Pure Chemical Industries, Osaka, Japan), Uald was measured by RIA (SPAC-S Aldosterone Kit [16]; Fuji Rebio, Tokyo, Japan), and PRA was measured by enzyme immunoassay (PRA enzyme immunoassay kits [18]; Yamasa, Choshi, Japan). In the recumbent position, the reference range of PAC was 3.0 to 15.9 ng/dL, and that of PRA was 0.2 to 2.7 ng/mL/h. The reference range of Uald was 10 μg/day or less.
Statistical analysis
Continuous variables were expressed as the median and interquartile range. Categorical variables were expressed as absolute numbers and percentages. Data between 2 groups were compared using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Correlations were evaluated using Spearman correlation coefficients. Statistical analyses were performed, and the receiver operating characteristic (ROC) curves drawn, using EZR statistical software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [19]. The optimal cutoff point of each value was set at the closest point to the upper left corner of the ROC curve. A 2-tailed P-value < 0.05 was considered statistically significant.
Results
Clinical characteristics of studied patients
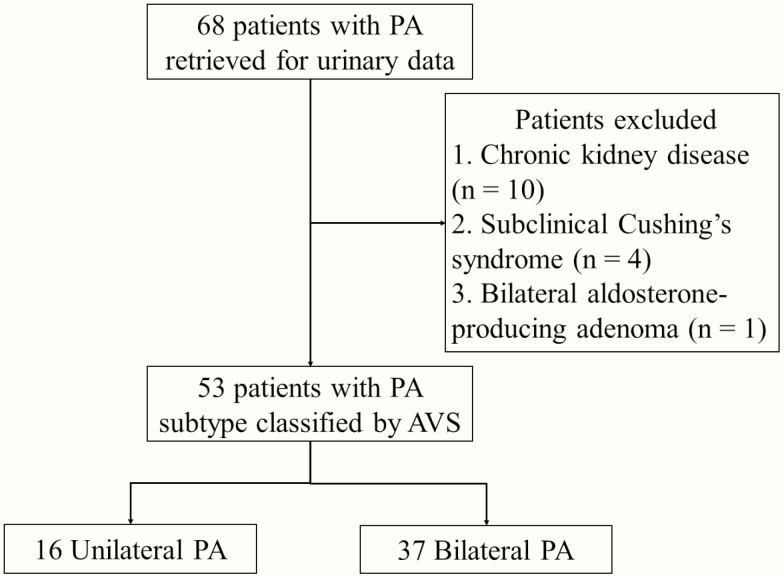
We identified 68 patients with PA who underwent AVS and had data available from Uald and PAC after saline infusion. We analyzed 53 out of 68 patients with PA. The reasons for exclusion of 15 patients from the analysis were as follows: chronic kidney disease with a decreased estimated glomerular filtration rate <60 mL/min/1.73 m2 (n = 10), complicated subclinical Cushing’s syndrome (n = 4), and bilateral aldosterone-producing adenoma with histopathological diagnosis (n = 1). All studied patients underwent AVS successfully and did not take diuretics or mineralocorticoid receptor antagonists. Of the 53 total patients, 16 were diagnosed with unilateral subtype and the remaining 37 with bilateral subtype according to AVS findings (Fig. 1). Clinical and biochemical characteristics of the unilateral and bilateral PA groups are shown in Table 1.
Figure 1.
Patient flowchart. Abbreviations: PA, primary aldosteronism and AVS, adrenal venous sampling.
Table 1.
Clinical and Biochemical Characteristics of Patients According to Subtypes
| Unilateral PA | Bilateral PA | P value | |
|---|---|---|---|
| (n = 16) | (n = 37) | ||
| Age, years | 46 (44, 48) | 49 (41, 53) | 0.510 |
| Male sex, n (%) | 11 (69) | 18 (49) | 0.235 |
| Systolic BP, mmHg | 135 (130, 144) | 138 (125, 150) | 0.977 |
| Diastolic BP, mmHg | 83 (80, 89) | 85 (81, 94) | 0.427 |
| Antihypertensive drugs, n | 1.0 (1, 2) | 1.0 (1, 2) | 0.870 |
| Adrenal nodule on CT, n (%) | 12 (75) | 9(24) | <0.001 |
| Serum potassium, mmol/L | 2.9 (2.5, 3.4) | 3.7 (3.6, 3.8) | <0.001 |
| eGFR, mL/min/1.73m2 | 82.3 (69.1, 88.4) | 78.9 (72.0, 88.0) | 0.954 |
| Urinary sodium, mEq/daya | 201 (140, 257) | 220 (171, 258) | 0.401 |
| Urinary potassium, mEq/daya | 62 (51, 71) | 42 (34, 50) | <0.001 |
| Urinary creatinine, g/daya | 1.30 (1.20, 1.55) | 1.35 (1.20, 1.60) | 0.991 |
| Plasma aldosterone, ng/dL | 35.1 (29.1, 44.8) | 16.7 (12.6, 18.9) | <0.001 |
| Plasma renin activity, ng/mL/h | 0.20 (0.20, 0.33) | 0.40 (0.30, 0.70) | 0.009 |
| Uald, μg/day | 26.9 (24.5, 33.0) | 9.4 (6.3, 11.6) | <0.001 |
| Plasma aldosterone after SSIT, ng/dL | 30.9 (20.8, 35.1) | 9.3 (7.5, 12.3) | <0.001 |
Data are expressed as medians with interquartile ranges unless otherwise indicated.
Abbreviations: BP, blood pressure; CT, computed tomography; eGFR, estimated glomerular filtration rate; PA, primary aldosteronism; SSIT, seated saline infusion test; Uald, urinary aldosterone.
aFive patients (2 unilateral PA, 3 bilateral PA) with no data available were excluded from this analysis.
ROC analysis of Uald and PAC after saline infusion
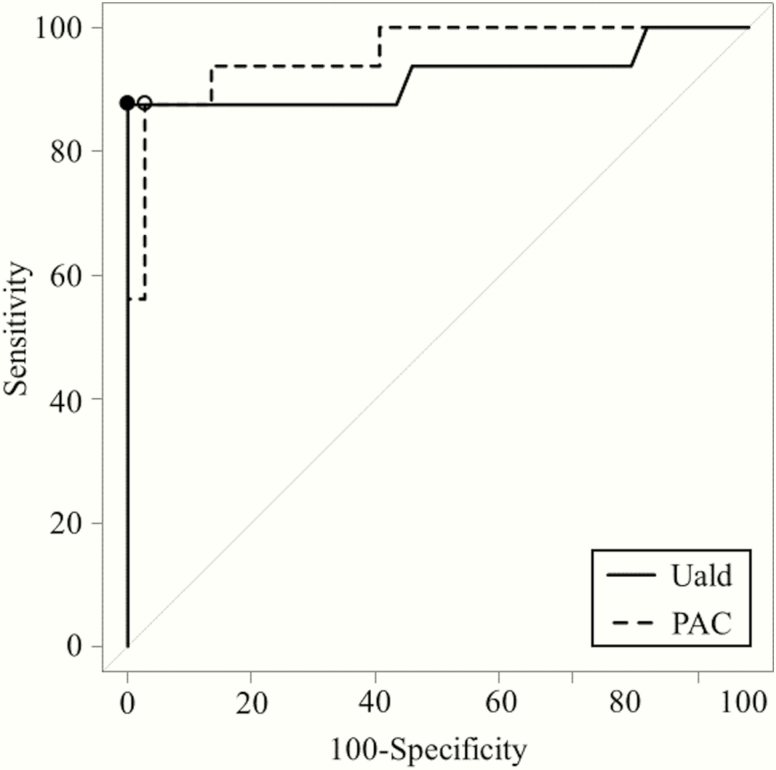
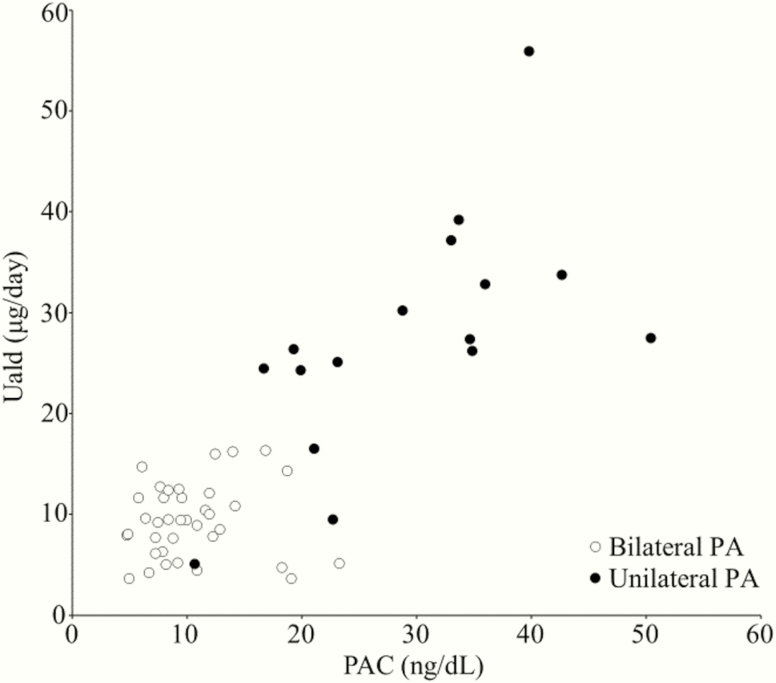
ROC curves were plotted to compare the diagnostic accuracy of Uald and PAC after saline infusion for diagnosing unilateral PA by comparing the differences in the areas under the ROC curve (AUC). Fig. 2 shows the results of the ROC curve analysis of Uald and PAC after saline infusion. The AUC for both tests were higher than the area under the diagonal line. The AUC were 0.921 (95% confidence interval [CI], 0.808-1) and 0.958 (95% CI, 0.901–1) for the 24-hour Uald and PAC after saline infusion, respectively, without a significant difference (P = 0.370). According to the ROC analysis, the predicted optimal cutoff value of the Uald was 16.5 μg/day (sensitivity, 87.5% [95% CI, 73.9-87.5%]; specificity, 100% [95% CI, 94.1-100%]), and that of the PAC after saline infusion was 19.3 ng/dL (sensitivity, 87.5% [95% CI, 72.0-92.5%]; specificity, 97.3% [95% CI, 90.6-99.5%]). The results for each subtype of PA at these optimal cutoff values are given in Table 2. Fig. 3 shows the relationship between 24-hour Uald and PAC after saline infusion. In studied patients with PA, Uald was positively correlated with PAC after saline infusion (r = 0.617; P < 0.001).
Figure 2.
Receiver operating characteristic (ROC) curves based on urinary aldosterone level (Uald) (solid line) and plasma aldosterone concentration (PAC) (dotted line) after saline infusion for the prediction of unilateral subtype in 53 patients with primary aldosteronism. Black point: Uald optimal cutoff was 16.5 μg/day (area under the ROC curve [AUC] 0.921, sensitivity 87.5%; specificity 100%). White point: PAC optimal cutoff was 19.3 ng/dL (AUC 0.958, sensitivity 87.5%; specificity 97.3%).
Table 2.
Numbers (%) of Positive and Negative Uald and PAC After Saline Infusion Results at Optimal Cutoff for Each Subtype
| Cutoff | Uald (μg/day) | PAC (ng/dL) | |||
|---|---|---|---|---|---|
| ≥16.5 | ≥19.3 | ||||
| Total | Positive | Negative | Positive | Negative | |
| All PA | 53 | 14 (26.4) | 39 (73.6) | 15 (28.3) | 38 (71.7) |
| Unilateral PA | 16 | 14 (87.5) | 2 (12.5) | 14 (87.5) | 2 (12.5) |
| Bilateral PA | 37 | 0 (0.0) | 37 (100.0) | 1 (2.7) | 36 (97.3) |
Abbreviations: PA, primary aldosteronism; PAC, plasma aldosterone concentration; Uald, urinary aldosterone.
Figure 3.
Relationship between urinary aldosterone level (Uald) and plasma aldosterone concentration (PAC) after saline infusion in 53 patients with primary aldosteronism (PA) (16 unilateral and 37 bilateral PA). In studied patients with PA, Uald was positively correlated with PAC after saline infusion (r = 0.617; P < 0.001).
Discussion
In this study, we demonstrated that Uald after infusing a sufficient and equal volume of saline had similar utility for identifying unilateral and bilateral PA as PAC after SSIT. Of 16 patients with unilateral PA, 14 were positive for the optimal cutoff in Uald and PAC as determined by ROC analysis. In this study, Uald was correlated with PAC after saline infusion under the condition of the same salt loading among patients. PAC or Uald levels after intravenously sufficient sodium-loading can be useful in diagnosing PA, and this supported the evidence of utility of Uald in the clinical practice guideline [11]
The oral sodium-loading test is used to assess 24-hour urinary sodium content for adequate oral salt intake in the clinical practice guidelines [11, 20]. Bravo et al demonstrated that urinary sodium levels after saline infusion (25 mL/kg) for 3 days in PA patients exceeded 250 mEq/day [21]. Based on this result, a 24-hour urinary sodium measurement of at least 200 to 250 mEq ensured that the patients had adequate salt supplementation in the oral sodium-loading test [21, 22]. In this study, although sodium excretion might not have been in equilibrium, the median urinary sodium excretion was a little over 200 mEq/day under both the orally and the intravenously sufficient sodium load. Therefore, given sufficient sodium loading, urinary sodium excretion may be irrelevant in subtype diagnosis of PA.
Although diagnostic accuracy of subtype diagnosis of PA at the optimal cutoff of Uald and PAC after saline infusion is high, these tests cannot substitute for AVS. In the results of optimal cutoff between Uald (16.5 μg/day) and PAC (19.3 ng/dL) after saline infusion, discrepancies were found in 2 of 16 patients with unilateral PA (one was a false negative in Uald, and the other was a false negative in PAC after saline infusion), and 1 patient with unilateral PA had false negative results with optimal cutoff for both tests. Moreover, discrepancies were found in 1 of 37 bilateral PA patients in the results of these tests (false positive in PAC after saline infusion) (Table 2). There is no denying that with both tests, a small proportion of unilateral PA patients can be overlooked. Our results demonstrated that patients with low PAC or Uald after saline infusion had low probability of unilateral PA, suggesting that these patients were lower priority of performing AVS than those with high aldosterone levels after saline infusion. In our study, the measurements of both PAC and Uald after saline infusion did not improve the ability to predict subtype diagnosis of PA compared to measurements of only one each. PAC measurement requires only one blood collection but refers to the level of hormone at the spot, while Uald measurement requires urine collection but refers to the total volume of the day. These measurements can both be properly used, depending on patients and facilities.
Our study design was limited by the small sample size and its retrospective nature at a single center. Due to the small sample size, multivariate analysis was not performed and confidence intervals were broadened to some extent in this study. Further prospective studies with larger sample sizes from multiple institutions are required to assess the reliable diagnostic value of Uald in PA. In addition, the studied patients were hospitalized and urine samples were collected for 24 hours, so the accuracy of urinalysis is generally assured. However, Uald in the outpatient clinic have been reported to vary even within the same patient and were not corrected by urinary sodium and/or creatinine concentrations [23]. The reproducibility of Uald was not evaluated in this study. Furthermore, Uald is mostly measured by RIA in routine clinical laboratories. Endocrine clinical practice guidelines describe the possibility that Uald measured by RIA may have lower diagnostic accuracy [11]. In this study, Uald was measured by RIA, but it is worth noting that unilateral PA can be predicted with high sensitivity and specificity in patients with normal renal function. If measurement methods that can avoid interference due to nonspecific reactions and cross-reactions, such as liquid chromatography tandem mass spectrometry, are more generally used, higher diagnostic accuracy can be expected. Finally, subtype diagnosis of PA in this study was based on AVS results. Although AVS is the standard procedure for subtype classification [11], the criteria used to determine the lateralization of aldosterone excess are not standardized across centers [24]. In this study, we used the lateralization index cutoff >4, which is the strict cutoff for unilateral diagnosis [11]. Some patients diagnosed as having the bilateral subtype by these criteria may in fact have the unilateral subtype, which reduces the sensitivity of the cutoff value proposed in this study.
In conclusion, we demonstrated the correlation between Uald and PAC after saline infusion. We reassessed Uald in PA patients under sufficient sodium loading and believed that our results support as an evidence of sodium-loading test in clinical guidelines.
Acknowledgments
We gratefully acknowledge the medical staff in the Departments of Urology, Radiology, and Pathology of the National Hospital Organization Kyoto Medical Center.
Financial Support: Japan Society for the Promotion of Science KAKENHI, Grant Number 17K16173.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- AUC
area under the receiver operating characteristic curve
- AVS
adrenal venous sampling
- CI
confidence interval
- CT
computed tomography
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
- RIA
radioimmunoassay
- ROC
receiver-operating characteristic
- SSIT
seated saline infusion test
- Uald
urinary aldosterone levels
Contributor Information
Hironobu Umakoshi, Email: umakoshi@med.kyushu-u.ac.jp.
Tetsuya Tagami, Email: ttagami@kuhp.kyoto-u.ac.jp.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607-618. [DOI] [PubMed] [Google Scholar]
- 2. Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045-1050. [DOI] [PubMed] [Google Scholar]
- 3. Rossi GP, Cesari M, Cuspidi C, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62-69. [DOI] [PubMed] [Google Scholar]
- 4. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62(2):331-336. [DOI] [PubMed] [Google Scholar]
- 5. Ohno Y, Sone M, Inagaki N, et al. ; Nagahama Study; JPAS Study Group Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension. 2018;71(3):530-537. [DOI] [PubMed] [Google Scholar]
- 6. Kaneko H, Umakoshi H, Ishihara Y, et al. Seated saline infusion test in predicting subtype diagnosis of primary aldosteronism. Clin Endocrinol (Oxf). 2019;91(6):737-742. [DOI] [PubMed] [Google Scholar]
- 7. Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):4113-4124. [DOI] [PubMed] [Google Scholar]
- 8. Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol. 1999;276(1):E43-E49. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi H, Haketa A, Ueno T, et al. Subtype prediction in primary aldosteronism: measurement of circadian variation of adrenocortical hormones and 24-h urinary aldosterone. Clin Endocrinol (Oxf). 2016;84(6):814-821. [DOI] [PubMed] [Google Scholar]
- 10. Schirpenbach C, Seiler L, Maser-Gluth C, et al. Confirmatory testing in normokalaemic primary aldosteronism: the value of the saline infusion test and urinary aldosterone metabolites. Eur J Endocrinol. 2006;154(6):865-873. [DOI] [PubMed] [Google Scholar]
- 11. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 12. Wu S, Yang J, Hu J, et al. Confirmatory tests for the diagnosis of primary aldosteronism: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2019;90(5):641-648. [DOI] [PubMed] [Google Scholar]
- 13. Nishikawa T, Omura M, Satoh F, et al. ; Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J. 2011;58(9):711-721. [DOI] [PubMed] [Google Scholar]
- 14. Shimamoto K, Ando K, Fujita T, et al. ; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253-390. [DOI] [PubMed] [Google Scholar]
- 15. Umakoshi H, Tsuiki M, Takeda Y, et al. ; JPAS Study Group Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(3):900-908. [DOI] [PubMed] [Google Scholar]
- 16. RRID:AB_2801309, https://scicrunch.org/resolver/AB_2801309.
- 17. RRID:CVCL_D151, https://scicrunch.org/resolver/CVCL_D151.
- 18. RRID:AB_2801275, https://scicrunch.org/resolver/AB_2801275.
- 19. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reznik Y, Amar L, Tabarin A. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: confirmatory testing. Ann Endocrinol (Paris). 2016;77(3):202-207. [DOI] [PubMed] [Google Scholar]
- 21. Bravo EL, Tarazi RC, Dustan HP, et al. The changing clinical spectrum of primary aldosteronism. Am J Med. 1983;74(4):641-651. [DOI] [PubMed] [Google Scholar]
- 22. Young WF Jr, Hogan MJ, Klee GG, Grant CS, van Heerden JA. Primary aldosteronism: diagnosis and treatment. Mayo Clin Proc. 1990;65(1):96-110. [DOI] [PubMed] [Google Scholar]
- 23. Ceral J, Malirova E, Ballon M, Solar M. The role of urinary aldosterone for the diagnosis of primary aldosteronism. Horm Metab Res. 2014;46(9):663-667. [DOI] [PubMed] [Google Scholar]
- 24. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151-160. [DOI] [PubMed] [Google Scholar]