Abstract
Background
Joubert syndrome (JS) is an inherited ciliopathy characterized by a complex midbrain–hindbrain malformation and multiorgan involvement. Renal disease, mainly juvenile nephronophthisis (NPH), was reported in 25–30% patients although only ∼18% had a confirmed diagnosis of chronic kidney disease (CKD). NPH often remains asymptomatic for many years, resulting in delayed diagnosis. The aim of the study was to identify a biomarker able to quantify the risk of progressive CKD in young children with JS.
Methods
Renal features were investigated in 93 Italian patients, including biochemical tests, ultrasound and 1-deamino-8D-arginine vasopressin test in children with reduced basal urine osmolality. A subset of patients was followed-up over time.
Results
At last examination, 27 of 93 subjects (29%) presented with CKD, ranging from isolated urinary concentration defect (UCD) to end-stage renal disease. Both normal and pathological urine osmolality levels remained stable over time, even when obtained at very early ages. Follow-up data showed that the probability of developing CKD can be modelled as a function of the urine osmolality value, exceeding 75% for levels <600 mOsm/kg H2O, and significantly increased in patients with an early diagnosis of isolated UCD.
Conclusions
We conclude that the frequency of CKD in JS increases with age and is higher than previously reported. Urine osmolality represents an early sensitive quantitative biomarker of the risk of CKD progression.
Keywords: 1-deamino-8D-arginine vasopressin test, early diagnosis, Joubert syndrome, nephronophthisis, urine osmolality
INTRODUCTION
Joubert syndrome (JS) is an autosomal recessive or X-linked congenital ataxia diagnosed on brain imaging by the pathognomonic ‘molar tooth sign’ [1]. JS is clinically and genetically heterogeneous, with mutations in >35 genes [2–7]. Yet, genotype–phenotype correlations are known only for few genes, and prognostic indicators are lacking [1, 8].
Kidney involvement is a frequent complication of JS, occurring in 25–30% of patients [1, 8–10]. In a recent study on 97 JS individuals, 29 (30%) presented renal phenotypes, including nephronophthisis (NPH) (20%), unilateral cystic dysplastic kidneys (3%) or undetermined cystic kidney disease (7%). Most of these phenotypes were detected by ultrasound, and only 18 of 29 (62%) patients had a confirmed diagnosis of chronic kidney disease (CKD) [10].
NPH is a distinctive clinical–pathological entity characterized by disruption of tubular basement membranes, progressive tubular atrophy, interstitial fibrosis and small cortico-medullary cysts, within small or normal-sized kidneys [11]. In most cases, NPH remains asymptomatic or paucisymptomatic in the first decade, manifesting only with polyuria, polydipsia and mild anaemia, but it inexorably progresses during adolescence or early adulthood to CKD, and finally to end-stage renal disease (CKD5). Renal failure is a frequent cause of death in JS, as dialysis and renal transplantation may be hindered by the occurrence of severe comorbidities [12].
Since JS is mostly diagnosed in very young children with neurological symptoms, when their renal function is still normal, tests to predict the risk of developing progressive renal disease would be extremely useful, to allow an early diagnosis and management of patients at risk [1, 13, 14]. Kidney ultrasound is sensitive in diagnosing cystic kidney diseases while, in isolated NPH, it may show increased echogenicity and/or small cortico-medullary cysts, or it may remain negative. Moreover, this test relies on the operator’s experience and does not predict the risk of developing CKD. To date, a reliable early quantitative biomarker for renal insufficiency in JS is lacking.
Here, we present a comprehensive assessment and long-term follow-up of renal functioning in a large cohort of Italian JS subjects, and evaluate the prognostic value of urine osmolality in predicting the risk of developing adverse renal outcome.
MATERIALS AND METHODS
Patients’ assessment
Ninety-three Italian JS children (from 76 families) were selected within an ongoing research project on cerebellar and brainstem congenital defects. All patients or their parents gave written informed consent. The study was approved by the Ethical Committee of the Bambino Gesù Children Hospital (#46LB/28-01-2014) in accordance with the Declaration of Helsinki.
Inclusion criteria were (i) a neuroradiologically confirmed diagnosis of JS; and (ii) a complete investigation of renal function at first examination, including (a) serum and urine electrolytes and (b) two-step assessment of urinary concentration ability, as follows. First, spontaneous urine osmolality was measured on the first-morning urine sample. If osmolality was ≥800 mOsm/kg H2O, no further tests were performed. Otherwise, patients underwent a challenging test with 1-deamino-arginine vasopressin (DDAVP) [15]. Briefly, hourly urine samples were collected over a 4-h period after 20 µg intranasal administration of DDAVP. The highest value among four was used to define the maximum urinary osmolality. During the test, patients had free access to water and were monitored and weighed. Children were not given water unless they clearly expressed thirst. The test was not performed in patients presenting with renal insufficiency or unable to exhibit thirst. Serum electrolytes were tested at the beginning and end of the test.
A urinary concentration defect (UCD) was diagnosed when maximum urine osmolality was <600 mOsm/kg H2O, whereas values between 600 and 800 mOsm/kg H2O were considered borderline [16]. Estimated glomerular filtration rate (eGFR) was calculated by using appropriate formulas according to age, namely the Schwartz formula in children and the Modification of Diet in Renal Disease equation in adults [17–19]. CKD categories were classified using KDOQI guidelines [14]. Most patients underwent kidney ultrasound, either at first examination or at follow-up.
Owing to intellectual impairment, polyuria could not be measured accurately in most patients and was broadly defined as the passage of large volumes of urine with increased frequency, associated with nycturia, enuresis and polydipsia.
Retinopathy was diagnosed based on abnormal findings at fundus oculi and/or electroretinogram. Liver involvement was defined as any type of liver abnormality, including repeatedly altered liver function tests, abnormal liver ultrasound or hepatic fibrosis detected with liver biopsy.
Sixty patients were followed-up one or more times over the years.
Seventy probands underwent next-generation sequencing of 122 ciliopathy genes, including 42 JS- and NPH-associated genes (Supplementary data, Table S1), as described [20]. Potentially, pathogenic variants were confirmed by Sanger sequencing and segregation analysis was performed.
Statistical analysis
Continuous variables were analysed with two-sided t-test if they passed the Kolmogorov–Smirnov normality test, and with two-sided Mann–Whitney U test otherwise. The normally and non-normally distributed variables were described by using mean and standard deviation (SD), or median and interquartile range (IQR), respectively. A logistic regression model was used to estimate the probability of developing decreased eGFR based on the presence of UCD (adjusting for age at osmolality assessment). A similar approach was employed to investigate the probability of presenting with polyuria, kidney ultrasound abnormalities and extra-renal signs as a function of CKD, and to explore genotype–phenotype correlations. Binomial-response general linear models were fit using the bias-reduction method [21]. Results were statistically significant for P < 0.05. Survival analysis was performed with Kaplan–Meier estimator. A receiver operating characteristic (ROC) curve was used to determine the diagnostic accuracy of urine osmolality assessment and to calculate the cut-off value for optimal sensitivity and specificity. Statistical analysis was performed using R [22].
RESULTS
Clinical characterization
Ninety-three Italian JS patients (55 males) were included in the study. Patients were divided in five subgroups: (i) ≥18 years with normal renal function (NRF ≥ 18); (ii) <18 years with normal renal function (NRF < 18); (iii) isolated UCD with normal eGFR (iUCD); (iv) mildly to severely decreased eGFR (CKD2–CKD4); and (v) end-stage renal disease (CKD5) [14]. The age cut-off was chosen as juvenile NPH mostly becomes symptomatic in the late first or early second decade [11], and therefore we assumed that subjects ≥18 years with normal renal function had negligible risk of kidney disease.
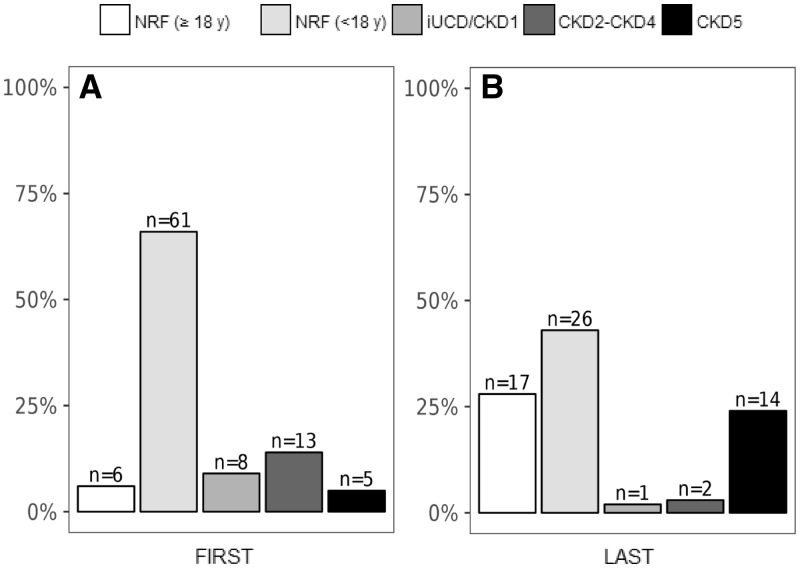
At first examination, median (IQR) age was 8 years (9; min: 1; max: 52; 14 patients ≥18 years). Sixty-seven patients had NRF, 8 had iUCD and 18 patients had CKD2–CKD5 (Figure 1A).
FIGURE 1.
Assessment of renal function at first and last examination. Renal status of all 93 patients at first evaluation (A). Renal status of 60 patients with available follow-up data at last examination (B). NRF, normal renal function; iUCD/CKD1, iUCD with normal eGFR; CKD2–CKD4, CKD with mildly to severely decreased eGFR; CKD5, end-stage renal disease.
Sixty patients were evaluated more than once, with median (IQR) follow-up of 9 years (6; min: 1; max: 14). At their last examination [median (IQR) age: 15 years (11); min: 4; max: 36], 43 out of 44 patients still had NRF (although those ≥18 years had raised from 4 to 17), 1 patient <18 years had iUCD, and the number of subjects with CKD2–CKD5 had increased from 9 to 16 (Figure 1B, Supplementary data, Figure S1). The remaining 33 subjects were assessed only once. Overall, the prevalence of CKD at last available examination was 29% (27/93).
When comparing clinical and instrumental findings between subjects with NRF and those with renal involvement (iUCD, CKD2–CKD4 and CKD5), the prevalence of polyuria and of abnormal kidney ultrasound was significantly higher in the second group, as expected. In particular, ultrasound was pathological in 21 of 23 subjects with CKD, showing isolated kidney hyperechogenicity in 18 and bilateral cysts in 3 (of whom 2 also presented increased echogenicity). Conversely, ultrasound was unremarkable in 60 subjects with NRF, whereas the remaining 2 (aged 5.9 and 14 years at last examination) presented kidney hyperechogenicity (Table 1).
Table 1.
Clinical and laboratory features of patients at last follow-up
| Total | NRF | CKD | Coefficient (95% CI)a | P-value | |
|---|---|---|---|---|---|
| Number of patients, n/N (%) | 93 | 66/93 (71) | 27/93 (29)b | – | – |
| Age (years) | 14 ± 9c | 15 ± 10c | 12 ± 6c | – | 0.16 |
| Kidney assessment, n/N (%) | |||||
| Polyuria | 22/88 (25) | 2/62 (3) | 20/26 (77) | 4.34 (2.95–6.60) | <0.001*** |
| Abnormal kidney ultrasound | 23/85 (27)d | 2/62 (3) | 21/23 (91) | 5.34 (3.72–8.14) | <0.001*** |
| Other features | |||||
| Retinopathy | 33/92 (36) | 17/65 (26) | 16/27 (59) | 1.38 (0.46–2.39) | 0.004** |
| Coloboma | 12/93 (13) | 9/66 (14) | 3/27 (11) | −0.15 (−1.80 to 1.10) | 0.83 |
| Liver involvement | 17/91 (19) | 12/65 (19) | 5/26 (19) | 0.09 (−1.19 to 1.18) | 0.88 |
For each variable, the % is calculated on the effective number of patients for whom information was available.
The coefficient stands for the regression coefficient β between renal function and each independent variable; bincluding two patients with iUCD and 25 with decreased eGFR; cvalues are expressed as mean ± SD; dincluding three patients with cystic kidney dysplasia. **P-value ≤0.01; ***P-value ≤0.001.
CKD, chronic kidney disease; NRF, normal renal function.
In respect of extra-renal involvement, only retinopathy significantly associated with renal impairment, with a frequency more than twice as high as in NRF subjects.
Genetic tests revealed mutations in 38 out of 70 analysed probands (54%). Genes mutated in both CKD and NRF groups include CEP290 (n = 5 CKD, 1 NRF), RPGRIP1L (n = 2 CKD, 1 NRF), TMEM67 (n = 1 CKD, 4 NRF) and AHI1 (n = 1 CKD, 3 NRF) (Supplementary data, Tables S2 and S3). Genes whose mutations were found only in the NRF group are C5orf42 (n = 5) and CC2D2A (n = 4). Other genes (NPHP1, NPHP4, KIAA0586, INPP5E, TMEM17, TMEM216, TMEM237 and TCTN1) were mutated only in single probands, of whom only those with NPHP4 or TMEM216 mutations had CKD.
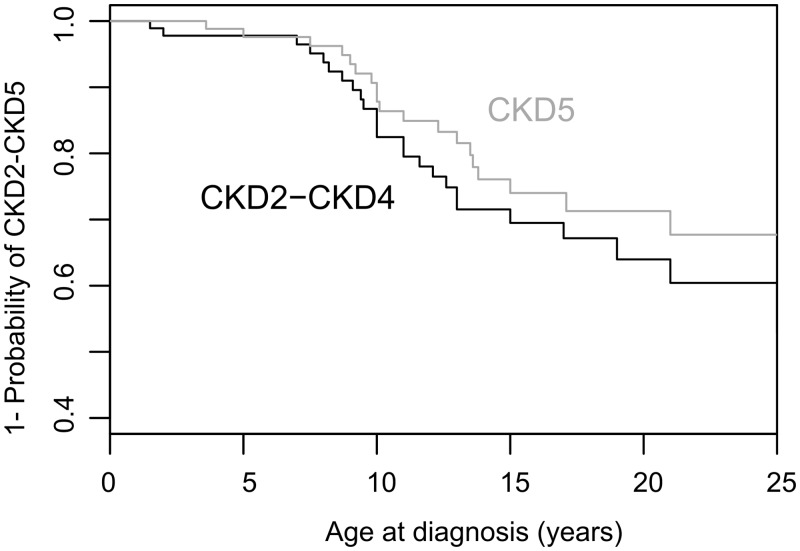
The evolution of renal function was assessed by Kaplan–Meier survival curves (Figure 2). Because in most patients, renal function was not evaluated before JS diagnosis, the age of each CKD category corresponds to the first available medical records, and does not always represent the actual age of onset of renal damage. Specifically, some patients were recruited after developing CKD5. With these limitations, patients developed CKD (CKD2–CKD5) at median age of 10 years.
FIGURE 2.
Survival analyses. Kaplan–Meyer curves representing the disease-free survival for CKD2–CKD4 and CKD5, respectively. The figure shows age at first diagnosis, which does not necessarily correspond to the actual age at onset. CKD2–CKD4, CKD with mildly to severely decreased eGFR; CKD5, end-stage renal disease.
In the 15 families with multiple affected members, the renal phenotype was fully concordant (NRF in 10 families and renal involvement in 5) (Supplementary data, Figure S2).
Assessment of urinary osmolality
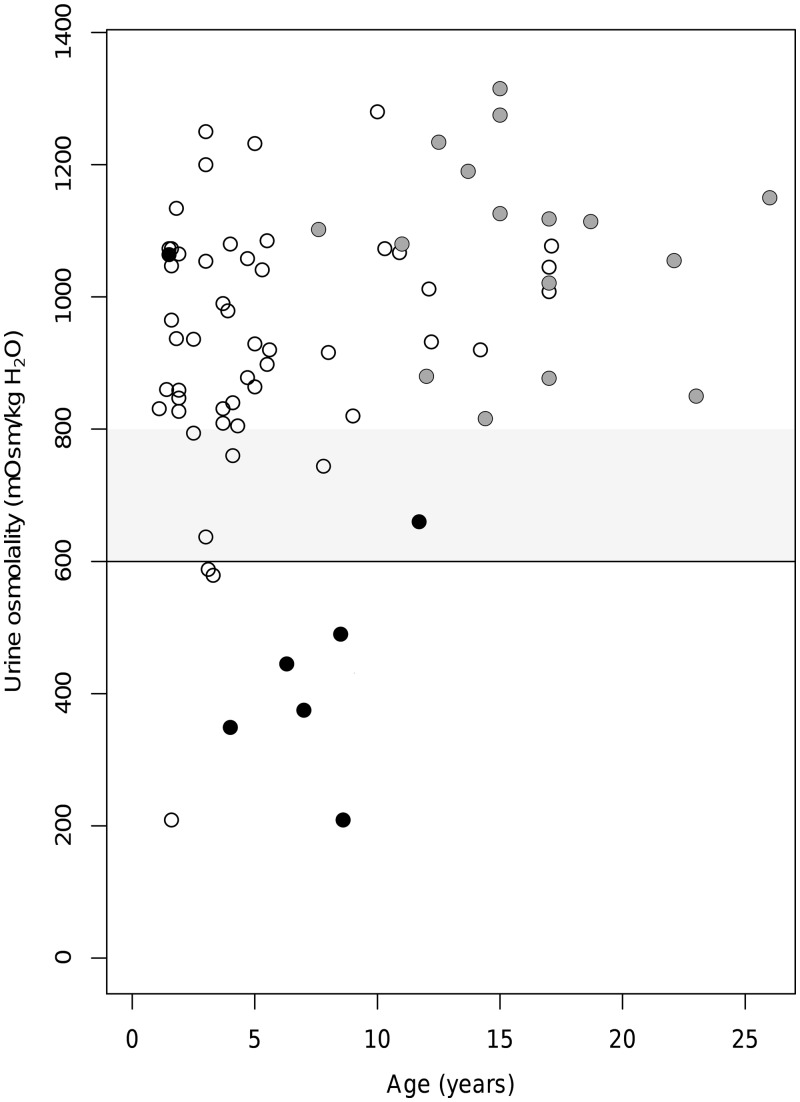
To evaluate the significance of isolated tubular defects in JS, we focused on the 77 patients who at first examination had either NRF (n = 67) or iUCD (n = 8). Their urine osmolality values at first examination were plotted against their age (Figure 3). Notably, UCD was already detectable in very young children, the youngest being 2 years old. Patients with iUCD had significantly higher magnesuria compared with those with NRF. No other differences were observed between the two groups (Table 2).
FIGURE 3.
Assessment of urine osmolality and evolution of renal function. For each patient, urine osmolality at first examination was plotted against age. Normality values are set at or >600 mOsm/kg H2O (black line). The grey zone between 600 and 800 mOsm/kg H2O defines borderline values. Only subjects with normal eGFR at first examination were included. Grey circles represent patients with NRF who were ≥18 years at time of last examination. Black circles represent patients with normal, borderline or abnormal urinary osmolality at first examination who later developed overt renal insufficiency with abnormal eGFR (CKD2–CKD5). White circles represent patients with NRF who were still <18 years at time of last examination.
Table 2.
Biochemical profile of patients with normal eGFR, with and without urine concentration defect
| NRF (n = 67) | iUCD (n = 8) | P-value | |
|---|---|---|---|
| Age, median (IQR), years | 5 (3–13) | 5 (3–7) | 0.45 |
| Serum Na, mean ± SD, mEq/L | 140.0 ± 2.5 | 139.9 ± 1.9 | 0.88 |
| Serum K, mean ± SD, mEq/L | 4.4 ± 0.5 | 4.1 ± 0.6 | 0.21 |
| Serum Cl, mean ± SD, mEq/L | 104.3 ± 3.0 | 103.1 ± 1.4 | 0.08 |
| Serum Ca, median (IQR), mg/dL | 9.6 (9.1–10.0) | 9.4 (9.4–9.7) | 0.78 |
| Serum P, mean ± SD, mg/dL | 4.9 ± 0.8 | 5.1 ± 1.0 | 0.55 |
| Serum Mg, mean ± SD, mg/dL | 2.2 ± 0.2 | 2.1 ± 0.3 | 0.48 |
| Serum UA, mean ± SD, mg/dL | 4.1 ± 1.2 | 4.4 ± 1.2 | 0.44 |
| FeNa, median (IQR), % | 0.5 (0.3–0.7) | 0.8 (0.5–2.1) | 0.13 |
| FeUA, mean ± SD, % | 9.4 ± 4.1 | 8.8 ± 4.5 | 0.73 |
| Ca/Cr, mean ± SD, mg/mg | 0.14 ± 0.12 | 0.09 ± 0.09 | 0.36 |
| Mg/Cr, mean ± SD, mg/mg | 0.15 ± 0.08 | 0.22 ± 0.08 | 0.04* |
| TRP, median (IQR), % | 91.0 (88.0–93.0) | 90.0 (88.0–92.0) | 0.68 |
| TmP/eGFR, mean ± SD, mg/dL | 4.3 ± 1.1 | 4.6 ± 1.1 | 0.51 |
| CrCl, mean ± SD, mL/min/1.73 m2 | 122.1 ± 30.6 | 107.7 ± 20.5 | 0.24 |
Italics are used for not-normally distributed variables. *P-value ≤0.05. Ca/Cr, urinary calcium/creatinine ratio; CrCl, creatinine clearance; FeNa, fractional excretion of sodium; FeUA, fractional excretion of uric acid; iUCD, isolated urine concentration defect; Mg/Cr, urinary magnesium/creatinine ratio; NRF, normal renal function; TmP/eGFR, maximum reabsorption of phosphates per unit volume of glomerular filtration rate; TRP, tubular reabsorption of phosphates.
In 18 patients, urine osmolality was reassessed after 5 ± 3 years (range: 2–12 years). None of the 12 patients with first values ≥800 mOsm/kg H2O had a pathological test when reevaluated. Three of four patients with first osmolality <600 mOsm/kg H2O showed abnormal results in subsequent tests. The fourth patient (first value 579 mOsm/kg H2O at 3 years) had normal urine osmolality 4 years later. Two children with initial borderline results (aged 3 and 12 years) underwent a second test after about 4 years: the follow-up osmolality was normal in one, borderline in the second patient (Supplementary data, Figure S3).
Predictive value of urinary osmolality testing
A main aim of this study was to assess the validity of urine osmolality in predicting the risk of developing CKD in JS. We therefore focused on a subset of 23 patients with normal eGFR at the first examination, who either developed CKD2–CKD5 later or maintained NRF in adulthood.
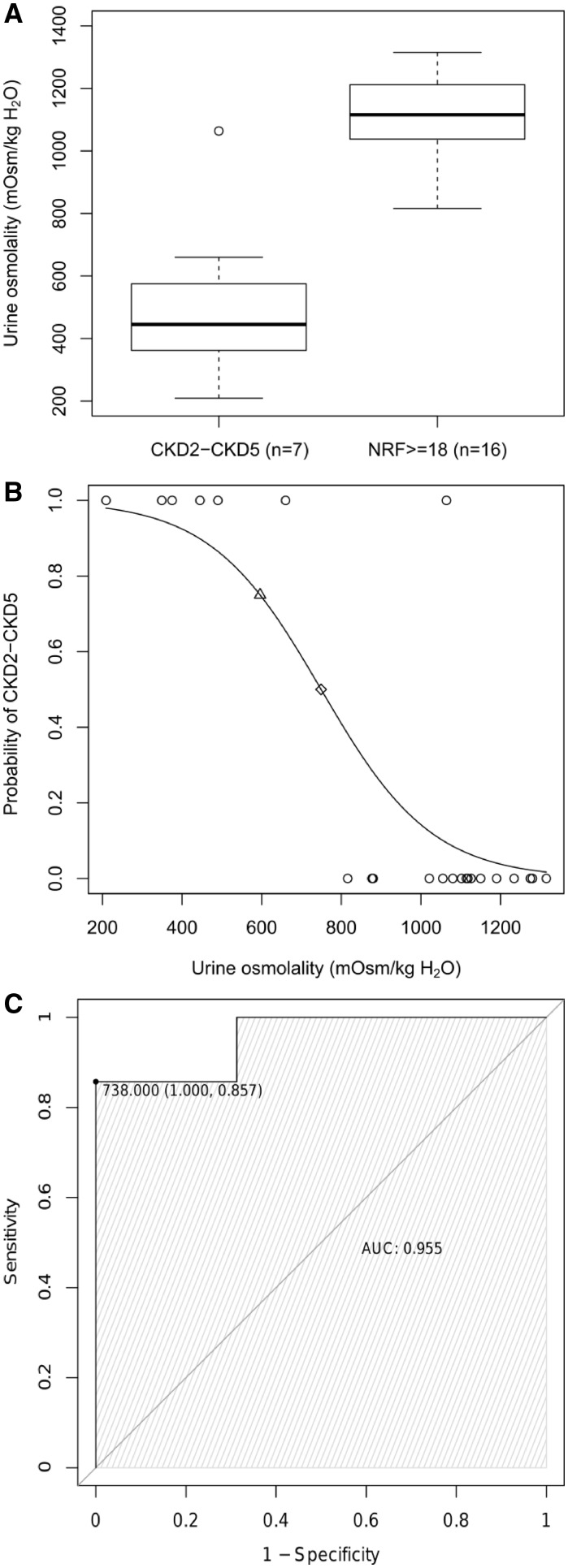
Seven subjects had abnormal eGFR at follow-up (CKD2–CKD5). Of them, five had iUCD at their first examination, one had borderline osmolality values and one had normal osmolality (Figure 3, black circles). Conversely, all 16 patients who maintained preserved eGFR after age 18 years had normal osmolality at first examination (Figure 3, grey circles). In these two groups (7 CKD2–CKD5 versus 16 NRF ≥ 18), we compared the distribution of urine osmolality values at first examination. In line with the above results, data did not overlap with the exception of a single patient with normal initial urinary concentration ability that later developed CKD5. Mean osmolality values significantly differed between the two groups (513 mOsm/kg H2O versus 1102 mOsm/kg H2O, P < 0.001) (Figure 4A). Logistic regression analysis showed that the probability of developing CKD2–CKD5 significantly correlated with the initial urine osmolality values [β-coefficient =−0.007, 95% confidence interval (95% CI) −0.023 to −0.003; P < 0.01], with only a small confounding effect of age at osmolality testing. Specifically, the probability of adverse renal outcome was >50% for values <749 mOsm/kg H2O, and >75% for values <596 mOsm/kg H2O (Figure 4B). Finally, ROC analysis demonstrated a high diagnostic accuracy of urine osmolality (AUC = 0.96, 95% CI 0.86–1.00). The optimal cut-off value for predicting progression to CKD2–CKD5 was 738 mOsm/kg H2O, corresponding to 86% sensitivity and 100% specificity (Figure 4C). All these results remained significant after including in the calculations the 26 NRF patients <18 years at last follow-up (data not shown).
FIGURE 4.
Predictive value of urinary osmolality. (A) Box and whisker plots of urine osmolality for patients with normal eGFR at first examination who at follow-up examination fell in the two following groups: NRF ≥ 18 (≥18 years with normal renal function); CKD2–CKD5. The central box represents the distance between the lower and the upper quartiles, the middle line is the median and the whiskers extend from the minimum to the maximum osmolality value, excluding outside and far out values, which are displayed as separate points. (B) Probability of developing adverse renal outcome (CKD2–CKD5) based on prediction from logistic regression model. Each circle represents a single patient. The risk is predicted to be >50% for urine osmolality values <749 mOsm/kg H2O (diamond) and >75% for urine osmolality values <596 mOsm/kg H2O (triangle). (C) ROC plot for urine osmolality comparing NRF ≥ 18 and CKD2–CKD5 groups. The area under is the curve is 0.96 (95% CI 0.86–1.00). The value of 738 mOsm/kg H2O is the best threshold for urine osmolality (defined as the value associated with minimum false-negative and false-positive rates). All figures refer to a subset of 23 patients with normal eGFR at the first examination, who either developed CKD2–CKD5 later or maintained normal NRF in adulthood.
DISCUSSION
Kidney involvement is a severe and insidious complication of JS. Renal disease may long remain unrecognized, and diagnosis is often made late, when renal function is impaired and complications such as anaemia and growth retardation have occurred and are only partially reversible. Moreover, a late diagnosis results in more rapid progression towards renal failure.
Since renal disease was not fully recognized until recently, JS patients were frequently diagnosed with advanced CKD. The probability curves for CKD2–CKD4 and for CKD5 observed in our cohort are similar in slope and are separated only by 1–2 years (Figure 2), suggesting that once renal impairment develops, patients progress relatively quickly to end-stage renal disease.
A recent study reported renal abnormalities in 30% children with JS, of whom only ∼60% (i.e. 18% of the whole cohort) had developed CKD [10]. The latter finding is likely an underestimation as, similar to other studies, this cohort included many young children, at risk of developing CKD later in life [8, 23]. Accordingly, when considering only 60 subjects for whom renal follow-up was available in our study, the number of patients with reduced eGFR approximately doubled between the first and the last examination, rising from 15% to 27% (Supplementary data, Figure S1). Hypothetically, this estimation could have also been biased by the fact that patients with abnormal renal findings were more likely to be followed-up over time. However, due to the severity of their disease, very few patients were lost at follow-up and the remaining patients were reanalysed irrespectively of the initial finding. Another potential bias of our study relates to the referral of some patients by nephrologists, which may have enriched the population in subjects with NPH. Yet, the majority of patients enrolled by nephrologists had been referred to them by neurologists to perform systematic evaluations of the renal function. We cannot rule out, however, that this may have marginally influenced the observed frequencies. Overall, it appears reasonable to estimate that renal insufficiency will occur in ∼25–30% of patients with JS.
Regardless of the exact prevalence, the early identification of children at risk of adverse renal outcome may be very important to delay progression to CKD and to avoid complications. Specifically, these patients require appropriate fluid and caloric intake, and early treatment of bone mineral disease, anaemia and growth retardation [13, 14]. Of notice, many patients with JS have severe neurological defects and may not sense or express thirst, which put them at potential risk of dehydration and fast progression of CKD.
Our study clearly shows that low urine osmolality after DDAVP stimulation identifies patients at risk of developing progressive CKD. In our cohort, an iUCD could be detected in patients as young as 2–3 years of age. These children showed no other pathological glomerular or tubular finding, but had on average higher magnesuria, compared with patients with normal concentration ability. This finding is particularly interesting since magnesium handling is nearly exclusively operated at the distal tubule, and isolated magnesuria have been reported in other conditions characterized by abnormalities in tubulo-interstitial development, such as in HNF1B mutations [24]. In principle, other markers of tubular dysfunction such as NGAL could also be useful for identifying patients at risk, but these could not be assessed in this study. Also, we could not collect enough data about low-molecular weight proteinuria, which would have indicated a proximal tubular dysfunction. Nonetheless, patients with iUCD did not present proteinuria by standard urine analysis, suggesting that proximal tubular reabsorption of proteins was preserved and that initial tubular defect was more distal.
When available, follow-up assessment of urinary osmolality showed that most normal and pathological values, even if obtained in very young children, remained in the same normal or pathological ranges after several years. Values within or close to the borderline range (600–800 mOsm/kg H2O) had a less predictable outcome. These observations reflect well the clinical evolution in the long term. Specifically, five out of seven subjects with iUCD at first examination (as well as one patient with borderline values) developed CKD at follow-up. Conversely, of the 17 subjects with normal osmolality that were reassessed in adult age, all but one had normal renal function. This single outliner was diagnosed with JS at age 2 months, had normal urinary concentration ability tested at 3 years and was then lost at follow-up. When reevaluated at 11 years, he was found to have CKD3 and progressed to CKD5 1 year later.
Overall, our study indicates that urine osmolality is a good predictor of the probability of developing renal insufficiency. In practical terms, we propose to measure the first-morning urine osmolality in all JS patients around the age of 3 years. If urine osmolality is repeatedly found <800 mOsm, then a DDAVP test is useful to assess the renal concentration ability. Although exceptions cannot be excluded, when patients can concentrate their urine >800 mOsm, the family can be reassured, as long as a control test is planned later (around 8 years of age). Borderline osmolality values (600–800 mOsm/kg H2O) should be rechecked earlier, as some patients may develop renal disease. Conversely, values <600 mOsm indicate a probable nephropathy. A confirmatory test should be planned in the following months and, if abnormal, appropriate water intake and renal monitoring should be started.
In our experience, a DDAVP test can be performed safely in an outpatient setting, as long as patients do not receive forced fluids. Children should not be encouraged to drink beyond thirst after DDAVP administration, and the test should never be given to children fed through a nasogastric or gastrostomy tube. One limitation of our protocol is that DDAVP was administered nasally, and some patients may not have absorbed the drug appropriately, which may possibly explain the variable outcomes of patients with borderline values.
To date, only renal ultrasound is routinely performed as an early diagnostic instrument of renal involvement in JS. However, this test may remain negative in patients with NPH until they develop advanced CKD. In our cohort, although a pathological ultrasound was significantly associated with CKD, discrepancies were observed between ultrasound findings at first examination and the evolution of kidney function. For instance, two patients with normal ultrasound subsequently developed renal insufficiency, whereas two other children diagnosed with kidney hyperechogenicity at ∼4 years of age (but with normal osmolality) still maintained a normal eGFR several years later. Furthermore, three out of eight patients presenting with iUCD at first examination had normal ultrasound at the same time.
This study also confirms the previously reported association of retinal and renal involvement in JS [8, 23], and the concordance of renal phenotype within families [23]. Pathogenic mutations in known JS-associated genes were identified in ∼54% probands with renal disease, with a high prevalence of mutations in CEP290, a gene that we first reported to be significantly associated to the retinal–renal phenotype [25]. This is also in line with a recent study describing the clinical and genetic correlates of a large cohort of 440 JS patients [8]. In our cohort, mutations in TMEM67 and AHI1 seem to be slightly overrepresented in patients with NRF compared with those with renal disease, at difference to what shown by Fleming and collaborators [10], who reported a similar mutation frequency of these genes in the two groups. A possible explanation for this discrepancy could lie in the definition of ‘kidney disease’ used by the authors, which also include subjects with abnormal renal ultrasound as an isolated finding, regardless of kidney function. However, these differences between studies can also be explained by the small number of patients with mutations in a given gene. Hence, comparison did not reach statistical significance.
In conclusion, we observed that nearly 30% patients with JS eventually develop renal insufficiency. Besides ultrasound abnormalities, an iUCD often represents the earliest sign of an underlying renal disease and predicts evolution towards CKD. A positive family history for renal disease and the presence of retinopathy also convey a high risk of renal involvement. These findings are relevant for the clinical management and genetic counselling of JS patients, and we suggest that urinary osmolality should be included in the diagnostic workup of children with JS.
FUNDING
This work was funded by the European Research Council (ERC Starting Grant 260888), the Telethon Foundation Italy (Grant GGP13146), the Pierfranco and Luisa Mariani Foundation (PADAPORT project) and the Italian Ministry of Health (Ricerca Finalizzata 2013 NET-2013-02356160, Ricerca Corrente ‘Neuroscienze Sperimentali’ and 5x1000 Anno 2016 to Fondazione Santa Lucia). The research was also supported by a Grant of the Italian Ministry of Education, University and Research (MIUR) to the Department of Molecular Medicine of the University of Pavia under the initiative ‘Dipartimenti di Eccellenza (2018–2022)’. This publication is distributed under the terms of open access policies implemented by the Italian Ministry of Education, University and Research (MIUR). S.N. is the recipient of a PhD bursary financed by AISJAC (Associazione Italiana Sindrome di Joubert e Atassie Congenite).
AUTHORS’ CONTRIBUTIONS
S.N., E.M.V. and F.E. designed the study, analysed data and wrote the manuscript. A.M. and M.R. performed NGS and mutation analysis. L.F., R.Battini, E.B., R.Borgatti, G.C., S.D., E.F., R.F., G.M.G., L.G., V.L., R.R., S.S., G.S., G.Z. and F.E. recruited patients and gathered detailed clinical information for the study. All authors critically reviewed the article.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
Supplementary Material
REFERENCES
- 1. Romani M, Micalizzi A, Valente EM.. Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet Neurol 2013; 12: 894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitchison HM, Valente EM.. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 2017; 241: 294–309 [DOI] [PubMed] [Google Scholar]
- 3. Vilboux T, Malicdan MC, Roney JC. et al. CELSR2, encoding a planar cell polarity protein, is a putative gene in Joubert syndrome with cortical heterotopia, microophthalmia, and growth hormone deficiency. Am J Med Genet A 2017; 173: 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stephen J, Vilboux T, Mian L. et al. Mutations in KIAA0753 cause Joubert syndrome associated with growth hormone deficiency. Hum Genet 2017; 136: 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck BB, Phillips JB, Bartram MP. et al. Mutation of POC1B in a severe syndromic retinal ciliopathy. Hum Mutat 2014; 35: 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li C, Jensen VL, Park K. et al. MKS5 and CEP290 dependent assembly pathway of the ciliary transition zone. PLoS Biol 2016; 14: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Mori R, Romani M, D’Arrigo S. et al. Hypomorphic recessive variants in SUFU impair the sonic hedgehog pathway and cause Joubert syndrome with cranio-facial and skeletal defects. Am J Hum Genet 2017; 101: 552–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bachmann-Gagescu R, Dempsey JC, Phelps IG. et al. Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet 2015; 52: 514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brancati F, Dallapiccola B, Valente EM.. Joubert Syndrome and related disorders. Orphanet J Rare Dis 2010; 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming LR, Doherty DA, Parisi MA. et al. Prospective evaluation of kidney disease in Joubert syndrome. Clin J Am Soc Nephrol 2017; 12: 1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stokman M, Lilien M, Knoers N.. Nephronophthisis In: Adam MP, Ardinger HH, Pagon RA. et al. (ed). GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle; 1993–2018, 2016 [PubMed] [Google Scholar]
- 12. Dempsey JC, Phelps IG, Bachmann-Gagescu R. et al. Mortality in Joubert syndrome. Am J Med Genet A 2017; 173: 1237–1242 [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 15. Aronson AS, Svenningsen NW.. DDAVP test for estimation of renal concentrating capacity in infants and children. Arch Dis Child 1974; 49: 654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rees L, Brogan PA, Bockenhauer D. et al. Paediatric Nephrology. 2nd edn.Oxford, UK: Oxford University Press, 2012 [Google Scholar]
- 17. Schwartz GJ, Haycock GB, Edelmann CM Jr. et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976; 58: 259–263 [PubMed] [Google Scholar]
- 18. Schwartz GJ, Muñoz A, Schneider MF. et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. https://www.kidney.org/content/mdrd-study-equation. (10 July 2018, date last accessed)
- 20. Roosing S, Romani M, Isrie M. et al. Mutations in CEP120 cause Joubert syndrome as well as complex ciliopathy phenotypes. J Med Genet 2016; 53: 608–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80: 27–38 [Google Scholar]
- 22.R Development Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing, 2017
- 23. Vilboux T, Doherty DA, Glass IA. et al. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med 2017; 19: 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adalat S, Woolf AS, Johnstone KA. et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 2009; 20: 1123–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brancati F, Barrano G, Silhavy JL. et al. CEP290 mutations are frequently identified in the oculo-renal form of Joubert syndrome–related disorders. Am J Hum Genet 2007; 81: 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.