Background:
The dapivirine vaginal ring reduced the risk of HIV infection by approximately 30% in Phase III trials. To ensure higher levels of protection against HIV and sexually transmitted infections, women should be counseled to use condoms when using the vaginal ring. This article evaluates the compatibility of male condoms with a placebo vaginal ring.
Methods:
This was a 2-period crossover, randomized, noninferiority trial. Couples in 2 sites in the United States were randomized to male condom use, with and without a placebo silicone vaginal ring, and asked to use 4 male condoms in each period. The primary noninferiority end points were total clinical failure and their component failure events (clinical breakage or slippage). Frequencies and percentages were calculated for each failure mode and differences in performance of the 2 periods using the male condom without the ring as reference. Noninferiority was defined using a 3% margin at the 5% significance level. Safety and acceptability were also assessed.
Results:
Seventy couples were enrolled, and 68 completed the trial with a total of 275 male condoms used in each period. Total condom clinical failure rates were 2.2% and 4.0% in the presence and absence of the vaginal ring, respectively, with a difference of −1.9% (95% confidence interval: −5.3% to 1.5%), thereby demonstrating noninferiority when used with the ring. There was no difference in safety between the 2 periods.
Discussion:
Concurrent use of the placebo silicone vaginal ring had no significant effect on male condom functionality or safety outcomes.
Key Words: vaginal ring, male condom, HIV prevention
INTRODUCTION
The dapivirine silicone vaginal ring containing 25 mg of dapivirine, a nonnucleoside reverse transcriptase inhibitor with potent antiviral activity against HIV type 1 (HIV-1), has been developed to increase HIV prevention options for women.1 Two phase-III trials, IPM 027 (The Ring Study) and MTN-020 (ASPIRE), demonstrated that the dapivirine vaginal ring (DVR) used monthly reduced women's overall risk of HIV-1 infection by approximately 31% compared with a placebo ring. Potentially greater risk reduction can be associated with increased adherence to ring use.2,3
The DVR could provide an additional HIV prevention option for women in sub-Saharan Africa where the need for woman-initiated HIV prevention is greatest.1 Currently, both male and female condoms are promoted in this region of the world as part of the HIV, sexually transmitted infection (STI), and pregnancy prevention strategies of the Joint United Nations Programme on HIV and AIDS.4 Consistent and correct use of the male latex condom reduces the risk of STIs and HIV transmission,5 and male condoms protect against pregnancy 98% of the time when used correctly and consistently, and 87% of the time during common use.6 In Africa, male and female condoms are distributed (often free of charge) through government facilities, nongovernmental organizations, social marketing groups, and in the private sector.4 This has resulted in an increase in the use of male condoms in sub-Saharan Africa.7,8
Trials of the DVR promoted the use of male condoms, and participants were counseled to use them for protection against HIV and STI infection.2,3 As a result, approximately 40% of women reported that they used male condoms always or some of the time throughout a phase-III DVR trial (unpublished data IPM 027). Microbicide gel trials have also previously reported an increase in male condom use from baseline.9 However, no data were collected on male condom functionality with the vaginal ring inserted in DVR trials. Once the DVR is available, women will always be counseled to use either male or female condoms with the DVR for maximum risk reduction of HIV-1 infection and STIs, and thus, the function of both male and female condoms with the vaginal ring needs to be evaluated.
Condom compatibility laboratory studies were conducted to evaluate whether dapivirine itself has any effect on the physical properties of a variety of types of male and female condoms. There are 3 major chemical components to the ring—polydimethylsiloxane (PDMS) rubber, silicone oil (dimethicone), and dapivirine. Silicone oils are the most commonly used lubricants applied to male and female condoms during manufacture.10 Based on the ubiquitous use of PDMS silicones with condoms, it can be concluded that there is no effect of these formulation components (totaling more than 99.6% by weight of the dosage form) on condom function. The dapivirine drug substance (comprising 0.3125% by weight of the formulation) has been shown to have no impact on condom functionality when applied as a gel formulation and tested according to American Society for Testing and Materials (ASTM) test method standards (unpublished data available on request). However, no clinical data on concomitant use of male condoms and DVRs are available.
A male condom functionality clinical trial to assess the functional performance of male condoms in the presence and absence of a placebo silicone vaginal ring was conducted to determine whether these products are compatible and acceptable to use together.
MATERIALS AND METHODS
This was an open-label, randomized, 2-period, crossover, noninferiority trial to assess the functionality of male condoms with a silicone vaginal ring (placebo ring containing no dapivirine) conducted at 2 research centers in the United States.
Study Objectives
The primary objective was to assess the total clinical failure rate of male condoms during vaginal intercourse in the presence and absence of the vaginal ring.
The primary noninferiority end points were self-reported total male condom clinical failure and their component failure events (clinical breakage during intercourse or withdrawal or complete slippage off the penis). For the purpose of this article, total male condom failure is reported as total clinical failure and nonclinical breakage as defined by the World Health Organization and regulatory agencies. Definitions of each failure mode analyzed in this trial are as follows:11,12
Clinical breakage: condom breaks or tears during intercourse or withdrawal from the vagina.
Nonclinical breakage: breakage noticed before intercourse or occurring after withdrawal of the male condom from the vagina. Nonclinical breakage is without potential adverse clinical consequences.
Clinical slippage: when a male condom slips completely off the penis during sexual intercourse or withdrawal from the vagina.
Total clinical failure: the sum of male condoms that clinically break or slip, which result in the reduction of the male condom protective function.
Secondary trial objectives included the following:
To assess the safety and tolerability of male condoms during vaginal intercourse in the presence and absence of the placebo vaginal ring.
To assess user acceptability of male condoms during vaginal intercourse in the presence and absence of the placebo vaginal ring.
Adherence to ring use was assessed by the occurrence of self-reported vaginal ring expulsion or removal associated with the use of male condoms.
Safety was assessed by evaluating the number, severity, relatedness, and duration of adverse events (AEs). For this trial, AEs were defined as any self-reported urogenital discomfort that arose during condom or condom/ring use that lasted more than an hour, any other urogenital or nonurogenital medical problem that could be related to condom or ring use, or any serious AE (SAE). Events that resolved within one hour of condom use were recorded. Standard acceptability measures were collected.
Study Population
The target population was 70, healthy, monogamous, sexually active couples in the United States who were either novice or experienced users of male condoms. The participants were recruited from 2 research centers (Northern California and Southern California).
Key inclusion criteria were as follows:
Mutually monogamous healthy heterosexual couples; current relationship ≥3 months; at low risk for HIV-1 infection; who could give written informed consent and were willing to comply with the study procedures;
Age ≥18 and ≤45 years (females) or ≥18 and ≤55 years (males) at time of the screening visit;
Not at risk for pregnancy, that is, female is surgically sterile, using an intrauterine device or using effective hormonal contraception, or has a vasectomized partner. The use of vaginal contraceptive rings was not allowed;
Low risk of acquiring HIV infection;
Sexually active and agree to have at least 8 acts of penile–vaginal intercourse using a trial condom over 2 periods of up to 4 weeks each;
Agree to use only the male condoms and lubricant provided by trial personnel and not to use other vaginal products except menstrual absorption products and nontrial lubricants during the trial;
Did not use genital piercing jewellery.
During the trial, the female participant attended all scheduled trial visits. The male partner was only required to attend the screening and enrollment visit, whereas the female partner returned both completed condom/ring use forms and acceptability questionnaires. Each couple was asked to use 8 male condoms in total—4 while the ring was inserted vaginally and 4 without the ring. Couples were asked to complete a condom log at home after each condom use episode. After completing all 4 condoms use episodes in each of the trial periods, the female participant returned to be interviewed about their experiences. Condom logs were used to capture data on condom function and safety. Interviewer-assisted questionnaires were used to gather acceptability data.
Study Products
The 2 trial products are described in Figure 1.
FIGURE 1.

Description of trial products.
Ethical Considerations
The trial was approved by the Institutional Review Boards of the California Family Health Council Inc, now known as Essential Access Health. The trial is registered with clinicaltrials.gov (NCT01755741). After reading and signing the informed consent and qualifying for trial inclusion, couples were enrolled in the trial. Participants in the trial were reimbursed for time and travel expenses.
Randomization
Based on a predetermined randomization schedule, 36 of the 70 enrolled couples were assigned to sequence A where the female partner wore the vaginal ring continuously during the first trial period, where after she crossed over to the second trial period during which no ring was worn. The other 34 couples were assigned to sequence B where the female partner only wore the vaginal ring during the second trial period, after crossing over from the first period during which no ring was worn. There was no washout between the 2 trial periods. A statistical programmer (not involved in the trial) developed the random allocation sequence, using a validated statistical program in SAS Software.13
Sample Size and Statistical Methods
Assuming a total clinical failure rate for the male condom when used with or without the vaginal ring of 2%,7–9 and an intracouple correlation of 0.15, 67 couples using 4 female condoms during each trial period (268 uses in both the presence and absence of the vaginal ring) would provide approximately 85% power (alpha equal to 0.05) to conclude noninferiority with a 3.0% margin. To allow for up to a 5% early discontinuation rate, enrollment of 70 couples was planned.
The hypothesis for the primary end points, total clinical failure and their component failure events, was that function of the male condom with the silicone ring inserted was “noninferior” to male condom use without the silicone ring inserted with regard to the rate of clinical failure events within a margin of 3.0%; that is, the upper limit of the 2-sided 95% confidence interval (CI) for the difference in the occurrence of events (male condom with ring − male condom without ring) was required to be below 3.0%.
The main analysis for primary and secondary end points was according to the assigned condom use sequence among participants who provided relevant follow-up data on at least one completed act of vaginal intercourse using a male trial condom with/without the vaginal ring. The statisticians were blinded to the study arms until the database was locked.
RESULTS
Recruitment and Baseline
Seventy couples were enrolled in the trial (35 couples per research center). In total, 68 couples completed the trial with each male partner using at least 1 condom in each of the 2 periods (Fig. 2). Both the couples who withdrew (one enrolled in sequence A and one in B) reported using all 4 condoms but did not attend the crossover visit. The full analysis population included all couples who engaged in at least one sexual encounter (completed act of vaginal intercourse) using a trial condom both with and without the vaginal ring. The safety population included all couples that were randomized in the trial. Twenty-five couples in this study participated in a “companion” study that evaluated the performance of female condoms when used with or without a silicone placebo vaginal ring. The results of this companion trial are presented in this journal edition.
FIGURE 2.
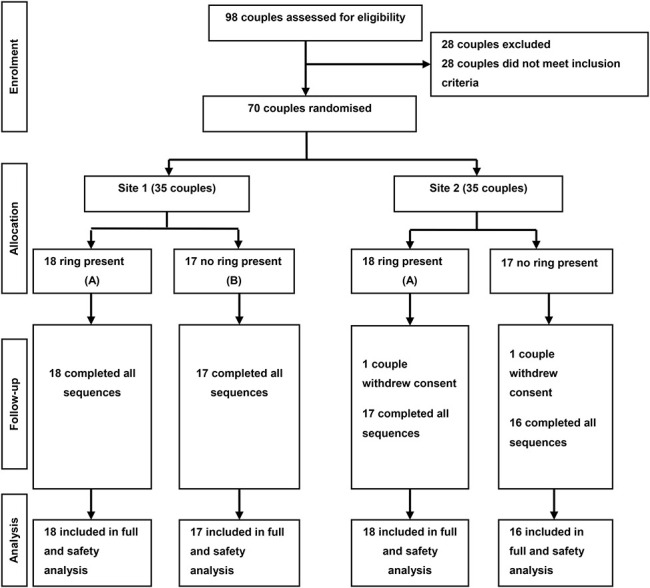
CONSORT flowchart.
Demographic characteristics are summarized in Table 1. The overall mean age across the 2 genders was very similar (women: 27.1 years; men: 28.1 years). Participants were generally well educated with most male and female participants reporting post high school experience with 43.5% overall completing a first degree or higher. One-third were employed full or part time, and 18.6% were students. Few participants (12.1%) were unemployed (Table 1).
TABLE 1.
Demographics and Baseline Characteristics—Safety Population
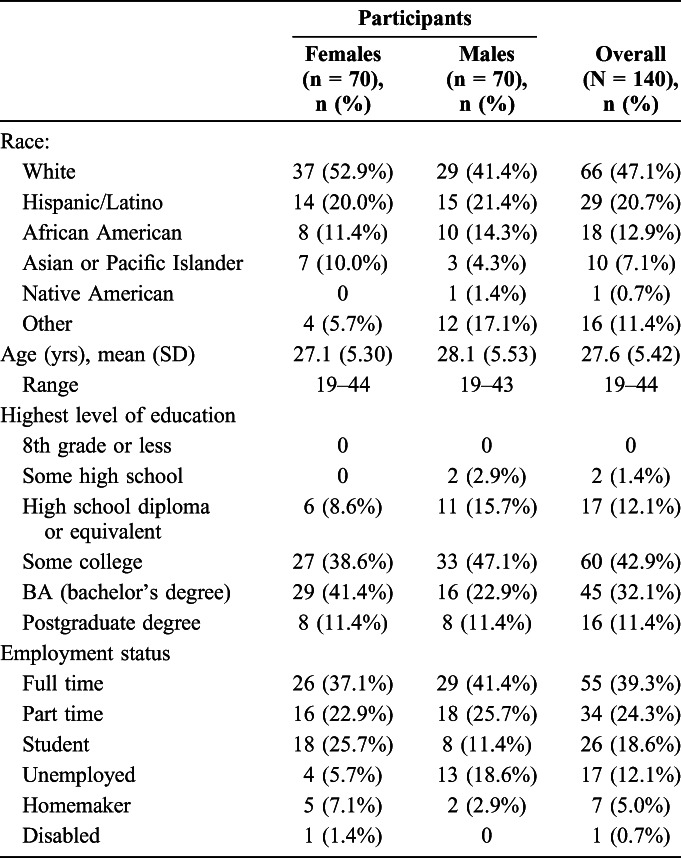
In the past 3 months, all but one male and one female participant had used the male condom at least once, with 74.9% (n = 52) of female participants and 84.3% (n = 59) of male participants using the male condom at least 11 times.
Condom Use and Functionality of Condoms
In total, 550 of the 552 dispensed male condoms were used in this trial by couples in the full analysis population (275 in the presence and 275 in the absence of a silicone placebo vaginal ring). Two events of nonclinical breakage were noted. One condom tore when the package was opened (intended to be used with the ring). The second event occurred after it was put on the penis. This condom tore while donning the condom, which was to be used without the ring. These 2 condoms were not used for sex and thus do not contribute to clinical breakage.
Noninferiority was demonstrated between the 2 periods for both male condom clinical failure modes. Total male condom clinical failure in the presence of a silicone placebo vaginal ring was 2.2%, and it was 4.0% in the absence of the vaginal ring (Table 2). The difference between the total clinical failure probability (when used with the ring) and the total clinical failure probability (when used without the ring), calculated using a generalized estimating equation procedure, was −1.9% (95% CI: −5.3% to 1.5%). The upper bound of the CI was less than 3.0%; therefore, the null hypothesis was rejected, which stated that the total clinical failure rate differed by at least 3.0%.
TABLE 2.
Male Condom Functionality
There was no report of vaginal ring expulsion during intercourse. There were 5 reports of vaginal ring removal and expulsion. Three participants removed the vaginal ring, and 2 participants reported ring expulsions sometime after using all 4 condoms.
Acceptability
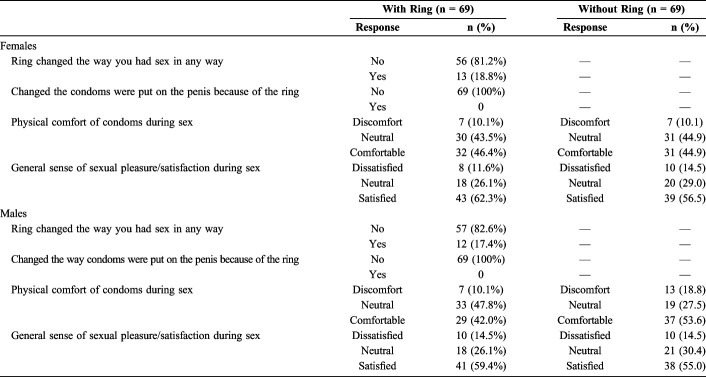
For both genders, the majority of participants (81.2% of women and 82.6% of men) felt that the vaginal ring did not change the way in which they had intercourse (Table 3). Those who said it did change the way they had sex often reported that they were more careful, cautious, or nervous when having sex. Others mentioned that they tried different positions during sex because some positions were uncomfortable. No participants changed the way of placing the male condom on the penis because of concurrent vaginal ring use.
TABLE 3.
Male Condom Acceptability
More than half of men (n = 38, 55.1%) and one-third of women (n = 23, 33.3%) reported feeling the vaginal ring during sex. Equal proportions of male and female participants (10.1%) found that using the male condoms in combination with the vaginal ring was physically uncomfortable. In comparison, 10.1% of female participants and, slightly more, male participants (18.8%) found that using the male condom was uncomfortable even when the vaginal ring was not used.
Levels of unsatisfactory pleasure while using the male condom, whether with or without the vaginal ring, were low: 11.6% (8/69) female participants and 14.5% (10/69) male participants with vaginal ring use, and 14.5% (10/69) for both female and male participants without the vaginal ring.
Safety
No treatment-emergent AEs (TEAE) were reported by any of the female participants. Two TEAEs were reported by 2 male participants, both for penile pain. One of these events occurred when a condom was used in the absence of the vaginal ring. The other event occurred during condom use in the presence of the vaginal ring and was considered by the investigator to be related to condom and ring use. Both events resolved without sequelae, and the couples completed the trial.
No postbaseline abnormal pelvic/urogenital examination findings were reported during the period of vaginal ring use; 2 abnormalities were reported for 2 female participants during the period without vaginal ring use (yeast vaginitis and suspected yeast vaginitis). No SAE were reported.
DISCUSSION
Use of the male condom together with a silicone vaginal ring did not result in increased condom failure rates, as was shown by the noninferiority demonstrated for all modes of condom clinical failure when used with the ring, compared with the use without the ring. The total clinical failure and component failure rates reported in this trial were generally slightly lower than the rates reported in other latex male condom failure studies,14,15 where total condom failure rates of between 3.8% and 13.3% were reported. A potential reason for this observation is that all but one couple in this trial had previously used male condoms, were well educated, and were well trained and counseled on male condom use as part of the trial procedures.
Concomitant use of the male condom with the vaginal ring had no impact on sexual pleasure for either male or female participants compared with the use without the vaginal ring. A comprehensive review of vaginal ring acceptability reported that between 70% and 90% of female users and 48%–97% of partners felt the ring during sex, although it is not known if the male partner was using a condom.16 Our study data showed similar levels of reporting, with 66.7% of women feeling the ring compared with 44.9% of men.
The rates of condom use (male and female) at last sex show a positive trend in many regions with some Latin American and European countries reporting rates among 15 to 24 year olds of more than 80%, although lower increase of around 30% have been recorded in some African countries.4 In particular, condom use at last higher-risk sex has increased over the past 3 decades in most countries across the world and is as high as 80%–90% in some countries.17 DVR vaginal rings do not protect against STIs and pregnancy and if introduced into a population with high rates of condom use at last high-risk sex, it would be important to support the use of both methods. In particular, male condom slippage and breakage rates are reported to be common,14,15 and so the 2 methods combined could potentially increase protection from HIV if there is a condom failure. Even if only a small portion of couples using the male condom opt to use a vaginal ring as additional protection, it would be important for providers to have evidence that the 2 products are compatible, so they can counsel those who choose to use both products.
Some women participating in vaginal ring studies have reported that they did not inform their partners they were using the ring.18 As male condom use was promoted in DVR trials, it is likely that some partners using condoms may not have known that the ring was being used. It is therefore important that this study was conducted to ensure that male condoms are not compromised by vaginal ring use.
Assessment of safety data demonstrated that the silicone vaginal ring was well tolerated and that condom use had no adverse impact on the safety and tolerability of the ring.
In conclusion, the data demonstrated that the use of the male condom with a silicone vaginal ring has no impact on condom functionality, acceptability, or safety of the ring. This provides reassurance that users of the DVR can be advised that concomitant use of male condoms is safe and that the function of the male condom will not be affected detrimentally.
Limitations
Blinding of participants and investigator site staff was not possible; however, allocation concealment was used to ensure that this limitation was minimized. Additionally, this trial was based exclusively on self-reported measures of condom use by participants.
ACKNOWLEDGMENTS
The authors thank all research nurses, interviewers and data entry staff in both participating research centers who collected and entered the data.
Footnotes
Supported by Norad (Norwegian Agency for Development Cooperation).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.A Long-Acting Ring to Protect Women against HIV. Available at: http://www.ipmglobal.org/sites/default/files/attachments/publication/ring_backgrounder_sept_2017.pdf. Accessed August 15, 2019. [Google Scholar]
- 2.Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNFPA, WHO and UNAIDS: Position Statement on Condoms and the Prevention of HIV, Other Sexually Transmitted Infections and Unintended Pregnancy. Available at: http://www.unaids.org/en/resources/presscentre/featurestories/2015/july/20150702_condoms_prevention. Accessed June 1, 2019. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Condom Fact Sheet In Brief. https://www.cdc.gov/condomeffectiveness/docs/condomfactsheetinbrief.pdf. Accessed June 1, 2019. [Google Scholar]
- 6.World Health Organization Department of Reproductive Health and Research (WHO/RHR), Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP). Family Planning: A Global Handbook for Providers (2018 Update) [Internet]. Baltimore, MD and Geneva, Switzerland: CCP and WHO; 2018. Available at: http://apps.who.int/iris/bitstream/10665/260156/1/9780999203705-eng.pdf?ua=1. Accessed June 20, 2019. [Google Scholar]
- 7.Beksinska M, Smit J, Mantell J. Progress and challenges in male and female condom use in South Africa. Sex Health. 2012;9:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleland J, Ali MM. Sexual abstinence, contraception, and condom use by young African women: a secondary analysis of survey data. Lancet. 2006;368:1788–1793. [DOI] [PubMed] [Google Scholar]
- 9.Marlow HM, Tolley EE, Weaver MA, et al. Changes in condom use during a microbicide clinical trial in Pune, India. AIDS Care. 2012;24:539–543. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Use and Procurement of Additional Lubricants for Male and Female Condoms: WHO/UNFPA/FHI360: Advisory Note. World Health Organization; 2012. Available at: https://apps.who.int/iris/handle/10665/76580. Accessed March 6, 2020. [Google Scholar]
- 11.International Organization for Standardization, ISO/CD 29943-1: Condoms-Guidance on Clinical Studies-Part 1: Male Condoms in Use Failure Modes Studies Based on Self-Reports 2010. Geneva, Switzerland: International Organization for Standardization.
- 12.Taylor D. Issues in the design, analysis and interpretation of condom functionality studies. Contraception. 2009;80:237–244. [DOI] [PubMed] [Google Scholar]
- 13.SAS Institute Inc. SAS/STAT Software, Version 9.1. Cary, NC: 2003. Available at: http://www.sas.com/. Accessed February 1, 2019. [Google Scholar]
- 14.Steiner M, Piedrahit C, Joanis C, et al. Condom breakage and slippage rates among study participants in eight countries. Int Fam Plan Perspect. 1994;20:55–58. [Google Scholar]
- 15.Duerr A, Gallo MF, Warner L, et al. Assessing male condom failure and incorrect use. Sex Transm Dis. 2011;38:580–586. [DOI] [PubMed] [Google Scholar]
- 16.Griffin JB, Ridgeway K, Montgomery E, et al. Vaginal ring acceptability and related preferences among women in low- and middle-income countries: a systematic review and narrative synthesis. PLoS One. 2019;14:e0224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNAIDS. A Condom Crisis at the Centre of the HIV Prevention Crisis [Internet]. 2018. Available at: https://www.unaids.org/en/resources/presscentre/featurestories/2018/july/20180723_condoms-AIDS2018. Accessed March 24, 2020. [Google Scholar]
- 18.Laborde ND, Pleasants E, Reddy K, et al. Impact of the dapivirine vaginal ring on sexual experiences and intimate partnerships of women in an HIV prevention clinical trial: managing ring detection and hot sex. AIDS Behav. 2018;22:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]