Abstract
BACKGROUND
Despite improvements in treatment, prostate cancer (PC) remains the second-leading cause of cancer death in men. Radiotherapy is among the first-line treatments for prostate cancer, but a significant number of patients relapse. Recent evidence supports the idea that PC is initiated by a subset of cells, termed cancer stem cells (CSCs). CSCs have also been implicated in radioresistance in various malignancies, but their role in PC has not yet been investigated.
METHODS
We compared the relative radiosensitivity of isolated CSCs to the total population of their corresponding cell lines, and examined the relative numbers of CSCs in irradiated cell lines following long-term recovery and in recurrent human prostate cancer.
RESULTS
Here, we show that while irradiation does not immediately favor increased survival of CSCs, irradiated PC cell lines showed an increase in CSC properties with long-term recovery. These data suggest that, although CSCs are initially damaged by radiation, they possess a greater capacity for recovery and regrowth.
CONCLUSIONS
The combination of radiotherapy with a CSC-targeted therapeutic strategy may prevent tumor recurrence.
Keywords: prostate cancer, cancer stem cells, radiotherapy, resistance
INTRODUCTION
Prostate cancer (PC) is the most common malignant neoplasm in men in developed countries [1]. Early detection of tumors by screening for prostate specific antigen (PSA) and improved treatments have greatly improved the 5-year disease-specific survival rate for PC [1]. Despite the improved survival rates, PC remains the second-leading cause of cancer death, producing an estimated 32,050 deaths in 2010 in the United States [2]. Localized PC is treated by surgery or radiation therapy. Following radiation, however, 20%–50% of patients experience biochemical failure, suggesting a significant proportion of patients will have recurrence and eventually die of the disease [3–5].
The cancer stem cell (CSC) model has gained increasing recent attention. According to this model, tumors are initiated from, and maintained by, a subpopulation of tumor cells. The defining properties of CSCs are self-renewal and differentiation, resulting in the generation of heterogeneous tumor cells [6,7]. CSCs have been isolated from a variety of different tumors [8–12], including prostate cancers [13,14].
Various CSC surface markers, including CD44, CD133 and integrin α2β1, are used alone or in combination to isolate prostate CSCs (pCSCs) [13–16]. Using these markers, we and others have shown that CSCs express high levels of stemness genes, and exhibit enhanced proliferative potential, self-renewal capacity, and tumorigenicity compared to non-CSC populations. Furthermore, isolated CSCs grow as prostatospheres when cultured under non-adherent conditions in defined media [13,14]. Prostatospheres, which are generated from rare cells (0.1%–8% of the total cell population), also demonstrate self-renewal and high proliferative potential (up to eight passages in culture) [17]. Moreover, we have recently shown that prostatospheres isolated from the prostate cancer cell lines, LNCaP and DU-145, or from patient samples by growth under non-adherent conditions are more tumorigenic than total cell populations [18].
CSCs in various organs, including breast, brain and stomach, are more resistant to radiation therapies than non-CSCs [19–22]. Breast CSCs, characterized by CD44 expression and low or absent CD24 expression (CD24−/low/CD44+), show greater clonogenic survival than total cell populations after irradiation [22]. In glioblastoma, CD133+ CSCs are dramatically increased after irradiation, and radioresistant glioblastomas exhibit a higher percentage of CD133-expressing CSCs [19]. Brain CSCs preferentially activate the DNA damage checkpoint in response to radiation and repair radiation-induced DNA damage more effectively than non-CSCs [19,20]. In atypical teratoid/rhabdoid brain tumors, the number of CD133+ cells is positively correlated with the degree of radioresistance [21]. In contrast, recent studies have suggested that radioresistance may not be a general property of CSCs [23,24]; among CSCs isolated from nine cell lines of brain, breast, colon, and pancreas cancers by fluorescence-activated cell sorting (FACS), only those from one breast cancer cell line (MDA-MB-231) showed radioresistance [23]. However, the radioresistance of prostate CSCs (pCSCs) has not yet been reported.
MATERIALS AND METHODS
Cell Culture and Prostatosphere Preparation
The PC cell lines LNCaP and DU-145 from American Type Culture Collection (Manassas, VA, USA) were maintained according to the supplier’s instructions. Prostatospheres were generated according to a previously described method [18], which included B-27 supplementation (Gibco, Grand Island, NY, USA). Prostatospheres of LNCaP and DU-145 cells were generated by culturing for 6 and 8 days, respectively.
Prostate Cancer Patients and Pathology
This study was approved by the Asan Medical Center Institutional Review Board. Tumor samples were obtained from prostate cancer patients who had received radiation therapy to the prostate at the Department of Radiation Oncology at Asan Medical Center. The primary inclusion criterion was patients whose tumor tissues had been harvested for pathologic examination before and after radiation therapy since the opening of the hospital (June 1986 to November 2010). Tissue samples were formalin fixed and paraffin embedded. Each tumor was graded and staged according to the Gleason system [25] and the 2010 anatomic stage/prognostic grouping proposed by the American Joint Committee on Cancer [26], respectively.
Irradiation
Prostate cancer cells were irradiated in a Mark I 68-A irradiator at a dose of approximately 1.8 Gy/min for the time required to generate the indicated doses. Corresponding controls were sham-irradiated. In experiments using multiple doses of irradiation, DU-145 and LNCaP cells were irradiated for 5 consecutive days with the therapeutic radiation dose of 2 Gy. Following the final dose of radiation, the cultures were either plated for measurement of growth, expression of CSC markers and biological properties (Fig. 3), or allowed to recover for 33–35 days and then assessed (Fig. 4).
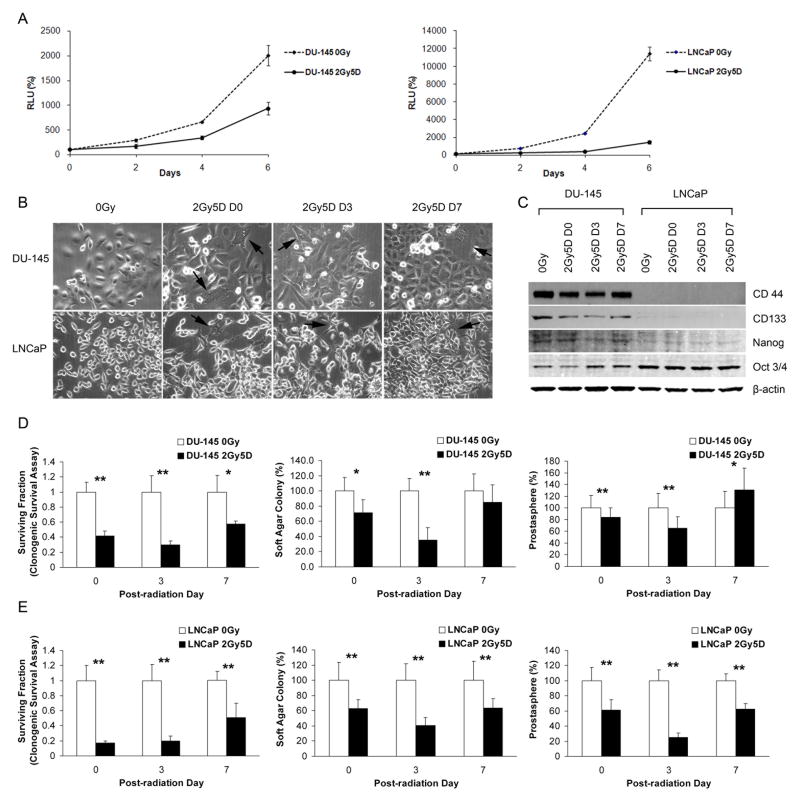
Fig. 3.
CSC properties of PC cell lines shortly after irradiation with daily 2 Gy doses for 5 days (2Gy5D). (A) Growth rates following irradiation, normalized to day 0. Bars represent means ± SDs. (B) Phase-contrast images of radioresistant DU-145 and LNCaP cultures, photographed on the indicated days. Large, elongated or stellate, multinucleated cells are noted (arrows). (Original magnification: x200) (C) Western blot analyses of CD44, CD133, Nanog, and Oct 4. (D and E) Clonogenic survival (left column), soft-agar (middle column), and prostatosphere (right column) assays were performed using irradiated DU-145 (D) and LNCaP (E) cells. All values are normalized to non-irradiated controls (0 Gy). Bars represent means ± SDs (*p < 0.05; **p < 0.001).
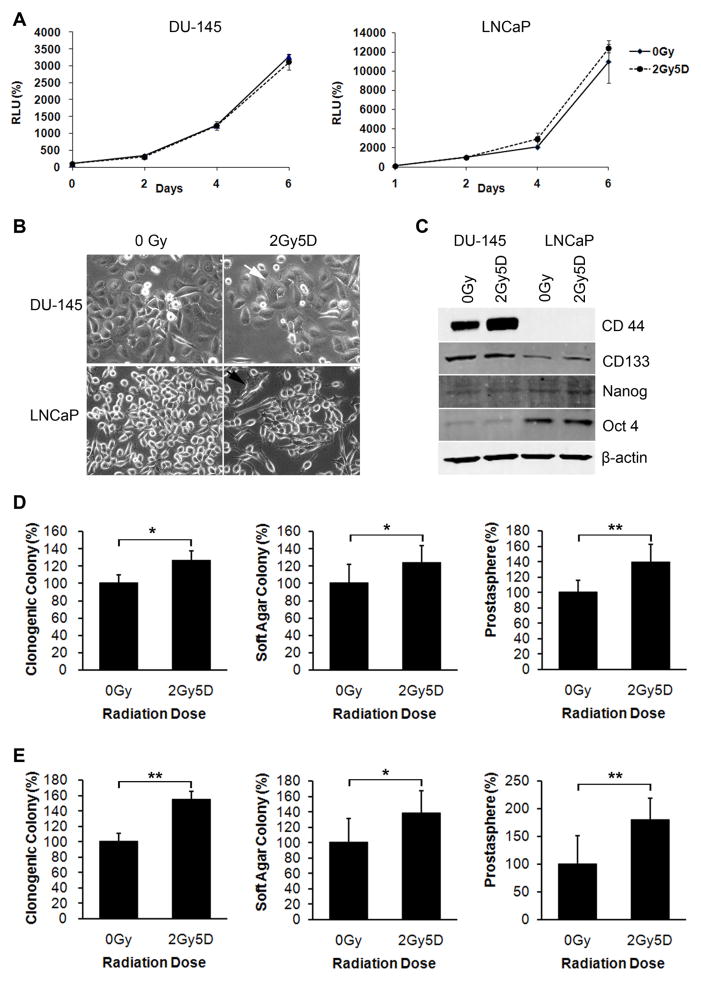
Fig. 4.
CSC properties of recurrent PC cells. (A) Growth rates of recurrent DU-145 and LNCaP cells following irradiation with daily 2-Gy doses for 5 days (2Gy5D). Data is normalized to day 0. Bars represent means ± SDs. (B) Elongated or stellate, multinucleated giant cells (arrows) in recurrent DU-145 and LNCaP cultures. (Original magnification: x200) (C) Western blot analyses of CD44, CD133, Nanog, and Oct-3/4 in recurrent DU-145 and LNCaP cells. (D and E) Clonogenic survival (left column), soft-agar (middle column), and prostatosphere (right column) assays were performed using recurrent DU-145 (D) and LNCaP (E) cells. Bars represent means ± SDs (*p < 0.05; **, p < 0.001).
Prostatosphere Quantification
Single cells were obtained from prostatospheres, generated as described above, by enzymatic dissociation with trypsin-EDTA. Spheres were counted using a GelCount colony counter (Oxford Optronix, Oxford, UK). Each experiment was repeated at least three times.
Flow Cytometry
FACS was performed as previously described [14]. Since most DU-145 cells express CD44, the brightest 5% of cells were sorted for CD44bright and the dimmest 5% (including those negative for CD44) were sorted for CD44low/negative.
Immunocytochemistry for γ-H2AX Foci
Two hours after irradiation, cells grown on chamber slides (Nalge Nunc International Inc, Naperville, IL, USA) were fixed in 2% formaldehyde for 10 minutes, washed twice in phosphate-buffered saline (PBS), then blocked with PBS/10% fetal bovine serum (FBS). After the blocking, slide-mounted cells were incubated for 1 hour with an anti-phospho-histone H2A.X (Ser 139) antibody (Millipore, Temecula, CA, USA), diluted 1:100 in PBS/10% FBS containing 0.1% saponin, followed by three washes with PBS/10% FBS. Slides were then incubated with secondary antibody for 1 hour, washed three times in PBS/10% FBS, mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc. Burlingame, CA, USA), and visualized with a fluorescence microscope (Axiovert 200M; Carl Zeiss, Thornwood, NY, USA). Nuclei containing ≥10 immunoreactive foci were considered positive, as previously described [27].
Cell Growth
Cell growth was measured by trypan blue exclusion or using a CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Cells were plated in triplicate at 3,350 cells/well in 24-well plates (trypan blue exclusion) or at 500 cells/well in 96-well plates (CellTiter-Glo). Each experiment was repeated three times.
Clonogenic Survival
A known number of cells were plated in triplicate in 6-well plates. After 7–11 days in culture, colonies with >50 cells were counted with a GelCount colony counter (Oxford Optronix, Oxford, UK). Clonogenic surviving fraction (SF) was calculated as follows: SF = (number of colonies formed at a given dose/number of cells plated at a given dose) x (control number of cells plated/control number of colonies formed) [28]. Each experiment was repeated three times.
Growth in Soft Agar
Anchorage-independent growth was studied by performing soft-agar assays using CytoSelect (Cell Biolabs, Inc. San Diego, CA, USA) according to the manufacturer’s instruction, with minor modification. Cells (200 cells/well) were plated in six replicates in 96-well plates. After 7–14 days, colonies were counted with a GelCount colony counter (Oxford Optronix). Each experiment was repeated at least three times.
Western Blotting
Total protein was extracted with RIPA buffer (Thermo Scientific, Rockford, IL, USA) and measured using a BCA Kit (Thermo Scientific). Proteins were resolved on 4%–20% Tris-Glycine gels (Invitrogen, Carlsbad, CA, USA) and transferred to polyvinylidene fluoride membranes. Membranes were blocked in 5% nonfat dry milk with 0.1% Tween-20 (PBST) for 1 hour, and then incubated with primary antibodies at 4°C overnight. After washing three times with PBST, membranes were incubated with secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) for 1 hour, and then scanned (Odyssey IR Imaging System; LI-COR Biosciences). The following primary antibodies were used: anti-CD44 (AbD Serotec, Raleigh, NC, USA), anti-CD133 (Abcam, Cambridge, MA, USA), anti-Oct4 (Novus Biologicals, Littleton, CO, USA), anti-Nanog (Abcam), anti-rH2AX (Millipore), and anti-β-actin (Abcam).
Immunohistochemistry
Human prostate cancer tissues harvested before and after radiation were compared for the expression of CD44 and CD133 by immunohistochemistry. Immunohistochemical staining was performed using an autostainer (Ventana Medical Systems, Inc., Tucson, AR, USA). The primary antibodies used in this investigation were CD44 (standard form, 1:100 dilution; Dako, Glostrup, Denmark) and CD133 (CD133/1 [AC133], 1:50 dilution; Miltenyi Biotec, Auburn, CA, USA). Nonspecific immunopositivity was reduced using an endogenous biotin blocking kit (Ventana Medical Systems Inc., Tucson, AR, USA). Diaminobenzidine was used as a chromogen, and the tissues were counterstained with hematoxylin. Normal urothelium from the urinary bladder and normal kidney were used as positive controls for CD44 and CD133, respectively. Negative controls omitting primary antibodies were included. The percentage of cells positive for membrane expression of CD133 or CD44 was measured by manual counting of immunostained cells under a microscope.
Statistical Analysis
Results were expressed as means ± SDs of at least triplicate determinations. Statistical outliers, determined using Grubb’s test (α = 0.05; calculated at http://www.graphpad.com/quickcalcs/Grubbs1.cfm), were removed from the analysis. Statistical comparisons were based on two-tailed Student’s t-tests. Differences were regarded as statistically significant at p-values < 0.05.
RESULTS
PC Cell Lines Regenerate After High-Dose Irradiation
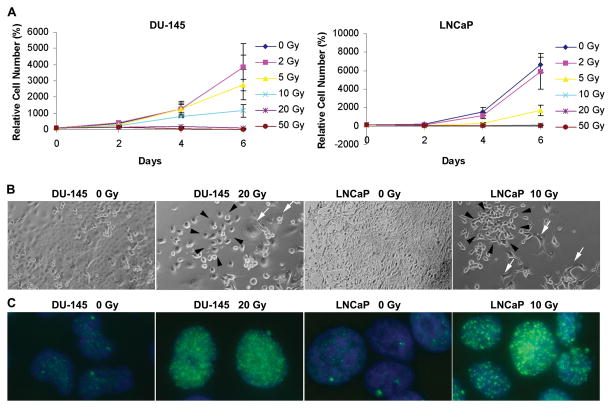
To determine radiation sensitivity, we treated DU-145 and LNCaP with different, single doses of radiation and then determined viable cells at multiple time points. The numbers of viable cells were reduced in a dose-dependent manner (Fig. 1A) whereas those of non-viable cells were increased (Supplemental Fig. 1). LNCaP cells were more sensitive to radiation than DU-145 cells. A small number of repopulating colonies were observed in LNCaP and DU-145 cultures irradiated with 10 Gy and 20 Gy, respectively (Fig. 1B). Neither cell line formed repopulating colonies at 50 Gy. Therefore, doses up to 10 Gy for LNCaP and 20 Gy for DU-145 were chosen.
Fig. 1.
Radiation-induced growth inhibition and DNA damage. (A) Viable cell numbers of LNCaP and DU-145 cells following irradiation, normalized to the number of cells plated. (B) Phase-contrast images of irradiated DU-145 and LNCaP cultures. Note repopulating colonies (black arrows) and degenerating cells (white arrows). (Original magnification: x100) (C) Immunocytochemistry of radiation-induced γ-H2AX foci (green), 2 hours post-irradiation. Images from DU-145 and LNCaP cells irradiated with 20 and 10 Gy, respectively. (Original magnification: x 600.)
To confirm radiation-induced DNA damage, we examined formation of γ-H2AX foci, which represent DNA double-strand breaks induced by irradiation [29], 2 hours after radiation. As expected, irradiation markedly increased γ-H2AX foci in cultures of DU-145 and LNCaP cells (Fig. 1C). Taken together, these data indicate that a small number of cells survive high-dose irradiation, despite the persistence of DNA damage 2 hours after irradiation.
CSCs Isolated from Prostate Cancer Cell Lines Show Variable Responses to Irradiation
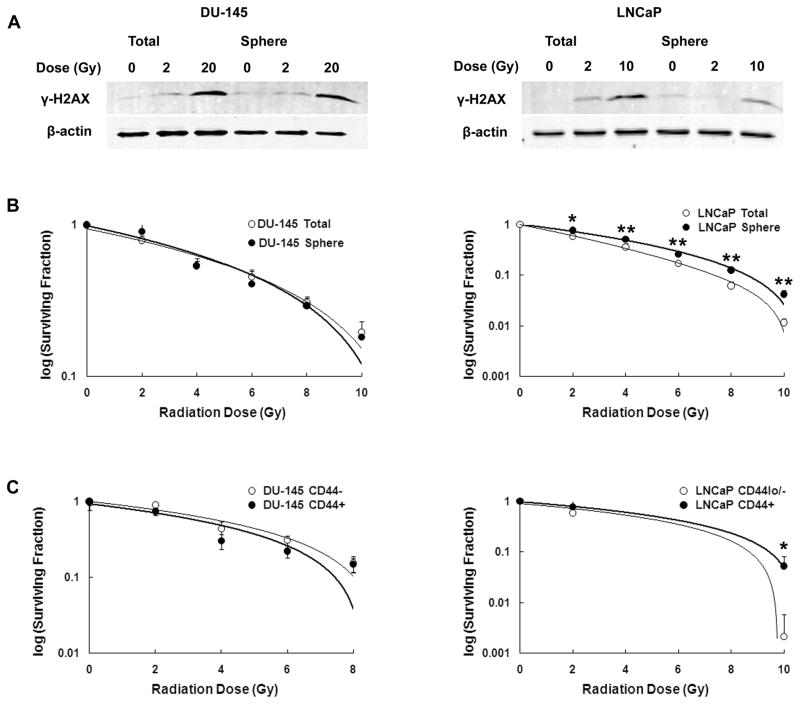
To determine whether radiation induces DNA damage to a similar extent in pCSCs and non-CSCs, we measured γ-H2AX phosphorylation in prostatospheres and total cell populations after a single dose of radiation (Fig. 2A and Supplemental Fig. 2). Spheres isolated from the LNCaP cell line showed less DNA damage than the total cell population, suggesting that they are more resistant to irradiation-induced damage. However, for DU-145, there was no difference in γ-H2AX levels in prostatospheres compared to the total cell population.
Fig. 2.
Radiation effects on pCSCs. (A) Western analysis of γ-H2AX. (B) Clonogenic survival fractions of total cell populations and prostatospheres of irradiated DU-145 and LNCaP cells. (C) Clonogenic cell-survival fractions of CD44low/− and CD44hi/+ populations (LNCaP and DU-145) irradiated with the indicated doses. Bars represent means ± SDs (*p < 0.05; **p < 0.001).
To determine if prostatospheres were more radioresistant than total cell populations, we performed clonogenic cell survival assays [30]. These assays demonstrated dose-dependent reductions in clonogenic survival in both total cell populations and prostatospheres of DU-145 and LNCaP after irradiation. Consistent with the decreased level of DNA damage following radiation, LNCaP prostatospheres showed higher survival fractions than the total cell population at all doses examined (Fig. 2B). However, no differences in survival fractions between the total cell population and prostatospheres were noted for the DU-145 cell line at any radiation dose.
Because proliferation following irradiation is mediated, in part, by the epidermal growth factor receptor (ErbB) signaling pathway [31] and EGF receptor levels are increased in recurrent PC following irradiation [32], we tested if EGF present in the defined media used to grow prostatospheres could account for their increased radioresistance. Culturing LNCaP prostatospheres in the absence of EGF did not decrease their radioresistance (Supplemental Fig. 3), indicating that the increased radioresistance of prostatospheres is not attributable to the EGF signaling pathway.
In addition to their propensity to form prostatospheres, CSCs can be identified by their high level of CD44 expression. Therefore, we determined clonogenic survival in irradiated CD44hi/+ and CD44lo/− populations. Similar to the results of prostatosphere experiments, LNCaP CD44hi/+ cells showed a statistically significant increase in survival fraction compared to CD44lo/− cells following irradiation with 10 Gy (Fig. 2C). Again, DU-145 cells showed no difference in survival fraction between putative CSCs (CD44hi/+) and more differentiated (CD44lo/−) cells. These results suggest that whereas some CSCs may be more resistant to radiation, this may not be a general phenomenon.
CSC Properties Are Not Enhanced Immediately Following Irradiation
To mimic radiotherapeutic schedules of prostate cancer patients, we irradiated total PC cell lines with 2 Gy daily for 5 days. In contrast to a single dose of 2 Gy irradiation (Fig. 1A), daily irradiation for 5 days with 2 Gy markedly reduced the growth rates of DU-145 and LNCaP cells (Fig. 3A). Compared to non-irradiated controls, irradiated DU-145 and LNCaP cells were modestly enlarged with occasional cells showing cytoplasmic processes and multinucleation. Morphological changes were most prominent on the day of the last irradiation dose (day 0 (D0), Fig. 3B).
If CSCs are more radioresistant than non-CSCs, the relative abundance of CSCs should increase following irradiation. To determine if the proportion of CSCs was higher following irradiation, we measured expression of the stem cell surface markers, CD44 and CD133, as well as the stemness-related transcription factors, Nanog and Oct-3/4, in DU-145 and LNCaP cells. In DU-145 cells, the expression of CD44 and CD133 in irradiated cells decreased immediately following irradiation (D0) compared to non-irradiated controls, was further reduced on day 3 (D3), but recovered on day 7 (D7) (Fig. 3C). However, Nanog and Oct-3/4 expression were not altered in DU-145 or LNCaP cells. Levels of CD44 and CD133 expression in LNCaP cells were too low to be evaluated by Western blotting (Fig. 3C).
We further investigated the relative numbers of CSCs by determining the biological properties of clonogenic survival, sphere formation, and anchorage-independent growth. Similar to the Western blot results obtained with surface markers, there was an initial decrease followed by partial to complete recovery at 7 days. Specifically, irradiated DU-145 cells showed smaller clonogenic surviving fractions than non-irradiated controls during the 7-day period (Fig. 3D). Prostatosphere-forming ability and anchorage-independent growth of irradiated DU-145 cells were decreased until day 3, then increased on day 7 to a value higher than (prostatosphere-forming ability) or equal to (anchorage-independent growth) that of non-irradiated controls. Compared to non-irradiated controls, values for irradiated LNCaP cells were smaller but followed similar trends to those of DU-145 cells in clonogenic survival, prostatosphere, and soft agar assays during the 7-day period (Fig. 3E).
Taken together, these results suggest that the CSC population, while initially decreased as determined by both surface marker expression as well as biological activity, was nearly restored or slightly elevated at 7 days following irradiation compared to non-irradiated cells.
Long-Term Recovery Following Irradiation Leads to Increased Levels of CSCs in PC Cell Lines
The greatest risk of PC recurrence is 1.5–3.5 years after radiotherapy, which may be the period of time required to establish the proper microenvironment for tumor regrowth and the regeneration of a tumor mass from a small initial number of radioresistant cells [5]. To study the effects of recovery and regrowth following irradiation, we irradiated DU-145 and LNCaP cells for 5 days at a dose of 2 Gy and then allowed cells to recover. Irradiated DU-145 and LNCaP cultures initially showed marked cell death, but a few repopulating colonies, termed recurrent, developed approximately 22 days post-irradiation, and cultures were fully repopulated after 33–35 days.
The growth rates measured after full repopulation of DU-145 and LNCaP cells were similar to those of the non-irradiated controls (Fig. 4A). Even more than 1 month after irradiation, radiation-induced morphological changes persisted in recurrent DU-145 and LNCaP cells (Fig. 4B).
To determine the percentage of CSCs in recurrent cultures, we examined the levels of CSC markers in these cells. At the protein level, a marked increase in CD44 expression was observed in recurrent DU-145 cultures compared to that in the non-irradiated controls (Fig. 4C). Similar to the expression patterns observed immediately following irradiation, Nanog and Oct-3/4 expression was unchanged in recurrent DU-145 cells compared to that in the non-irradiated controls. Recurrent LNCaP cells exhibited slightly increased CD133 expression and no difference in Nanog and Oct-3/4 expression (Fig. 4C).
Multiple doses of radiation not only resulted in an increase in CSC surface marker expression, but also enhanced the biological properties of CSCs (Fig. 4D and 4E). Irradiation resulted in increases in clonogenic survival, prostatosphere formation, and anchorage-independent growth in recurrent DU-145 and LNCaP cells (Fig. 4D and E).
Taken together, these results demonstrate that CSC properties are enhanced in recurrent PC cell lines after long-term recovery.
Radiotherapy Increases the CD44+ Cell Population in Prostate Cancer Patients
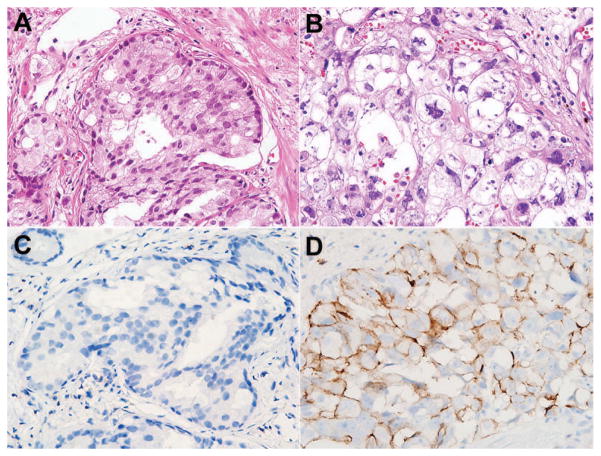
To extend the in vitro results to primary human prostate cancer, we evaluated the expression of stem cell markers in patients with cancer recurrence following radiation therapy. Because post-radiation biopsy is not a routine procedure, we were able to identify only five qualifying cases out of the 643 prostate cancer patients treated with radiation therapy at the Asan Medical Center in 24 years. On pathologic examination, these tumors were all acinar adenocarcinomas with Gleason scores of 7 to 9 at the time of diagnosis (Fig. 5A). In all cases, radiation therapy induced changes in tumor cell morphology: loss of glandular pattern; abundant, pale, vacuolated cytoplasm; nuclear pleomorphism; pyknosis; and chromatin clumping (Fig. 5B). CD44+ tumor cells were not identified in pre-radiation biopsies (Fig. 5C); however, three cases showed CD44+ tumor cells in post-radiation biopsies with a wide range of expression (2%–35%; Fig. 5D and Table 1). Interestingly, the two cases with the highest CD44 expression had accompanying distant organ metastases. None of the pre- or post-radiation biopsies expressed CD133.
Fig. 5.
Radiation effects on morphology and CD44 expression in primary human prostate cancer (case 5). Compared to the prostate cancer before radiotherapy (A), radiation-induced atypia, pleomorphic hyperchromatic nuclei, and pale voluminous cytoplasm are evident in the tumor biopsied after radiation (B). No CD44-expressing tumor cells were detected in the prostate cancer tissues before radiation (C), but is strongly expressed at the cell membrane of tumor cells after radiation (D). (A and B, hematoxylin-eosin stain; C and D, immunohistochemistry; original magnification, x400.)
Table 1.
Clinicopathologic Features of Primary Prostatic Adenocarcinoma Patients Before and After Radiation
| Pre-radiation biopsy
|
Radiation (Gy) |
Post-radiation biopsy
|
Metastasis | Follow up |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age (years) |
PSA (ng/ml) |
Tumor stage |
Prognostic group |
Gleason score |
CD44 (%) |
CD133 (%) |
Age (years) |
PSA (ng/ml) |
CD44 (%) |
CD133 (%) |
|||
| 1 | 66 | 10.4 | T2c | IIB | 7 | 0 | 0 | 69 | 71 | 9.1 | 0 | 0 | None | 13 Mo, AWD* |
| 2 | 61 | 43.5 | T3 | III | 9 | 0 | 0 | 76 | 64 | 7.5 | 0 | 0 | None | 20 Mo, AWD* |
| 3 | 57 | 21.6 | T2b | IIB | 7 | 0 | 0 | 65 | 60 | 3.2 | 2 | 0 | None | 5 Mo, AWOD* |
| 4 | 78 | 9.8 | T2c | IIB | 7 | 0 | 0 | 60 | 82 | 17.2 | 10 | 0 | Bone | 36 Mo, AWD* |
| 5 | 63 | 14.5 | T4 | IV | 8 | 0 | 0 | 70 | 67 | 3.1 | 35 | 0 | Bone, liver, lung | 6 Mo, DOD* |
Abbreviations: PSA, prostate specific antigen; AWD, alive with disease; AWOD, alive without disease; DOD, dead of disease.
DISCUSSION
Because we did not measure the repair capacity of pCSCs directly, the radioresistance mechanism cannot be defined yet. However, it appears that low radiosensitivity and high repair capacity of pCSCs contribute to the increase of pCSCs following long-term recovery. LNCaP pCSCs enriched for by isolation of both prostatospheres and CD44 positivie cells were less damaged by radiation as shown by low γ-H2AX phosphorylation and the high clonogenic survival as compared to total cells (Fig. 2). If low radiosensitivity was the only mechanism leading to increased survival, the relative abundance of CSCs should increase immediately after irradiation. Instead, the CSC properties were decreased immediately after radiation (Fig. 3) but increased after long-term recovery (Fig. 4 and Fig. 5). This suggests that radiation initially damages pCSCs but they are better able to recover from radiation-induced damage suggesting a greater DNA repair capacity. However, further experiments will need to be conducted to directly determine if the pCSCs do contain increased DNA-repair capacity.
Similarly, in glioblastoma, Bao et al. observed initial DNA damage in both CSCs and non-CSCs after radiation, but found that CSCs possessed a preferential ability to repair this damage and exhibited an overall survival advantage [19]. Although further experiments are necessary to determine if DNA repair mechanisms are increased in prostate CSCs, our evidence indicates that CSCs recover from radiation and are increased in recurrent cultures and human prostate cancer tissues.
Immediately following irradiation, the CSC properties of DU-145 and LNCaP cells were reduced maximally on day 3 (Fig. 3). This phenomenon might be partly explained by radiation-induced mitotic death. Several mechanisms have been implicated in mitotic death, such as the failure of spindle formation in M phase, loss of the G2 checkpoint leading to “mitotic catastrophe,” or improper chromosome segregation due to damage and loss of genetic material [33]. As mitotic death by its very nature is normally delayed, occurring over a period of days, it may be responsible for the low CSC properties on day 3 after irradiation [33].
Interestingly, the radioresistance of isolated CSCs was not uniformly higher than that of the total cell population (Fig. 2). CSCs isolated from DU-145 cells through sorting of CD44-expressing cells or prostatosphere formation did not show increased radioresistance compared to the total cell population. However, LNCaP CSCs, isolated by either method, were more radioresistant than the total cell population. This discrepancy may be due to the isolation methods chosen, intrinsic differences in the CSCs themselves, or the result of comparing purified CSCs to the total cell population in cell lines that have vastly different percentages of CSCs. As we have demonstrated previously, LNCaP cells have a very low percentage of CD44+ cells (approximately 0.04%) whereas greater than 70% of DU-145 cells express CD44 [14]. Therefore, a difference in the radiation-sensitivity of CSCs compared to the total cell population may not be readily apparent in the DU-145 cell line, where 70% of the total cell line has the CSC phenotype. In contrast, the difference in radiation resistance would be more apparent in a comparison of LNCaP CSCs to a total population where only 0.04% of the population has the CSC phenotype. Alternatively, there may be intrinsic differences in the CSCs isolated from LNCaP and DU-145 cultures. These cell lines represent hormone-sensitive and hormone-refractory prostate cancer, respectively; thus, there might be a biological difference in CSCs due to differences in hormone-responsivity status. Additional experiments will be needed to test this possibility. Despite the differences observed in isolated CSCs, both cell lines showed an increase in the CSC phenotype after long-term recovery (Fig. 4).
Previous studies have shown that decreased CD44 expression correlates with an unfavorable prognosis, with frequent postoperative PSA recurrence and poor survival [27,34,35]. However, a recent study reported an increase in CD44+ tumor cells in metastatic prostate cancer tissues compared to matched primary tumors [36]. In our study, the five cases for which before and after samples were available were recurrent tumors belonging to the poor prognostic group. CD44 expression was not detected initially in the five patients. However, CD44+ cells were evident post-radiation therapy, with significant CD44 expression in three cases accompanying distant organ metastasis. This finding is in accord with previous studies and supports the CSC population-increasing effect of radiation, although further studies with a larger number of patients will be required to confirm this. None of the five cases expressed CD133, suggesting that the CD133-positive CSC population in primary prostate cancer tissues is too rare or CD133 expression is too low to be detected by immunohistochemistry.
The recovery of the CSC population and the increase in the CSC phenotype/biological activity has important therapeutic implications. Because irradiation does not eliminate the CSC population, a combination of radiotherapy and a CSC-targeting agent may provide the maximum benefit to patients.
CONCLUSIONS
Irradiation of PC cell lines with doses currently employed in the clinic leads to increased numbers of CSCs upon long-term recovery. We also determined that pCSCs are initially damaged by irradiation and show a recovery period of approximately 1 week, during which time properties of self-renewal and anchorage-independent growth are reduced. Taken together, our data indicate that a combinatorial approach using irradiation and a CSC-targeted therapeutic, immediately following irradiation, may be advantageous.
Supplementary Material
Phase-contrast images and the numbers of non-viable DU-145 and LNCaP cells after radiation. Before and after radiation with a single dose of 2 or 20 Gy, phase-contrast images (A and C) and the numbers of non-viable (B and D) DU-145 (A and B) and LNCaP cells (C and D) were obtained at various time points (0, 2, 12, 24, and 48 h) in high-power fields (magnification, ×400). Representative degenerating non-viable cells are demarcated by arrows.
γ-H2AX levels were examined by Western blotting at two time points (2 and 6 h) after administering single radiation dose of 20 Gy. γ-H2AX expression was increased at 2 h after irradiation compared to that in non-radiated cells (0 h) but decreased at 6 h after irradiation.
Epidermal growth factor does not contribute to increased radioresistance of LNCaP spheres. Clonogenic survival fractions for LNCaP prostatospheres grown in the presence of EGF (●) or absence of EGF (○).
Acknowledgments
Grant sponsor: NCI, NIH; Grant number: N01-CO-12400 and intramural research fund; Grant sponsor: National Research Foundation of Korea (NRF); Grant number: 2010-0013063.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Conflicts of Interest Notification: The authors report no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr, Miller DW, Adams JA, Shipley WU. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen PL, D’Amico AV, Lee AK, Suh WW. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer. 2007;110:1417–1428. doi: 10.1002/cncr.22941. [DOI] [PubMed] [Google Scholar]
- 5.Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, Michalski JM, Pisansky TM, Sandler HM, Shipley WU, Zelefsky MJ, Zietman AL. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. International journal of radiation oncology, biology, physics. 2003;57:915–928. doi: 10.1016/s0360-3016(03)00632-1. [DOI] [PubMed] [Google Scholar]
- 6.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer research. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 7.Ward RJ, Dirks PB. Cancer stem cells: at the headwaters of tumor development. Annual review of pathology. 2007;2:175–189. doi: 10.1146/annurev.pathol.2.010506.091847. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 9.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer research. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 10.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 12.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 13.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 14.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. British journal of cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer research. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 16.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer research. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell research. 2009;19:683–697. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 18.Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL. Genomic profiling of tumor initiating prostatospheres. BMC Genomics. 2010;11:324. doi: 10.1186/1471-2164-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 20.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes & development. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiou SH, Kao CL, Chen YW, Chien CS, Hung SC, Lo JF, Chen YJ, Ku HH, Hsu MT, Wong TT. Identification of CD133-positive radioresistant cells in atypical teratoid/rhabdoid tumor. PloS one. 2008;3:e2090. doi: 10.1371/journal.pone.0002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. Journal of the National Cancer Institute. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 23.Al-Assar O, Muschel RJ, Mantoni TS, McKenna WG, Brunner TB. Radiation response of cancer stem-like cells from established human cell lines after sorting for surface markers. International journal of radiation oncology, biology, physics. 2009;75:1216–1225. doi: 10.1016/j.ijrobp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Dittfeld C, Dietrich A, Peickert S, Hering S, Baumann M, Grade M, Ried T, Kunz-Schughart LA. CD133 expression is not selective for tumor-initiating or radioresistant cell populations in the CRC cell lines HCT-116. Radiother Oncol. 2009 doi: 10.1016/j.radonc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. The American journal of surgical pathology. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 26.Edge SB, Fritz AG, Byrd DR, Greene FL, Compton CC, Trotti A. AJCC Cancer Staging Manual. 7. New York: Springer-Verlag; 2010. [Google Scholar]
- 27.Aaltomaa S, Lipponen P, Ala-Opas M, Kosma VM. Expression and prognostic value of CD44 standard and variant v3 and v6 isoforms in prostate cancer. Eur Urol. 2001;39:138–144. doi: 10.1159/000052428. [DOI] [PubMed] [Google Scholar]
- 28.Hermann RM, Wolff HA, Jarry H, Thelen P, Gruendker C, Rave-Fraenk M, Schmidberger H, Christiansen H. In vitro studies on the modification of low-dose hyper-radiosensitivity in prostate cancer cells by incubation with genistein and estradiol. Radiation oncology (London, England) 2008;3:19. doi: 10.1186/1748-717X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.PUCK TT, MARCUS PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. Methods in molecular medicine. 2005;110:21–28. doi: 10.1385/1-59259-869-2:021. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Ullrich RK, Contessa JN, Dent P, Mikkelsen RB, Valerie K, Reardon DB, Bowers G, Lin PS. Molecular mechanisms of radiation-induced accelerated repopulation. Radiation oncology investigations. 1999;7:321–330. doi: 10.1002/(SICI)1520-6823(1999)7:6<321::AID-ROI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Skvortsova I, Skvortsov S, Stasyk T, Raju U, Popper BA, Schiestl B, von Guggenberg E, Neher A, Bonn GK, Huber LA, Lukas P. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics. 2008;8:4521–4533. doi: 10.1002/pmic.200800113. [DOI] [PubMed] [Google Scholar]
- 33.Halperin EC, Perez CA, Brady LW, Wazer DE, Freeman C, Prosnitz LR. Perez and Brady’s Principles and Practice of Radiation Oncology. 5. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.Kallakury BV, Sheehan CE, Ross JS. Co-downregulation of cell adhesion proteins alpha- and beta-catenins, p120CTN, E-cadherin, and CD44 in prostatic adenocarcinomas. Hum Pathol. 2001;32:849–855. doi: 10.1053/hupa.2001.26463. [DOI] [PubMed] [Google Scholar]
- 35.Vis AN, Noordzij MA, Fitoz K, Wildhagen MF, Schroder FH, van der Kwast TH. Prognostic value of cell cycle proteins p27(kip1) and MIB-1, and the cell adhesion protein CD44s in surgically treated patients with prostate cancer. J Urol. 2000;164:2156–2161. [PubMed] [Google Scholar]
- 36.Eaton CL, Colombel M, van der Pluijm G, Cecchini M, Wetterwald A, Lippitt J, Rehman I, Hamdy F, Thalman G. Evaluation of the frequency of putative prostate cancer stem cells in primary and metastatic prostate cancer. Prostate. 2010;70:875–882. doi: 10.1002/pros.21121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phase-contrast images and the numbers of non-viable DU-145 and LNCaP cells after radiation. Before and after radiation with a single dose of 2 or 20 Gy, phase-contrast images (A and C) and the numbers of non-viable (B and D) DU-145 (A and B) and LNCaP cells (C and D) were obtained at various time points (0, 2, 12, 24, and 48 h) in high-power fields (magnification, ×400). Representative degenerating non-viable cells are demarcated by arrows.
γ-H2AX levels were examined by Western blotting at two time points (2 and 6 h) after administering single radiation dose of 20 Gy. γ-H2AX expression was increased at 2 h after irradiation compared to that in non-radiated cells (0 h) but decreased at 6 h after irradiation.
Epidermal growth factor does not contribute to increased radioresistance of LNCaP spheres. Clonogenic survival fractions for LNCaP prostatospheres grown in the presence of EGF (●) or absence of EGF (○).