Abstract
Current understanding of the impact of coronavirus disease-2019 (COVID-19) on arrhythmias continues to evolve as new data emerge. Cardiac arrhythmias are more common in critically ill COVID-19 patients. The potential mechanisms that could result in arrhythmogenesis among COVID-19 patients include hypoxia caused by direct viral tissue involvement of lungs, myocarditis, abnormal host immune response, myocardial ischemia, myocardial strain, electrolyte derangements, intravascular volume imbalances, and drug sides effects. To manage these arrhythmias, it is imperative to increase the awareness of potential drug-drug interactions, to monitor QTc prolongation while receiving COVID therapy and provide special considerations for patients with inherited arrhythmia syndromes. It is also crucial to minimize exposure to COVID-19 infection by stratifying the need for intervention and using telemedicine. As COVID-19 infection continues to prevail with a potential for future surges, more data are required to better understand pathophysiology and to validate management strategies.
Key Words: arrhythmias, channelopathies, COVID-19, myocarditis, QT prolongation, SARS-CoV-2, torsades de pointes
Abbreviations and Acronyms: AAD, antiarrhythmic drug; ARDS, acute respiratory distress syndrome; BrS, Brugada syndrome; COVID-19, coronavirus disease-2019; ICD, implantable cardioverter-defibrillator; LQTS, long QT syndrome; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TdP, torsades de pointes; TTE, transthoracic echocardiography
Central Illustration
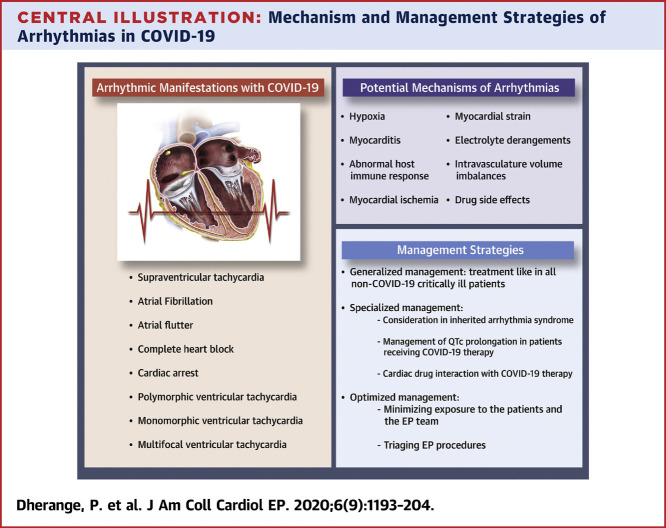
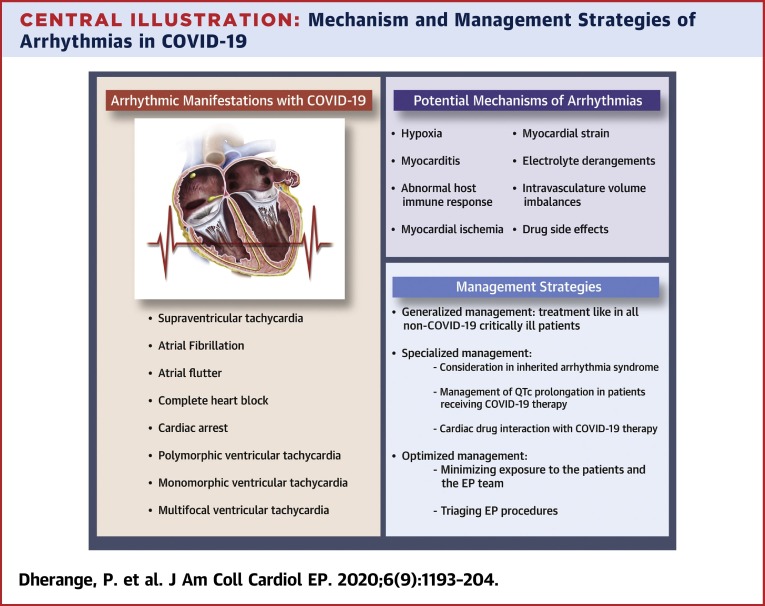
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which causes coronavirus disease-2019 (COVID-19), was first officially reported in Wuhan, Hubei Province, in China on December 31, 2019 (1). Spreading rapidly, COVID-19 has now been classified as a pandemic with far-reaching impact on the world’s health and economic sectors. Millions of coronavirus cases have been reported so far (2), and several efforts are underway to better define the epidemiology of the disease (3). Although many cases remain asymptomatic, the most common symptoms include cough, fever, and fatigue. A minority of patients experience complications such as acute respiratory distress syndrome (ARDS) and other end organ damage (4). Of particular concern is cardiac injury, defined as elevation of high-sensitivity troponin I above the 99th percentile, which appears common. One case series of 146 hospitalized COVID-19 patients in China reported a cardiac injury incidence of 20% and a higher mortality of 51.2% compared with 4.5% in patients with cardiac injury versus without cardiac injury (5). Similarly, in another case series, cardiac injury was manifested in 27.8% of 187 COVID-19 patients, and presence of cardiac injury was associated with fatal outcomes, with a significantly higher mortality of 59.6% compared with patients without cardiac injury (8.9%) (6). In an observational cohort study of German patients who recently recovered from COVID-19 illness and underwent cardiac magnetic resonance with a median duration of 71 days since COVID-19 diagnosis, 78% of the patients were found to have cardiac involvement and 60% had ongoing inflammation, irrespective of the severity and overall course of the illness (7). One aspect of cardiac injury and overall critical illness is an increased risk for cardiac arrhythmias. In this review, we present the current state of knowledge in the potential mechanism and manifestations of cardiac arrhythmias in COVID-19–infected patients to provide a resource for clinicians during this rapidly evolving crisis (Central Illustration ).
Central Illustration.
Mechanism and Management Strategies of Arrhythmias in COVID-19
Data on Arrhythmia From Previous Epidemics and the Current COVID-19 Outbreak
Because we currently have limited data on the current SARS-CoV-2 outbreak, it is worthwhile to understand from previous historic coronavirus epidemics due to SARS-CoV and Middle Eastern respiratory syndrome coronavirus. In a case series of 121 patients diagnosed with SARS-CoV-2, 71.9% were found to have tachycardia independent of hypotension and fever, and 14.9% were found to have bradycardia as a transient event (8). Tachycardia was not further characterized into arrhythmias, although 1 patient was found to have transient atrial fibrillation. It was noted that the tachycardia persisted in 40% of patients at follow up after discharge. In a case series of 70 patients with laboratory-confirmed Middle Eastern respiratory syndrome coronavirus in 2014 in Saudi Arabia, cardiac arrhythmias including tachyarrhythmias and severe bradyarrhythmia requiring temporary pacemaker occurred in 15.7% of patients (9). Though influenza belongs to a different family of viruses, it is worthwhile to note its role in arrhythmias, as both COVID-19 and influenza are believed to cause myocarditis and arrhythmias (10). Influenza has been noted to cause various tachyarrhythmias, heart block, and ventricular fibrillation, though most have been presumed to be secondary to myocarditis (11). In a survey-based study conducted by Madjid et al. (12), patients with an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy with defibrillator were noted to have more ICD shocks delivered during influenza season than during other periods of the year. Also, patients with high influenza activity were reported more likely to have a ventricular arrhythmia treated with shock and with antitachycardia pacing (12).
Our initial understanding on incidence of arrhythmias in the SARS-CoV-2 outbreak was from retrospective studies from Wuhan, China. In a case series of 137 patients of coronavirus in tertiary hospitals in the Hubei province in China, nearly 7.3% reported palpitations as the initial symptom (13). In another similarly sized study, 16.7% of patients were reported to have arrhythmias, with a higher incidence of 44.4% versus 6.9% in intensive care unit (ICU) patients compared with non-ICU patients (14). A low prevalence of arrhythmias is noted among clinically stable COVID-19 patients in a single-day snapshot survey-based data (15). A case series by Guo et al. (6) reported an occurrence of 5.9% of sustained episodes of ventricular tachycardia or ventricular fibrillation in 187 hospitalized COVID-19 patients.
The data on arrhythmia characterization in COVID-19 patients in the United States are now emerging. In a retrospective case series involving 393 COVID-19 patients admitted in 2 hospitals in New York City, 28 (7.1%) patients were found to have atrial arrhythmias, whereas only 1 (0.3%) patient who was mechanically ventilated had ventricular arrhythmias (16). With the current data, arrhythmias are noted to be more frequent in critically ill patients. Among 115 COVID-19 patients, atrial arrhythmias were noted in 16.5% of the patients, who were all admitted to the ICU (17).
There are several manifestations of arrhythmias observed in COVID-19 patients. Bradyarrhythmias have been described by several authors in the literature. A case of transient complete heart block requiring cardiopulmonary resuscitation was reported in a patient with COVID-19 (18). Another case of transient high-grade atrioventricular (AV) block was reported, which was thought to be as a result of subclinical myocarditis (19). In a case series by He et al. (20), 1 of the 2 patients with COVID-19 was found to have transient complete heart block along with temporary occurrence of S1Q3T3, which suggests transient pulmonary artery hypertension secondary to ARDS. Along with cases of bradyarrhythmias, tachyarrhythmias including atrial fibrillation, atrial flutter, supraventricular tachycardias, as well as ventricular arrhythmias, have been reported in patients with COVID-19. A case of multifocal ventricular tachycardia was preceded by electrocardiography (ECG) findings of ST-segment elevation in a patient with COVID-19 (20). A case of acute bilateral pulmonary embolism in a COVID-19 patient later developed sustained monomorphic ventricular tachycardia with syncope requiring cardioversion (21). Also, some of the “repurposed drugs'' used for the management of COVID-19 have known risk of QTc prolongation, which could lead to torsades de pointes (TdP) (22). Brugada-like ECG pattern on presentation was reported in a COVID-19 case who later had a brief episode of supraventricular tachycardia (23). A case of atrial flutter with 2:1 AV block was seen as the presenting rhythm in a male with symptoms of COVID-19 who was later tested to be positive for the novel coronavirus (24).
A recent case series published from New York highlights cases of cardiac arrhythmias in COVID-19 infection, which include high-grade AV block, new onset atrial fibrillation, polymorphic ventricular tachycardia due to a ventricular premature contraction causing a long-short sequence, and cardiac arrest with pulseless electrical activity (25). These manifestations suggest various potential mechanisms for arrhythmogenesis.
Possible Mechanism for Arrhythmogenesis
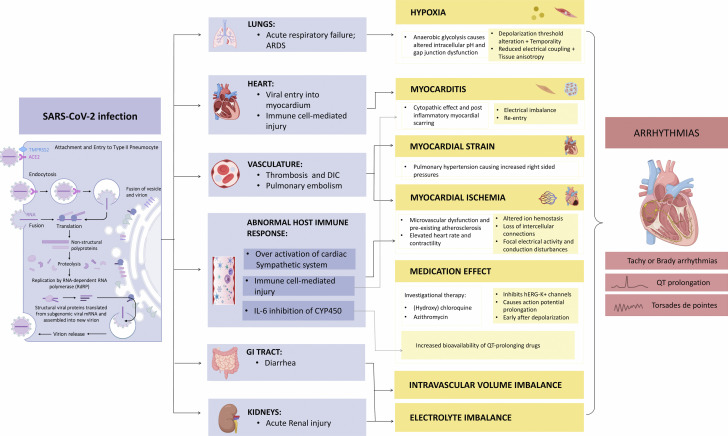
Numerous potential mechanisms increase the risk of cardiac arrhythmias during COVID-19 infection. These include various modes of injury to myocardium as well as extracardiac processes that can exacerbate arrhythmias in patients with a pre-existing proclivity. In the setting of a viral infection, chronic cardiovascular diseases can become unstable due to increased metabolic demand and reduced cardiac reserve (26). This imbalance, in association with direct myocardial damage and increased inflammatory response, could increase the risk of acute coronary syndrome, heart failure, and cardiac arrhythmias (27,28). Arrhythmias in COVID-19 may result either primarily due to hypoxia caused from direct viral tissue involvement of lungs, myocarditis, or abnormal host immune response, or secondarily as a result of myocardial ischemia, myocardial strain due to pulmonary hypertension, electrolyte derangements, intravascular volume imbalances, and drug side effects. Arrhythmias are not merely due to the direct effect of COVID-19 infection, but rather are likely as a result of systemic illness (29). The potential mechanisms of arrhythmia in COVID-19 are outlined in Figure 1 .
Figure 1.
Potential Mechanisms of Arrhythmia and COVID-19
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) virus enters host cells, leading to viral infection and immune system activation and affecting the lungs, heart, and other organ systems. Risk of arrhythmias may be broadly mediated by hypoxia, myocarditis, myocardial strain, myocardial ischemia, medication effects, intravascular volume imbalance, and electrolyte imbalance. ACE2 = angiotensin-converting enzyme 2; ARDS = acute respiratory distress syndrome; CYP450 = cytochrome P450; DIC = disseminated intravascular coagulation; GI = gastrointestinal; IL = interleukin; mRNA = messenger RNA.
The progression of COVID-19 has been hypothesized to be across 3 overlapping yet distinct stages. This includes stage 1, for early infection with constitutional symptoms, and stage 2, in which there is viral entry and replication in the type II pneumocytes, causing direct viral cytotoxicity and activation of inflammatory immune response, leading to ARDS and hypoxia. If the immune response is unable to clear the virus in a productive and protective fashion, stage 3 ensues, which is a hyperinflammatory state, and there is multiorgan dysfunction due to a cytokine storm (10).
Hypoxia caused due to direct viral involvement in the lung tissue
Acute respiratory failure as a result of lung injury from novel coronavirus infection can lead to hypoxia. Following from our understanding of hypoxia-induced cellular damage as it is rooted in myocardial ischemia due to coronary occlusion, hypoxia can activate anaerobic glycolysis, reducing intracellular pH and thus increasing cytosolic calcium levels. This, in turn, can facilitate early and late depolarizations, as well as cause and temporal alterations in the action potential duration (30). Hypoxia also causes an increase in the extracellular potassium levels, which decreases the threshold for depolarization, accelerating electrical conduction (30). Additionally, Hypoxemia can also cause reduced electrical coupling and tissue anisotropy due to dephosphorylation of connexin 43 in the gap junctions (31).
Myocarditis
SARS-CoV-2 myocarditis has been reported several times in the literature so far (32,33). The proposed mechanism for the pathophysiology of myocarditis includes direct myocardial injury as a consequence of direct viral tissue involvement (34) or due to extrapulmonary migration of infected alveolar macrophages, and can potentially predispose to enhanced arrhythmic risk by disrupting electrical conduction (30). SARS-CoV-2 utilizes the spike protein to bind to angiotensin-converting enzyme 2 (ACE2) receptors on the myocardial cell membrane (35). In theory, when the SARS-CoV-2 attaches to the ACE2 receptors found on the myocardium, there is down-regulation of the ACE2 receptors, which causes unopposed angiotensin II accumulation, leading to adverse myocardial remodeling by its action on angiotensin II type 1 receptors (36). Intracellular presence of the virus also inhibits stress granule formation via its accessory protein, promoting its replication and causing cell damage. Based on prior in-vitro studies on SARS-CoV, amiodarone and other cationic amphiphilic drugs like dronedarone, verapamil, and the calcium-channel blocker bepridil seem to target this part of the viral life cycle by accumulating in the late endosomes or lysosomes and increasing the pH of these organelles, thereby affecting coronavirus replication (37).
Another possible mechanism is via cell-mediated cytotoxicity in which primed CD8+ T lymphocytes migrate to the cardiomyocytes and cause myocardial inflammation. Via cytokine storm, proinflammatory cytokines, which are released into the circulation, augment T lymphocyte activation, which further releases more cytokines resulting in a positive feedback loop of immune activation and myocardial injury (32). Myocarditis can cause arrhythmia in the acute stage due to a direct cytopathic effect, causing electrical imbalance, ischemia from microvascular dysfunction, and gap junction dysfunction from impaired myocardial expression of connexins, or due to ion-channel impairment, especially seen in patients with overlapping inflammatory channelopathies. In viral myocarditis, host and viral factors can cause structural as well as electrophysiological remodeling, which can cause abnormal calcium handling and down-regulation of potassium channels, leading to prolonged repolarization and abnormal conduction. Prolonged repolarization can induce triggered activity, whereas a combination with abnormal conduction (which involves reduced conduction velocity, decreased refractoriness, and increased diffusion of conduction in the myocardium) can cause either circus-type re-entry or phase 2 re-entry without an obstacle (38). Arrhythmia can also be seen in the post-inflammatory stage, in which variable degrees of myocardial scar can be present promoting re-entrant arrhythmias (33).
Tavazzi et al. (39) describe a case of COVID-19 presenting with cardiogenic shock and new onset cardiomyopathy with echocardiography showing a dilated left ventricle (LV), severe and diffuse LV hypokinesia (LV ejection fraction 34%), and coronary angiography showing normal coronaries. Endomyocardial biopsy demonstrated cytopathic interstitial inflammatory cells, but no viral particles were observed in the myocyte. This observation suggests that myocardial localization was either due to a viremia phase or due to migration of infected alveolar macrophages in extrapulmonary tissues (39).
Abnormal host immune response
Along with a direct role in myocardial injury (40), cytokines including interleukin (IL)-6, tumor necrosis factor-α, and IL-1 can modulate the expression and function of potassium and calcium channels (inflammatory cardiac channelopathies) and cause prolongation of ventricular action potential (41,42). Inflammatory cytokines are a well-studied trigger for arrhythmia, especially in patients with long QT syndrome (LQTS), due to overactivation of the cardiac sympathetic system using the hypothalamus-mediated inflammatory reflex and peripheral-mediated activation of the stellate ganglion pathway (43), which can lead to QT prolongation (44). Furthermore, IL-6 inhibits cytochrome P450 (CYP450), which increases the bioavailability of QT-prolonging drugs (45).
Myocardial ischemia
Potential causes for myocardial ischemia are microvascular dysfunction and a hyperinflammatory state, leading to activation of inflammatory cells within a pre-existing atherosclerotic plaque, causing vasoconstriction due to dysregulation of the coronary vascular endothelial function. In one study, microvascular dysfunction was found to be in the form of disseminated intravascular coagulation due to hyperinflammation and immune activation (46). IL-6 and tumor necrosis factor-α can both cause depletion of the coagulation and fibrinolytic system, leading to bleeding as well as thrombosis (47). The other postulated mechanism of microvascular dysfunction causing myocardial ischemia is via infection-mediated vasculitis. SARS-CoV-2 attaches to the ACE receptors, which are also expressed in arterial and venous endothelial cells (48). Vasculitis can thus be triggered either as a result of hypersensitivity reaction induced due to direct viral entry in the myocardial endothelial cells or due to indirect immunological response (49). Myocardial injury with ST-segment elevation has also been reported in patients with COVID-19 infection (50). Cytokine surge and inflammatory mediators can also cause acute coronary syndrome due to activation of inflammatory cells within a pre-existing atherosclerotic plaque as well as vasoconstriction due to dysregulation of the coronary vascular endothelial function (51). Pathogen-associated molecular patterns are formed with viral products in the systemic circulation, which can cause activation of innate immune response as well as activation of inflammasome resulting in the formation of biologically active cytokines (52).
Myocardial strain
Right myocardial strain can be seen as a result of pulmonary embolism, which has been reported as the most common thrombotic complication in COVID-19 patients (53), or due to pulmonary hypertension, causing increased right sided pressures secondary to severe ARDS, sepsis, or heart failure (54). On the one hand, increased right atrial pressure along with increased sympathetic tone in patients with pulmonary hypertension and hypoxia can predispose to increased risk of atrial tachyarrhythmias (55). On the other hand, a case of temporary occurrence of S1Q3T3, which has been previously described in this review, was found to have transient complete heart block suggested to be due to transient pulmonary artery hypertension (20).
Electrolyte derangements
Arrhythmias due to electrolyte abnormalities as well as its effect on pre-existing arrhythmias has been well studied (56). In a case series, electrolyte disturbances were reported in 7.2% of 416 hospitalized patients with COVID-19 infection (5). These were attributed to COVID-19–associated diarrhea or from renal injury, among other cases. In a retrospective analysis by Diao et al. (57), acute kidney injury was reported in 27% of 85 patients hospitalized with COVID-19 infection. A case report of a COVID-19 patient presenting with atrial arrhythmias previously described in this review was noted to have severe electrolyte disturbances including hypokalemia, hypomagnesemia, and hypophosphatemia (24).
Intravascular volume imbalances
Fluid disturbances are commonly encountered in critically ill patients. Intravascular volume imbalances in COVID-19 could either be ascribed to sepsis caused from ARDS or due to heart failure. The most commonly encountered arrhythmia in the critical care setting, which can be caused due to the previously described mechanism among others, is atrial fibrillation (58).
Drug side effects
Many of the “off-label” COVID-19 therapies including (hydroxy)chloroquine, lopinavir/ritonavir and azithromycin have either known or possible risk of TdP due to their effect on QT prolongation (59). (Hydroxy)chloroquine and azithromycin inhibit the hERG-K+ channel, which causes prolongation of action potential, and this along with unopposed inward Na+ and Ca2+ currents trigger early after depolarization that can lead to TdP. In a cohort study of 90 hospitalized COVID-19 patients, the administration of hydroxychloroquine with or without azithromycin was associated with an increased risk of QTc prolongation and risk of TdP (60). Antimalarial drugs (hydroxy)chloroquine can cause not only this fatal side effect of QT prolongation, but also advanced AV conduction block (28). Currently, there are not enough data on adverse cardiac effects with some other COVID-19 therapies including ribavirin, remdesivir, and tocilizumab.
Management of Arrhythmias With COVID-19
The goal of managing arrhythmias in patients with COVID-19 is to treat safely by minimizing exposure and by being mindful of drug-drug interactions. There are not enough clinical studies to guide management of arrhythmia in confirmed COVID-19 cases. Treatment of any bradyarrhythmias or tachyarrhythmias in a COVID-19 patient should be similar to treatment as in any patient with arrhythmia due to infection or transient metabolic disturbances. Special consideration should be given to management of arrhythmias in patients with inherited arrhythmia syndrome and management of QTc prolongation while receiving COVID therapy.
Management of arrhythmias in suspected or confirmed COVID-19 cases
The European Society of Cardiology (61) as well as the Latin American Heart Rhythm Society in collaboration with the Colombian, Argentinian, Brazilian, and Mexican Societies of Cardiac Electrophysiology (62) have provided a consensus document for management of various arrhythmias in a confirmed COVID-19 patients, focusing on the transient nature of arrhythmias, drug-drug interactions, and limiting exposure and personal protective equipment (PPE) use. The recommendations are summarized subsequently.
Bradyarrhythmias
Bradycardia including sinus or AV block in COVID-19 patients can be seen because of drug side effects with use of (hydroxy)chloroquine, lopinavir/rotinavir, and azithromycin. AV blocks could also be seen in myocarditis if there is involvement of the conduction system. Intubated patients can experience transient bradycardia during tracheal secretion suctioning or during proning of the patient due to transient increased vagal tone. In patients with persistent bradycardia, use of isoprenaline and atropine can be considered prior to temporary pacemaker implantation (61). Temporary pacemaker implantation is considered a reasonable option prior to implanting a permanent device due to the transient nature of bradyarrhythmias, nature of critical illness, risk of bacterial superinfection, and risk of device infection. However, evaluation of a permanent pacemaker should be reassessed after recovery from COVID-19 infection (62).
Atrial tachyarrhythmias (supraventricular tachycardia and atrial fibrillation or flutter)
It is important to identify and treat secondary causes of atrial tachyarrhythmias like hypoxia, metabolic and electrolyte imbalances, proarrhythmic effect of drugs, or myocardial ischemia.
In patients with supraventricular tachycardia, intravenous adenosine can be used for acute termination, although further validation is needed. Electrical cardioversion can be considered in patients with refractory cases and should be postponed in stable and asymptomatic patients. One should have a low threshold to initiate maintenance therapy with beta-blockers (BBs) or calcium-channel blockers (CCBs) if BBs are contraindicated; however, BB and CCB drug interactions with antiviral drugs should be evaluated prior to starting these medications to avoid bradycardia and QT prolongation (61,62).
In patients with recurrent atrial fibrillation and flutter who are hemodynamically stable, consider discontinuing antiarrhythmic drugs (AADs) especially sotalol, flecainide and likely amiodarone and propafenone given serious drug-drug interactions with antiviral drugs and to start rate controlling medications with BBs or CCBs unless contraindicated, with or without digoxin. Similarly, patients with new onset of atrial fibrillation or flutter with a stable rhythm can be treated with rate control. Rhythm control strategy should be reserved for patients with hemodynamically unstable patients or in congestive heart failure patients (61,62). There is a high risk of immediate recurrence of arrhythmia without the use of any AAD as maintenance therapy, which can be minimized by treating secondary causes of arrhythmias prior to attempting rhythm control strategy.
Rhythm control strategy can be achieved with synchronized cardioversion or AAD medications like amiodarone; however, close monitoring with caution is recommended with the use of amiodarone in patients undergoing treatment with fingolimod. Propafenone or flecainide is preferred in structurally normal hearts who are on fingolimod (63). Transthoracic echocardiography (TTE) is recommended only in hemodynamically unstable patients. Thus, it is imperative to note that the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65–74 years, sex category) score may underestimate risk of stroke. Myocardial injury should be considered if new onset LV dysfunction is noted on TTE, which would necessitate escalation of immunosuppressants in patients with myocarditis (61,62). Cardiac computed tomography can be considered to rule out left atrial appendage thrombus prior to cardioversion if indicated, instead of transesophageal echocardiography to avoid aerosol-generating procedures (25). In terms of anticoagulation, the consensus is to start early to obviate the need for transesophageal echocardiography if rhythm control is needed. Data suggest that COVID-19 infection can be associated with a hypercoagulable state that might increase the risk for thromboembolism, especially in patients with markedly elevated D-dimer levels and severely ill patients admitted in the ICU (64,65). One should reassess rate versus rate control strategy and the need for anticoagulation after recovering from COVID-19 infection.
Ventricular Arrhythmias
It is important to identify and treat secondary causes like hypoxia, metabolic or electrolyte imbalances, and proarrhythmic effects of drugs. In patients with ventricular tachycardia storm not related to the previously mentioned causes, the first choice of therapy is amiodarone or intravenous lidocaine, especially if underlying myocardial infarction is suspected, in addition to sympathetic blockade with esmolol, sedation, and possible intubation. In patients with polymorphic ventricular tachycardia or ventricular fibrillation with prolonged QTc interval, replace electrolytes to target potassium level >4.5, start intravenous infusion of magnesium and isoprenaline infusion, and stop QT-prolonging antiviral meds. Temporary pacemaker implantation using flotation guided technique at bedside in emergency situations can be considered to overdrive terminate ventricular tachycardia and incase of recurrent TdP or bradycardia (61). One should also assess the need for escalation of therapies, including the use of extracorporeal membrane oxygenation (62). Consider TTE in patients with new onset malignant ventricular arrhythmia if not related to QT prolongation. After recovery from COVID-19, evaluate the need for secondary prevention ICD and catheter ablation. If transient cardiomyopathy due to myocarditis is suspected, consider wearable defibrillator devices (61).
Channelopathies and Inherited Arrhythmia Syndrome
In patients with LQTS, monitor the QT interval and cardiac rhythm due to high risk of QT prolongation seen in COVID-19. Hypokalemia should be avoided in patients with COVID-19–associated diarrhea. It is recommended to maintain a potassium level at the higher range of normal. The effect of fever is not much evident with LQTS (except with specific LQTS2 mutation). Whereas in patients with Brugada syndrome (BrS), fever can unmask the ECG manifestation of type 1 BrS, and thus it is recommended that all patients with COVID-19 self-medicate with paracetamol or acetaminophen if they develop fever. High-risk BrS patients who develop fever (>38.5°C) despite treatment should be evaluated in the emergency room and kept under observation if ECG shows type 1 pattern; otherwise, the patient can be discharged home to limit exposure to SARS-CoV-2 in the hospital. Patients with catecholaminergic polymorphic ventricular tachycardia have adrenergic-related ventricular arrhythmias; thus, catecholamine infusions should be administered with great caution in critically ill patients. BBs and flecainide should be continued while monitoring interactions with antiviral drugs. Patients with short QT syndrome do not seem to be at particular risk with COVID-19 (66).
QT liability with COVID-19 therapy
Experimental drugs used for treatment of COVID-19 hold a significant risk for QTc prolongation. A case series by Ramireddy et al. (67) reported an increase in QTc interval in 12% of patients receiving hydroxychloroquine, azithromycin, or both; however, no TdP was seen. In a randomized controlled trial, CloroCOVID-19 trial (Chloroquine Diphosphate for the Treatment of Severe Acute Respiratory Syndrome Secondary to SARS-COV2), studying the effect of low-dose (450 mg twice daily for 1 day, then 450 mg daily for 4 days) versus high-dose (600 mg twice daily for 10 days) chloroquine in addition to azithromycin in severe COVID-19 patients showed lethality in the higher dose group with an odds ratio of 3.6 (95% confidence interval: 1.2 to 10.6) and thus recommended against the use of high-dose chloroquine in severe cases (68). Traditionally, the Tisdale risk score, which has been used to calculate the risk for QTc prolongation in hospitalized patients, could also be used to risk-stratify COVID-19 patients with a goal to aggressively monitor increased risk patients (69). It consists of ≥68 years of age, female sex, use of loop diuretics, potassium level ≤3.5 mEq/l, baseline QTc interval ≥450 ms, acute myocardial infarction, number of QT-prolonging medications, sepsis, and heart failure. A score of ≤6 predicts low risk, 7 to 10 medium risk, and ≥11 high risk of drug-associated QT prolongation (70).
An algorithm (59) proposed by Mayo Clinic for patients in whom QTc-prolonging drugs like (hydroxy)chloroquine ± azithromycin or lopinavir/ritonavir are under consideration recommend to obtain a pre-treatment baseline QTc measurement, baseline electrolytes (K+, Ca+2, and Mg+2), to determine if any home QTc-prolonging medication can be discontinued and to document high-risk cardiovascular and comorbid conditions. QTc interval can be measured using standard 12-lead ECG, telemetry, or mobile ECG devices. However, if using a handheld ECG device, either a multilead handheld ECG device or a single-lead handheld ECG device in at least 3 lead positions are recommended as a single position may lead to under-reporting of the QTc interval (71). A QTc measurement of <460 ms in pre-pubertal patients, <470 ms in post-pubertal male patients, and <480 ms in post-pubertal female patients is considered low risk to prescribe or continue therapy. In patients with QTc interval >500 ms at baseline or an increase in QTc measurement of ≥500 ms or if the change in QT interval is ≥60 ms, 2 to 3 h after a dose of hydroxychloroquine or other QT-prolonging agent, we recommend re-evaluation of the risk of TdP, discontinuation of other QTc-prolonging medications, correction of all electrolyte abnormalities, and placing of the patient on continuous telemetry, with consideration of a wearable defibrillator or placement of external defibrillator patches. In patients with a wide QRS either due to bundle branch block or ventricular pacing, wide QRS-adjusted QTc interval can be calculated to account for the wide QRS: [wide QRS-adjusted QTc interval = QTc interval − (QRS interval − 100 ms)] (59).
A case report by Mitra et al. (72) reports a possible benefit of addition of lidocaine or mexiletine to allow combination therapy with azithromycin and chloroquine in patients with a prolonged QT interval. Class Ib antiarrhythmic agents, due to their effect on the late sodium current by blocking the INa-L channel, have been shown to slightly shorten the QT interval and suppress TdP in acquired LQTS. Other known therapies to alleviate risk of TdP, such as maintaining potassium and magnesium levels and heart rates >70 beats/min have also been described (72).
Cardiac drug interaction with COVID-19 therapy
Another important consideration during management of COVID-19 patients is drug-drug interaction in the treatment of COVID-19, especially with concomitant use of antiarrhythmics and anticoagulants. For instance, the levels of hepatically eliminated AADs including amiodarone, propafenone, flecainide, quinidine, and some novel oral anticoagulants including apixaban and rivaroxaban, which are metabolized mainly by different CP450 isoenzymes (e.g., CYP3A4, CYP2C9), can be increased by coadministration of lopinavir/ritonavir, as it inhibits CYP3A4 (66,73). Table 1 summarizes the potential interactions of drugs used for COVID-19 therapy with cardiac drugs.
Table 1.
Possible Interactions of Drugs Used for COVID-19 Therapy With Cardiac Drugs
| Treatment | Select Drug-Drug Interactions∗ |
|---|---|
| Azithromycin | ↓ in HR, ↑ PR interval, ↑↑ in QTc interval with very low risk of TdP. AAD drug interactions:
|
| (Hydroxy) chloroquine | ↓ in HR, ↑ PR interval, ↑↑ in QTc interval with very low risk of TdP. AAD drug interactions:
|
| Lopinavir/ritonavir | ↑ PR interval, ↑↑ in QTc interval with low risk of TdP. AAD interactions:
|
| Remdesivir | Unknown |
| Fingolimod/siponimod | ↓↓↓in HR, ↑↑ in PR interval, ↑ in QTc prolongation with unknown risk of TdP. AAD interactions:
|
| Ribavirin | Unknown |
| Tocilizumab | No ECG changes described AAD interactions:
|
| Methylprednisolone | Unknown |
| Interferon alfa-1 | Unknown |
↑/↓ = mild increase/decrease; ↑↑/↓↓ = moderate increase/decrease; ↑↑↑/↓↓↓ = severe increase/decrease; AAD = antiarrhythmic drug; BB = beta-blocker; CCB = calcium-channel blocker; COVID-19 = coronavirus disease-2019; ECG = electrocardiography; HR = heart rate; TdP = torsades de pointes
Severe: drugs should not be administered; moderate: potential interaction, need dose adjustments/close monitoring; mild: weak intensity interaction, need for dose adjustments/close monitoring is unlikely to be required.
Optimization of resources and minimizing exposure
In the COVID-19 pandemic, the U.S. health care system may face another huge challenge due to shortage of PPE, which are routinely used by health care providers to protect themselves from infectious conditions and toxic substances. The fundamental goal is to avoid elective procedures in order to conserve precious resources like PPE and hospital beds and also minimize unnecessary exposure to COVID-19. A practical guide from a New York City Hospital Network describes an approach used to overcome these challenges due to the surge of COVID-19 activity in New York by ramping down and restructuring of the electrophysiology services (74).
The nature of procedures in the field of electrophysiology are mainly nonemergent, except for some cardiac arrhythmias, which require urgent interventions. The Heart Rhythm Society COVID-19 task force (75) has put together a consensus document which divides electrophysiological procedures into urgent or emergent, semi-urgent, and nonurgent or elective procedures. Procedures are considered emergent if they would reduce the risk of clinical deterioration, hospitalization, or death. Patients requiring electrophysiology procedures should be screened for fever and COVID-19 symptoms and should undergo COVID-19 testing. In locations with limited testing availability or with concern for community spread, it may be prudent to consider at least droplet precautions for all electrophysiology procedures. N95 masks should be used by all operating physicians and a surgical mask for all patients (74). If needing intubation which is an aerosol generating procedure, it is recommended to consider performing in a negative pressure room either in the electrophysiology lab or inpatient ICU before bringing the patient to electrophysiology lab. Anesthesia data recommends to limit personnel in the procedure room during intubation or extubation and to follow institutional protocols for the length of time before other personnel can return to the room after intubation which could be between 15 and 30 min based on frequency of air exchanges and the type of PPE used (76). Conscious sedation can be considered by EP attending with backup anesthesia services without compromising patient safety (74). Electrocautery is another aerosol generating procedure that is routinely used during device implantation procedures, and the smoke produced can have a significant amount of viral load. Surgical data recommend using low power electrocautery settings and using adequate PPE including goggles or face shield for eye protection as it is considered possible that SARS-CoV-2 can be transmitted to the conjunctiva by aerosol, although there is no evidence (77). PlasmaBlade (Medtronic, Minneapolis, Minnesota) is a soft tissue dissection device other than electrocautery that is routinely used especially during generator changes to limit lead damage, produces less smoke, and in theory could be used as a substitute for electrocautery; however, there is no evidence to support this. Apart from considerations for electrophysiology procedures, the Heart Rhythm Society COVID-19 task force document (75) also provides guidance regarding management of patients in the outpatient setting via telemedicine as well as regarding minimizing in-person cardiovascular implantable electronic device interrogation via remote device monitoring.
Conclusions
Our understanding of the impact of COVID-19 on arrhythmias continues to evolve as new data emerge. Current data suggest that arrhythmias are more frequent in the critical care settings. The increasing risk of arrhythmias in COVID-19 patients is likely as a result of systemic illness and not merely as a result of direct effect of the viral infection. To manage arrhythmias in patients with COVID-19, it is imperative to increase the awareness of potential drug-drug interactions, to monitor QTc prolongation while receiving COVIDm-19 therapy, and provide special considerations for patients with inherited arrhythmia syndromes. It is also crucial to minimize exposure to COVID-19 infection by stratifying the need for intervention and using telemedicine. As COVID-19 infection continues to prevail with a potential for future surges, more data are required to better understand pathophysiology and to validate management strategies.
Acknowledgment
Graphic design supplemented by BioRender.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Bruce Lerman, MD, served as Guest Editor for this paper. William Stevenson, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Clinical Electrophysiologyauthor instructions page.
References
- 1.Jee Y. WHO IHR emergency committee for the COVID-19 outbreak. Epidemiology and Health. 2020;42 doi: 10.4178/epih.e2020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 United States cases by county. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map Available at:
- 3.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiology. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 July 27 doi: 10.1001/jamacardio.2020.3557. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu C.-M., Wong R.S.-M., Wu E.B., et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad M., Omrani A.S., Baig K., et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. J Am Coll Cardiol Basic Trans Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estabragh Z.R., Mamas M.A. The cardiovascular manifestations of influenza: a systematic review. Int J Cardiol. 2013;167:2397–2403. doi: 10.1016/j.ijcard.2013.01.274. [DOI] [PubMed] [Google Scholar]
- 12.Madjid M., Connolly A.T., Nabutovsky Y., Safavi-Naeini P., Razavi M., Miller C.C. Effect of high influenza activity on risk of ventricular arrhythmias requiring therapy in patients with implantable cardiac defibrillators and cardiac resynchronization therapy defibrillators. Am J Cardiol. 2019;124:44–50. doi: 10.1016/j.amjcard.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Fang Y.-Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala S., Peretto G., De Luca G., et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol. 2020 Jun 16 doi: 10.1111/pace.13987. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colon C.M., Barrios J.G., Chiles J.W., et al. Atrial arrhythmias in COVID-19 patients. J Am Coll Cardiol EP. 2020 May 28 doi: 10.1016/j.jacep.2020.05.015. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azarkish M., Laleh Far V., Eslami M., Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020;41:2131. doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kir D., Mohan C., Sancassani R. HEART BRAKE-an unusual cardiac manifestation of coronavirus disease 2019 (COVID-19) J Am Coll Cardiol Case Rep. 2020;2:1252–1255. doi: 10.1016/j.jaccas.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J., Wu B., Chen Y., et al. Characteristic ECG manifestations in patients with COVID-19. Can J Cardiol. 2020 Mar 29 doi: 10.1016/j.cjca.2020.03.028. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birchak J., Khan A., Singh G., Schuger C., Maskoun W. An unusual case of sustained ventricular tachycardia from acute pulmonary embolism. J Am Coll Cardiol. 2020;75:2820. [Google Scholar]
- 22.Naksuk N., Lazar S., Peeraphatdit T.B. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. 2009:215–221. doi: 10.1177/2048872620922784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidovich M.I. Transient Brugada-like ECG pattern in a patient with coronavirus disease 2019 (COVID-19) J Am Coll Cardiol Case Rep. 2020;2:1245–1249. doi: 10.1016/j.jaccas.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seecheran R., Narayansingh R., Giddings S., et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochav S.M., Coromilas E., Nalbandian A., et al. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020 Jun 22 doi: 10.1016/j.hrthm.2020.06.016. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 31.Kolettis T.M. Coronary artery disease and ventricular tachyarrhythmia: pathophysiology and treatment. Curr Opin Pharmacol. 2013;13:210–217. doi: 10.1016/j.coph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020 May 5 doi: 10.1016/j.hrthm.2020.05.001. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peretto G., Sala S., Rizzo S., et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019;16:793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Lindner D., Fitzek A., Bräuninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 Jul 27 doi: 10.1001/jamacardio.2020.3551. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aimo A., Baritussio A., Emdin M., Tascini C. Amiodarone as a possible therapy for coronavirus infection. Eur J Prev Cardiol. 2020 Apr 16 doi: 10.1177/2047487320919233. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tse G., Yeo J.M., Chan Y.W., Lai E.T., Yan B.P. What is the arrhythmic substrate in viral myocarditis? Insights from clinical and animal studies. Front Physiol. 2016;7:308. doi: 10.3389/fphys.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puntmann V.O., Taylor P.C., Barr A., Schnackenburg B., Jahnke C., Paetsch I. Towards understanding the phenotypes of myocardial involvement in the presence of self-limiting and sustained systemic inflammation: a magnetic resonance imaging study. Rheumatology (Oxford) 2010;49:528–535. doi: 10.1093/rheumatology/kep426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazzerini P.E., Capecchi P.L., Laghi-Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovasc Med. 2015;2:26. doi: 10.3389/fcvm.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazzerini P.E., Laghi-Pasini F., Boutjdir M., Capecchi P.L. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. doi: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 43.Lazzerini P.E., Capecchi P.L., Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208. [DOI] [PubMed] [Google Scholar]
- 44.Yanowitz F., Preston J.B., Abildskov J.A. Functional distribution of right and left stellate innervation to the ventricles: production of neurogenic electrocardiographs changes by unilateral alteration of sympathetic tone. Circ Res. 1966;18:416–428. doi: 10.1161/01.res.18.4.416. [DOI] [PubMed] [Google Scholar]
- 45.Lazzerini P.E., Acampa M., Capecchi P.L., et al. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res. 2015;67:332–339. doi: 10.1002/acr.22455. [DOI] [PubMed] [Google Scholar]
- 46.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt B.J. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370:847–859. doi: 10.1056/NEJMra1208626. [DOI] [PubMed] [Google Scholar]
- 48.Huertas A., Montani D., Savale L., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56:2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19—A case series. N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 52.Veerdonk FL van de, van de Veerdonk F.L., Netea M.G., Dinarello C.A., Joosten L.A.B. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Klok F.A., M J H, van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ñamendys-Silva S.A., Santos-Martínez L.E., Pulido T., et al. Pulmonary hypertension due to acute respiratory distress syndrome. Braz J Med Biol Res. 2014;47:904–910. doi: 10.1590/1414-431X20143316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wanamaker B., Cascino T., McLaughlin V., Oral H., Latchamsetty R., Siontis K.C. Atrial arrhythmias in pulmonary hypertension: pathogenesis, prognosis and management. Arrhythm Electrophysiol Rev. 2018;7:43–48. doi: 10.15420/aer.2018.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surawicz B. Role of electrolytes in etiology and management of cardiac arrhythmias. Prog Cardiovasc Dis. 1966;8:364–386. doi: 10.1016/s0033-0620(66)80011-7. [DOI] [PubMed] [Google Scholar]
- 57.Diao B., Wang C., Wang R., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 Mar 5 doi: 10.1038/s41467-021-22781-1. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosch N.A., Cimini J., Walkey A.J. Atrial fibrillation in the ICU. Chest. 2018;154:1424–1434. doi: 10.1016/j.chest.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mercuro N.J., Yen C.F., Shim D.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 May 1 doi: 10.1001/jamacardio.2020.1834. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance Available at:
- 62.Saenz L.C., Miranda A., Speranza R., et al. Recommendations for the organization of electrophysiology and cardiac pacing services during the COVID-19 pandemic: Latin American Heart Rhythm Society (LAHRS) in collaboration with: Colombian College Of Electrophysiology, Argentinian Society of Cardiac Electrophysiology (SADEC), Brazilian Society Of Cardiac Arrhythmias (SOBRAC), Mexican Society Of Cardiac Electrophysiology (SOMEEC) J Interv Card Electrophysiol. 2020 Apr 29 doi: 10.1007/s10840-020-00747-5. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rattanawong P., Shen W., El Masry H., et al. Guidance on acute management of atrial fibrillation in COVID-19. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C.-I., Postema P.G., Arbelo E., et al. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Heart Rhythm. 2020 Mar 31 doi: 10.1016/j.hrthm.2020.03.024. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramireddy A., Chugh H.S., Reinier K., et al. Experience with hydroxychloroquine and azithromycin in the COVID-19 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borba M.G.S., Val F.F.A., Sampaio V.S., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 69.Malviya A. Ventricular arrhythmia risk due to chloroquine/hydroxychloroquine treatment for COVID-19: should it be given. Indian Heart J. 2020;72:131–132. doi: 10.1016/j.ihj.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson T.F., Kovacs R.J., Stecker E.C. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. Eur Heart J. 2020;9:215–221. [Google Scholar]
- 71.Cheung C.C., Davies B., Gibbs K., Laksman Z.W., Krahn A.D. Multi-lead QT screening is necessary for QT measurement: implications for management of patients in the COVID-19 era. J Am Coll Cardiol EP. 2020;6:878–880. doi: 10.1016/j.jacep.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitra R.L., Greenstein S.A., Epstein L.M. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: Possible benefits of intravenous lidocaine. HeartRhythm Case Rep. 2020;6:244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heidbuchel H., Verhamme P., Alings M., et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 74.Rubin G.A., Wan E.Y., Saluja D., et al. Restructuring electrophysiology during the COVID-19 pandemic: a practical guide from a New York City hospital network. Crit Pathw Cardiol. 2020 Apr 27 doi: 10.1097/HPC.0000000000000225. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lakkireddy D.R., Chung M.K., Gopinathannair R., et al. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020 Apr 1 doi: 10.1016/j.hrthm.2020.03.028. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wald S.H., Arthofer R., Semple A.K., Bhorik A., Lu A.C. Determination of length of time for “post-aerosol pause” for patients under investigation or positive for COVID-19. Anesth Analg. 2020 Apr 27 doi: 10.1213/ANE.0000000000004921. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirschmann M.T., Hart A., Henckel J., Sadoghi P., Seil R., Mouton C. COVID-19 coronavirus: recommended personal protective equipment for the orthopaedic and trauma surgeon. Knee Surg Sports Traumatol Arthrosc. 2020;28:1690–1698. doi: 10.1007/s00167-020-06022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]