Abstract
Knockout (KO) mice missing the sweet taste receptor subunit T1R3 or the signaling protein TRPM5 have greatly attenuated sweetener preferences. Yet both types of KO mice develop preferences for glucose but not fructose in 24-h tests, which has been attributed to the postoral reinforcing actions of glucose. Here we probed for residual sugar taste sensitivity in KO mice. Unlike wildtype (WT) mice, food-restricted T1R3 KO and TRPM5 KO mice displayed little attraction for 8% glucose and 8% fructose in 1-min, two-bottle choice tests. However, in 1-h tests about half of the T1R3 KO mice displayed a significant preference for glucose over fructose (78–84%), while WT mice showed either no or weak preferences (41–56%) for glucose. Following one-bottle training sessions, WT mice display greater glucose preferences although still weaker than those observed in T1R3 KO mice. In contrast, TRPM5 KO mice were indifferent to sugars in 1-h tests but developed a strong preference for glucose over fructose in 24-h tests. T1R3 taste cells contain the sodium glucose cotransporter 1 (SGLT1) and the ATP-gated K+ (KATP) metabolic sensor, which may mediate the unlearned glucose preference displayed by T1R3 KO mice. Unlike WT mice, many T1R3 KO mice strongly preferred glucose to a non-metabolizable glucose analog (α-methyl-D-glucopyranoside, MDG) in initial 1-h choice tests. Glucose and MDG are both ligands for SGLT1 which indicates that SGLT1 sensing does not mediate the glucose preference of T1R3 KO mice. Instead, KATP sensing and/or other oral sensors are implicated. The MDG findings also argue against postoral sensing as the primary source of the initial glucose preference displayed by T1R3 KO mice. Why only half of the T1R3 KO mice showed this preference in 1-h tests remains to be determined. All T1R3 KO mice preferred glucose to fructose in 24-h tests, which appears to be due to both oral and postoral glucose sensing.
Keywords: Sweet taste, Fructose, α-methyl-D-glucopyranoside, saccharin, SGLT1, KATP channel, Postoral learning
1. Introduction
In mammals, the attraction to sugars and non-nutritive sweeteners is primarily mediated by the T1R2+T1R3 taste receptor and downstream signaling elements including, among others, the Ca2-activated cation channel TRPM5 [8]. The critical importance of the T1R2+T1R3 receptor to the appetite for sweets is documented by the greatly reduced avidity for sweet tastants in knockout (KO) mice missing one or both of the T1R2 and T1R3 receptor subunits [10,50]. In particular, T1R3 KO mice are indifferent to sucrose in brief (5-sec, 1-min) licking tests and fail to prefer dilute sugar solutions in 24-h sugar vs. water choice tests [10,18,24,45,50,53]. However, T1R3 KO mice develop significant preferences for concentrated sucrose solutions in 24-h tests [7,10,24,50,53]. This 24-h sucrose preference of T1R3 KO mice has been attributed to a learned association between the T1R3-independent orosensory properties (e.g., odor, texture) and the postoral nutritive effects of the sugar solutions [50,55]. Supporting this view, we observed that T1R3 KO mice, like normal C57BL/6 wildtype (B6 WT) mice, learned a strong preference (92%) for a flavored solution (the CS+, e.g., grape) paired with intragastric (IG) self-infusions of 16% sucrose over a different flavored solution (the CS−, e.g., cherry) paired with IG water infusions [37]. We subsequently reported that sugar-naive T1R3 KO and TRPM5 KO mice given 24-h choice tests with sugar (0.5–32%) vs. water displayed significant preferences for concentrated (8–32%) glucose solutions but not for fructose solutions [54]. The differential preference response of KO mice to glucose and fructose can be explained by the different postoral reinforcing actions (i.e., appetition) of the two sugars [31]. That is, B6 WT mice acquire strong preferences for a flavored saccharin solution paired with IG glucose infusions but not for flavored saccharin solution paired with IG fructose infusions [32,40,52]. These and other findings indicate that postoral conditioning effects of sucrose are primarily due to the actions of glucose released by sucrose digestion in the gut [5].
While the critical importance of the T1R2+T1R3 receptor for oral sugar preference and acceptance is well established, there is also evidence for T1R2+T1R3-independent glucose taste sensors. One study reported that oral stimulation with glucose promoted the release of GLP-1 from taste cells in WT and T1R3 KO mice [22]. More recently, Glendinning et al. [18] observed that oral glucose but not fructose elicited cephalic phase insulin release (CPIR) in WT and T1R3 KO mice. In contrast, the CPIR response was not observed in SUR1 KO mice, which are missing a critical component of the KATP channel that serves as a glucose sensor [15]. Other potential glucose sensors located in taste cells include the sodium-glucose cotransporter (SGLT1) and other glucose transporters (GLUTs) [42,48]. Schier et al. [29] recently reported evidence that T1R2+T1R3-independent sugar sensing can mediate a learned discrimination between glucose and fructose. WT and T1R2+T1R3 double knockout (DKO) mice were trained to drink glucose and fructose solutions in daily one-bottle sessions, which allowed the mice to associate the orosensory features of the sugar solutions with their differential postoral appetition effects. In a subsequent brief-access (10 sec) lick test the WT and DKO mice displayed enhanced lick responses for glucose relative to fructose. How the DKO mice discriminated between the two sugar solutions was not determined, but findings obtained with WT mice suggested the involvement of olfactory cues. A subsequent study by Glendinning et al. [17] confirmed that odor cues contribute to the learned differential licking response of B6 mice to glucose and fructose solutions during brief-access lick tests.
In view of the findings that T1R3 KO mice develop preferences for glucose but not fructose in 24-h tests and the recent evidence for oral glucose taste sensing in T1R3 KO mice, the present study probed for residual glucose taste preferences in these KO mice. This was accomplished by conducting brief- and short-term preference tests with glucose vs. fructose solutions. Brief access lick tests (5 sec to 1 min) with naive mice evaluate the orosensory attractiveness to taste solutions in the absence of post-oral feedback. Based on prior brief access tests, food-restricted T1R3 KO mice, unlike WT mice, were expected to be unresponsive to glucose and fructose in a 1-min choice test [18,24,46,50,53]. Short-term tests (1 h) also evaluate the orosensory attractiveness of taste solutions but in this case post-oral appetition effects can enhance the response to glucose but not fructose [52]. However, in order for the animal to experience post-oral sugar appetition they must first spontaneously drink the sugar solution. The present study determined if the residual glucose taste sensing of T1R3 KO mice would induce an unconditioned attraction to glucose in food-restricted T1R3 KO mice given 1-h rather than 1-min two-bottle access to glucose vs. fructose. If so, their selective consumption of glucose in the 1-h test would produce a postoral conditioned enhancement of their oral glucose preference. We also determined if residual glucose sensing was dependent upon the TRPM5 ion channel by comparing the 1-h sugar preference responses of TRPM5 KO and T1R3 KO mice.
2. Experiment 1. Glucose vs. Fructose preferences in T1R3 KO, TRPM5 KO and WT female mice
2.1. Methods
2.1.1. Subjects
Naïve T1R3 KO (n=10) [10] and TRPM5 KO (n=10) [9] mice were derived from mice produced by homologous recombination in C57BL/6J embryonic stem cells and maintained on this background. Naïve B6 WT mice were bred from stock obtained from the Jackson Laboratories (Bar Harbor, ME). All mice were females, as in our prior 24-h study of glucose and fructose preferences [54]. The animals were singly housed in plastic tub cages in a room maintained at 22°C with a 12:12-h light:dark cycle and given ad libitum access to chow (5001; PMI Nutrition International) and water except where noted. Experimental protocols were approved by the institutional animal care and use committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.1.2. Apparatus
Brief (1-min) and short (1-h) lick tests were conducted in 10 clear plastic chambers (15 × 15 × 32 cm) with a stainless-steel perforated floor. Fluid was available from two stainless-steel sipper spouts through slots (5 × 20 mm, 32 mm apart) in a stainless-steel plate at the front of the cage. The sipper spouts were attached to 50-mL glass tubes with a rubber stopper. The tubes were mounted on individual motorized bottle holders (ENV-252M; Med Associates, Fairfax, VT) that positioned the spouts 1 mm in front of the cage at the start of a trial and retracted them at the end of the trial. Licks were monitored with electronic lickometers (ENV-250B; Med Associates) interfaced to a microcomputer. Intakes were not measured because the low intakes precluded accurate measurements.
2.1.3. Procedure
The mice were initially water restricted overnight and trained to drink water from two sipper tubes during a 5-min session in the test chambers. On the next two days the water-restricted mice were given 1-min, two-bottle tests with 8% glucose vs. 8% fructose (food grade, Tate and Lyle, Honeyville Food Products); Table 1 summarizes the test sequence for this and subsequent experiments. In these and all subsequent 1-min and 1-h subsequent two-bottle tests the animals were first given 5-sec access to the left sipper tube, and then 5-sec access to the right sipper tube prior to the choice test. The mice were then returned to their home cages and 1 h later the 1-min test was repeated with the left-right position of the sipper tubes reversed. The timing of each 5-sec and 1-min period for each mouse began with its 10th lick. The latency to lick was measured as the time between presentation of the lick tubes to the 10th lick. (The 10th rather than first “lick” was used because occasionally a mouse made contact with the sipper tube with its nose or paw.) If the mouse did not emit 10 licks in the 5-sec or 1-min period, the sipper tube was retracted after 1 min or 2 min, respectively. The next 5-sec period began for all mice ~15 s after the last sipper tube was retracted. The mice were maintained water-restricted during these test days by giving them 1-h access to water following the last training/test session. Following the second sugar training session, the animals were given ad libitum access to water but restricted rations that maintained them at ~90% of their ad libitum body weight. Fixed-size chow pellets (0.5 or 1 g, F0171, F0173; Bio-Serv, Frenchtown, NJ) allowed for precise adjustment of daily food rations.
Table 1.
Experimental Procedures
| Experimental Days | Dep. State* | Solutions | |
|---|---|---|---|
| 1-min Tests | Experiment 1: TRPM5 KO, T1R3 KO, WT Groups | ||
| 1 – 2 | WR Two-Bottle Test | Water restricted | 8% glucose vs. 8% fructose |
| 3 | FR Two-Bottle Test | Food restricted | 8% glucose vs. 8% fructose |
| 1-h Tests | |||
| 4 – 5 | Two-Bottle Test 1 | Food restricted | 8% glucose vs. 8% fructose |
| 6 – 9 | One-Bottle Train | Food restricted | 8% glucose and 8% fructose |
| 10 – 11 | Two-Bottle Test 2 | Food restricted | 8% glucose vs. 8% fructose |
| 24-h Home Cage Tests | |||
| 12 – 15 | One-Bottle Train | Food restricted | TRPM5 KO, WT: 8% glucose and 8% fructose |
| 16 – 17 | Two-Bottle Test | Food restricted | TRPM5 KO, WT: 8% glucose vs. 8% fructose |
| 12 – 13 | Two-Bottle Test | Food ad lib | T1R3 KO Lo, Hi: 0.2% saccharin vs. water |
| 1-min Tests | Experiment 2: T1R3 KO, WT Groups | ||
| 1 – 2 | WR Two-Bottle Test | Water restricted | 8% glucose vs. 8% fructose |
| 3 | FR Two-Bottle Test | Food restricted | 8% glucose vs. 8% fructose |
| 1-h Tests | |||
| 4 – 5 | Two-Bottle Test 1 | Food restricted | 8% glucose vs. 8% fructose |
| 6 – 9 | One-Bottle Train | Food restricted | 8% glucose and 8% fructose |
| 10 – 11 | Two-Bottle Test 2 | Food restricted | 8% glucose vs. 8% fructose |
| 13 – 14 | Two-Bottle Test 3 | Food restricted | 0.2% saccharin vs. water |
| 24-h Home Cage Tests | |||
| 15 – 16 | Two-Bottle Test | Food ad lib | 8% glucose vs. 8% fructose |
| 17 – 18 | Two-Bottle Test | Food ad lib | 8% fructose vs. water |
| 1-min Tests | Experiment 3: T1R3 KO, WT Groups | ||
| 1 – 2 | WR Two-Bottle Test | Water restricted | 8% glucose vs. 8.6% MDG |
| 3 | FR Two-Bottle Test | Food restricted | 8% glucose vs. 8.6% MDG |
| 1-h Tests | |||
| 4 – 5 | Two-Bottle Test 1 | Food restricted | 8% glucose vs. 8.6% MDG |
| 6 – 9 | One-Bottle Train | Food restricted | 8% glucose and 8.6% MDG |
| 10 – 11 | Two-Bottle Test 2 | Food restricted | 8% glucose vs. 8.6% MDG |
| 13 – 14 | Two-Bottle Test 3 | Food restricted | 0.2% saccharin vs. water |
Deprivation states described in Methods. All 1-min and 1-h tests conducted in lick test chambers; 24-h tests conducted in home cages.
While food-restricted, the mice were given two 1-min glucose (G) vs. fructose (F) test sessions, followed on subsequent days by two 1 h/day test sessions (Test 1) with glucose vs. fructose. They were next given four 1 h/day one-bottle training sessions with the two sugars (F,G,F,G, in that order) which allowed them to associate the orosensory and postoral properties of each sugar. This was followed by another two 1 h/day, two-bottle sessions (Test 2) with glucose vs. fructose. Timing for 1-h tests began for all mice when the sipper tubes were inserted in front of the test cages, although the latency to the 10th lick was recorded. The 1-h tests were conducted in the test chambers. As described below and in Table 1, additional 24-h, one- and two-bottle tests were conducted in home cages with food-restricted TRPM5 KO and WT mice. As a phenotype test of their sweet ageusia [18], the T1R3 KO mice were given 24-h two-bottle tests with 0.2% saccharin (sodium saccharin, Sigma-Aldrich, St. Louis, MO) vs. water in their home cages with food ad libitum. Fluid intakes were recorded in the 24-h sessions by weighing the fluid bottles at the start and end of the sessions; intakes were corrected for spillage. Fluid spillage was estimated by measuring the change in weight of two sugar bottles kept on an empty cage for 24 h.
The 1-min and 1-h lick data and 24-h intake data were averaged for the two two-bottle or one-bottle sessions, which controlled for side preferences. Genotype differences in sugar acceptance were evaluated using a mixed model ANOVA with genotype as a between group factor and sugar (glucose vs. fructose) as a within-group factor. Within-group differences in sweetener intakes were evaluated with simple main effects tests. Two-bottle sugar preferences were calculated as percent glucose preference (e.g., glucose licks/total licks x 100) and evaluated in separate ANOVAs or t-tests.
2.1. Results
In Test 1, six of the T1R3 KO mice completed between 558 and 2787 total licks across the 1-h test. This rate of licking was comparable to that of the B6 WT mice, who completed between 892 and 2017 total licks across the 1-h test. In contrast, the remaining four T1R3 KO mice only completed 54–110 total licks across the 1-h test; this rate of licking was within the range of the TRPM5 KO mice, which completed between 50 and 455 total licks across the 1-h test. Based on the apparent bimodality in lick rate for sugars across the T1R3 KO mice, we henceforth assigned them to the T1R3 KO Hi and Lo lick groups. Supplemental Fig. S1 shows the distribution of Test 1 total licks for the individual mice in this and subsequent experiments. The statistical analysis focused on comparing the behavior of the T1R3 KO Hi lick group (n=6) with that of the TRPM5 KO (n=10) and WT (n=10) groups. Experiment 2 included a large group of T1R3 KO that allowed for analyses of T1R3 KO Hi and Lo lick subgroups.
1-min Tests
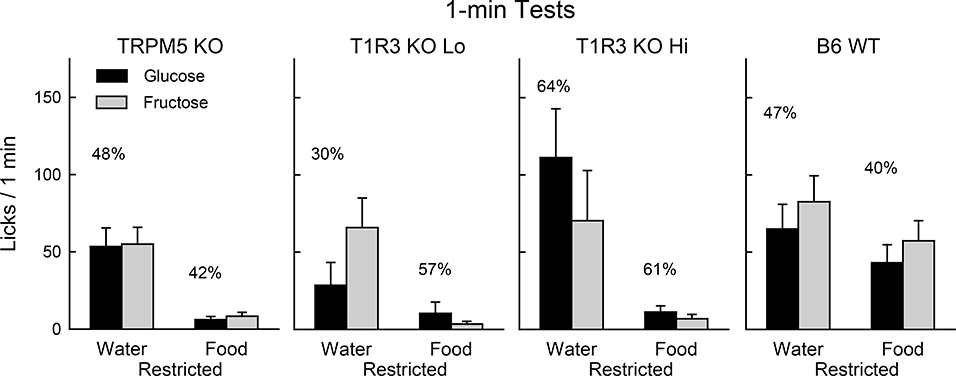
When water restricted, the T1R3 KO Hi, TRPM5 KO, and WT mice licked similar amounts for the two sugars and there were no group differences in sugar preferences (Fig. 1). Significant group differences emerged, however, when the mice were tested while food restricted. Now the T1R3 KO Hi and TRPM5 KO mice emitted very few licks compared to the WT mice [F(2,23) = 11.8, P < 0.001] (Fig. 1). The T1R3 KO Hi mice tended to lick more glucose and the TRPM5 KO and WT groups more fructose but these differences were not significant and there were no group differences in percent glucose preference. When water deprived, T1R3 KO Hi mice licked more than the KO Lo mice, but the two subgroups were similar in their low lick rates when food restricted (Fig. 1).
Fig. 1.
Experiment 1. Mean (+SE) 8% glucose and 8% fructose licks during 1-min two-bottle tests conducted under water- or food-restriction for TRPM5 KO (n=10), T1R3 KO Lo (n=4), T1R3 KO Hi (n=6), and B6 WT (n=10) mice. The mice had no prior experience with sugars. Numbers atop bars represent the mean percent preference for that sugar.
1-h Tests
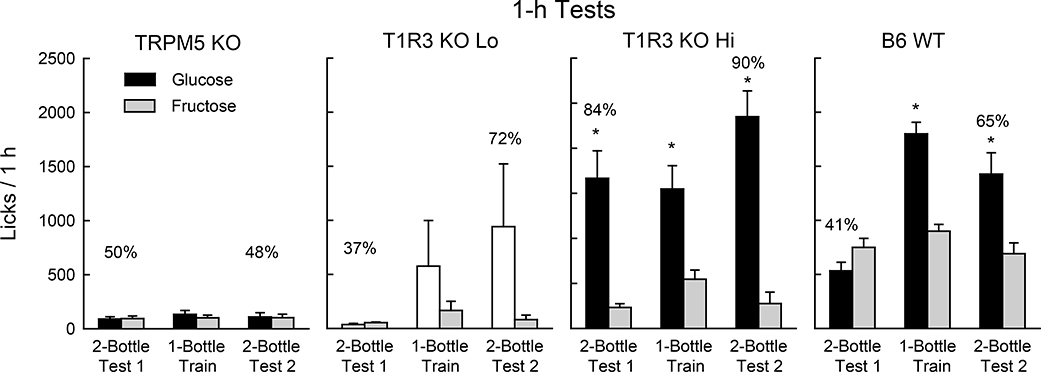
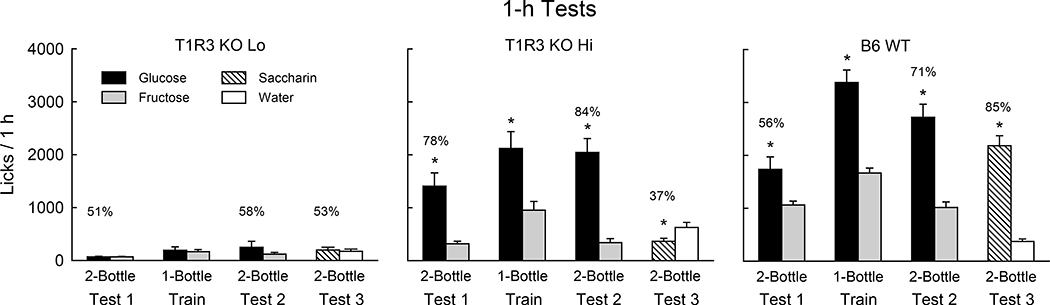
When lick tests were extended to 1 h/day, significant group differences emerged (Fig. 2). In Test 1, the T1R3 KO Hi group licked substantially more than did the TRPM5 KO mice and even more than the WT mice [F(2,23) = 33.1, P < 0.001]. (Even including all 10 mice, the T1R3 KO group licked more sugar than the TRPM5 KO group, 1175.1 vs. 182.8 licks/h, P < 0.001). T1R3 KO Hi and WT groups differed, however, in that the KO mice licked significantly (P < 0.01) more for glucose than fructose, whereas the WT mice licked slightly more for fructose [Group x Sugar interaction, F(2,23) = 32.8, P < 0.001]. Note that the T1R3 KO Hi mice licked more glucose than fructose in the first and second 1-h sessions of Test 1 (1666.3 vs. 282.0, 2005.5 vs. 182.8 licks/h, P < 0.01). In contrast, the WT mice licked more fructose than glucose in the first session (836.6 vs. 379.0, P < 0.01) but not in the second session (661.6 vs. 687.5 licks/h). Overall, the Test 1 glucose percent preference of the T1R3 KO Hi mice exceeded that of the WT mice (84% vs. 41%, t(14) = 6.1, P < 0.001). In the one-bottle training sessions, the T1R3 KO Hi and WT mice licked much more than did the TRPM5 KO mice [F(2,23) = 63.9, P < 0.001]. The WT and T1R3 KO Hi groups licked similar amounts and both licked significantly more (P < 0.01) for glucose than fructose. In the second two-bottle test (Test 2), the T1R3 KO Hi and WT mice again licked substantially more than did the TRPM5 mice [F(2,23) = 59.5, P < 0.001] and now both groups licked (P < 0.001) more for glucose than fructose. The T1R3 KO Hi mice, however, licked more (P < 0.05) glucose than did the WT mice and displayed a greater glucose preference (90% vs. 65%, t(14) = 2.9, P < 0.05).
Figure 2.
Experiment 1. Mean (+SE) 8% glucose and 8% fructose licks during 1-h two-bottle tests and one-bottle training sessions under food-restriction for TRPM5 KO (n=10), T1R3 KO Lo (n=4), T1R3 KO Hi (n=6) and B6 WT (n=10) mice. Numbers atop bars represent the mean percent preference for that sugar. Two of the T1R3 KO Lo mice developed an avidity for glucose in the 1-bottle and 2-bottle Test 2 sessions which accounts for the increase in the glucose means and SEs. Significant (P < 0.05) within group differences between glucose and fructose licks indicated by *.
As previously noted, the four mice in the T1R3 KO Lo subgroup licked very little sugar in Test 1, but two of the mice developed an attraction to glucose in the one-bottle sessions which led to a significant preference for glucose over fructose in Test 2 (1849.5 vs. 86.0, licks/h, 94%). The remaining two mice remained indifferent to glucose and fructose in Test 2 (31.3 vs. 76.8 licks/h, 49%).
24-h Tests
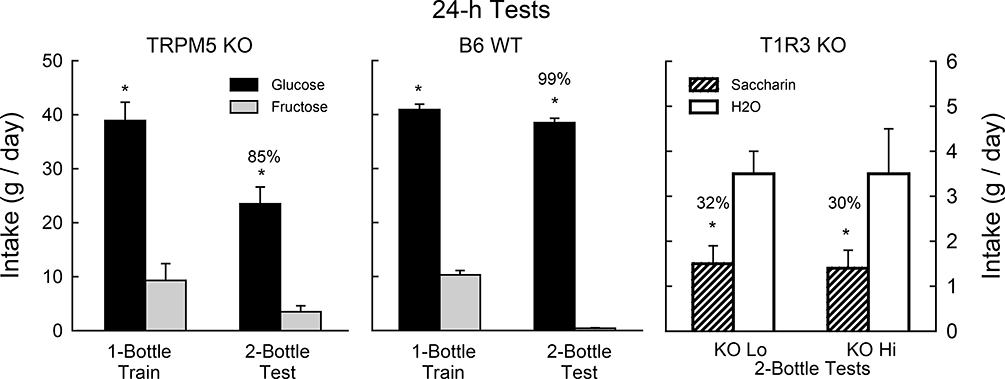
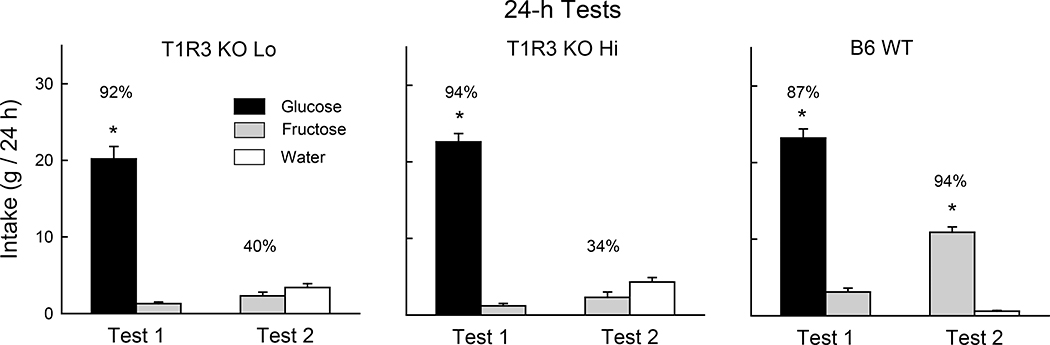
In our prior study, TRPM5 KO mice acquired a significant preference for 8% glucose but not for 8% fructose in 24-h tests with sugar vs. water [54]. We therefore determined if the current TRPM5 KO mice, which were indifferent to glucose and fructose in 1-min and 1-h tests, would come to prefer glucose when given 24-h experience with the two sugars. During initial 24-h one-bottle sessions, the food-restricted TRPM5 KO mice, like WT mice, consumed significantly more glucose than fructose [F(1,18) = 290.2, P < 0.001] and the groups did not differ in their overall intakes (Fig. 3). In the subsequent 24-h, two-bottle test, both groups consumed significantly more glucose than fructose [F(1,18) = 249.0, P < 0.001]. The TRPM5 KO mice, however, consumed less glucose than did the WT mice; the fructose intakes of the two groups did not differ [Group x Sugar interaction, F(1,18) = 20.7, P < 0.001]. In addition, the glucose percent preference of the TRPM5 group was less than that of the WT group (85% vs. 99%, t(18) = 2.9, P < 0.01).
Figure 3.
Experiment 1. Left panels: Mean (+SE) 8% glucose and 8% fructose intakes during 24-h one-bottle training days and two-bottle test under food-restriction for TRPM5 KO (n=10) and B6 WT (n=10) mice. Right panel: Mean (+SE) 0.2% saccharin and water intakes during 24-h two-bottle test for T1R3 KO Lo (n=4) and KO Hi (n=6) mice. Numbers atop bars represent the mean percent preference for that solution. Significant (P < 0.05) within group differences between solution intakes indicated by *.
In the 24-h saccharin vs. water test conducted with T1R3 KO mice, the KO Hi and KO Lo subgroups both consumed less saccharin than water (F(1,8) = 7.6, P < 0.05) and they did not differ in their saccharin licks or percent preferences (32% vs. 30%) (Fig. 3). This confirms prior findings that T1R3 KO mice avoid saccharin, presumably due to its bitter taste component [16,43].
2.3. Discussion
When water restricted, the KO mice licked as much as the WT mice. This indicated that their thirst motivation was intact and their taste ageusia did not impair licking responses in the test cages. However, when food-restricted, the KO mice licked at very low rates. This is attributed to their greatly reduced attraction to the taste of the sugars rather than impaired hunger motivation. In other experiments, food-restricted T1R3 KO mice readily licked for maltodextrin solutions, which have an attractive taste not mediated by the T1R2+T1R3 sweet receptor [45,53]. When the sugar tests were extended to 1 h/day, all the TRPM5 KO mice licked at very low rates. However, in the 24-h one and two-bottle tests the TRPM5 KO mice licked substantially more glucose than fructose. This is consistent with prior findings of TRPM5 KO mice preferring glucose but not fructose in 24-h sugar vs. water tests [54]. With prolonged training, the KO mice learned to associate the TRPM5-independent orosensory properties of glucose with the sugar’s potent postoral reinforcing effects.
In contrast to the TRPM5 KO group, 6 of the 10 T1R3 KO mice licked substantially during the 1-h Test 1. Furthermore, T1R3 KO Hi mice significantly preferred glucose to fructose in the very first 1-h session whereas the WT mice preferred fructose in this session. The T1R3 KO Hi and WT mice then licked more glucose than fructose in the 1-h training sessions, and both groups preferred glucose in Test 2, although the preference was greater for the T1R3 KO Hi group. The increased glucose preference displayed in Test 2 by T1R3 KO Hi and WT mice confirms prior findings that separate experience with the two sugars enhances the preference for glucose over fructose [1,40].
Why the six T1R3 KO Hi mice licked considerably more in Test 1 than the four T1R3 KO Lo mice (1898.7 vs. 89.6 total licks/1 h) is not clear. The Hi and Lo subgroups did not differ in their total licks during the 1-min, food-restricted test (18.1 vs. 13.9). The T1R3 KO Hi and Lo subgroups also did not differ in their lack of preference for saccharin in the 24-h test (Fig. 3). This indicates that their differential 1-h response to glucose and fructose does not represent a generalized difference in their sweetener preferences. As noted above, following one-bottle training, two of the four T1R3 KO Lo mice displayed a strong glucose preference in Test 2.
3.1. Experiment 2: Glucose vs. fructose preferences in male and female T1R3 KO and WT mice
In view of the bimodal 1-h sugar licking responses observed in Experiment 1, the second experiment studied a much larger number of T1R3 KO mice (n=32) so that the behavioral responses of high and low licking KO subgroups could be analyzed in greater detail. In addition, both male and female were included to determine if there are sex differences in the avidity for sugars in T1R3 KO mice.
3.1.1. Methods
Naïve T1R3 KO (female n=19, male n=13) and WT mice (female n=12, male n=12) were given 1-min and 1-h sugar tests as in the first experiment. Additional 1-h and 24-h tests were conducted with 8% glucose, 8% fructose and 0.2% saccharin as described in the Results section.
3.1.2. Results
Based on the Test 1 lick totals observed with the T1R3 KO, TRPM5 KO and B6 WT mice in the first experiment, the T1R3 KO mice in this and subsequent experiments were divided into Lo and Hi subgroups if their total Test 1 licks were less or more than 500 total licks/h, respectively (see Fig. S1). The mice in the T1R3 KO Lo group (n=18) emitted 55–314 total licks/h in Test 1 compared to the T1R3 KO Hi mice (n=14) which licked 516–3707 total licks/h. Statistical analyses compared the responses of these two subgroups with that of the B6 WT mice (n=24) which had 878–5158 total licks/h in Test 1. Additional analyses were conducted with sex as a factor.
1-min Tests
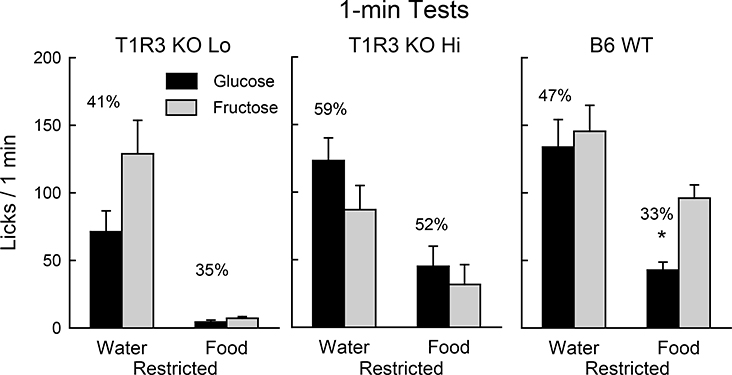
When water restricted, the T1R3 KO Lo and T1R3 KO Hi mice licked similar amounts for the two sugars but somewhat less than did the WT mice [F(2,53) = 4.0, P < 0.05]; licks for glucose and fructose did not significantly differ in the three groups (Fig. 4). The three groups displayed similar latencies to lick (6.6, 8.7, 6.9 sec). When food restricted, however, significant group differences in licks and latencies emerged. The T1R3 KO Lo mice licked less than the T1R3 KO Hi mice, which in turn licked less than the WT mice (11.7 vs. 59.9 vs. 139.0 total licks/min, F(2,53) = 38.8, P < 0.001). In addition, whereas the two T1R3 KO groups did not significantly differ in their glucose and fructose licks, the WT mice licked less (P < 0.01) for glucose than fructose [Group x Sugar interaction, F(2,53) = 8.5, P < 0.01]. The latencies to lick also differed for the T1R3 KO Lo, T1R3 KO Hi, and WT mice (70.7 > 43.2 > 7.7 sec, respectively; F(2,53) = 24.5, P < 0.001).
Figure 4.
Experiment 2. Mean (+SE) 8% glucose and 8% fructose licks during 1-min two-bottle tests conducted under water- or food-restriction for T1R3 KO Lo (n=18), T1R3 KO Hi (n=14) and B6 WT (n=24) mice. The mice had no prior experience with sugars. Numbers atop bars represent the mean percent preference for that sugar. Significant (P < 0.05) within group differences in glucose and fructose licks indicated by *.
1-h Tests
As shown in Fig. 5, mice in the T1R3 KO Lo subgroup licked very little in Test 1, and 16 of the 18 mice continued to lick very little sugar in the 1-bottle training sessions and 2-bottle Test 2. Two of the KO Lo mice, however, displayed an increased attraction to sugars during 1-bottle sessions and subsequently a strong glucose over fructose preference in Test 2 (1382.5 vs. 202.3 licks/h, 85.2%). In contrast, the T1R3 KO Hi mice licked as much glucose as did the WT mice in Test 1 (Fig. 5). The WT mice licked more fructose than did the KO Hi mice, so their total licks exceeded that of the KO Hi group [F(1,36) = 6.4, P < 0.05]. Also, the latency to initiate licking was shorter for the WT mice than for the KO Hi mice which, in turn, was shorter than for the KO Lo mice (5.4 vs. 73.1 vs. 578.2 sec, F(2,53) = 574.5, P < 0.001). The KO Hi and WT mice licked more for glucose than fructose with the difference being greater for the KO Hi mice, although the Group x Sugar interaction was not significant. Nevertheless, the percent glucose preference of the KO Hi group exceeded that of the WT group in Test 1 (78% vs. 56%, t(36) = 3.6, P < 0.001).
Figure 5.
Experiment 2. Mean (+SE) 8% glucose and 8% fructose licks during 1-h two-bottle Tests 1 and 2, and one-bottle training sessions under food-restriction for T1R3 KO Lo (n=18), T1R3 KO Hi (n=14) and B6 WT (n=24) mice. The 0.2% saccharin vs. water Test 3 included 23 B6 WT mice. Numbers atop bars represent the mean percent preference for that solution. Significant (P < 0.05) within group differences between glucose vs. fructose or saccharin vs. water licks indicated by *.
A detailed analysis of the two 1-h sessions that constituted Test 1 revealed even greater group differences between T1R3 KO Hi and WT mice (Fig. S2). In the first 1-h session the T1R3 KO Hi mice licked more (P < 0.05) glucose than fructose as early as 15 min into the session. In contrast, the WT mice licked more (P < 0.05) for fructose than glucose at 15 min although by the end of the 1-h session they licked equally for the two sugars. There were considerable individual differences in sugar licks and preferences, however, particularly in the WT group. In the first 1-h session, 11 of the 14 (78%) KO Hi mice licked more glucose than fructose whereas only 10 of 24 (42%) WT mice did so; mean 1-h glucose preferences were 71% and 48%, respectively, for the KO Hi and WT groups [t(36) = 3.0, P < 0.001] (Fig. S2). In the second test session, 13 of 14 KO Hi mice licked more glucose than fructose but only 15 of 24 WT mice did so; mean glucose preferences were now 79% and 61%, respectively for the T1R3 KO Hi and WT groups [t(36) = 2.5, P < 0.05].
In the subsequent one-bottle tests both the T1R3 KO Hi and WT groups licked substantially more (175% and 107%, respectively) for glucose than fructose [F(1,36) = 100.2, P < 0.001]; overall total 1-h licks were higher for the WT group than the KO Hi group [F(1,36) = 12.1, P < 0.01] (Fig. 5). In two-bottle sugar Test 2, both groups licked more for glucose than fructose [F(1,36) = 66.2, P < 0.001] and total licks were again higher for the WT group [F(1,36) = 10.8, P < 0.01] (Fig. 5). The KO Hi mice, however, displayed a stronger glucose preference than did the WT mice (84% vs. 71%, t(36) = 2.5, P < 0.05]. In contrast to the results of the food-restricted (FD) 1-min Test and 1-h Test 1, the latencies to lick in Test 2 did not significantly differ for the WT and KO Hi mice (3.7 vs. 31.3 sec; the KO Hi group latency was 8.7 sec excluding one unusual KO Hi mouse with a latency of 324.1 sec). Also in contrast to the FD 1-min Test, the total licks emitted by the WT and KO Hi groups during the first min of Test 2 did not significantly differ (246.2 vs. 222.0 licks/min), although the percent glucose licks in the first min were higher for the KO Hi mice than for the WT mice (76.0 vs. 55.6%, t(29) = 2.3, P < 0.05). The 1-min data are based on 11 KO Hi and 20 WT mice; the 1-min lick data for the remaining mice were lost due to a computer storage problem.
In view of the substantially different 1-h sugar preferences displayed by the T1R3 KO Lo and Hi subgroups, two-bottle Test 3 determined if they differed in their preference for 0.2% saccharin vs. water. As shown in Fig. 5, whereas the WT mice licked substantially more for saccharin than water, both T1R3 KO Hi and Lo subgroups failed to do so [Group x Sweetener interaction, F(2,52) = 74.2, P < 0.001]. In fact, the T1R3 KO Hi mice licked more (P < 0.01) for water than saccharin. (The saccharin and subsequent sugar test data for one female WT mouse were discarded because of illness.)
24-h Tests
In our prior study [54] T1R3 KO mice strongly (~90%) preferred 8% glucose to water but did not prefer 8% fructose to water in 24-h, two-bottle tests. Given that the T1R3 KO Lo mice in the present experiment were indifferent to 8% glucose and fructose in 1-h tests, a 2-day home cage test was conducted with these mice to determine if they would prefer glucose to fructose when given 24-h test sessions; food was ad libitum in this test. As shown in Fig. 6, T1R3 KO Lo mice, as well as T1R3 KO Hi and WT mice consumed substantially more glucose than fructose in the 24-h test [F(2,52) = 453.1, P < 0.001]. The percent glucose preferences of the T1R3 KO Lo, T1R3 KO Hi and WT groups did not significantly differ (92%, 94%, 87%, respectively). Overall, the T1R3 KO Lo mice consumed slightly less (P < 0.05) sugar than did the WT mice, while the KO Hi and WT mice did not differ in total sugar intakes [F(2,52) = 4.5, P < 0.05]. The sugar preferences of the two T1R3 KO subgroups were further analyzed by comparing individual mouse intakes in the first and second days of the 24-h test (Supplemental Fig. S3). On both test days, Lo and Hi subgroups consumed more glucose than fructose and there were no group differences [F(1,30 = 337.1, P < 0.001]. In particular, on the first day, 16 of 18 Lo mice and 13 of 14 Hi mice preferred glucose to fructose, and on day 2 all mice in both subgroups strongly preferred glucose. Overall, the subgroups consumed more glucose on day 2 than day 1 [Day x Sugar interaction, F(1,30) = 15.1, P < 0.01] and percent glucose preference increased from the first to second day [F(1,30) = 5.8, P < 0.05].
Figure 6.
Experiment 2. Mean (+SE) 8% glucose vs. fructose intakes and 8% fructose vs. water intakes during 24-h, two-bottle Tests 1 and 2 with food ad libitum for T1R3 KO Lo (n=18), T1R3 KO Hi (n=14) and B6 WT (n=23) mice. Numbers atop bars represent the mean percent preference for that sugar. Significant (P < 0.05) within group differences between glucose vs. fructose intakes and fructose vs. water intakes indicated by *.
In a final 2-day test, the mice were given the choice of 8% fructose vs. water. Whereas the WT mice consumed more (P < 0.001) fructose than water, both T1R3 KO subgroups consumed slightly more water than fructose [Group x Sugar interaction, F(2,52) = 55.8, P < 0.001] (Fig. 6). The percent fructose preference of the WT mice also exceeded that of the T1R3 KO Lo and T1R3 KO Hi subgroups (94% vs. 40% and 34%, F(2,52) = 32.7, P < 0.001). This indicates that the prior experience of the T1R3 KO mice with glucose and fructose did not induce a preference for fructose over water.
Sex Differences
Overall, there were few sex differences in the sweetener responses of the mice. The proportion of T1R3 KO Hi lickers were similar for the female (8/19 = 0.42) and male (6/13 = 0.46) mice and there were no sex differences in glucose and fructose licks/intakes in the 1-min, 1-h, and 24-h tests conducted with food-restricted and ad libitum mice. In the 1-min, water-restricted test total licks were higher (P < 0.05) in male than female mice, and in the 1-h saccharin vs. water and 24-h fructose vs. water mice KO Hi and WT male mice licked more (P < 0.05) sweetener than did female mice.
3.3. Discussion
Consistent with the first experiment, the food-restricted T1R3 KO mice licked considerably less sugar than WT mice in the 1-min choice test but many T1R3 KO mice licked substantial amounts in 1-h two-choice Test 1. In fact, T1R3 KO Hi mice displayed a stronger glucose preference than did the WT mice in Test 1. The KO Hi mice licked more sugar than did the KO Lo mice in the 1-min test, which may be related to their propensity to lick more in the 1-h test. In contrast to their different 1-h lick responses, the KO Lo and KO Hi subgroups displayed similar 24-h sugar intakes and glucose preferences, so that they did not fundamentally differ in their avidity for glucose over fructose except for their tendency to lick in the 1-h test periods. The finding that 16 of the T1R3 KO Lo mice consumed little or no fructose (mean 1.1 g/24 h) in the first 24-h test day suggests that oral glucose sensing, not postoral conditioning was responsible for their glucose preference. The finding that T1R3 KO Lo mice preferred glucose in 24-h but not 1-min or 1-h tests indicates that the T1R3-independent glucose sensor generates a relatively weak signal compared to the T1R2+T1R3 sweet receptor. Nevertheless, postoral glucose appetition presumably stimulated their glucose intakes on day 1 and 2 of testing [52,54] (see General Discussion).
Consistent with the first experiment, both the T1R3 KO Hi and KO Lo subgroups did not prefer saccharin to water in Test 3 nor did they prefer fructose to water in the final 24-h test. These findings further demonstrate the specificity of glucose preferences displayed by the T1R3 KO mice. A new finding of this experiment was that male and female T1R3 KO mice are similar in their propensity to display glucose preferences in 1-h choice tests.
4. Experiment 3: Glucose and α-methyl-D-glucopyranoside preferences in female T1R3 KO and WT mice
The 1-h glucose preferences displayed by many of the T1R3 KO mice in Experiments 1 and 2 appear to represent the actions of a glucose-specific taste receptor. One potential glucose sensor is SGLT1, which is expressed in T1R2+T1R3 taste cells and binds to glucose but not fructose [47,48]. Consistent with this interpretation, lingual application of the SGLT1 inhibitor phloridizin reduced the chorda tympani nerve response to glucose in mice [47]. SGLT1 is of particular interest because in the intestinal tract it mediates, at least in part, the postoral appetition actions of glucose [38,52]. Experiment 3 investigated the possible involvement of taste cell SGLT1 in the glucose preference displayed by T1R3 KO mice by comparing their preference responses to isomolar (0.44 M) concentrations of glucose (8%) and α-methyl-D-glucopyranoside (8.6% MDG). MDG is a non-metabolizable analog of glucose that, like glucose, binds to SGLT1 as well as to the T1R2+T1R3 sweet receptor [11,13,20]. Furthermore, in flavor conditioning studies IG infusions of 8% MDG conditioned similar preferences as did IG 8% glucose in B6 mice [52]. In a 3-min choice test, B6 mice preferred isomolar MDG to glucose, suggesting that it has a sweeter taste [3]. If T1R3 KO mice, like WT mice, prefer MDG as much or more than glucose this would support the idea that a lingual SGLT1 taste pathway mediates the residual glucose preference of the KO mice.
4.1.1. Methods
Naïve female T1R3 KO (n=14) and WT mice (n=10) were tested as in Experiment 2 but with isomolar 8% glucose and 8.6% MDG (Sigma Chemical Co., St. Louis, MO). Based on total licks emitted in the 1-h Test 1, the T1R3 KO mice were divided into KO Hi (n=10, 599–3031 total licks/h) and KO Lo (n=4, 68–375 total lick/h) subgroups (see Fig. S1). Because of the small size of the KO Lo subgroup, statistical analysis focused on the T1R3 KO Hi and WT groups. The mice were given 1-min and 1-h lick tests with the two sugars as in prior experiments. They were also tested for their 1-h preference for 0.2% saccharin vs. water.
4.1.2. Results and Discussion
1-min Tests
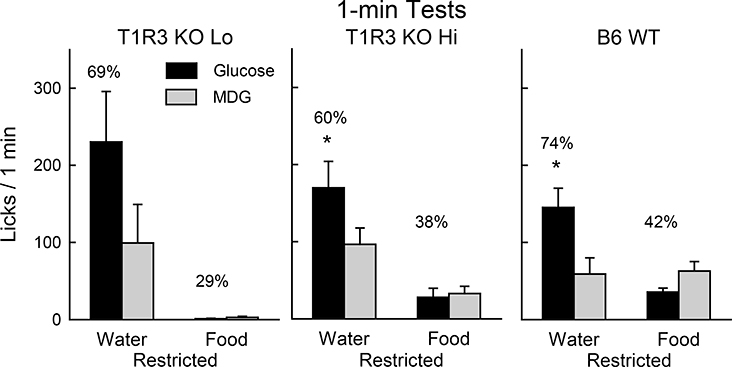
When water restricted, the T1R3 KO Hi and WT groups did not differ in total licks and both groups licked more for glucose than MDG [F(1,18) = 11.0, P < 0.01] (Fig. 7). When food restricted, the WT mice licked more than the T1R3 KO Hi mice, although this difference was marginal (P = 0.064), and both groups tended to lick more for MDG than glucose. The T1R3 KO Hi and WT groups had similar latencies to lick (6.7, 10.2 sec) when water restricted but the T1R3 KO Hi mice had longer latencies than WT mice when food restricted (66.1 vs. 11.8 sec, t(18) = 2.9, P < 0.01). Note that like the KO Hi and WT groups, the T1R3 KO Lo mice licked more glucose than MDG when water deprived, but licked very little sugar when food deprived (Fig. 7).
Figure 7.
Experiment 3. Mean (+SE) 0.44 M glucose (8%) vs. α-methyl-D-glucopyranoside (8.6% MDG) licks during 1-min two-bottle tests conducted under water- or food-restriction for T1R3 KO Lo (n=4), T1R3 KO Hi (n=10) and B6 WT (n=10) mice. The mice had no prior experience with sugars. Numbers atop bars represent the mean percent preference for that sugar. Significant (P < 0.05) within group differences between glucose vs. MDG licks indicated by *.
1-h Tests
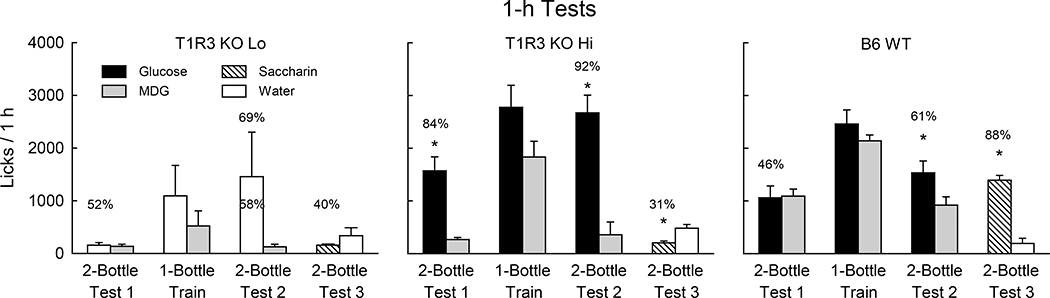
When given 1-h access to the two sugars in Test 1, the T1R3 KO Hi and WT groups did not differ in their total licks but the T1R3 KO Hi mice licked more for glucose than MDG while the WT mice licked similar amounts for the two sugars (Group x Sugar interaction, F(1,18) = 16.2, P < 0.001) (Fig. 8). Consequently, the percent glucose preference of the T1R3 KO Hi mice exceeded that of the WT mice (84% vs. 46%, t(18) = 6.7, P < 0.001). On the other hand, the latency to lick was longer in the T1R3 KO Hi group than in the WT group (150.1 vs. 13.4 sec, t(18) = 2.53, P < 0.05). In the first 1-h session of Test 1, 6 of 10 T1R3 KO Hi mice licked substantially more glucose than MDG while the remaining four T1R3 KO Hi mice licked very little for either sugar (Fig. S4). In the second session all 10 T1R3 KO Hi mice licked more glucose than MDG. In contrast, only 2 of 10 WT mice licked more glucose than MDG in session 1 and 6 of 10 WT mice in session 2 did so. In the subsequent 1-bottle sessions, the T1R3 KO Hi mice licked more (P < 0.001), by 60%, for glucose than MDG while the WT mice licked only slightly more (15%) for glucose than MDG [Group x Sugar interaction, F(1,18 = 5.0, P < 0.05] (Fig. 8). In two-bottle Test 2 both groups now licked more for glucose than MDG [F(1,18) = 50.4, P < 0.001] although the T1R3 KO Hi mice licked more (P < 0.01) for glucose than did WT mice (Group x Sugar interaction, [F(1,18) = 17.0, P < 0.001]. The percent glucose preference of the T1R3 KO Hi mice also exceeded that of the WT mice (92% vs. 61%, t(18) = 4.0, P < 0.001). In contrast to Test 1, the latencies to lick in Test 2 were similar for the T1R3 KO Hi and WT mice (5.7 vs. 5.1 sec).
Figure 8.
Experiment 3. Mean (±SE) 0.44 M glucose (8%) vs. α-methyl-D-glucopyranoside (8.6% MDG) licks during 1-h two-bottle Tests 1 and 2, and one-bottle training sessions, and during 0.2% saccharin vs. water 2-bottle Test 3 under food restriction for T1R3 KO Lo (n=4), T1R3 KO Hi (n=10) and B6 WT (n=10) mice. Two of T1R3 KO Lo mice developed an avidity for glucose in the 1-bottle and 2-bottle Test 2 sessions which accounts for the increase in the glucose means and SEs. Numbers atop bars represent the mean percent preference for that sugar. Significant (P < 0.05) within group differences between glucose vs. fructose or saccharin vs. water licks indicated by *.
In a final two-bottle test, the WT mice licked significantly more for saccharin than water while the T1R3 KO Hi mice licked more water than saccharin [F(1,18) = 196.2, P < 0.001]; overall, the WT mice licked substantially more than T1R3 KO Hi mice in this test [F(1,18) = 42.2, P < 0.001] (Fig. 8). The percent saccharin preference was much greater for WT than T1R3 KO Hi mice (88% vs. 31%, t(18) = 12.8, P < 0.001).
As previously noted, the four mice in the T1R3 KO Lo subgroup licked very little sugar in Test 1. However, two of the mice developed an attraction to glucose in the one-bottle sessions, which led to a significant preference for glucose over MDG in Test 2 (2869.0 vs. 195.5 licks/h, 94%). The remaining two mice remained indifferent to glucose and MDG in Test 2 (44.5 vs. 56.8 licks/hr, 45%). The four T1R3 KO Lo mice either avoided or were indifferent to saccharin (percent intake 21% - 55%) in Test 3 (Fig. 8).
Because both glucose and MDG bind to SGLT1, the profound preference for glucose over MDG displayed by the T1R3 KO Hi mice argues against a critical role of SGLT1 in the glucose over fructose preference displayed by the T1R3 KO Hi mice in Experiments 1 and 2. This does not exclude the involvement of SGLT1, but if so the critical glucose signal would be downstream from the SGLT1 channel. As discussed below, the glucose preference over MDG also does not support a critical role for postoral conditioning in the glucose over fructose preference displayed by the T1R3 KO Hi mice in Experiments 1 and 2. Given that water-restricted KO and WT mice in prior experiments did not differ in their licking response to glucose and fructose, the glucose preference over MDG displayed by the water-restricted T1R3 KO Lo, T1R3 KO Hi and B6 WT mice was unexpected. The reason why thirsty but not hungry mice preferred glucose to MDG in 1-min tests is not known.
5. General Discussion
The present findings replicate prior studies in showing that food-restricted sweet ageusic T1R3 KO and TRPM5 KO mice show little avidity for sugar solutions in brief access lick tests [24,46,50,53]. However, when the test was extended to 1 h, a substantial number of T1R3 KO mice were attracted to glucose and preferred it to fructose. In contrast, the WT mice showed no or at best a weak (41–56%) preference for glucose in 1-h Test 1. Following one-bottle training, during which they consumed more glucose than fructose, the WT mice displayed a more substantial glucose preference (65–71%), although still weaker than that of the T1R3 KO Hi mice (84–90%). With 24-h training and testing, all mice (TRPM5 KO, T1R3 KO, and WT) displayed near-total preferences for glucose over fructose. The findings suggest that mice have a T1R3-independent glucose taste sensor that mediates the 1-h glucose preference of T1R3 KO mice. The behavioral actions of this glucose taste sensor appear to be masked in WT mice because their T1R2+T1R3 sweet receptors are stimulated by both fructose and glucose. In fact, as discussed below, fructose appears to stimulate the sweet receptor more than isomolar glucose. The postoral actions of glucose enhanced the preference for this sugar in all mice but this does not readily account for the initial 1-h glucose preference displayed by T1R3 KO Hi mice.
An unusual but consistent finding of this study is that ~50% of the T1R3 KO mice studied showed an avidity to glucose during the first 1-h test with this sugar in the various experiments. This is not likely due to some residual functioning of the T1R3 receptor in these mice given that their sweetener response was specific to glucose; the T1R3 KO Hi and KO Lo mice did not differ in their low avidity to saccharin and fructose. Alternatively, the T1R3 KO Hi and Lo mice may have differed in the sensitivity of their putative glucose taste sensor. This possibility cannot be discarded. However, the nearly identical glucose preference responses displayed in Experiment 2 by the T1R3 KO Lo and Hi mice during the first 24-h glucose vs. fructose test session indicates that, over this time scale, the glucose responsiveness of the KO Lo and KO Hi mice was nearly identical.
We previously reported that T1R3 KO mice displayed significant preferences for 8% to 32% glucose but not fructose in 24-h sugar vs. water tests [54]. We attributed this preference to the T1R3 KO mice learning to associate T1R3-independent orosensory properties of glucose solutions (odor or texture) with the potent postoral appetition actions of the sugar. This interpretation was supported by our findings that, in 24-h sessions, T1R3 KO and WT mice acquired strong preferences for a flavored solution paired with IG sucrose (a glucose + fructose disaccharide) [37] and that WT mice learned to prefer flavors paired with IG glucose but not fructose [32,52]. Subsequent studies demonstrated that in WT mice, IG glucose but not fructose rapidly stimulated the intake of and preference for flavored saccharin solutions in 1 h/day sessions [51,52]. Yet, several findings suggest that postoral conditioning was not the primary cause of the initial glucose preference displayed by T1R3 KO Hi mice in the 1-h tests of the present experiments, although it would have enhanced their attraction to the sugar in the initial and subsequent sessions. First, most T1R3 KO Hi mice licked glucose and very little fructose in the first 1-h two-bottle session (Fig. S2), which indicates that oral sensing focused the initial sampling of the sugar solutions. This interpretation is not inconsistent with our earlier finding that IG glucose rapidly (within 15 min) stimulated oral intake because the mice in those experiments had only one flavored solution available to drink [51,52]. The ability of animals to learn a glucose-paired flavor preference when offered a two-bottle choice of different flavors has not been investigated in mice but there are data in rats [4,12,28]. Most relevant here are findings obtained with food-restricted rats given 20 h/day tests with CS+ and CS− solutions (grape or cherry saccharin) paired with IG 16% glucose and IG water, respectively. Although some rats (5 of 9) displayed a preference for the CS+ flavor early in the first 20-h test while consuming very little CS− (42.0 vs. 2.5 g/20 h), the remaining four rats did not develop a CS+ preference until many hours later (or until the second or third 20-h session) and consumed only slightly more CS+ than CS− in the first session (29.4 vs. 22.4 g/20 h).
Second, TRPM5 KO mice failed to display glucose preferences in the 1-h tests, yet, like T1R3 KO mice, they develop glucose preferences in 24-h sugar vs. water tests [54]. Furthermore, water-restricted TRPM5 KO mice learn to prefer CS+ flavored water paired with IG glucose infusions over a CS− flavored water paired with IG water during 1-h training sessions [33]. Conceivably, postoral sugar conditioning may be less effective in TRPM5 KO than T1R3 KO mice but there is no evidence that this is the case. Instead, TRPM5 KO mice may fail to show an early, unconditioned preference for glucose in 1-h sessions because the TRPM5 channel is part of the downstream signaling pathway used by the putative glucose taste sensor. In this case, the glucose preference displayed by TRPM5 KO mice in 24-h tests presumably represents a postoral conditioned attraction to other orosensory features of glucose (e.g., odor) [17,29].
The differential response displayed by T1R3 KO and TRPM5 KO mice in the 1-h sugar preference tests led us to reanalyze our prior 24-h glucose data obtained with these KO strains [54]. As indicated in Fig. S5, during the very first 24-h 8% glucose vs. water session, 7 of 9 T1R3 KO mice strongly preferred glucose, and all preferred glucose on the second day, with group preferences of 81% and 94% on days 1 and 2, respectively. In contrast, TRPM5 KO mice were indifferent to glucose on the first day (50% preference) and only on the second day did a glucose preference (88%) emerge. This analysis is consistent with notion that the 8% glucose preference of T1R3 KO mice was guided by oral glucose sensing while that of the TRPM5 KO mice was due primarily to postoral glucose conditioning.
In addition to glucose, T1R3 KO mice also develop preferences for sucrose in 24-h sugar vs. water tests [53]. To assess if there is an oral component to their sucrose preference, we determined if T1R3 KO mice would prefer sucrose to water in 1 h/day tests. As shown in Fig. S6, food-restricted KO mice showed no avidity to 8% sucrose in a 1-h test, yet a KO Hi subgroup subsequently displayed a 90% preference for glucose over water. Thus, like 8% fructose, 8% sucrose does not appear to have an inherently attractive taste component to T1R3 KO mice. In view of these findings, we re-analyzed our previously published 24-h sucrose preference data obtained with T1R3 KO mice [53]. As shown in Fig. S7, the daily test data indicate that the T1R3 KO mice were indifferent to 8% sucrose on both test days, and significantly preferred 16% sucrose to water only on the second test day with this solution. These data suggest that postoral sugar conditioning rather than oral sucrose sensing is responsible for the sucrose preference of the T1R3 KO mice. It is possible, however, that oral sensing of the glucose released by sucrose hydrolysis in the mouth contributed to the 16% sucrose preference displayed by the T1R3 KO mice. Consistent with this idea is evidence that oral sucrose hydrolysis accounts for the CPIR stimulated by this sugar in mice [15].
In Experiment 3, T1R3 KO mice displayed an early and robust 1-h preference for glucose over MDG, which provides a third source of evidence against a postoral conditioning explanation of their glucose over fructose preference. This is based on our findings that IG infusions of 8% MDG and 8% glucose in B6 mice condition similar CS+ flavor preferences (70%), relative to a CS− flavor paired with IG water infusions [52]. Thus, T1R3 KO mice would not be expected to display a significant glucose preference over MDG if the preference was meditated by postoral appetition signals. The findings that WT mice did not consume more glucose than MDG in 1-h Test 1 or in subsequent one-bottle training sessions is consistent with findings indicating that both forms of glucose bind to the T1R2 +T1R3 sweet receptor [3,13,20] and both have postoral appetition actions [52]. Nevertheless, the WT mice subsequently preferred glucose to MDG by 64% in Test 2 which indicates that the postoral appetition effect of glucose is somewhat greater than that of MDG. This is consistent with the recent finding that B6 mice trained to associate one CS+ flavor with IG infusions of 8% glucose and a different CS+ flavor with IG infusions of 8% MDG subsequently displayed a 67% preference for the glucose-paired CS+ over the MDG-paired CS+ [49]. Also relevant here are the findings that TRPM5 KO mice, following 24-h one-bottle training with 8% glucose or MDG, preferred both sugars to water although their glucose preference exceeded that for MDG (85% vs. 68%) [35]. Glucose may condition even stronger preferences than MDG with 24-h tests or at higher concentrations (12%, 16%) because the accumulation of the nonmetabolizable MDG in the body may generate inhibitory signals that reduce its reward effects [49,52].
Since MDG and glucose are both ligands for SGLT1, the finding that T1R3 KO mice strongly preferred glucose to MDG does not support the idea that SGLT1 taste cell sensing is responsible for their preference for glucose over fructose. Instead, the differential response of T1R3 KO mice to glucose and non-metabolizable MDG suggests that the residual glucose taste displayed by T1R3 KO mice may be mediated by glucose metabolism activating KATP signaling in sweet taste cells [15,21]. The regulatory sulfonylurea receptor (Sur1) is an essential part of the KATP signaling pathway. The finding that Sur1 KO mice show reduced chorda tympani nerve response to glucose and no CPIR to oral glucose documents a role of Sur1 in glucose taste signaling [15,21]. However, Sur1 KO mice display normal concentration-dependent licking responses to glucose which indicates that, in the presence of functional T1R2+T1R3 receptors, the KATP signaling pathway does not influence sugar avidity [15]. It may be, though, that in the absence of normal T1R2+T1R3 signaling, the KATP pathway modulates sugar avidity. It would be of interest to determine the glucose vs. fructose preferences of double KO mice missing both T1R3 and Sur1 taste signaling units. The possible involvement of taste cell KATP signaling in the glucose preference of T1R3 KO mice raises the question of the role of CPIR, if any, in this glucose preference [15]. One approach to this question would be to determine if suppressing the CPIR with atropine treatment attenuated glucose preference in T1R3 KO mice [41].
Other orosensory receptors may influence sugar preferences. In particular, rodents have a taste for glucose polymers (maltooligosaccharides) that is distinct from their taste for sugars, although the identity of the putative glucose polymer taste receptor remains unknown [27,30,45,46,53]. In rats, some evidence suggests that glucose, but not fructose, weakly activates the putative glucose polymer taste receptor [27] but whether this is the case for mice is less certain [46]. Recent findings indicate that olfactory signals also contribute to the sugar preferences of T1R3 KO and B6 WT mice [17,29,55]. In particular, the experience-induced preference of T1R3 KO mice for sucrose solutions is significantly attenuated by olfactory bulbectomy [55]. Furthermore, the experience-induced enhanced brief-access licking response to glucose relative to fructose is greatly reduced or absent in WT mice made anosmic by bulbectomy, bulbotomy or zinc sulfate treatment [17,29]. However, bulbectomy did not alter the brief access licking response of sugar-naive mice to glucose and fructose [17]. This suggests that the unconditioned glucose preference displayed by T1R3 KO mice relative to fructose or MDG in initial 1-h sessions was not mediated by olfactory cues. Nevertheless, olfactory cues may have contributed to the experience-induced increase in glucose preference, first min licks, and decreased lick latency displayed by T1R3 KO mice in Test 2 which followed one-bottle training sessions.
We previously reported that sugar-naive T1R3 KO mice displayed preferences for 8–32% but not 0.5–4% glucose in 24-h choice tests versus water [54]. In a subsequent test series, the KO mice preferred 0.5–4% as well as 8–32% glucose, indicating that they had acquired a preference for T1R3-independent sensory features which extended to dilute glucose. In a third test series, the same T1R3 KO mice also significantly preferred 0.5–32% fructose to water, which contrasts with the failure of sugar-naive T1R3 KO mice to prefer fructose even with extensive 24-h testing with this sugar [54]. Taken together, these findings indicate that extensive experience with glucose conditions a preference for T1R3-independent sensory features of glucose that generalize to fructose. This cross-sugar generalization may explain an early report of a “residual” glucose preference in T1R3 KO mice [10]. In that report, T1R3 KO mice did not differ from WT mice in their 24-h preference for dilute to concentrated glucose solutions. However, the T1R3 KO mice had previously been given 24-h tests with sucrose, which may have been responsible for their subsequent preference for glucose over water. Yet in the present study, T1R3 KO mice with prior experience with both glucose and fructose did not prefer fructose to water in a subsequent 24-h choice test (Fig. 6). Thus, depending upon the type of prior experience (glucose only vs. glucose vs. fructose), T1R3 KO mice may treat the flavors of glucose and fructose as similar or distinct (see also [29]). Recent studies indicate that extensive sugar exposure may condition an attraction to sugar odor cues that mediate, in part, the discrimination between glucose and fructose [17,29]. It is also possible that extensive experience with glucose alone conditions an odor preference that supports the preference for fructose [54]. Future studies should investigate the effects of anosmia treatments on glucose experience-induced fructose preferences in T1R3 KO mice.
In the initial 1-min and/or 1-h choice tests, WT mice either preferred fructose to glucose or were indifferent to the two sugars. Other behavioral and electrophysiology data suggest that fructose is somewhat sweeter than isomolar glucose [14,18,26,29,42]. Behavioral data obtained in short- and long-term tests may not reflect this difference in sweet taste because the potent postoral actions of glucose selectively enhance the response to this sugar. In an unpublished experiment we compared 1-h oral glucose vs. fructose preferences of B6 mice in which the postoral actions of the two sugars were equated. This was accomplished by fitting the mice with an IG catheter and giving them the choice between 8% glucose paired with matched IG infusions of fructose vs. 8% fructose paired with matched IG 8% glucose infusions. Thus, the tastes of both sugars were paired with the postoral actions of a glucose + fructose mixture. In an initial 1-h choice test (Test 1), the mice consumed more fructose than glucose and continued to do so in Test 2 which followed one-bottle training sessions; the fructose preferences were 73% and 68% in Tests 1 and 2, respectively (Fig. S8). Thus, in the absence of differential postoral effects, B6 mice significantly preferred fructose to glucose, consistent with the idea that fructose has a sweeter taste. Other mice had each sugar paired with IG infusions of the same sugar and they consumed more fructose in Test 1, but substantially more glucose in Test 2 following one-bottle training sessions; their fructose preferences were 66% and 20% in Tests 1 and 2, respectively (Fig. S8). Thus, the postoral actions of glucose converted the initial preference for fructose to a strong glucose preference in these mice. The preference shift displayed by these mice is similar to that displayed by the B6 WT mice in Experiments 1 and 2. Consistent with these findings, Myers et al.[25] recently reported that rats trained to drink fructose and glucose solutions paired with infusions of the opposite sugar licked more fructose than glucose in brief access tests while rats trained with infusions of the same sugar licked more glucose than fructose.
In summary, this study demonstrates that in the absence of a functioning T1R2+T1R3 sweet receptor, many T1R3 KO mice strongly prefer glucose to fructose, suggesting the action of a glucose-specific taste sensor. This glucose sensor appears to require the action of the TRPM5 channel, given that TRPM5 KO mice did not display glucose preferences in 1-h tests. Conceivably, the 24-h glucose preference displayed by TRPM5 KO mice may involve the oral glucose sensor, and this requires additional study. The preference displayed by T1R3 KO mice for glucose over MDG indicates that taste cell SGLT1 signaling is not responsible for the glucose preference since both sugars are SGLT1 ligands. Instead, KATP taste signaling may mediate the glucose preference of T1R3 KO mice, but this remains to be established and other sensors may also be involved. The signal arising from the glucose-specific receptor is relatively weak, at least before significant experience, compared with the T1R2+T1R3 sweet receptor as indicated by the minimal licking response of T1R3 KO and T1R2+T1R3 KO mice to glucose in brief-access lick tests [46,50], the failure of T1R3 KO mice to prefer glucose to fructose in 1-min tests, and the failure of sugar-naive T1R3 KO mice to prefer dilute (0.5–4%) glucose solutions to water in 24-h choice tests [54]. The significant fructose preference over glucose displayed by B6 mice when the postoral actions of the two sugars are equated confirms other findings indicating that fructose more effectively stimulates the T1R2+T1R3 receptor than does glucose (see also [25]). However, glucose has a more potent postoral conditioning action in B6 mice, which explains why they acquire a strong glucose preference over fructose [32,52]. Postoral glucose appetition can also account for the glucose (and sucrose) preferences displayed by TRPM5 KO and other near-total ageusic KO mice in long-term tests (CALHM1 KO, IP3R KO, P2X2/P2X3 DKO, TRPM4 KO) [6,19,34,39].
A major limitation of the current study is that we are not certain why only about half of the T1R3 KO displayed robust glucose preferences in the 1-h tests. Conceivably, T1R3 KO Hi and Lo mice differ in their oral glucose taste sensitivity. Prior studies of outbred rats revealed substantial differences in sucralose preferences, which appears related to differences in their sweet and bitter taste sensitivities [23,36,44]. Note however, that these preference differences were stable traits that were observed in both short- and long-term tests [23]. In contrast, while T1R3 KO Hi and Lo mice differed in their glucose vs. fructose avidity in short-term tests, they were identical in their sugar avidity in 24-h tests (Experiment 2). Alternatively, it may be differences in their propensity to sample the sugar solutions in 1-h tests that led T1R3 KO Hi but not Lo mice to consume enough glucose during the 1-h sessions to activate their low-affinity oral glucose sensor. Conceivably, if the mice were water as well food restricted prior to the 1-h sessions, more or all of the T1R3 KO mice would have expressed strong glucose preferences. Further research is needed to address this issue.
Supplementary Material
Acknowledgment
This research was supported by grant DK-31135 from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Robert F. Margolskee for providing us with the T1R3 KO and TRPM5 KO breeding stock, and John I. Glendinning and two anonymous reviewers for their insightful comments on this paper.
References
- [1].Ackroff K, Sclafani A. Flavor preferences conditioned by sugars: Rats learn to prefer glucose over fructose. Physiol. Behav 50 (1991) 815–824. [DOI] [PubMed] [Google Scholar]
- [2].Ackroff K, Sclafani A. Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol. Behav 129 (2014) 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning in rats by glucose but not a non-metabolizable glucose analog. Physiol. Behav 133 (2014) 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol. Behav 72 (2001) 691–703. [DOI] [PubMed] [Google Scholar]
- [5].Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: Maltose is more reinforcing than sucrose. Physiol. Behav 64 (1998) 535–541. [DOI] [PubMed] [Google Scholar]
- [6].Banik BD, Martin LE, Freichel M, Torregrossa AM, Medler KF. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc. Natl. Acad. Sci. U. S. A 115 (2018) E772–E781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol. Genomics 41 (2010) 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chaudhari N, Roper SD. The cell biology of taste. J. Cell Biol 190 (2010) 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B Jr., Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem. Senses 31 (2006) 253–264. [DOI] [PubMed] [Google Scholar]
- [10].Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301 (2003) 850–853. [DOI] [PubMed] [Google Scholar]
- [11].Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol. Behav 106 (2012) 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Drucker DB, Ackroff K, Sclafani A. Flavor preference produced by intragastric Polycose infusions in rats using a concurrent conditioning procedure. Physiol. Behav 54 (1993) 351–355. [DOI] [PubMed] [Google Scholar]
- [13].Glaser D, Wanner M, Tinti JM, Nofre C. Gustatory responses of pigs to various natural and artificial compounds known to be sweet in man. Food Chem. 68 (2000) 375–385. [Google Scholar]
- [14].Glendinning JI, Beltran F, Benton L, Cheng S, Gieseke J, Gillman J, Spain HN. Taste does not determine daily intake of dilute sugar solutions in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 299 (2010) R1333–R1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Glendinning JI, Frim YG, Hochman A, Basile A, Sclafani A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am. J. Physiol. Regul. Integr. Comp. Physiol 312 (2017) R597–R610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Glendinning JI, Gilman J, Zamer H, Margolskee RF, Sclafani A. The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol. Behav 107 (2012) 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Glendinning JI, Maleh J, Ortiz G, Touzani K, Sclafani A. Olfaction contributes to the learned avidity for glucose relative to fructose in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol in press (2020) [DOI] [PubMed] [Google Scholar]
- [18].Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, Vasselli JR, Sclafani A. Sugar-induced cephalic phase insulin release is mediated by a T1r2+T1r3-independent taste pathway in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 309 (2015) R552–R560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1, 4, 5-trisphosphate receptor. J. Biol. Chem 282 (2007) 37225–37231. [DOI] [PubMed] [Google Scholar]
- [20].Jakinovich W Jr., Goldstein IJ. Stimulation of the gerbil’s gustatory receptors by monosaccharides. Brain Res. 110 (1976) 491–504. [DOI] [PubMed] [Google Scholar]
- [21].Jyotaki M, Yee K, and Margolskee RF The ATP-gated K+ channel mediates taste responses to sugars in mice. Chem Senses 41 (2016) E271–E272. [Google Scholar]
- [22].Kokrashvili Z, Yee KK, Ilegems E, Iwatsuki K, Li Y, Mosinger B, Margolskee RF. Endocrine taste cells. Br. J. Nutr 111 Suppl. 1 (2014) S23–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Loney GC, Torregrossa A, Smith JC, Sclafani A, Eckel LA. Rats display a robust bimodal preference profile for sucralose. Chem. Senses 36 (2011) 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murovets VO, Bachmanov AA, Zolotarev VA. Impaired glucose metabolism in mice lacking the Tas1r3 taste receptor gene. PLoS One 10 (2015) e0130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Myers KP, Summers YM, Geyer-Roberts E, Schier AL. The role of post-ingestive feedback in the development of an enhanced appetite for the orosensory properties of glucose over fructose in rats. Nutrients 12 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ninomiya Y, Imoto T, Sugimura T. Sweet taste responses of mouse chorda tympani neurons: Existence of gurmarin-sensitive and -insensitive receptor components. J. Neurophysiol 81 (1999) 3087–3091. [DOI] [PubMed] [Google Scholar]
- [27].Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neurosci. Biobehav. Rev 11 (1987) 187–196. [DOI] [PubMed] [Google Scholar]
- [28].Puerto A, Deutsch JA, Molina F, Roll P. Rapid rewarding effects of intragastric injections. Behav. Biol 18 (1976) 123–134. [DOI] [PubMed] [Google Scholar]
- [29].Schier LA, Inui-Yamamoto C, Blonde GD, Spector AC. T1R2+T1R3-independent chemosensory inputs contributing to behavioral discrimination of sugars in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 316 (2019) R448–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sclafani A. Starch and sugar tastes in rodents: An update. Brain Res. Bull 27 (1991) 383–386. [DOI] [PubMed] [Google Scholar]
- [31].Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite 71 (2013) 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol. Behav 106 (2012) 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am. J. Physiol. Regul. Integr. Comp. Physiol 302 (2012) R1119–R1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sclafani A, Ackroff K. Maltodextrin and fat preference deficits in “taste-blind” P2X2/P2X3 knockout mice. Chem. Senses 39 (2014) 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sclafani A, Ackroff K. Flavor preference conditioning by different sugars in sweet ageusic Trpm5 knockout mice. Physiol. Behav 140 (2015) 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sclafani A, Clare R. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem. Senses 29 (2004) 523–528. [DOI] [PubMed] [Google Scholar]
- [37].Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 299 (2010) R1643–R1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sclafani A, Koepsell H, Ackroff K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am. J. Physiol. Regul. Integr. Comp. Physiol 310 (2016) R631–R639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sclafani A, Marambaud P, Ackroff K. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol. Behav 126 (2014) 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sclafani A, Vural AS, Ackroff K. CAST/Ei and C57BL/6J mice differ in their oral and post-oral attraction to glucose and fructose. Chem. Senses 42 (2017) 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sclafani A, Xenakis S. Atropine fails to block the overconsumption of sugar solutions by hypothalamic hyperphagic rats. J. Comp. Physiol. Psychol 95 (1981) 708–719. [DOI] [PubMed] [Google Scholar]
- [42].Sukumaran SK, Yee KK, Iwata S, Kotha R, Quezada-Calvillo R, Nichols BL, Mohan S, Pinto BM, Shigemura N, Ninomiya Y, Margolskee RF. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc. Natl. Acad. Sci. U. S. A 113 (2016) 6035–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tordoff MG. Gene discovery and the genetic basis of calcium consumption. Physiol. Behav 94 (2008) 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Torregrossa AM, Loney GC, Smith JC, Eckel LA. Examination of the perception of sweet- and bitter-like taste qualities in sucralose preferring and avoiding rats. Physiol. Behav 140 (2015) 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: Implications for saccharide taste receptors in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 296 (2009) R855–R865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Treesukosol Y, Smith KR, Spector AC. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J. Neurosci 31 (2011) 13527–13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yasumatsu-Nakano K, Ito K, Ohkuri T, Iwata S, Margolskee RF, and Ninomiya Y Analysis of sweet taste responses via SGLT1 in mouse chorda tympani and glossopharyngeal nerves. Chemical Senses 41 (2016) E227. [Google Scholar]
- [48].Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc. Natl. Acad. Sci. U. S. A 108 (2011) 5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang L, Han W, Lin C, Li F, de Araujo IE. Sugar metabolism regulates flavor preferences and portal glucose sensing. Front. Integr. Neurosci 12 (2018) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115 (2003) 255–266. [DOI] [PubMed] [Google Scholar]
- [51].Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol 301 (2011) R1635–R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am. J. Physiol. Regul. Integr. Comp. Physiol 305 (2013) R840–R853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am. J. Physiol. Regul. Integr. Comp. Physiol 296 (2009) R866–R876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of T1r3 and Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem. Senses 38 (2013) 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem. Senses 34 (2009) 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.