Abstract
Galectins have been implicated in inhibiting B cell receptor (BCR) signaling in mature B cells but promoting pre-BCR signaling during early development. Galectins bind to branched N-glycans attached to cell-surface glycoproteins to control the distribution, clustering, endocytosis and signaling of surface glycoproteins. During T cell development, N-glycan branching is required for positive selection of thymocytes, inhibiting both death by neglect and negative selection via enhanced surface retention of the CD4/CD8 co-receptors and limiting TCR clustering/signaling, respectively. The role of N-glycan branching in B cell development is unknown. Here we report that N-glycan branching is absolutely required for development of mature B cells in mice. Elimination of branched N-glycans in developing B cells via targeted deletion of N-acetylglucosaminyl transferase I (Mgat1) markedly reduced cellularity in the bone marrow and/or spleen, and inhibited maturation of pre-, immature and T2 transitional B cells. Branching deficiency markedly reduced surface expression of the pre-BCR/BCR co-receptor CD19 and promoted spontaneous death of pre-B cells and immature B cells in vitro. Death was rescued by low dose pre-BCR/BCR stimulation but exacerbated by high dose pre-BCR/BCR stimulation as well as anti-apoptotic BclxL over-expression in pre-B cells. Branching deficiency also enhanced Nur77 induction, a marker of negative selection. Together these data suggest that as in T cells, N-glycan branching promotes positive selection of B cells by augmenting pre-BCR/BCR signaling via CD19 surface retention while limiting negative selection from excessive BCR engagement.
INTRODUCTION
T and B cells (or lymphocytes) undergo similar developmental events and checkpoints that involve the dynamic expression of transcription factors, response to extracellular environmental cues such as cytokines, and the somatic rearrangement, expression, and screening of their antigen receptors in order to generate a functionally diverse, self-tolerant lymphocyte repertoire (1–4). Abnormalities in lymphocyte development and selection give rise to congenital immunodeficiencies, leukemias/lymphomas, and autoimmune diseases (5). In the bone marrow, developing B cells rearrange immunoglobulin (Ig) μ heavy (H)-chain and light (L)-chain genes to express surface bound IgM that complexes with immunoreceptor tyrosine-based activation motif (ITAM) containing Igα and Igβ (CD79A and CD79B) signaling proteins to form the B cell receptor (BCR) on immature B cells (6–11). BCR binding to antigen directs immature B cell fate – when the BCR is engaged by self-antigen, immature B cells die by clonal deletion (negative selection by apoptosis) or undergo receptor editing (continuous L-chain rearrangement) to reduce/eliminate autoreactivity (12). Immature B cells continue selection through transitional developmental stages (T1 and T2) in the bone marrow and the spleen, but predominantly maturate into follicular (FO) or marginal zone (MZ) B cells in the spleen and co-express surface bound IgM and IgD (13). Approximately 25% of developing B cells become mature B cells in the bone marrow (14). Unengaged BCRs induce tonic signaling to drive developmental progression and survival of immature B cells in a CD19 dependent manner (15). CD19 is a transmembrane glycoprotein first expressed in pro-B cells in the bone marrow and augments BCR signaling at multiple points during B cell development, maturation, and differentiation (16–20). Mice with targeted deletion of CD19 have greatly reduced B cell numbers, impaired B cell function, and defective immune responses (21, 22).
The branching of Asparagine (N)-linked glycans with N-acetylglucosamine by the N-acetylglucosaminyl transferases Mgat1, Mgat2, Mgat4a/b and Mgat5 sequentially increase production of ligands for the galectin family of sugar-binding proteins (23, 24). At the cell surface, interactions of galectins with branched N-glycans attached to glycoproteins generates a macromolecular lattice, thereby controlling receptor localization, mobility, clustering, and surface retention to impact cell function/differentiation and disease states (25–33). Our group demonstrated that N-glycan branching regulates thymic positive selection by defining the upper and lower bounds of TCR affinity for peptide-MHC (33). Using a T cell specific (Lck driven cre) knockout of the N-glycan branching enzyme Mgat1, we observed markedly reduced thymic and splenic T cell numbers. Mgat1 deletion enhanced thymocyte death by neglect via decreased CD4/CD8 co-receptor surface retention (i.e., enhanced CD4/CD8 endocytosis) and associated reduced Lck induced Erk signaling, which are important for augmenting low affinity TCR engagement. N-glycan deficient thymocytes simultaneously exhibited increased death by negative selection due to excessive Ca2+ flux driven by enhanced TCR clustering. Thus, during T cell development N-glycan branching provides a mechanism for decoupling CD4/CD8 co-receptor and TCR signaling to maintain the appropriate range of TCR signal intensity necessary for thymocyte positive selection and generation of functional circulating CD4+ and CD8+ T cells.
Various studies have demonstrated glycosylation to be important in B cell development, selection, and maturation. This includes the sialic acid-binding immunoglobulin-type lectin (Siglec) and B cell inhibitory co-receptor CD22 (34–37), the sialyltransferase ST6Gal1 (38), fucosylation (39, 40), and galectins (41–46). The role of N-glycan branching, however, has not been investigated. Here, we provide evidence that N-glycan branching promotes positive selection of B cells by enhancing low affinity BCR engagement via promoting CD19 surface levels while also reducing high affinity BCR engagement to prevent negative selection.
MATERIALS AND METHODS
Mice
Mgat1f/f (006891), Mgat2f/f (006892), CD19-cre (006785), CD23-Cre (028197), and Bim−/− (004525) mice were obtained from Jackson Laboratory. Eμ-BclxL mice were transferred to us by Dr. Brian Iritani from the Department of Comparative Medicine at the University of Washington, Seattle. Mgat1f/f/tetO-cre/ROSA-rtTA mice were previously described (33). Inter-breeding generated all other mice. Mice used were 5–7 weeks old but otherwise selected randomly for experiments and approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Flow cytometry
Flow cytometric analysis was performed as previously described (25, 33, 47). The fluorophore conjugated mouse specific antibodies from Thermo Fisher/eBioscience were CD43 (R2/60), IgM (II/41), B220 (RA3–6B2), CD19 (eBio1D3), CD23 (B3B4), and CD21 (8D9). For flow cytometric analysis of glycan expression, cells were stained with 2 ug/mL L-PHA-FITC or biotinylated L-PHA followed by streptavidin conjugated fluorophore (Vector Labs and Thermo Fisher Scientific, respectively). Fluorophore conjugated Annexin-V was from BD Pharmingen, and fluorophore conjugated Nur77 from Cell Signaling Technologies. Samples were acquired on the Attune NxT (Thermo Fisher Scientific) flow cytometer. Data analysis was performed using FlowJo software.
Cell culture and stimulation
For in vitro experiments, isolated bone marrow cells were cultured in RPMI-1640 media (Thermo Fisher Scientific) supplemented with 10% heat inactivated fetal bovine serum (VWR), 2 uM L-glutamine and 100 U/mL penicillin/streptomycin (Gibco), and 50 uM β-mercaptoethanol (Gibco). Cells were cultured at 2.5 ×105 cells per well in a 96-well plate in triplicates without stimulation, or in the presence of functional grade goat anti-mouse IgM F(ab’)2 (polyclonal, Thermo Fisher Scientific) at various concentrations.
Statistical Analysis
Prism 8 software was used for all statistical testing. For comparison of 3 or more groups, we used the non-parametric Kruskal-Wallis test with false discovery rate correction (Benjamini, Krieger and Yekutieli) for multiple comparisons (Fig. 1e,f, 3d–g, 4b,c). The Mann-Whitney test was used when comparing only two groups, with 1-tailed tests in Fig. 2, 3c and 4a as we predicted a priori the direction of the effect of Mgat1 deletion based on our previous T cell data (33) as well as the results of Kruskal-Wallis tests in Figure 1.
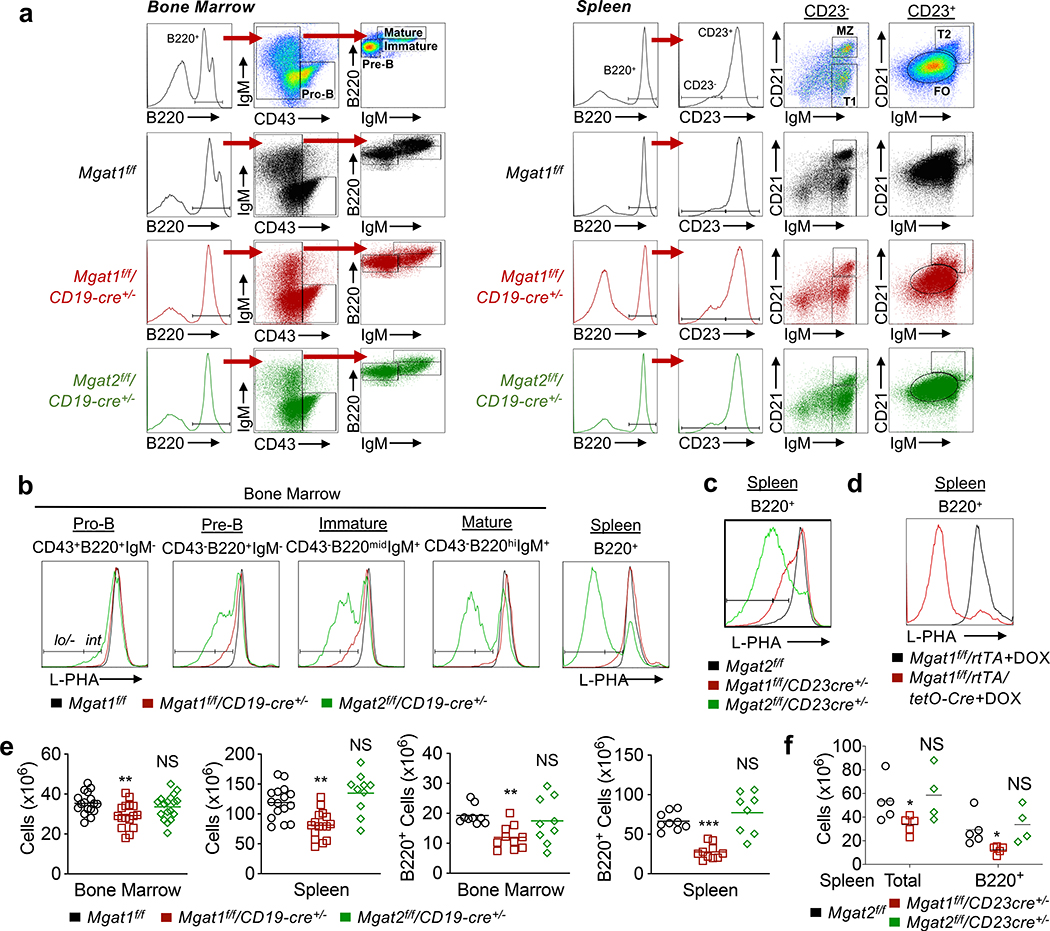
Figure 1. Late B cell development requires N-glycan branching.
(a-d) Flow cytometric analysis with indicated markers on bone marrow B cell subsets and/or splenic B cells from mice of the indicated genotypes. (e,f) Total cellularity or B220+ cell numbers (calculated by multiplying percentage of B220+ cells acquired by flow cytometry by total cellularity) of bone marrow and/or spleens from mice of the indicated genotypes. Each dot represents one mouse. Kruskal-Wallis test with false discovery rate correction (Benjamini, Krieger and Yekutieli) for multiple comparisons (e,f). Bars indicate mean. NS, not significant;
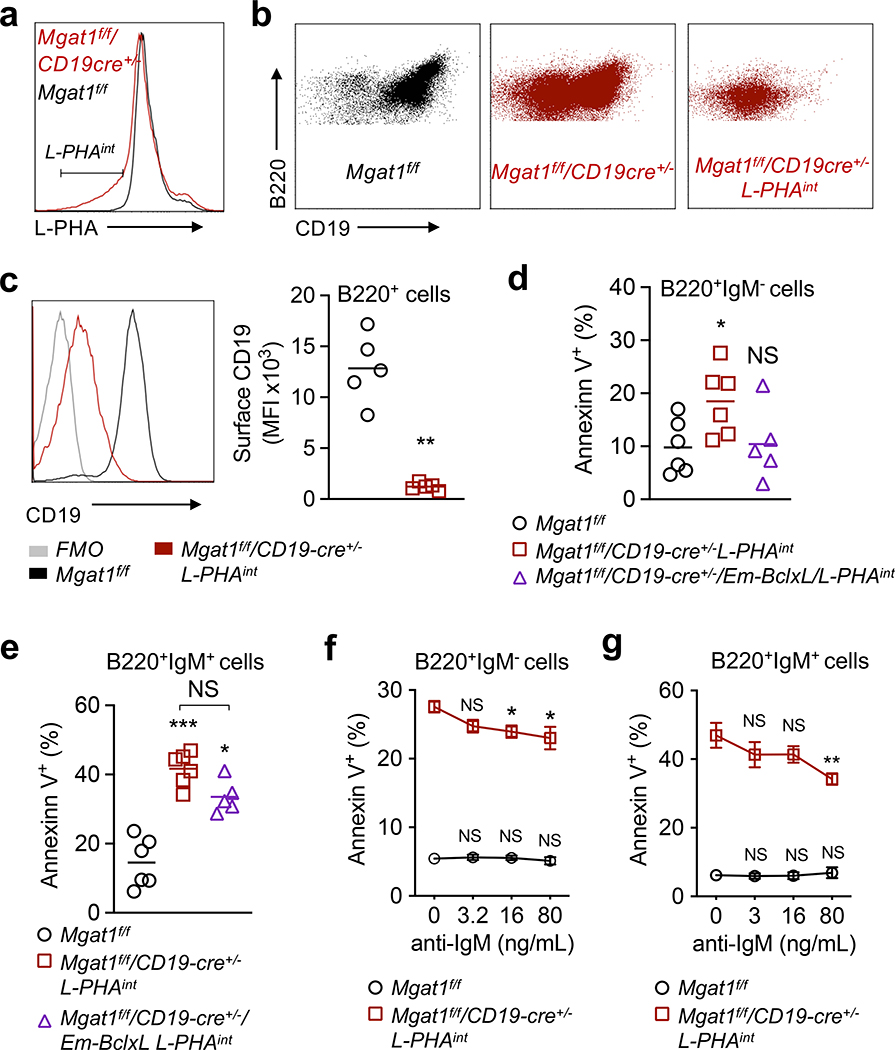
Figure 3. N-glycan branching promotes immature B cell positive selection via CD19 surface expression.
(a-c) Flow cytometric analysis of ex vivo B220+ bone marrow B cells for the L-PHAint gate (a) and CD19 surface levels (b,c). Each dot represents one mouse, unpaired one-tailed Mann-Whitney t-test (c). **P < 0.01. (d-g) Annexin V binding to bone marrow B cells cultured for 1 day without BCR stimulation (d,e) and with low doses of anti-IgM F(ab’)2 (f,g), gated on B220+IgM- (d,f) and B220+IgM+ (e,g) cells. Cells from control mice are gated on all B cells, while all others are gated on the L-PHAint population as indicated below each panel. n ≥ 3. Each dot represents one mouse (d,e). Kruskal-Wallis test with false discovery rate correction (Benjamini, Krieger and Yekutieli) for multiple comparisons (d-g), with comparison to zero anti-IgM (f,g). Error bars indicate mean ± s.e.m. ***P < 0.001; *P < 0.05.
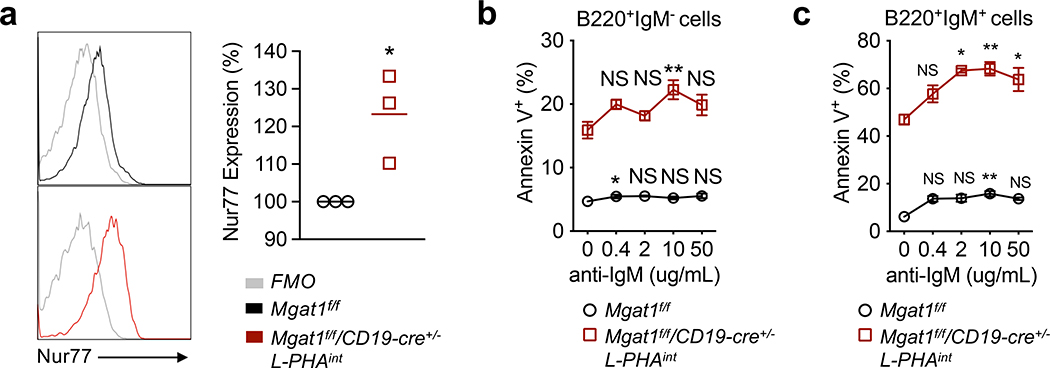
Figure 4. N-glycan branching limits immature B cell negative selection by limiting BCR engagement.
(a) Flow cytometric analysis of ex vivo B220+IgM+ bone marrow B cells for intracellular staining of Nur77. Each dot represents one mouse of the indicated genotypes; unpaired one-tailed Mann-Whitney t-test. *P = 0.05. (b-c) Annexin V binding to bone marrow B cells following a 1-day culture with high doses of anti-IgM F(ab’)2, gated on B220+IgM= (b) and B220+IgM+ (c) cells. n = 3. Kruskal-Wallis test with false discovery rate correction (Benjamini, Krieger and Yekutieli method) for multiple comparisons, with comparison to zero anti-IgM for each genotype. Error bars indicate mean ± s.e.m. *P < 0.05.
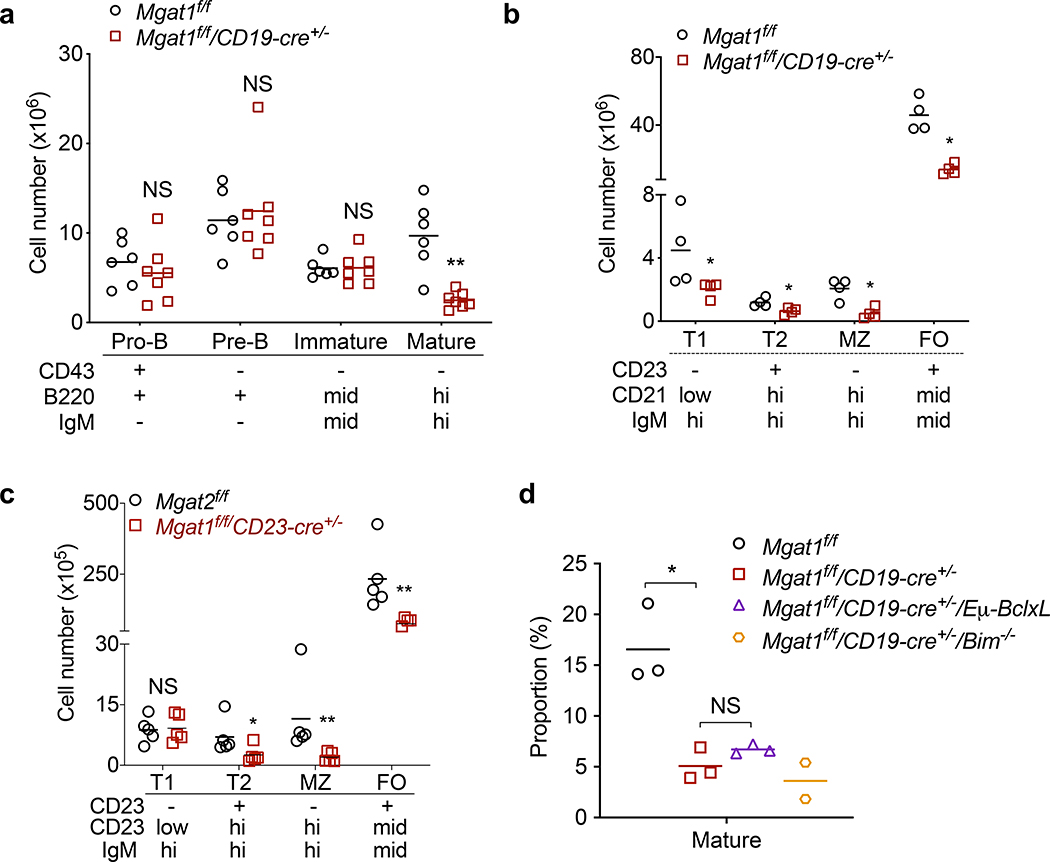
Figure 2. N-glycan branching promotes transition of immature to mature B cells in bone marrow and B cell maturation in the spleen.
(a-d) Flow cytometric analysis of total number of B cell subtypes in the bone marrow (a) and spleen (b,c) using indicated markers, and proportion of mature B cells in spleen (d) from mice of the indicated genotypes. Each dot represents one mouse, unpaired one-tailed Mann-Whitney t-test. Bars indicate mean. NS, not significant; **P < 0.01; *P < 0.05.
RESULTS
N-glycan branching is required for B cell generation
To investigate a role for N-glycan branching in B cell development, we generated B cell specific Mgat1 and Mgat2 deficient mice using CD19-cre. Mgat1 deletion completely eliminates while Mgat2 deletion reduces N-glycan branching (Supplemental Fig 1a). CD19 expression is initiated at the pro-B cell stage (16, 17). The plant lectin L-PHA (Phaseolus vulgaris leukoagglutinin) binds to β1,6GlcNAc-branched N-glycans generated by the sequential action of the Mgat1, Mgat2 and Mgat5 branching enzymes (Supplemental Fig 1a) (25, 31, 33). The loss of L-PHA binding directly identifies cells deleted for Mgat1, Mgat2 or Mgat5 (25, 31, 33). Therefore, we utilized L-PHA flow cytometry to assess deletion of Mgat1 and Mgat2 in the bone marrow and spleen of B220+ B cells (Fig. 1a). In Mgat2f/f/CD19-cre+/− mice, pro-B cells displayed a small population with reduced L-PHA binding (L-PHAint) that became a larger population of L-PHAlo/- cells while transitioning to the mature B cell stage and entering the periphery (Fig. 1b). In contrast, only a small proportion of L-PHAint pre-B and immature B cells were observed in Mgat1f/f/CD19-cre+/− mice, with the population largely disappearing at the mature B cell stage and in peripheral splenic B cells (Fig. 1b). The lack of L-PHAlo/- B cells in Mgat1f/f/CD19-cre+/− mice does not result from L-PHA binding to a glycan structure other than β1,6GlcNAc-branched N-glycans, as disrupting their biosynthesis via deletion of Mgat2 (i.e. Mgat2f/f/CD19-cre+/− mice) results in a large population of L-PHAlo/- B cells. As CD19-cre has a deletion efficiency of only ~75–80% in bone marrow (48), the population of L-PHA+ B cells in Mgat1f/f/CD19-cre+/− mice likely results from inefficient CD19-cre mediated excision of Mgat1 and continued differentiation/expansion of this L-PHA+ cell population (Fig. 1b).
To further investigate these results, we generated Mgat1f/f/CD23-cre+ and Mga2f/f/CD23-cre+ mice where CD23-cre will delete Mgat1 and Mgat2 in immature transitional 2 (T2) B220+ B cells. These cells remain sensitive to BCR regulated selection and apoptosis (49, 50). CD23-cre driven deletion phenocopied the results of CD19-cre, with Mgat1f/f/CD23-cre+ displaying L-PHAint but not L-PHAlo/- B220+ splenic B cells while ~90% of B220+ splenic B cells from Mgat2f/f/CD23-cre+ mice were L-PHAlo/- cells (Fig. 1c).
To directly examine whether Mgat1 deletion impacts mature B cells, we examined doxycycline treated Mgat1f/f/tetO-cre/ROSA-rtTA adult mice (33). These mice displayed a large population of L-PHAlo/- B220+ splenic B cells (Fig. 1d). This confirms that L-PHA detects Mgat1 deleted B cells and indicates that Mgat1 deletion specifically impacts survival of developing B cells through to the T2 stage in the periphery, while having little impact on mature peripheral B cell survival.
Consistent with the conclusion that the complete but not partial loss of N-glycan branching leads to death of developing B cells, total cell number in the bone marrow and/or spleen as well as the number of B220+ B cells were markedly reduced in Mgat1f/f/CD19-cre+/− and Mgat1f/f/CD23-cre+ but not Mgat2f/f/CD19-cre+/− or Mgat2f/f/CD23-cre+ mice (Fig. 1e,f). Together, these results indicate that partial N-glycan branching levels are permissible for B cell development, while complete elimination prevents production of mature B cells in the bone marrow and spleen without impacting survival of peripheral mature B cell. This parallels our observations in T cells, where Mgat1 deletion in thymocytes completely blocked development of mature T cells, while deletion in peripheral mature T cells did not impact cell death (33).
N-glycan branching drives bone marrow development and splenic maturation of B cells
Next, we examined at what stage loss of N-glycan branching impacts B cell development and maturation. Cell surface markers and gating strategy used to identify specific B cell populations were as previously described (16, 51) and is depicted in Fig. 1a. We focused on Mgat1f/f/CD19-cre+/− and Mgat1f/f/CD23-cre+ mice as they displayed significant loss of B cells in the bone marrow and/or spleen. In the bone marrow of Mgat1f/f/CD19-cre+/− mice, cellularity at each developmental stage revealed little difference at the pro-, pre-, and immature stages, but a drastic loss in the number of mature B cells in (Fig. 2a). In the spleen, transitional T1, transitional T2, follicular (FO) and marginal zone (MZ) B cells are similarly reduced by Mgat1 deficiency (Fig. 2b). In Mgat1f/f/CD23-cre+ mice, the number of transitional T1 B cells was unchanged but there was a marked reduction in transitional T2, follicular (FO) and marginal zone (MZ) B cells (Fig. 2c), the former consistent with CD23 expression beginning at the T2 stage. These data suggest that branching is essential for B cell development, particularly during the transition from immature and T2 to mature B cells.
Inhibiting programmed cell death fails to rescue B cell death from the loss of N-glycan branching
In T cells, we previously observed that the death of developing thymocytes induced by Mgat1 deletion was rescued by over-expression of anti-apoptotic BclxL as well as targeted deletion of pro-apoptotic Bim (33). Therefore we investigated whether manipulating these programmed cell death pathways (i.e., the mitochondrial apoptotic pathway (52)) could similarly rescue death of N-glycan branching deficient B cells. We generated Mgat1f/f/CD19-cre+/−/Eμ-BclxL to induce B cell specific anti-apoptotic BclxL overexpression, as well as Mgat1f/f/CD19-cre+/−/Bim−/− for deletion of pro-apoptotic Bim. As Mgat1 deficiency primarily altered the transition from immature to mature B cells, we focused on changes in mature B cells in the bone marrow. Neither Mgat1f/f/CD19-cre+/−/Eμ-BclxL nor Mgat1f/f/CD19-cre+/−/Bim−/− displayed an increase in the proportion of mature B cells (Fig. 2d). Thus, BclxL overexpression, and potentially Bim deletion, fails to overcome the developmental block of N-glycan branching deficient immature B cells into mature B cells.
N-glycan branching promotes CD19 associated tonic signaling to B cell death by neglect
Pre- and immature B cells are subject to positive selection, with CD19 playing an important role in both pre-BCR signaling in pre-B cells (53) and BCR signaling in immature B cells (15). Only intermediate (i.e., tonic) pre-BCR/BCR signaling allows continued development into mature B cells. As N-glycan branching regulates surface retention of cell surface glycoproteins, we assessed whether Mgat1 deficiency alters positive selection by reducing surface expression of CD19. Indeed, L-PHAintB220+ B cells from the bone marrow of Mgat1f/f/CD19-cre+/− mice displayed a marked reduction in CD19 surface expression (Fig. 3a–c). To assess whether N-glycan branching deficiency drives death of developing B cells, we cultured bone marrow cells for one day in media without stimulation and assessed cell death by Annexin V labeling. Both L-PHAintB220+IgM- B cells and L-PHAintB220+IgM+ B cells from Mgat1f/f/CD19-cre+/− mice displayed significant increases in Annexin V binding relative to control cells (Fig. 3d,e), consistent with increased sensitivity to death from reduced CD19 dependent pre-BCR/BCR tonic signaling. BclxL over-expression in L-PHAintB220+IgM- cells from Mgat1f/f/CD19-cre+/− mice reversed the increase in cell death (Fig. 3f). However, in L-PHAintB220+IgM+ B cells BclxL overexpression displayed only a marginal and non-significant impact on cell death (Fig. 3e). To investigate whether increased death was from reduced pre-BCR/BCR tonic signaling, we assessed whether minimally enhancing pre-BCR/BCR signaling would reduce the death induced by N-glycan branching deficiency. Indeed, low doses of a polyclonal anti-IgM F(ab’)2 antibody, which should activate both pre-BCR and BCR via the common heavy chain, partially rescued L-PHAintB220+IgM- and L-PHAintB220+IgM+ B cells from death (Fig. 3f,g). Taken together, these data suggest that N-glycan branching promotes CD19 surface expression and associated pre-BCR/BCR tonic signaling to prevent death by neglect of developing B cells.
N-glycan branching inhibits negative selection of developing B cells
Low level stimulation of BCR reduced but did not fully rescue death of N-glycan branching deficient developing B cells. As developing B cells are also subject to death by negative selection via strong BCR signaling from encounter with high affinity self-antigen, this suggests that N-glycan branching may also regulate negative selection in immature B cells. Nur77 is a transcription factor that is expressed at higher levels in B cells subject to excessive antigen encounter and eventual deletion by negative selection (54–56). Ex vivo L-PHAintB220+IgM+ B cells displayed greater Nur77 levels compared to control (Fig 4a). This suggests N-glycan branching deficiency enhances BCR engagement and negative selection in vivo. To further support this hypothesis, we examined whether providing high BCR signaling further enhances the death of B cells deficient in N-glycan branching. Indeed, BCR stimulation with high doses of anti-IgM F(ab’)2 enhanced cell death of L-PHAintB220+IgM+ greater than L-PHAintB220+IgM- cells, as evidenced by Annexin V labeling (Fig. 4b,c). These data suggest that N-glycan branching also inhibits negative selection of immature B cells.
DISCUSSION
Here we report that N-glycan branching is required for the development but not survival of mature B cells. Loss of N-glycan branching impacts the survival of pre-B cells, immature B cells and T2 transitional peripheral B cells but not mature B cells, where only the former cells are subject to positive selection. The effects of N-glycan branching during B cell positive selection is similar to what we have observed in T cells (33), with N-glycan branching appearing to be similarly required to maintain the boundaries of pre-BCR/BCR signaling thresholds to permit positive selection by inhibiting both death by neglect and negative selection. Mechanistically, N-glycan branching is required for CD19 surface expression in developing B cells. CD19 is necessary for low affinity pre-BCR/BCR engagement to maintain tonic BCR signaling and prevent death by neglect (15). The mechanism by which N-glycan branching inhibits death by negative selection is less clear, but may be similar to that in T cells, namely limiting antigen induced BCR clustering to prevent excessive signaling and death by negative selection.
CD19 is coupled to both pre-BCR and BCR signaling mediated apoptosis pathways (15, 53). For immature B cells, lack of CD19 results in loss of basal phosphoinositide 3-kinase (PI3K) signaling, continued recombination-activating genes (RAG) expression, and L-chain receptor editing, thus inhibiting positive selection (15). Phosphatase and tensin homologue (PTEN) opposes PI3K activity; the absence of PTEN activity reverses the effects of CD19 loss to promote immature B cell positive selection (57, 58). Like CD19, BCR deletion results in loss of mature B cells (59, 60). Constitutive PI3K signaling was necessary and sufficient to rescue the survival of BCR deficient B cells, whereas other interventions such as constitutive nuclear factor-kappa B (NFκB) signaling, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK) kinase (MEK) signaling, or Bcl2 overexpression were not (59, 60). While Bcl2 and BclxL are both anti-apoptotic factors in programmed cell death, BclxL has been shown to be superior in promoting B cell survival (61); thus we chose to overexpress BclxL. In contrast to N-glycan branching deficient thymocytes, neither BclxL overexpression nor Bim deletion significantly impacted the loss of mature B cells in vivo induced by Mgat1 deletion. However, BclxL overexpression did rescue death of branching deficient pre-B cells but displayed little effect on survival of immature B cells in vitro. This suggests that the failure of BclxL over-expression to rescue the generation of mature B cells in Mgat1f/f/CD19-cre+/− mice is largely driven by the failure to rescue immature B cells rather than pre-B cells. Notably, branching deficiency induced a much greater loss of cell surface CD19 in developing B cells than CD4 and CD8 co-receptor loss induced in thymocytes (~90% versus ~25–50%, respectively) (33). Therefore, B cell N-glycan branching deficiency largely phenocopies CD19/BCR deficiency that could not be rescued with Bcl2 over-expression, whereas a partial loss of CD4/CD8 in thymocytes is a milder phenotype that could be rescued by altering apoptotic pathways. Future research examining mechanisms by which N-glycan branching deficiency affects PI3K and apoptosis in immature B cells would provide insight in how branching regulates CD19-BCR downstream signaling pathways and subsequent functional outcomes such as death.
CD19cre and CD23cre driven deletion of Mgat1 both resulted in generation of B cells with reduced (L-PHAint) but not absent N-glycan branching (L-PHAlo/-). Once the Golgi branching pathway is blocked (eg Mgat1 deletion), there is a gradual reduction in branching at the cell surface as 1) the enzyme is slowly lost from the Golgi and 2) glycoproteins at the cell surface with normal glycosylation are replaced slowly by new glycoproteins lacking branched N-glycans. Thus, L-PHAint cells represent this transition state. Further reductions in branching beyond this transition state triggers cell death, resulting in the absence of L-PHAlo/- B cells.
In contrast to Mgat1 deletion, Mgat2f/f/CD19-cre+/− and Mgat2f/f/CD23-cre+/− mice were grossly comparable to controls, yet we expected them to have an intermediate phenotype due to having a single N-glycan branch, which should allow formation of a partially intact galectin-glycoprotein lattice. However, in T cells we recently reported that loss of branching in Mgat2 deficient T cells is partially compensated for by poly-N-acetyllactosamine (poly-LacNAc) extension of the remaining single GlcNAc branch (Supplemental Fig 1a (31). Thus, N-glycan branching loss in B cells from Mgat2f/f/CD19-cre+/− mice may be compensated for by poly-LacNAc extension to maintain lattice integrity and appropriate BCR signaling thresholds for B cell positive selection. Indeed, unpublished data indicates that Mgat2 deficient peripheral B cells display the same increase in poly-LacNAc extension that we observed in Mgat2−/− T cells.
Our recent work on N-glycan branching in thymocytes (33) and now B cell positive selection gives great emphasis to the role branching plays in maintaining central tolerance. Future research in delineating the role of N-glycan branching in B cell tolerance may provide new insight on the role of B cells in diseases such as systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis.
Supplementary Material
KEY POINTS.
N-glycan branching is required for generation of mature B cells.
N-glycan branching inhibits development of pre-, immature and T2 B cells,
N-glycan branching promotes CD19 surface expression to inhibit death by neglect.
ACKNOWLEDGEMENTS
We thank Dr. Brian Iritani from the Department of Comparative Medicine at the University of Washington, Seattle, for transferring Eμ-BclxL mice to us.
Funding Sources: R01AI144403 and T32AI060573 from the National Institute of Allergy and Infectious Disease to MD and C.L.M, respectively.
REFERENCES
- 1.Ikuta K, Uchida N, Friedman J, and Weissman IL. 1992. Lymphocyte development from stem cells. Annu Rev Immunol 10: 759–783. [DOI] [PubMed] [Google Scholar]
- 2.Zuniga-Pflucker JC, and Lenardo MJ. 1996. Regulation of thymocyte development from immature progenitors. Curr Opin Immunol 8: 215–224. [DOI] [PubMed] [Google Scholar]
- 3.LeBien TW, and Tedder TF. 2008. B lymphocytes: how they develop and function. Blood 112: 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, Ideker T, Glass CK, and Murre C. 2010. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol 11: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MD, and Alder MN. 2006. The evolution of adaptive immune systems. Cell 124: 815–822. [DOI] [PubMed] [Google Scholar]
- 6.Pieper K, Grimbacher B, and Eibel H. 2013. B-cell biology and development. J Allergy Clin Immunol 131: 959–971. [DOI] [PubMed] [Google Scholar]
- 7.Niiro H, and Clark EA. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol 2: 945–956. [DOI] [PubMed] [Google Scholar]
- 8.Pelanda R, and Torres RM. 2012. Central B-cell tolerance: where selection begins. Cold Spring Harb Perspect Biol 4: a007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King LB, Norvell A, and Monroe JG. 1999. Antigen receptor-induced signal transduction imbalances associated with the negative selection of immature B cells. J Immunol 162: 2655–2662. [PubMed] [Google Scholar]
- 10.Reth M 1992. Antigen receptors on B lymphocytes. Annu Rev Immunol 10: 97–121. [DOI] [PubMed] [Google Scholar]
- 11.Monroe JG 2006. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol 6: 283–294. [DOI] [PubMed] [Google Scholar]
- 12.Nemazee D 2017. Mechanisms of central tolerance for B cells. Nat Rev Immunol 17: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai S, and Cariappa A. 2009. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol 9: 767–777. [DOI] [PubMed] [Google Scholar]
- 14.Cariappa A, Chase C, Liu H, Russell P, and Pillai S. 2007. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood 109: 2339–2345. [DOI] [PubMed] [Google Scholar]
- 15.Diamant E, Keren Z, and Melamed D. 2005. CD19 regulates positive selection and maturation in B lymphopoiesis: lack of CD19 imposes developmental arrest of immature B cells and consequential stimulation of receptor editing. Blood 105: 3247–3254. [DOI] [PubMed] [Google Scholar]
- 16.Hardy RR, Kincade PW, and Dorshkind K. 2007. The protean nature of cells in the B lymphocyte lineage. Immunity 26: 703–714. [DOI] [PubMed] [Google Scholar]
- 17.Stamenkovic I, and Seed B. 1988. CD19, the earliest differentiation antigen of the B cell lineage, bears three extracellular immunoglobulin-like domains and an Epstein-Barr virus-related cytoplasmic tail. J Exp Med 168: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter RH, and Fearon DT. 1992. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 256: 105–107. [DOI] [PubMed] [Google Scholar]
- 19.Chalupny NJ, Kanner SB, Schieven GL, Wee SF, Gilliland LK, Aruffo A, and Ledbetter JA. 1993. Tyrosine phosphorylation of CD19 in pre-B and mature B cells. Embo J 12: 2691–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uckun FM, Burkhardt AL, Jarvis L, Jun X, Stealey B, Dibirdik I, Myers DE, Tuel-Ahlgren L, and Bolen JB. 1993. Signal transduction through the CD19 receptor during discrete developmental stages of human B-cell ontogeny. J Biol Chem 268: 21172–21184. [PubMed] [Google Scholar]
- 21.Engel P, Zhou LJ, Ord DC, Sato S, Koller B, and Tedder TF. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3: 39–50. [DOI] [PubMed] [Google Scholar]
- 22.Rickert RC, Rajewsky K, and Roes J. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376: 352–355. [DOI] [PubMed] [Google Scholar]
- 23.Dennis JW, Nabi IR, and Demetriou M. 2009. Metabolism, cell surface organization, and disease. Cell 139: 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigorian A, Torossian S, and Demetriou M. 2009. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev 230: 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demetriou M, Granovsky M, Quaggin S, and Dennis JW. 2001. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409: 733–739. [DOI] [PubMed] [Google Scholar]
- 26.Brewer CF, Miceli MC, and Baum LG. 2002. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Current opinion in structural biology 12: 616–623. [DOI] [PubMed] [Google Scholar]
- 27.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, and Dennis JW. 2007. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129: 123–134. [DOI] [PubMed] [Google Scholar]
- 28.Chen IJ, Chen HL, and Demetriou M. 2007. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J Biol Chem 282: 35361–35372. [DOI] [PubMed] [Google Scholar]
- 29.Lee SU, Grigorian A, Pawling J, Chen IJ, Gao G, Mozaffar T, McKerlie C, and Demetriou M. 2007. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J Biol Chem 282: 33725–33734. [DOI] [PubMed] [Google Scholar]
- 30.Mkhikian H, Grigorian A, Li CF, Chen HL, Newton B, Zhou RW, Beeton C, Torossian S, Tatarian GG, Lee SU, Lau K, Walker E, Siminovitch KA, Chandy KG, Yu Z, Dennis JW, and Demetriou M. 2011. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun 2: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mkhikian H, Mortales CL, Zhou RW, Khachikyan K, Wu G, Haslam SM, Kavarian P, Dell A, and Demetriou M. 2016. Golgi self-correction generates bioequivalent glycans to preserve cellular homeostasis. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo L, Khim P, Mkhikian H, Mortales CL, and Demetriou M. 2017. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou RW, Mkhikian H, Grigorian A, Hong A, Chen D, Arakelyan A, and Demetriou M. 2014. N-glycosylation bidirectionally extends the boundaries of thymocyte positive selection by decoupling Lck from Ca(2)(+) signaling. Nature immunology 15: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 34.Nitschke L, Carsetti R, Ocker B, Kohler G, and Lamers MC. 1997. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol 7: 133–143. [DOI] [PubMed] [Google Scholar]
- 35.Otipoby KL, Andersson KB, Draves KE, Klaus SJ, Farr AG, Kerner JD, Perlmutter RM, Law CL, and Clark EA. 1996. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature 384: 634–637. [DOI] [PubMed] [Google Scholar]
- 36.Danzer CP, Collins BE, Blixt O, Paulson JC, and Nitschke L. 2003. Transitional and marginal zone B cells have a high proportion of unmasked CD22: implications for BCR signaling. Int Immunol 15: 1137–1147. [DOI] [PubMed] [Google Scholar]
- 37.Samardzic T, Marinkovic D, Danzer CP, Gerlach J, Nitschke L, and Wirth T. 2002. Reduction of marginal zone B cells in CD22-deficient mice. Eur J Immunol 32: 561–567. [DOI] [PubMed] [Google Scholar]
- 38.Irons EE, and Lau JTY. 2018. Systemic ST6Gal-1 Is a Pro-survival Factor for Murine Transitional B Cells. Front Immunol 9: 2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Liu Q, Pang Y, Jin J, Wang H, Cao H, Li Z, Wang X, Ma B, Chi Y, Wang R, Kondo A, Gu J, and Taniguchi N. 2012. Core fucosylation of mu heavy chains regulates assembly and intracellular signaling of precursor B cell receptors. J Biol Chem 287: 2500–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao D, Huang Y, Huang X, Wang W, Yan Q, Wei L, Xin W, Gerson S, Stanley P, Lowe JB, and Zhou L. 2011. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood 117: 5652–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonzi J, Bornet O, Betzi S, Kasper BT, Mahal LK, Mancini SJ, Schiff C, Sebban-Kreuzer C, Guerlesquin F, and Elantak L. 2015. Pre-B cell receptor binding to galectin-1 modifies galectin-1/carbohydrate affinity to modulate specific galectin-1/glycan lattice interactions. Nature communications 6: 6194. [DOI] [PubMed] [Google Scholar]
- 42.Gauthier L, Rossi B, Roux F, Termine E, and Schiff C. 2002. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proceedings of the National Academy of Sciences of the United States of America 99: 13014–13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi B, Espeli M, Schiff C, and Gauthier L. 2006. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol 177: 796–803. [DOI] [PubMed] [Google Scholar]
- 44.Mourcin F, Breton C, Tellier J, Narang P, Chasson L, Jorquera A, Coles M, Schiff C, and Mancini SJ. 2011. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood 117: 6552–6561. [DOI] [PubMed] [Google Scholar]
- 45.Espeli M, Mancini SJ, Breton C, Poirier F, and Schiff C. 2009. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood 113: 5878–5886. [DOI] [PubMed] [Google Scholar]
- 46.de Oliveira FL, Dos Santos SN, Ricon L, da Costa TP, Pereira JX, Brand C, Fermino ML, Chammas R, Bernardes ES, and El-Cheikh MC. 2018. Lack of galectin-3 modifies differentially Notch ligands in bone marrow and spleen stromal cells interfering with B cell differentiation. Sci Rep 8: 3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grigorian A, Lee SU, Tian W, Chen IJ, Gao G, Mendelsohn R, Dennis JW, and Demetriou M. 2007. Control of T Cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem 282: 20027–20035. [DOI] [PubMed] [Google Scholar]
- 48.Rickert RC, Roes J, and Rajewsky K. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 25: 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norvell A, Mandik L, and Monroe JG. 1995. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol 154: 4404–4413. [PubMed] [Google Scholar]
- 50.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, and Hardy RR. 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol 167: 6834–6840. [DOI] [PubMed] [Google Scholar]
- 51.Carsetti R 2004. Characterization of B-cell maturation in the peripheral immune system. Methods Mol Biol 271: 25–35. [DOI] [PubMed] [Google Scholar]
- 52.Kalkavan H, and Green DR. 2018. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otero DC, and Rickert RC. 2003. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol 171: 5921–5930. [DOI] [PubMed] [Google Scholar]
- 54.Zikherman J, Parameswaran R, and Weiss A. 2012. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 489: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers DR, Zikherman J, and Roose JP. 2017. Tonic Signals: Why Do Lymphocytes Bother? Trends Immunol 38: 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan C, Mueller JL, Noviski M, Huizar J, Lau D, Dubinin A, Molofsky A, Wilson PC, and Zikherman J. 2019. Nur77 Links Chronic Antigen Stimulation to B Cell Tolerance by Restricting the Survival of Self-Reactive B Cells in the Periphery. J Immunol 202: 2907–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anzelon AN, Wu H, and Rickert RC. 2003. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol 4: 287–294. [DOI] [PubMed] [Google Scholar]
- 58.Benhamou D, Labi V, Novak R, Dai I, Shafir-Alon S, Weiss A, Gaujoux R, Arnold R, Shen-Orr SS, Rajewsky K, and Melamed D. 2016. A c-Myc/miR17–92/Pten Axis Controls PI3K-Mediated Positive and Negative Selection in B Cell Development and Reconstitutes CD19 Deficiency. Cell Rep 16: 419–431. [DOI] [PubMed] [Google Scholar]
- 59.Lam KP, Kuhn R, and Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 60.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, and Rajewsky K. 2009. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang W, Weintraub BC, Dunlap B, Garside P, Pape KA, Jenkins MK, Goodnow CC, Mueller DL, and Behrens TW. 1998. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity 9: 35–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.