Abstract
The contribution of spatial processes to the spatial patterns of ecological systems is widely recognized, but spatial patterns in the ecology of plant‐herbivore interactions have rarely been investigated quantitatively owing to limited budget and time associated with ecological research. Studies of the level of browsing on various tree species reported either no spatial auto‐correlation or a small effect size. Further, the effects of disturbance events, such as hurricanes, which create large forest openings on spatial patterns of herbivory are not well understood.
In this study, we used forest inventory data obtained from the federal state of Baden‐Württemberg (Southern Germany) between 2001 and 2009 (grid size: 100 × 200 m) and thus, after hurricane Lothar struck Southern Germany in 1999. We investigated whether the browsing level of trees (height ≤ 130 cm) in one location is independent of that of the neighborhood.
Our analyses of 1,758,622 saplings (187.632 sampling units) of oak (Quercus), fir (Abies), spruce (Picea), and beech (Fagus) revealed that the browsing level is characterized by a short distance spatial auto‐correlation.
The application of indicator variables based on browsed saplings should account for the spatial pattern as the latter may affect the results and therefore also the conclusions of the analysis.
Keywords: beech, fir, forest inventory, Morans'I, oak, roe deer, spruce
Spatial auto‐correlation for both regeneration density and browsing level is rather the rule than an exception. Accounting for spatial pattern in forestry will have important consequences for the design of forest inventories and management practices.

1. INTRODUCTION
Although tree recruitment in the northern Hemisphere has been impacted by large herbivores for thousands of years (Sommer, Fahlke, Schmölcke, Benecke, & Zachos, 2009), anthropogenic changes may be the most important recent factor affecting forest development (Tinner et al., 2013; Whitlock, Colombaroli, Conedera, & Tinner, 2017) and ungulate density (Bradshaw, Hannon, & Lister, 2003) as well as the relationship between them. It is widely recognized that the population dynamic of large herbivores is impacted by forest changes (Gaillard et al., 2003; Gill, Johnson, Francis, Hiscocks, & Peace, 1996). Research conducted over the last decade has highlighted, that large herbivores affect tree recruitment and species composition and thus future forest development as well (Bernard et al., 2017; Hidding, Tremblay, & Côté, 2013; Kuijper, Cromsigt, et al., 2010; Nuttle, Ristau, & Royo, 2014).
Browsing by large herbivores is frequently perceived as a major challenge for tree recruitment in Europe (Kupferschmid & Heiri, 2019), North America (Devaney, Pullen, Cook‐Patton, Burghardt, & Parker, 2020; Saucier, Champagne, Côté, & Tremblay, 2019), and Asia (Tamura & Yamane, 2017). However, forestry management remains largely disconnected from the management of large herbivore populations (Beguin, Tremblay, Thiffault, Pothier, & Côté, 2016; Reimoser, 2003). During the 18th century, forest management in Europe focussed on the consequences of anthropogenic changes for sustainable forestry practices, such as the loss of tree recruitment area due to industrial development and the intensive utilization of domestic animals (see Carlowitz, 1713). Until the early 20th century, the effects of large herbivores on tree recruitment and thus on forest development were largely ignored because large herbivores were of relatively low abundance, both in North America (Leopold, Bean, & Norman, 1943) and in Europe (Breitenmoser, 1998; Jędrzejewska, Jędrzejewski, Bunevich, Miłkowski, & Krasiński, 1997). During the 1940s and 1950s, however, awareness of the effects of large herbivores on tree recruitment in the Northern Hemisphere increased (Aldous, 1944; Leopold et al., 1943). Among the approaches developed to quantify the utilization of forest plants by large herbivores (Aldous, 1944; Reimoser, 2000) was that of Zai (1964), who in 1964 proposed a determination of the percentage of browsed trees (number of trees with browsed terminal buds divided by the total number of trees—the browsing level) as a robust index of roe deer (Capreolus capreolus) browsing. However, it was not until the 1980s that the browsing level of terminal buds was linked to tree growth and tree survival (Eiberle & Nigg, 1984, 1987). Thereafter, browsing‐level assessments gained increasing attention (Reimoser & Gossow, 1996; Welch, Staines, Scott, & French, 1992).Thus, the browsing‐level approach of Zai (1964), with its minimal required effort and observer independence, seemed to be a promising method to quantify the impact of large herbivores on tree regeneration (Morellet & Guibert, 1999). However, Reimoser, Odermatt, Roth, and Suchant (1997) and Senn and Häsler (2005) argued that a specific browsing level cannot be seen as direct damage caused by herbivores nor can it be related to a specific damage to forestry caused by herbivores. Reimoser (2003) pointed out that a higher browsing level might be a consequence of: (a) an increased need of large herbivores to engage in damaging activities, (b) an increase in the numbers of large herbivores, or (c) a change in forest structure resulting in the increased vulnerability of the saplings.
During the last two decades, numerous studies have contributed to a more holistic picture of the factors affecting the browsing of trees (Gerhardt, Arnold, Hackländer, & Hochbichler, 2013), including the population size of large herbivores (Beguin et al., 2016; Bernes et al., 2018; Chollet et al., 2016), forest management (Beguin, Pothier, & Prévost, 2009; Reimoser, Partl, Reimoser, & Vospernik, 2009), plant species composition (Boulanger et al., 2017; Mysterud, Askilsrud, Loe, & Veiberg, 2010; Nishizawa, Tatsumi, Kitagawa, & Mori, 2016), disturbance events (Royo, Collins, Adams, Kirschbaum, & Carson, 2010), landscape composition (Royo, Kramer, Miller, Nibbelink, & Stout, 2017), the combined effects of two large herbivores on plant communities (Faison, DeStefano, Foster, Motzkin, & Rapp, 2016) and those of season and herbivore density (Giroux, Dussault, Tremblay, & Côté, 2016). Thus, any indicator variable drawing on information from browsed trees should be characterized by a high spatial and temporal variability (Kuijper et al., 2009; Kuijper, Cromsigt, et al., 2010; Kuijper, Jedrzejewska, et al., 2010). Even though earlier studies of ecological processes recognized the importance of spatial processes (Moran, 1950; Sokal & Oden, 1978b), the quantification of spatial patterns in ecological research is rare (Dormann, 2007). Given that tree regeneration occurs patchily (Yokozawa, Kubota, & Hara, 1999) or in waves (Wiegand, Moloney, & Milton, 1998) and that large herbivores select habitat patches (Moser, Schütz, & Hindenlang, 2006; Widmer et al., 2004), spatial auto‐correlation in both the sapling density and browsing level should be the rule rather than the exception. Indeed, several authors have observed that the regeneration of oak and beech trees is spatially clumped (Götmark & Kiffer, 2014; Kunstler, Curt, & Lepart, 2004). Kunstler and coauthors (Kunstler et al., 2004) concluded that the spatial patterns of trees are mainly affected by seed dispersal and the spatial variability of germination. This nonrandom distribution in the environment will presumably affect the habitat utilization of large herbivores, which in part feed on saplings (Kuijper et al., 2009). The decision to browse a tree is part of a hierarchical decision‐making process that incorporates various factors at different spatial scales (cf. Champagne, Moore, Côté, & Tremblay, 2018). On an individual level, browsing intensity may be largely determined by the amount and quality of the forage (Shipley & Spalinger, 1995), including its species richness (Ohse, Seele, Holzwarth, & Wirth, 2017). This relation holds in particular for roe deer (Capreolus capreolus), as a selective feeder with a small rumen capacity (Hofmann, 1989).
In Baden‐Württemberg (Southern Germany) the browsing of trees is mainly attributable to roe deer because other species of the family Cervidae are restricted to relatively small areas (Hagen, Haydn, & Suchant, 2018; Hagen, Kühl, Kröschel, & Suchant, 2019). The home range of roe deer individuals varies between 20 and 60 ha (0.2–0.6 km2) depending on the region, season, and landscape composition (Lovari, Serrao, & Mori, 2017; Morellet et al., 2013; Richard, Said, Hamann, & Gaillard, 2014). Within home range roe deer select not only high‐quality forage patches but also high‐quality plants within those patches (Moser et al., 2006), and food quantity and quality will likely impact roe deer numbers (Gaillard et al., 2003; Gill et al., 1996). In the study of Gill and coauthors (Gill et al., 1996), roe deer density correlated negatively with total conifer cover with a time lag of 6 years. Thus, based on the ecology of plant‐herbivore interactions, it can be expected that the browsing of trees will be characterized by spatial patterns. However, there have been no, or at best few (cf. Champagne et al., 2018; Morellet & Guibert, 1999; Ohse et al., 2017) attempts to quantify the spatial characteristics of the browsing level. Furthermore, the results of those studies indicated either no auto‐correlation (Champagne et al., 2018; Morellet & Guibert, 1999) or only negligibly small effect sizes (Ohse et al., 2017).
In the present study, data from forest inventories (grid size of 100 × 200 m) conducted in the German federal state of Baden‐Württemberg (predator‐free system; only red fox (Vulpes vulpes), which kills roe deer juveniles, are common in Baden‐Württemberg [Kämmerle, Niekrenz, & Storch, 2019]) between 2001 and 2009 were analyzed. We tested the assumption that the species‐specific browsing level of saplings (height ≤ 130 cm) at one location is independent of the species‐specific browsing level of saplings at neighboring locations. The spatial independence not only of the browsing level but also of the number of saplings per sampling unit (sapling density) was determined by calculating Moran's I (Moran, 1950). The results presented for four major tree species (Abies, Picea, Fagus, Quercus) in Europe highlight the need to quantify spatial patterns in plant‐herbivore ecology research and practice.
2. DATA AND METHODS
Since 1998, forest cultural undertakings (“Betriebe”) of the German federal state of Baden‐Württemberg has made use of forest inventory data to estimate timber production (Nothdurft, Borchers, Niggemeyer, Saborowksi, & Kändler, 2009). The inventory is conducted once per decade at the level of one “Betrieb” and collects data on the amount, age, and spatial distribution of tree species within a predefined grid (100 × 200 m, cf. Figure S1). The sampling units were marked by a steel pipe embedded in the ground in order to prevent visual detection. The collected information included the number of young trees (height ≤ 130 cm) within a sampling unit (circle with r = 1.5 m and an area of 7.1 m2) with and without browsing. A browsed tree was defined as a tree whose terminal bud was browsed during the last 3 years. Each sampling unit contained information describing a maximum of 90 saplings per tree species (density of 12.68 saplings per m2). In sampling units where the number of saplings exceeded this density, saplings deemed to be representative with respect to the height distribution of the regeneration and the overall browsing level were sampled. For this study, we analyzed data of four tree species, fir (Abies, number of saplings [N] = 238,471), Norway spruce (Picea, [N] = 715,120), beech (Fagus, [N] = 694,854), and oak (Quercus, [N] = 110,176) for the period 2001–2009 (Table 1). The annual sample covering several distinct regions in Baden‐Württemberg (cf. Figure 1) may or may not have appropriately represented the sapling density and the browsing level for the federal state of Baden‐Württemberg. We thus compared the browsing level determined in forest inventories with the results of the “Forstliches Gutachen Baden‐Württemberg” (cf. Figure 2), an official management tool to estimate both the browsing level and the possibility to reach forest management objectives. Since 1983, a survey has been conducted every third year for each hunting ground in Baden‐Württemberg (N ≈ 6,000, size of the hunting grounds ≈300–400 ha). In December 1999, hurricane Lothar struck Eastern France (Storms et al., 2006) and Southern Germany (Erb, Odenthal‐Kahabka, & Püttmann, 2004), creating large openings in the respective forests. In the federal state of Baden‐Württemberg, 30 million solid cubic meters were storm damaged (Erb et al., 2004). The resulting openings led to an increase in the number of saplings and thus to improved habitat quality for large herbivores in subsequent years (Storms et al., 2006; Widmer et al., 2004). Data of the “Forstliches Gutachten Baden‐Württemberg” suggested that the browsing intensity in Baden‐Württemberg declined to a local minimum in 2001. Thus, in this study, we used 2001 as the reference year (cf. Figure 2).
TABLE 1.
Moran's I value for the browsing level and the regeneration density as well as basic information about the number of sample unit and the number of sampled trees
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|---|
| Fir | |||||||||
| Number of sampling units | 3,526 | 6,930 | 7,728 | 2,522 | 2.637 | 2,922 | 2,441 | 2,437 | 4,550 |
| Browsing level | 0.12 | 0.14 | 0.14 | 0.21 | 0.31 | 0.3 | 0.26 | 0.23 | 0.32 |
| Estimate I100 | 0.18 | 0.16 | 0.14 | 0.13 | 0.28 | 0.14 | 0.05 | 0.12 | 0.12 |
| Standard deviation | 0.03 | 0.02 | 0.02 | 0.04 | 0.03 | 0.04 | 0.05 | 0.05 | 0.03 |
| p two sided | <.0001 | <.0001 | <.0001 | .0003 | <.0001 | .0002 | .23 | .01 | <.0001 |
| Estimate I200 | 0.04 | 0.07 | 0.07 | 0.06 | 0.15 | 0.03 | 0 | 0.04 | 0.07 |
| Standard deviation | 0.04 | 0.03 | 0.03 | 0.05 | 0.05 | 0.06 | 0.07 | 0.07 | 0.04 |
| p two sided | .31 | .01 | .009 | .26 | .001 | .64 | .93 | .5 | .04 |
| Total number of saplings | 24,136 | 56,776 | 64,729 | 18,613 | 12,490 | 12,524 | 11,870 | 12,928 | 24,405 |
| Mean sapling density | 6.85 | 8.19 | 8.34 | 7.38 | 4.74 | 4.29 | 4.86 | 5.34 | 5.36 |
| Estimate I100 | 0.22 | 0.25 | 0.23 | 0.12 | 0.16 | 0.07 | 0.09 | 0.07 | 0.11 |
| Standard deviation | 0.03 | 0.02 | 0.02 | 0.04 | 0.03 | 0.04 | 0.05 | 0.05 | 0.03 |
| p two sided | <.0001 | <.0001 | <.0001 | .0006 | <.0001 | .06 | .04 | .12 | <.0001 |
| Estimate I200 | 0.1 | 0.12 | 0.09 | 0.1 | 0.1 | 0.03 | 0 | 0.02 | 0.04 |
| Standard deviation | 0.04 | 0.03 | 0.03 | 0.05 | 0.06 | 0.07 | 0.07 | 0.07 | 0.04 |
| p two sided | .009 | <.0001 | .0008 | .04 | .03 | .57 | .93 | .71 | .26 |
| Spruce | |||||||||
| Number of sampling units | 6,114 | 9,176 | 11,890 | 5,169 | 3,414 | 5,469 | 3,319 | 5,074 | 4,727 |
| Browsing level | 0.03 | 0.02 | 0.03 | 0.07 | 0.08 | 0.07 | 0.03 | 0.03 | 0.03 |
| Estimate I100 | 0.1 | 0.12 | 0.23 | 0.21 | 0.32 | 0.1 | 0.04 | 0.06 | 0.02 |
| Standard deviation | 0.02 | 0.02 | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 |
| p two sided | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .0002 | .37 | .05 | .5 |
| Estimate I200 | 0.04 | 0.02 | 0.1 | 0.12 | 0.2 | 0.03 | 0 | 0.01 | 0.01 |
| Standard deviation | 0.03 | 0.02 | 0.02 | 0.03 | 0.04 | 0.04 | 0.07 | 0.05 | 0.04 |
| p two sided | .22 | .43 | <.0001 | .0005 | <.0001 | .37 | .93 | .73 | .87 |
| Total number of saplings | 67,897 | 122,154 | 231,414 | 80,737 | 28,392 | 55,564 | 20,990 | 56,642 | 51,330 |
| Mean sapling density | 11.01 | 13.31 | 19.46 | 15.62 | 8.31 | 10.16 | 6.32 | 11.16 | 10.86 |
| Estimate I100 | 0.26 | 0.27 | 0.22 | 0.14 | 0.2 | 0.17 | 0.05 | 0.11 | 0.12 |
| Standard deviation | 0.02 | 0.02 | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 |
| p two sided | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .29 | .0004 | <.0001 |
| Estimate I200 | 0.16 | 0.16 | 0.14 | 0.14 | 0.13 | 0.11 | 0.03 | 0.06 | 0.07 |
| Standard deviation | 0.03 | 0.02 | 0.02 | 0.03 | 0.04 | 0.04 | 0.07 | 0.05 | 0.04 |
| p two sided | <.0001 | <.0001 | <.0001 | .03 | .002 | .003 | .64 | .22 | .08 |
| Beech | |||||||||
| Number of sampling units | 10,256 | 11,804 | 9,827 | 5,648 | 5,649 | 9,525 | 8,274 | 7,654 | 5,888 |
| Browsing level | 0.09 | 0.11 | 0.13 | 0.17 | 0.16 | 0.18 | 0.1 | 0.12 | 0.18 |
| Estimate I100 | 0.19 | 0.15 | 0.19 | 0.17 | 0.21 | 0.17 | 0.11 | 0.17 | 0.11 |
| Standard deviation | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 |
| p two sided | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Estimate I200 | 0.09 | 0.06 | 0.06 | 0.08 | 0.11 | 0.08 | 0.05 | 0.08 | 0.03 |
| Standard deviation | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 |
| p two sided | <.0001 | .005 | .009 | .01 | .0001 | .003 | .04 | .003 | .35 |
| Total number of saplings | 114,331 | 119,092 | 102,478 | 57,008 | 41,352 | 75,272 | 66,482 | 68,950 | 49,889 |
| Mean sapling density | 11.15 | 10.09 | 10.43 | 10.09 | 7.32 | 7.9 | 8.03 | 9.01 | 8.47 |
| Estimate I100 | 0.34 | 0.34 | 0.21 | 0.21 | 0.17 | 0.13 | 0.23 | 0.17 | 0.06 |
| Standard deviation | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 |
| p two sided | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .02 |
| Estimate I200 | 0.23 | 0.19 | 0.11 | 0.12 | 0.08 | 0.05 | 0.11 | 0.12 | 0.01 |
| Standard deviation | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 |
| p two sided | <.0001 | <.0001 | <.0001 | .0002 | .003 | .03 | <.0001 | <.0001 | .66 |
| Oak | |||||||||
| Number of sampling units | 2,155 | 3,005 | 1,977 | 1,858 | 1,,895 | 2,022 | 1,521 | 1,079 | 1,902 |
| Browsing level | 0.15 | 0.19 | 0.18 | 0.31 | 0.29 | 0.19 | 0.19 | 0.24 | 0.34 |
| Estimate I100 | 0.11 | 0.14 | 0.08 | 0.1 | 0.2 | 0.1 | 0.04 | 0.01 | 0.1 |
| Standard deviation | 0.05 | 0.04 | 0.06 | 0.05 | 0.04 | 0.05 | 0.08 | 0.14 | 0.05 |
| p two sided | .02 | .0002 | .16 | .05 | <.0001 | .04 | .6 | .87 | .04 |
| Estimate I200 | 0.03 | 0.04 | 0.02 | 0.05 | 0.09 | 0.03 | 0.01 | 0.01 | 0.05 |
| Standard deviation | 0.08 | 0.06 | 0.1 | 0.08 | 0.06 | 0.08 | 0.14 | 0.37 | 0.08 |
| p two sided | .71 | .46 | .82 | .54 | .14 | .64 | .88 | .81 | .48 |
| Total number of saplings | 13,865 | 28,316 | 11,343 | 14,165 | 13,475 | 10,218 | 6,050 | 2,815 | 9,929 |
| Mean sapling density | 6.43 | 9.42 | 5.74 | 7.62 | 7.11 | 5.05 | 3.98 | 2.61 | 5.22 |
| Estimate I100 | 0.21 | 0.23 | 0.32 | 0.29 | 0.19 | 0.3 | 0.12 | 0.03 | 0.17 |
| Standard deviation | 0.05 | 0.04 | 0.05 | 0.05 | 0.04 | 0.05 | 0.07 | 0.11 | 0.05 |
| p two sided | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .11 | .72 | .0004 |
| Estimate I200 | 0.07 | 0.1 | 0.17 | 0.13 | 0.14 | 0.12 | 0.04 | 0.00 | 0.15 |
| Standard deviation | 0.08 | 0.06 | 0.1 | 0.08 | 0.06 | 0.08 | 0.13 | 0.00 | 0.08 |
| p two sided | .37 | .12 | .09 | .12 | .02 | .1 | .67 | .99 | .05 |
Bold values highlight a significant spatial auto‐correlation under the global p‐value of .05.
FIGURE 1.
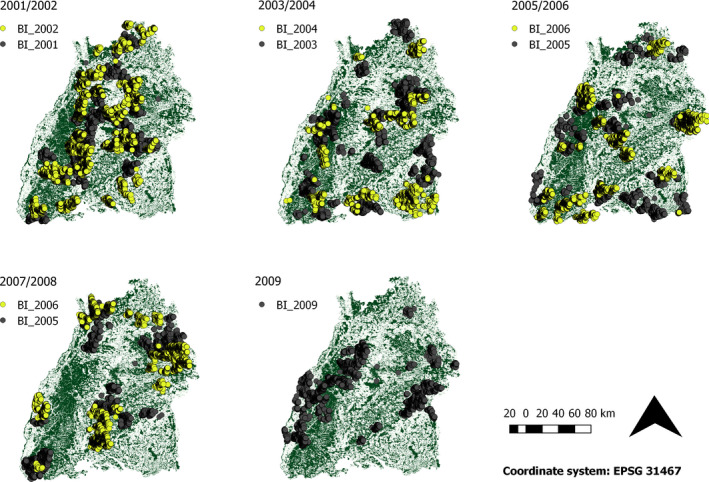
Sampling units of forest inventories (BI) in Baden‐Württemberg (2001–2009). The background shows the area covered by forest
FIGURE 2.
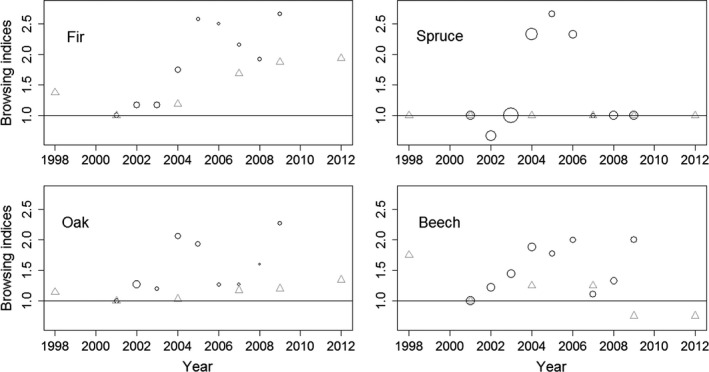
Temporal variation in the browsing of fir, spruce, beech, and oak. Open black circles show a browsing index based on data of forest inventories. To facilitate visual comparisons, all time series were normalized by the browsing level for 2001. The size of the circle represents the mean sapling density (the greater the circle the greater the mean number of saplings per sampling unit). Open gray triangles represent a browsing index derived from data of the Forstliches Gutachten Baden‐Württemberg—it represents the proportion of hunting grounds in Baden‐Württemberg that reported a high browsing level (>50%). To facilitate visual comparisons, time series were normalized by the value of 2001. We choose 2001 as a reference year as it represents a local minimum of the browsing level in Baden‐Württemberg
We calculated Moran's I for a predefined neighborhood d nb (d nb = 100 m, 200 m,…, 500 m that cover an area of 0.03 km2, 0.126 km2,…,0.785 km2) covering mean values of home range size published for roe deer to test for spatial independence:
| (1) |
where N is the number of spatial units, x the browsing level, x mean the mean browsing level, wij the weight according to the defined neighborhood (wij = 0 for i = j; wij = 0 for d(i, j) > d nb) and W the sum of all wij.
Thus, I was calculated as the correlation coefficient for pairs of points considered as neighbors.
A calculated value of I significantly less or greater than 0 negated the hypothesis that the browsing of young trees (height ≤ 130 cm) is a spatially independent process. The Bonferroni correction was used to correct for multiple testing effects. Statistical calculations were carried out using R version 3.4.4 (R Core Team 2018) and the R package spdep (Bivand et al., 2006).
3. RESULTS
Between 2001 and 2009, the browsing level of the four studied tree species (fir, spruce, beech, and oak) varied considerably, by a factor of 2 (Table 1 and Figure 2). Compared to the reference year 2001, high browsing levels were determined for all tree species during the period 2004–2006 (Figure 2). Sapling density peaked during 2001–2003 (cf. Table 1 and Figure 3—local maximum for oak in 2002, beech in 2001, spruce and fir in 2003). The estimates for I as well as the estimated p‐values showed strong inter‐annual variations for both the browsing level and the sapling density (Table 1, Figures 3 and S4). Both the browsing level and the sapling density were characterized by a positive spatial auto‐correlation (Figures 3 and S4). Thus, Moran's I (browsing level) was greatest for a neighborhood distance (d nb) of 100 m (Table 1, Figure 3) and decreased for an increasing d nb up to 500 m (Figures S2 and S3 show Moran's I for d nb up to 500 m) indicating that both variables were characterized by a short‐distance auto‐correlation. Although the parameter estimates for I did not exceed 0.32 (browsing level) and 0.34 (sapling density), the data did not support the hypothesis of spatial independence for either sapling density (d nb = 100 m, all tree species) or browsing level (d nb = 100 m, fir, spruce, and beech). The maximal values for I (browsing level) were calculated for the year 2005 and independently of the tree species (Figures 3 and S4) and corresponded to a rather high overall browsing level (Figure 2). Thus, the local maximum of both, the browsing level and the parameter estimate of Moran's I lagged 5–6 years behind hurricane Lothar. Maximal values for I (sapling density) were calculated for fir, spruce and beech during the years 2001 and 2002 (directly after Lothar created large openings) and for oak during the period 2003–2006 (Figures 3 and S4).
FIGURE 3.
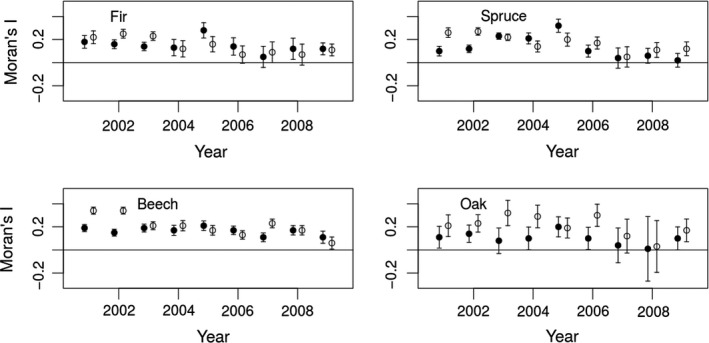
Moran's I for the browsing level (filled circles) and the sapling density (open circles) based on a neighborhood distance of 100 m. The bars cover twice the square root of the estimated variance
4. DISCUSSION
In this study, Moran's I (Moran, 1950) was calculated to test for the spatial independence of (a) the sapling density of four tree species (fir, spruce, beech, and oak ≤ 130 cm) and (b) the browsing level of those trees, using forest inventory data for Baden‐Württemberg (2001–2009). The results showed an auto‐correlation of the two variables for a distance of 100 m (and partly for larger neighborhood distances up to 300 m; cf. Figures S2 and S4).
The unambiguous demonstration of a spatial auto‐correlation for sapling density and browsing level was initially surprising. Although the ecology of plant‐herbivore interactions predicts the existence of spatial auto‐correlation in sapling density and in the browsing level of trees, previous research reported either the absence of spatial auto‐correlation in the browsing level (Champagne et al., 2018; Morellet & Guibert, 1999) or rather small values of Moran's I (Ohse et al., 2017). This mismatch between ecological prediction and recent findings might reflect the uncertainty in decision‐making processes owing to incomplete information (Hagen, Kramer‐Schadt, Fahse, & Heurich, 2014). The limited budget and time invested in ecological research have led to a rather limited spatial‐temporal resolution of the available data sets and thus in a low power to detect spatial auto‐correlations. For example, previous sampling units frequently corresponded to a single year (Morellet & Guibert, 1999), two years (Champagne et al., 2018) or three years (Ohse et al., 2017) and were sampled in relatively small study areas (<6 km2 Morellet & Guibert, 1999, Champagne et al., 2018; 75 km2 Ohse et al., 2017). Our inventory data for the year 2007 provide an example of how incomplete information can result in a failure to detect spatial auto‐correlations (Table 1). The estimated value of Morans I (year 2007) supports the hypothesis of spatial independence for both sapling density and browsing level. However, while this conclusion might be true for the sampled year/region, it does not imply that data on sapling density and browsing level can generally be regarded as spatially independent variables (Table 1, Figures 2 and S4). Differences in Moran's I between tree species may be due to the different dispersal strategies of the trees (Dormann, 2007; Yokozawa et al., 1999). The decrease in Moran's I (sapling density) for fir, spruce and beech throughout the period 2001–2009 likely reflected ecological processes initiated by hurricane Lothar in 1999. Only Moran's I (sapling density) for oak showed intensive year to year variations. Moran's I (browsing level) reached peak values in 2005, which coincided with the high values for the browsing level (cf. Figures 2 and 3). The difference between the maximum and minimum annual browsing levels of oak, fir, spruce, and beech was 20%, 19%, 6%, and 9%, respectively (Table 1). These differences together with Moran's I (browsing level) clearly show that browsing is a highly variable process both in time and in space. While this is well‐known in plant‐herbivore ecology (Beguin et al., 2016; Bernes et al., 2018; Sinclair & Krebs, 2002; Sokal & Oden, 1978a), our study is the first to show evidence that the browsing level of four major European tree species (fir, spruce, oak, and beech) is characterized by a significant short‐distance auto‐correlation. The fact that Moran's I of the browsing level and sapling density was more likely to be significant for a neighborhood distance of 100 m suggests that processes responsible for this spatial pattern were themselves characterized a by short‐distance spatial autocorrelation (Sokal & Oden, 1978b). The observed spatial pattern can be explained by four different responses (Sokal & Oden, 1978b): (1) to an environmental gradient (Model I): (2) to habitat patches that are heterogeneous among themselves but internally homogenous (Model II); (3) to the isolation caused by distance (Model III); and (4) to differences in historical factors (Model IV).
We suggest that the observed auto‐correlation of the sapling density is best explained by a combination of Model II, III, and IV. As for the observed spatial auto‐correlation of the browsing level, our results favor a combination of Model I, II, and III. Although forest practices are one major factor impacting the distribution of tree species and species composition in Germany (Model IV) hurricane Lothar created large openings in Baden‐Württemberg. These openings were homogenous (Model II) but separated from each other (Model III). The openings led to an increase in the overall sapling density between 2001 and 2003 (cf. Table 1 and Figure 2). High values were determined for Moran's I for the sapling density of oaks between 2003 and 2006, when the sampling units were characterized by relatively low to medium sapling densities (Table 1, Figure 2). Moran's I for the sapling densities of fir, spruce and beech were greatest for the years 2001 and 2002 and thus for years in which sapling density was highest (Models II and III). The high sapling density between 2001 and 2003 together with the inability of hunters to access hunting grounds in 2000 and 2001 (cf. Gaillard et al., 2003 for France) may have affected the population dynamic of roe deer in Baden‐Württemberg and in turn the overall browsing level (Model I). With respect to the results of Gill and coauthors, this might have affected the browsing of trees around the year 2005 (6 years after Lothar created the openings). In fact, not only the browsing level of oak, spruce, fir and beech (Figure 2) but also Moran's I of the browsing level (Figures 3 and S4) peaked during 2004–2006. These results suggest that hurricane Lothar initiated the following cascade: The storm‐damaged forest led to both an inability of hunters to access hunting grounds and an increase in the number of saplings → an increase in habitat suitability together with a decrease in hunting‐related mortality → an increase in roe deer numbers in subsequent years → and higher browsing levels. Thus, the spatial auto‐correlation of the browsing level for relatively short neighborhood distances (area of 0.03 km2 [Figure 3], 0.13 km2 [Figure S4] and 0.28 km2 [Figure S2] for spruce in 2005) might reflect not only the sapling density but also the selection process of roe deer within their home range, as the home range size varies between 0.2 and 0.6 km2 (Lovari et al., 2017; Morellet et al., 2013; Richard et al., 2014) and is smaller in forest areas (Lovari et al., 2017). If this was the case, then the analysis of datasets of browsed and unbrowsed trees using a grid size of 50 m or 25 m would be informative. Although definitively identifying the drivers of the spatial auto‐correlation in both the regeneration and the browsing level will be challenging, our findings highlight the importance of accounting for spatial patterns in plant‐herbivore ecology. In addition, the application of indicator variables based on browsed trees (cf. Chevrier et al., 2012; Maublanc, Bideau, Launay, Monthuir, & Gerard, 2016; Morellet et al., 2007; Pierson & De Calesta, 2015) should account for the spatial pattern in sapling density. It should also be noted that although forest inventories in Austria, Germany, and Switzerland are conducted using a grid size of 100 × 200 m or 100 × 100 m (Kupferschmid, 2018; Nothdurft et al., 2009; Ohse et al., 2017), the distance between the sample units used to obtain information on browsed trees is frequently <100 m (Ammer, 1996; Moser et al., 2006; Kuijper et al., 2009; Champagne et al., 2018) or ≤200 m (Heinze et al., 2011; Heuze, Schnitzler, & Klein, 2005; Kuijper, Jedrzejewska, et al., 2010; Morellet & Boscardin, 2001; Ohse et al., 2017; Partl, Szinovatz, Reimoser, & Schweiger‐Adler, 2002).
Thus, we suggest that every study using data on browsed trees should first investigate the existence and strength of spatial auto‐correlation. If the variable of interest is used as a target variable for any regression model or correlation analysis, then appropriate statistical methods should be applied (cf. Dormann et al., 2007). Otherwise, the assumption of independence of most standard statistical procedures will be violated and type I and II error rates might increase (Dormann et al., 2007; Legendre, 1993). Our study can be understood as a first step in a systematic investigation of short‐distance spatial autocorrelation phenomena in plant‐herbivore ecology. The insights obtained from those investigations will likely have important consequences for the design of forest inventories and the management practices derived from their results.
CONFLICT OF INTEREST
Rudi Suchant and Robert Hagen disclosed no conflict of interests.
AUTHOR CONTRIBUTION
Robert Hagen: Conceptualization (lead); Formal analysis (lead); Methodology (lead); Validation (equal); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Rudi Suchant: Conceptualization (supporting); Data curation (lead); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Project administration (lead); Supervision (lead); Validation (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting).
Supporting information
Figures S1‐S4
ACKNOWLEDGMENTS
We thank W. Ran for editorial support during the preparation of previous drafts of this manuscript and two anonymous reviewers and the associate editor for constructive comments. Open access funding enabled and organized by Projekt DEAL.
Hagen R, Suchant R. Evidence of a spatial auto‐correlation in the browsing level of four major European tree species. Ecol Evol. 2020;10:8517–8527. 10.1002/ece3.6577
DATA AVAILABILITY STATEMENT
Data visualized in Figures 2, 3 and S2–S4 were archived in Dryad (https://doi.org/10.5061/dryad.4xgxd256m). Data of forest inventories (raw data shown in Figure 1) will not be made publicly available as data contain sensitive information (human subject data in time and space) about timber production in the federal state of Baden‐Württemberg.
REFERENCES
- Aldous, S. E. (1944). A deer browse survey method. Journal of Mammalogy, 25(2), 130–136. 10.2307/1375010 [DOI] [Google Scholar]
- Ammer, C. (1996). Impact of ungulates on structure and dynamics of natural regeneration of mixed mountain forests in the Bavarian Alps. Forest Ecology and Management, 88, 43–53. 10.1016/S0378-1127(96)03808-X [DOI] [Google Scholar]
- Beguin, J. , Pothier, D. , & Prévost, M. (2009). Can the impact of deer browsing on tree regeneration be mitigated by shelterwood cutting and strip clearcutting? Forest Ecology and Management, 257, 38–45. 10.1016/j.foreco.2008.08.013 [DOI] [Google Scholar]
- Beguin, J. , Tremblay, J. , Thiffault, N. , Pothier, D. , & Côté, S. D. (2016). Management of forest regeneration in boreal and temperate deer – forest systems : Challenges, guidelines, and research gaps. Ecosphere, 7(10), 1–16. 10.1002/ecs2.1488 [DOI] [Google Scholar]
- Bernard, M. , Boulanger, V. , Dupouey, J.‐L. , Laurent, L. , Montpied, P. , Morin, X. , … Saïd, S. (2017). Deer browsing promotes Norway spruce at the expense of silver fir in the forest regeneration phase. Forest Ecology and Management, 400, 269–277. 10.1016/j.foreco.2017.05.040 [DOI] [Google Scholar]
- Bernes, C. , Macura, B. , Jonsson, B. G. , Junninen, K. , Müller, J. , Sandström, J. , & Macdonald, E. (2018). Manipulating ungulate herbivory in temperate and boreal forests: Effects on vegetation and invertebrates. A systematic review. Environmental Evidence, 7, 13 10.1186/s13750-018-0125-3 [DOI] [Google Scholar]
- Bivand, R. , Bernat, A. , Carvalho, M. , Chun, Y. , Dormann, C. F. , Dray, S. , … Millo, G. (2006). The spdep package, version 0.3‐13. [Google Scholar]
- Boulanger, V. , Dupouey, J.‐L. , Archaux, F. , Badeau, V. , Baltzinger, C. , Chevalier, R. , … Ulrich, E. (2017). Ungulates increase forest plant species richness to the benefit of non‐forest specialists. Global Change Biology, 24(2), e485–e495. 10.1111/gcb.13899 [DOI] [PubMed] [Google Scholar]
- Bradshaw, R. H. W. , Hannon, G. E. , & Lister, A. M. (2003). A long‐term perspective on ungulate‐vegetation interactions. Forest Ecology and Management, 181, 267–280. 10.1016/S0378-1127(03)00138-5 [DOI] [Google Scholar]
- Breitenmoser, U. (1998). Large predators in the Alps: The fall and rise of man’s competitors. Biological Conservation, 83(3), 279–289. 10.1016/S0006-3207(97)00084-0 [DOI] [Google Scholar]
- Carlowitz, H. C. (1713). Sylvicultura oeconomica In Braun J. F. (Ed.). [Google Scholar]
- Champagne, E. , Moore, B. D. , Côté, S. D. , & Tremblay, J. P. (2018). Spatial correlations between browsing on balsam fir by white‐tailed deer and the nutritional value of neighboring winter forage. Ecology and Evolution, 8(5), 2812–2823. 10.1002/ece3.3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier, T. , Saïd, S. , Widmer, O. , Hamard, J. P. , Saint‐Andrieux, C. , & Gaillard, J. M. (2012). The oak browsing index correlates linearly with roe deer density: A new indicator for deer management? European Journal of Wildlife Research, 58(1), 17–22. 10.1007/s10344-011-0535-9 [DOI] [Google Scholar]
- Chollet, S. , Padié, S. , Stockton, S. , Allombert, S. , Gaston, A. J. , & Martin, J. L. (2016). Positive plant and bird diversity response to experimental deer population reduction after decades of uncontrolled browsing. Diversity and Distributions, 22, 274–287. 10.1111/ddi.12393 [DOI] [Google Scholar]
- Devaney, J. L. , Pullen, J. , Cook‐Patton, S. C. , Burghardt, K. T. , & Parker, J. D. (2020). Tree diversity promotes growth of late successional species despite increasing deer damage in a restored forest. Ecology, e03063 10.1002/ecy.3063 [DOI] [PubMed] [Google Scholar]
- Dormann, C. F. (2007). Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Global Ecology and Biogeography, 16(2), 129–138. 10.1111/j.1466-8238.2006.00279.x [DOI] [Google Scholar]
- Dormann, C. F. , McPherson, J. , Araújo, M. , Bivand, R. , Bolliger, J. , Carl, G. , … Wilson, R. (2007). Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography, 30(5), 609–628. 10.1111/j.2007.0906-7590.05171.x [DOI] [Google Scholar]
- Eiberle, K. , & Nigg, H. (1984). Zur Ermittlung und Beurteilung der Verbissbelastung. Institut Für Wald‐ Und Holzforschung Der ETH Zürich, 193, 97–110. [Google Scholar]
- Eiberle, K. , & Nigg, H. (1987). Criteria for permissible browse impact on sycamore maple (Acer pseudoplatanus) in mountain forests. Experientia, 43(2), 127–133. 10.1007/BF01942830 [DOI] [Google Scholar]
- Erb, W. , Odenthal‐Kahabka, J. , & Püttmann, W. (2004). Orkan “Lothar”. Bewältigung der Sturmschäden in den Wäldern Baden‐Württembergs. Dokumentation, Analyse, Konsequenzen In Ministerium für Ernährung und Ländlichen Raum Baden‐Württemberg/Landesforstverwaltung Baden‐Württemberg/Forstliche Versuchs‐ und Forschungsanstalt (Ed.), Schriftenreihe der Landesforstverwaltung Baden‐Württemberg (Band 83) (pp. 1–443). Freiburg im Breisgau: Forstliche Versuchs‐ und Forschungsanstalt Baden Württemberg. [Google Scholar]
- Faison, E. K. , DeStefano, S. , Foster, D. R. , Motzkin, G. , & Rapp, J. M. (2016). Ungulate browsers promote herbaceous layer diversity in logged temperate forests. Ecology and Evolution, 6(13), 4591–4602. 10.1002/ece3.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, J.‐M. , Duncan, P. , Delorme, D. , Laere, G. V. , Pettorelli, N. , Maillard, D. , & Renaud, G. (2003). Effects of Hurricane Lothar on the population dynamics of European Roe Deer. The Journal of Wildlife Management, 67(4), 767–773. 10.2307/3802684 [DOI] [Google Scholar]
- Gerhardt, P. , Arnold, J. M. , Hackländer, K. , & Hochbichler, E. (2013). Determinants of deer impact in European forests – A systematic literature analysis. Forest Ecology and Management, 310, 173–186. 10.1016/j.foreco.2013.08.030 [DOI] [Google Scholar]
- Gill, R. M. A. , Johnson, A. L. , Francis, A. , Hiscocks, K. , & Peace, A. J. (1996). Changes in roe deer (Capreolus capreolus L.) population density in response to forest habitat succession. Forest Ecology and Management, 127(88), 31–41. [Google Scholar]
- Giroux, M.‐A. , Dussault, C. , Tremblay, J.‐P. , & Côté, S. D. (2016). Winter severity modulates the effect of using a habitat temporally uncoupled from browsing on a large herbivore. Ecosphere, 7(8), e01432. [Google Scholar]
- Götmark, F. , & Kiffer, C. (2014). Regeneration of oaks (Quercus robur/Q. petraea) and three other tree species during long‐term succession after catastrophic disturbance (windthrow). Plant Ecology, 215(9), 1067–1080. 10.1007/s11258-014-0365-4 [DOI] [Google Scholar]
- Hagen, R. , Haydn, A. , & Suchant, R. (2018). Estimating red deer (Cervus elaphus) population size in the Southern Black Forest : The role of hunting in population control. European Journal of Wildlife Research, 64(4), 1–8. 10.1007/s10344-018-1204-z [DOI] [Google Scholar]
- Hagen, R. , Kramer‐Schadt, S. , Fahse, L. , & Heurich, M. (2014). Population control based on abundance estimates: Frequency does not compensate for uncertainty. Ecological Complexity, 20, 43–50. 10.1016/j.ecocom.2014.07.006 [DOI] [Google Scholar]
- Hagen, R. , Kühl, N. , Kröschel, M. , & Suchant, R. (2019). Verbiss an Tanne und Eiche in Baden‐Württemberg: Ein Vergleich zwischen nadelbaum‐ und laubbaumdominierten Waldbeständen. Allgemeine Forst‐ Und Jagdzeitung, 7/8(190), 161–168. 10.23765/afjz0002044 [DOI] [Google Scholar]
- Heinze, E. , Boch, S. , Fischer, M. , Hessenmöller, D. , Klenk, B. , Müller, J. , … Halle, S. (2011). Habitat use of large ungulates in northeastern Germany in relation to forest management. Forest Ecology and Management, 261(2), 288–296. 10.1016/j.foreco.2010.10.022 [DOI] [Google Scholar]
- Heuze, P. , Schnitzler, A. , & Klein, F. (2005). Consequences of increased deer browsing winter on silver fir and spruce regeneration in the Southern Vosges mountains: Implications for forest management. Annals of Forest Science, 62(2), 175–181. [Google Scholar]
- Hidding, B. , Tremblay, J.‐P. , & Côté, S. D. (2013). A large herbivore triggers alternative successional trajectories in the boreal forest. Ecology, 94(12), 2852–2860. 10.1890/12-2015.1 [DOI] [PubMed] [Google Scholar]
- Hofmann, R. R. (1989). Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia, 78, 443–457. 10.1007/BF00378733 [DOI] [PubMed] [Google Scholar]
- Jędrzejewska, B. , Jędrzejewski, W. , Bunevich, A. N. , Miłkowski, L. , & Krasiński, Z. A. (1997). Factors shaping population densities and increase rates of ungulates in Białowieża Primeval Forest (Poland and Belarus) in the 19th and 20th centuries. Acta Theriologica, 42(4), 399–451. [Google Scholar]
- Kämmerle, J. L. , Niekrenz, S. , & Storch, I. (2019). No evidence for spatial variation in predation risk following restricted‐area fox culling. BMC Ecology, 19(1), 1–10. 10.1186/s12898-019-0235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper, D. P. J. , Cromsigt, J. P. G. M. , Churski, M. , Adam, B. , Jedrzejewska, B. , & Jedrzejewski, W. (2009). Do ungulates preferentially feed in forest gaps in European temperate forest? Forest Ecology and Management, 258, 1528–1535. 10.1016/j.foreco.2009.07.010 [DOI] [Google Scholar]
- Kuijper, D. P. J. , Cromsigt, J. P. G. M. , Jędrzejewska, B. , Miścicki, S. , Churski, M. , Jędrzejewski, W. , & Kweczlich, I. (2010). Bottom‐up versus top‐down control of tree regeneration in the Białowieża Primeval Forest, Poland . Journal of Ecology, 98(4), 888–899. 10.1111/j.1365-2745.2010.01656.x [DOI] [Google Scholar]
- Kuijper, D. P. J. , Jedrzejewska, B. , Brzeziecki, B. , Churski, M. , Jedrzejewski, W. , & Zybura, H. (2010). Fluctuating ungulate density shapes tree recruitment in natural stands of the Białowieza Primeval Forest, Poland. Journal of Vegetation Science, 21, 1082–1098. 10.1111/j.1654-1103.2010.01217.x [DOI] [Google Scholar]
- Kunstler, G. , Curt, T. , & Lepart, J. (2004). Spatial pattern of beech (Fagus sylvatica L.) and oak (Quercus pubescens Mill.) seedlings in natural pine (Pinus sylvestris L.) woodlands. European Journal of Forest Research, 123(4), 331–337. 10.1007/s10342-004-0048-0 [DOI] [Google Scholar]
- Kupferschmid, A. D. (2018). Selective browsing behaviour of ungulates influences the growth of Abies alba differently depending on forest type. Forest Ecology and Management, 429, 317–326. 10.1016/j.foreco.2018.06.046 [DOI] [Google Scholar]
- Kupferschmid, A. D. , & Heiri, C. (2019). Recovery of Abies alba and Picea abies saplings to browsing and frost damage depends on seed source. Ecology and Evolution, 9(6), 3335–3354. 10.1002/ece3.4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre, P. (1993). Spatial autocorrelation: Trouble or new paradigm? Ecology, 74(6), 1659–1673. [Google Scholar]
- Leopold, A. , Bean, E. F. , & Norman, C. F. (1943). Deer irruptions. Transactions of the Wisconsin Academy of Sciences, Arts, and Letters., 35, 351–366. [Google Scholar]
- Lovari, S. , Serrao, G. , & Mori, E. (2017). Woodland features determining home range size of roe deer. Behavioural Processes, 140, 115–120. 10.1016/j.beproc.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Maublanc, M. L. , Bideau, E. , Launay, C. , Monthuir, B. , & Gerard, J. F. (2016). Indicators of ecological change (IEC) as efficient tools for managing roe deer populations: A case study. European Journal of Wildlife Research, 62(2), 189–197. 10.1007/s10344-016-0992-2 [DOI] [Google Scholar]
- Moran, P. A. P. (1950). Notes on continuous stochastic phenomena. Biometrika, 37(1–2), 17–23. 10.1093/biomet/37.1-2.17 [DOI] [PubMed] [Google Scholar]
- Morellet, N. , Bonenfant, C. , Börger, L. , Ossi, F. , Cagnacci, F. , Heurich, M. , … Mysterud, A. (2013). Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. Journal of Animal Ecology, 82, 1326–1339. 10.1111/1365-2656.12105 [DOI] [PubMed] [Google Scholar]
- Morellet, N. , & Boscardin, Y. (2001). The browsing index: New tool uses browsing pressure to monitor deer populations. Wildlife Society Bulletin, 29(4), 1243–1252. [Google Scholar]
- Morellet, N. , Gaillard, J.‐M. , Hewison, A. J. M. , Ballon, P. , Boscardin, Y. , Duncan, P. , … Maillard, D. (2007). Indicators of ecological change: New tools for managing populations of large herbivores. Journal of Applied Ecology, 44(3), 634–643. 10.1111/j.1365-2664.2007.01307.x [DOI] [Google Scholar]
- Morellet, N. , & Guibert, B. (1999). Spatial heterogeneity of winter forest resources used by deer. Forest Ecology and Management, 123(1), 11–20. 10.1016/S0378-1127(99)00007-9 [DOI] [Google Scholar]
- Moser, B. , Schütz, M. , & Hindenlang, K. E. (2006). Importance of alternative food resources for browsing by roe deer on deciduous trees: The role of food availability and species quality. Forest Ecology and Management, 226, 248–255. 10.1016/j.foreco.2006.01.045 [DOI] [Google Scholar]
- Mysterud, A. , Askilsrud, H. , Loe, L. E. , & Veiberg, V. (2010). Spatial patterns of accumulated browsing and its relevance for management of red deer Cervus elaphus . Wildlife Biology, 16(2), 162–172. 10.2981/09-043 [DOI] [Google Scholar]
- Nishizawa, K. , Tatsumi, S. , Kitagawa, R. , & Mori, A. S. (2016). Deer herbivory affects the functional diversity of forest floor plants via changes in competition‐mediated assembly rules. Ecological Research, 31, 569–578. 10.1007/s11284-016-1367-6 [DOI] [Google Scholar]
- Nothdurft, A. , Borchers, J. , Niggemeyer, P. , Saborowksi, J. , & Kändler, G. (2009). Eine Folgeaufnahme einer Betriebsinventur als zweiphasige Stichprobe zur Stratifizierung. Allgemeine Forst‐ Und Jagdzeitung, 180(7–8), 133–140. [Google Scholar]
- Nuttle, T. , Ristau, T. E. , & Royo, A. A. (2014). Long‐term biological legacies of herbivore density in a landscape‐scale experiment: Forest understoreys reflect past deer density treatments for at least 20 years. Journal of Ecology, 102(1), 221–228. 10.1111/1365-2745.12175 [DOI] [Google Scholar]
- Ohse, B. , Seele, C. , Holzwarth, F. , & Wirth, C. (2017). Different facets of tree sapling diversity influence browsing intensity by deer dependent on spatial scale. Ecology and Evolution, 7(17), 6779–6789. 10.1002/ece3.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partl, E. , Szinovatz, V. , Reimoser, F. , & Schweiger‐Adler, J. (2002). Forest restoration and browsing impact by roe deer. Forest Ecology and Management, 159(1–2), 87–100. 10.1016/S0378-1127(01)00712-5 [DOI] [Google Scholar]
- Pierson, T. G. , & De Calesta, D. S. (2015). Methodology for estimating deer browsing impact. Human‐Wildlife Interactions, 9(1), 67–77. [Google Scholar]
- R Core Team (2018). R Foundation for Statistical Computing; https://r-project.org/ [Google Scholar]
- Reimoser, F. (2000). Anmerkungen zur Feststellung von Wildverbiß und zum Vergleich von Verbißkennzahlen. Zeitschrift Für Jagdwissenschaft, 46(1), 51–56. 10.1007/bf02240664 [DOI] [Google Scholar]
- Reimoser, F. (2003). Steering the impacts of ungulates on temperate forests. Journal for Nature Conservation, 10(4), 243–252. 10.1078/1617-1381-00024 [DOI] [Google Scholar]
- Reimoser, F. , & Gossow, H. (1996). Impact of ungulates on forest vegetation and its dependence on the silvicultural system. Forest Ecology and Management, 88, 107–119. 10.1016/S0378-1127(96)03816-9 [DOI] [Google Scholar]
- Reimoser, F. , Odermatt, O. , Roth, R. , & Suchant, R. (1997). Die Beurteilung von Wildverbiss durch Soll‐Ist‐Vergleich. Allgemeine Forst Und Jagdzeitung, 168(11–12), 214–227. [Google Scholar]
- Reimoser, S. , Partl, E. , Reimoser, F. , & Vospernik, S. (2009). Roe‐deer habitat suitability and predisposition of forest to browsing damage in its dependence on forest growth—Model sensitivity in an alpine forest region. Ecological Modelling, 220(18), 2231–2243. 10.1016/j.ecolmodel.2009.05.022 [DOI] [Google Scholar]
- Richard, E. , Said, S. , Hamann, J. L. , & Gaillard, J. M. (2014). Daily, seasonal, and annual variations in individual home‐range overlap of two sympatric species of deer. Canadian Journal of Zoology, 92(10), 853–859. 10.1139/cjz-2014-0045 [DOI] [Google Scholar]
- Royo, A. A. , Collins, R. , Adams, M. B. , Kirschbaum, C. , & Carson, W. P. (2010). Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity. Ecology, 91(1), 93–105. 10.1890/08-1680.1 [DOI] [PubMed] [Google Scholar]
- Royo, A. A. , Kramer, D. W. , Miller, K. V. , Nibbelink, N. P. , & Stout, S. L. (2017). Spatio‐temporal variation in foodscapes modifies deer browsing impact on vegetation. Landscape Ecology, 32(12), 2281–2295. 10.1007/s10980-017-0568-x [DOI] [Google Scholar]
- Saucier, V. , Champagne, E. , Côté, S. D. , & Tremblay, J. P. (2019). Combined effects of simulated browsing, warming and nutrient addition on forage availability for migratory caribou in Nunavik, Canada. Polar Biology, 42(8), 1561–1570. 10.1007/s00300-019-02543-y [DOI] [Google Scholar]
- Senn, J. , & Häsler, H. (2005). Wildverbiss: Auswirkungen und Beurteilung. Forum Für Wissen, 17–25. [Google Scholar]
- Shipley, L. A. , & Spalinger, D. E. (1995). Influence of size and density of browse patches on intake rates and foraging decisions of young moose and white‐tailed deer. Oecologia, 104(1), 112–121. 10.1007/BF00365569 [DOI] [PubMed] [Google Scholar]
- Sinclair, A. R. E. , & Krebs, C. J. (2002). Complex numerical responses to top‐down and bottom‐up processes in vertebrate populations. Philosophical Transactions of the Royal Society B: Biological Sciences, 357, 1221–1231. 10.1098/rstb.2002.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal, R. R. , & Oden, N. L. (1978a). Spatial antocorrelation in biology II. Some biological implications and four applications of evolutionary and ecological interest. Biological Journal of the Linnean Society, 10, 229–249. [Google Scholar]
- Sokal, R. R. , & Oden, N. L. (1978b). Spatial autocorrelation in biology I. Methodology. Biological Journal of the Linnean Society, 10, 199–228. [Google Scholar]
- Sommer, R. S. , Fahlke, J. M. , Schmölcke, U. , Benecke, N. , & Zachos, F. E. (2009). Quaternary history of the European roe deer Capreolus capreolus. Mammal Review, 39(1), 1–16. 10.1111/j.1365-2907.2008.00137.x [DOI] [Google Scholar]
- Storms, D. , Said, S. , Fritz, H. , Hamann, J. L. , Saint‐Andrieux, C. , & Klein, F. (2006). Influence of hurricane Lothar on red and roe deer winter diets in the Northern Vosges. France. Forest Ecology and Management, 237(1–3), 164–169. 10.1016/j.foreco.2006.09.043 [DOI] [Google Scholar]
- Tamura, A. , & Yamane, M. (2017). Response of understory vegetation over 10 years after thinning in an old‐growth cedar and cypress plantation overgrazed by sika deer in eastern Japan. Forest Ecosystems, 4(1), 1–10. 10.1186/s40663-016-0088-1 [DOI] [Google Scholar]
- Tinner, W. , Colombaroli, D. , Heiri, O. , Henne, P. D. , Steinacher, M. , Untenecker, J. , … Valsecchi, V. (2013). The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecological Monographs, 83(4), 419–439. 10.1890/12-2231.1 [DOI] [Google Scholar]
- Welch, D. , Staines, B. W. , Scott, D. , & French, D. D. (1992). Leader browsing by red and roe deer on young sitka spruce trees in Western Scotland. II. Effects on growth and tree form. Forestry, 65(I), 309–330. 10.1093/forestry/65.3.309 [DOI] [Google Scholar]
- Whitlock, C. , Colombaroli, D. , Conedera, M. , & Tinner, W. (2017). Land‐use history as a guide for forest conservation and management. Conservation Biology, 32(1), 84–97. 10.1111/cobi.12960 [DOI] [PubMed] [Google Scholar]
- Widmer, O. , Saïd, S. , Miroir, J. , Duncan, P. , Gaillard, J. M. , & Klein, F. (2004). The effects of hurricane Lothar on habitat use of roe deer. Forest Ecology and Management, 195(1–2), 237–242. 10.1016/j.foreco.2004.02.021 [DOI] [Google Scholar]
- Wiegand, T. , Moloney, K. A. , & Milton, S. J. (1998). Population dynamics, disturbance, and pattern evolution: Identifying the fundamental scales of organization in a model ecosystem. The American Naturalist, 152(3), 321–337. 10.1086/286172 [DOI] [PubMed] [Google Scholar]
- Yokozawa, M. , Kubota, Y. , & Hara, T. (1999). Effects of competition mode on the spatial pattern dynamics of wave regeneration in subalpine tree stands. Ecological Modelling, 118, 73–86. 10.1016/S0304-3800(99)00050-2 [DOI] [Google Scholar]
- Zai, L. E. (1964). Untersuchungen über Methoden zur Beurteilung von Rehwildverbiss in Waldbeständen Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich, 109(3), 197‐265 Zürich, Switzerland: Eidgenössische Technische Hochschule in Zürich; 10.3929/ethz-b-000251651 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S4
Data Availability Statement
Data visualized in Figures 2, 3 and S2–S4 were archived in Dryad (https://doi.org/10.5061/dryad.4xgxd256m). Data of forest inventories (raw data shown in Figure 1) will not be made publicly available as data contain sensitive information (human subject data in time and space) about timber production in the federal state of Baden‐Württemberg.


