Abstract
Novel 2019 coronavirus (COVID-19) infection usually causes a respiratory disease that may vary in severity from mild symptoms to severe pneumonia with multiple organ failure. Coagulation abnormalities are frequent, and reports suggest that COVID-19 may predispose to venous and arterial thrombotic complications. We report a case of acute lower limb ischemia and resistance to heparin as the onset of COVID-19 disease, preceding the development of respiratory failure. This case highlights that the shift of coagulation profile toward hypercoagulability was associated with the acute ischemic event and influenced the therapy.
Respiratory failure is the most common reason for emergency department (ED) admission of patients with the novel COVID-19 infection and usually the first sign of disease. Thrombotic complications are common in hospitalized patients for COVID-19 pneumonia, and coagulopathy has been associated with poor prognosis.1 , 2 Venous thrombosis and pulmonary embolism are the most represented complications, whereas thrombotic arterial events are relatively uncommon and reported in severe cases.1 , 3 , 4
We report the case of a patient with acute left lower limb ischemia (ALI) and resistance to unfractionated heparin (UFH) as clinical onset of COVID-19 disease, preceding the development of respiratory failure.
Clinical Case
A 53-year-old man was admitted to our ED for ALI, leg pain started the previous day associated with walking difficulties. Absence of blood flow in the entire left femoral-popliteal axis was detected at duplex scan ultrasonography. A subsequent thoracoabdominal computed tomography angiography scan confirmed thrombotic occlusion at the iliac level extended to femoropopliteal arteries and distal circulation, collaterally a focal thrombotic defect within the right tibioperoneal trunk was evident too, with reperfusion of the distal posterior tibial artery (Fig. 1 ). Clinical and imaging findings were consistent with the diagnosis of left ALI (Rutherford category IIb). Lung imaging showed diffuse bilateral interstitial infiltrating shadows, mainly at a subpleural level. Patient complained no recent respiratory symptoms or fever; oxygenation and vital signs were normal. Medical history showed hypertension on medical treatment and mild obesity (body mass index 33 kg/m2). The nucleic acid detection of COVID-19 was negative at the ED. An emergent surgical thromboembolectomy of the left lower limb was performed (standard embolectomy catheters, Le Maitre®; Burlington–MA; USA). After the removal of the iliac clot, forceful pulsatile inflow was established; conversely, minimum back-bleeding was observed from the superficial femoral artery. Diagnostic angiography revealed a patent femoropopliteal axis with occlusion of the anterior, posterior, and peroneal artery at the mid-calf level. The posterior tibial artery was perfused by collaterals at the ankle level, whereas a typical aspect of “desert foot” with absence of forefoot microcirculation was discovered, probably due to acute microvascular virus-related thrombosis. Then, selective regional intra-arterial thrombolysis was performed with the administration of 20 mg/20 min bolus of recombinant tissue plasminogen activator (Actilyse® – Boehringer Ingelheim Italia S.p.A.; Milan, Italy), with a 4F Berenstein catheter positioned in the lower popliteal artery.4 After thrombolysis, a partial recanalization of the peroneal artery until the ankle level was observed, together with detectable flow at continuous wave (CW) Doppler without tibial or forefoot vessel recanalization (Fig. 2 ). Anticoagulation with UFH infusion and prostacyclin therapy were started immediately in the operating room. Few hours after the procedure, leg pain worsened, and compartment syndrome was suspected. Emergent fasciotomy of the lateral compartment of the calf was performed at bedside. On the first postoperative (PO) day, a second COVID-19 nasopharyngeal swab was performed and resulted positive for the infection while respiratory function deteriorated requiring oxygen supplementation. On the fourth PO day, the patient was transferred to the intensive care unit (ICU) for further worsening of the respiratory function. Continuous positive airway pressure was performed with a helmet and maintained for 9 days. UFH infusion was chosen because of acute renal failure due to rhabdomyolysis (creatine phosphokinase 56.749 U/l and myoglobin 19.928 μg/l on the first PO day, acute kidney injury stage 2) and continued in accordance with our standard protocol. However, the therapeutic target was difficult to achieve (activated partial thromboplastin time ratio 2.5–3.0). Antithrombin levels were measured daily and supplemented accordingly. Increase in UFH requirement above 50.000 U/day raised concern about heparin resistance, and further evaluation of coagulation function was performed.5 Both intrinsic (intem) and extrinsic (extem) coagulation pathways were explored by rotational thromboelastometry (ROTEM) analysis. No sign of effective anticoagulation was detected (intem clotting time (CT) and clotting time formation were within normal range), and data suggested a hypercoagulability state (maximum clotting firmness (MCF) intem and extem were 75 and 76 mm, respectively).6 At the same time, anti-factor Xa (AFXa) assay was below the therapeutic range (0.08 U/ml). Therefore, UFH infusion was increased up to 79.200 U/day to obtain activated partial thromboplastin time ratio> 2.5. The ROTEM intem profile showed increased CT (300 s), whereas both MCF pathways (intem and extem) did not change. Afterward, AFXa resulted in the therapeutic range (0.43 U/ml). Platelets count and fibrinogen level were normal at ED admission, but the latter steadily increased after 2 days of hospital stay.
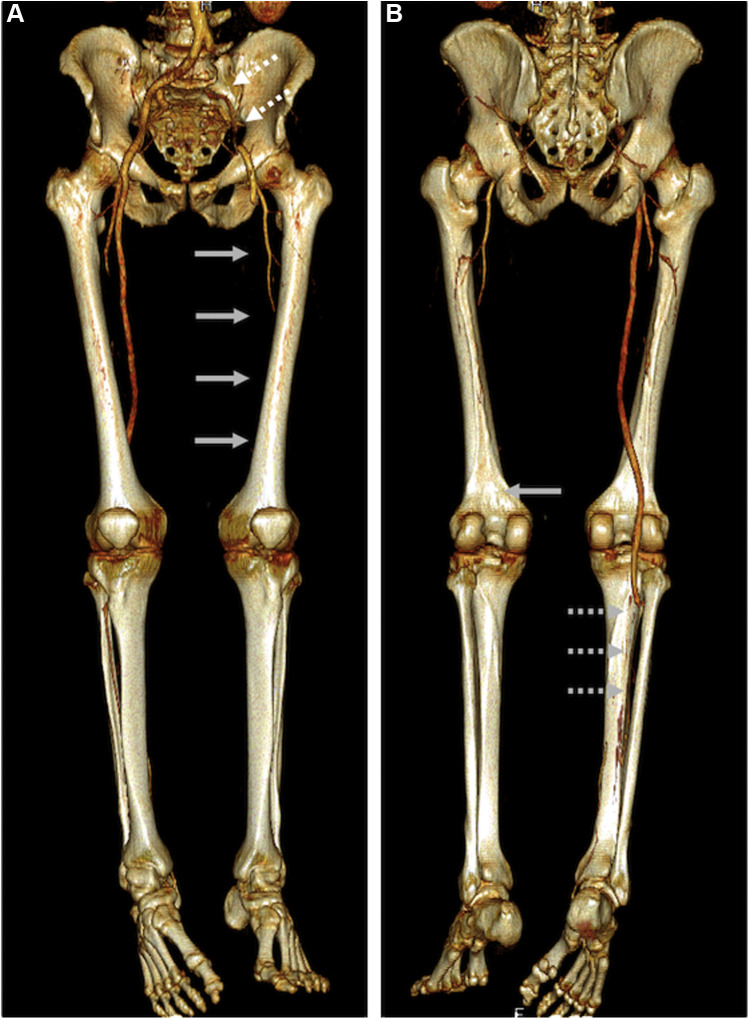
Fig. 1.
A, anterior CTA reconstruction showing occlusion of the left iliac axis (white dotted arrows), reperfusion of the common and deep femoral arteries and sequent occlusion at the origin of the superficial femoral artery (gray arrows); B, posterior CTA reconstruction showing left popliteal-tibial vessel occlusion (gray arrow) and right tibioperoneal trunk occlusion with reperfusion of the distal posterior tibial artery (gray dotted arrows).
Fig. 2.
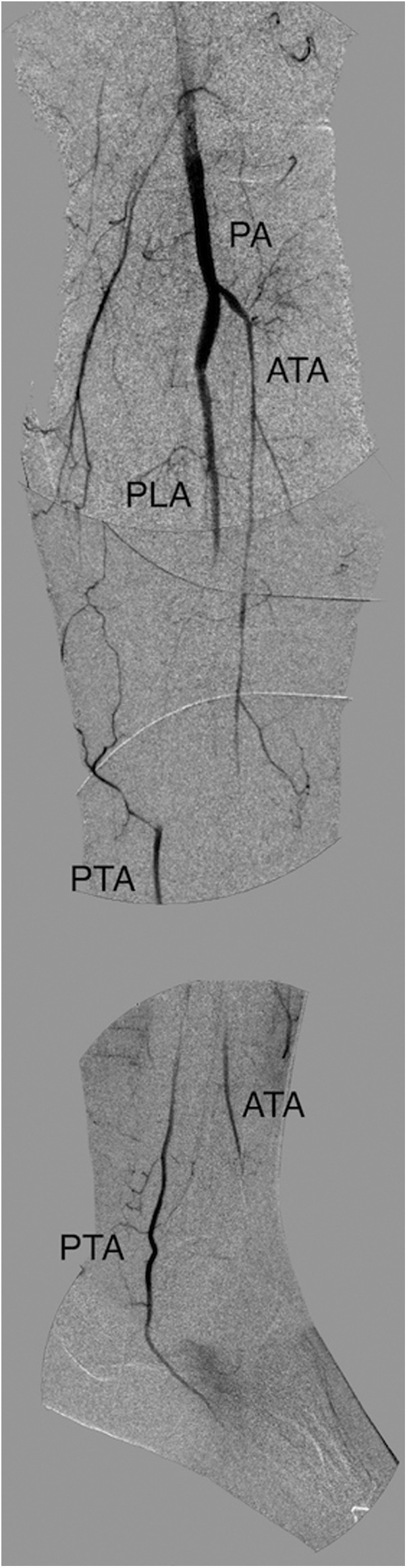
Final digital subtraction angiography (DSA) showing incomplete tibial vessel recanalization and absence of forefoot vessels (i.e., “desert foot”). PA, popliteal artery; ATA, anterior tibial artery; PLA, peroneal artery; PTA, posterior tibial artery.
Left lower limb perfusion slowly improved during the following days, with restoration of direct blood flow within the posterior and anterior tibial artery in the proximal foot (CW Doppler). Foot sensitivity and motility improved; however, a permanent superficial peroneal nerve impairment persisted. Skin cyanosis was limited at the forefoot, and dry necrosis developed at the distal phalanx. On the 18th PO day, the patient was transferred to infectious disease unit. On ICU discharge, no respiratory or renal support was required: cytolysis enzymes decreased during the days and renal function recovered (creatine phosphokinase 826 U/l, myoglobin 266 μg/l, creatinine 1.06 mg/dl). Continuous infusion of UFH was stopped and low-molecular-weight heparin (LMWH) was introduced (100 U/kg/die b.i.d.). No bleeding complication was observed. On the 40th PO day, the patient has been discharged to rehabilitation.
Discussion
Fever, cough, and respiratory failure are the most common symptoms complained by patients suspected for COVID-19 disease at ED admission. Nevertheless, we describe a case of acute lower limb ischemia as an atypical presentation of COVID-19 infection. In this case, a patient with low cardiovascular risk developed an acute lower limb ischemia before the onset of respiratory symptoms. No signs of preexisting atherosclerotic arterial disease were detected at CT scan or during surgery. Previous case reports of ALI and peripheral arterial disease have been described in critically ill patients with COVID-19,7 as well as coronary artery disease and ischemic stroke as rare manifestations usually associated with respiratory distress.8 Deep venous thrombosis and pulmonary embolism are the most common complications, even during LMWH prophylaxis.8 The hemostatic system is markedly shifted toward the procoagulant state in critically ill patients with COVID-19 predisposing to thrombotic events.9 Moreover, coagulation abnormalities have been identified as a persistent feature during the disease and associated to increased mortality.1 , 2 Although this patient had low risk of arterial thrombotic event (hypertension and mild obesity), he showed coagulation abnormalities described in COVID-19 infection as high fibrinogen level and hypercoagulability state at ROTEM analysis (increased MCF). Maximal clot amplitude at thromboelastography analysis above normal value was reported in more than 80% of patients with COVID.9 Even in absence of preexisting atherosclerotic arterial disease, the procoagulant and inflammatory state induced by COVID-19 probably acts like a trigger in arterial thrombosis, especially of microcirculation vessels. A recent study reported higher failure rate of revascularization surgery in patients with ALI and COVID-19 pneumonia.4 Frequent early recurrent thrombosis and absence of forefoot microcirculation are also described. Intraoperative fibrinolysis and prompt anticoagulation with intravenous UHF have been advocated to improve microcirculation and prevent rethrombosis.4 Effective anticoagulation target was difficult to achieve despite the increase of UFH doses. Heparin resistance has been associated with high fibrinogen, factor VIII, and von Willebrand factor in patients with COVID-19.10 Surgical thromboembolectomy, intraoperative fibrinolysis, vasodilation therapy (prostacyclin), optimization of anticoagulation with antithrombin monitoring and supplementation, and AFXa determination to confirm suboptimal heparin level were successful in restoring perfusion and avoided major amputation in the case presented.
Finally, at admission in absence of respiratory symptoms, CT scan showed multifocal bilateral peripheral ground glass areas consistent with characteristic COVID-19 pulmonary findings. First, nasopharyngeal swab was negative; however, reverse transcription polymerase chain reaction (RT-PCR) screening has a limit in sensitivity at initial presentation (60%–79%).11 , 12 Typical CT scan with bilateral peripheral ground glass opacifications led the physicians to repeat the RT-PCR test that confirmed COVID-19 diagnosis. Pathological CT scan findings may be present before respiratory symptoms onset, as it occurred in the reported case.13 Respiratory impairment and moderate acute respiratory distress syndrome could be treated successfully with noninvasive ventilation, whereas the major disease features were associated with hypercoagulability and arterial thrombotic occlusion event, which was the first reason for ED admission.
Thrombotic events associated with COVID-19 infection warrant advance coagulation monitoring to resolve acute problems and improve our understanding of patients' coagulation state. Arterial thrombotic events causing ALI associated with COVID-19 infection are mainly located within microcirculation vessels, with associated high rate of recurrent thrombosis. Therefore, we decided to shift our policy toward a more aggressive regimen of prompt and full anticoagulation with intravenous UFH and selective intra-arterial thrombolysis. New insights toward advance coagulation monitoring and patients’ hypercoagulable state during COVID-19 pandemic are needed to fully understand the pathogenesis of thrombotic effects.
Footnotes
Declarations of interest: none.
References
- 1.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X. Abnormal coagulation Parameters are associated with poor Prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Xiao M., Zhang S. Coagulopathy and Antiphospholipid Antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellosta R., Luzzani L., Natalini G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020 doi: 10.1016/j.jvs.2020.04.483. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durrani J., Malik F., Ali N. To Be or not to Be a case of heparin resistance. J Community Hosp Intern Med Perspect. 2018;8:145–148. doi: 10.1080/20009666.2018.1466599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crochemore T., Corrêa T.D., Lance M.D. Thromboelastometry profile in critically ill patients: a Single-Center, Retrospective, Observational Study. PLoS One. 2018;13:e0192965. doi: 10.1371/journal.pone.0192965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B., She J., Wang Y. Venous thrombosis and Arteriosclerosis Obliterans of lower Extremities in a Very severe patient with 2019 novel coronavirus disease: a case report. Thromb Thrombolysis. 2020;50:229–232. doi: 10.1007/s11239-020-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodigiani C., Iapichino G., Carenzo L., Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an Academic hospital in milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A report of thromboelastography findings and Other Parameters of Hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors J.M., Levy J.H. COVID-19 and its Implications for thrombosis and Anticoagulation. Blood. 135, 2020:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y., Zhang H., Xie J. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J.L., Luo L., Luo Z.D. Diagnostic performance between CT and initial Real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168:105980. doi: 10.1016/j.rmed.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salehi S., Abedi A., Balakrishnan S. Coronavirus disease 2019 (COVID-19): a Systematic Review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]