Abstract
Background
The combination of Hydroxychloroquine (HCQ) and azithromycin showed effectiveness as a treatment for COVID-19 and is being used widely all around the world. Despite that those drugs are known to cause prolonged QT interval individually there is no study assessing the impact of this combination on electrocardiography (ECG). This study aimed to assess the impact of a 5-day course of HCQ and azithromycin combination on ECG in non-ICU COVID19(+) patients.
Methods
In this retrospective observational study, we enrolled 109 COVID19(+) patients who required non-ICU hospitalization. All patients received 5-day protocol of HCQ and azithromycin combination. On-treatment ECGs were repeated 3-6 h after the second HCQ loading dose and 48-72 h after the first dose of the combination. ECGs were assessed in terms of rhythm, PR interval, QRS duration, QT and QTc intervals. Baseline and on-treatment ECG findings were compared. Demographic characteristics, laboratory results were recorded. Daily phone call-visit or bed-side visit were performed by attending physician.
Results
Of the 109 patients included in the study, the mean age was 57.3 ± 14.4 years and 48 (44%) were male. Mean baseline PR interval was 158.47 ± 25.10 ms, QRS duration was 94.00 ± 20.55 ms, QTc interval was 435.28 ± 32.78 ms, 415.67 ± 28.51, 412.07 ± 25.65 according to Bazett's, Fridericia's and Framingham Heart Study formulas respectively. ∆PR was −2.94 ± 19.93 ms (p = .55), ∆QRS duration was 5.18 ± 8.94 ms (p = .03). ∆QTc interval was 6.64 ± 9.60 ms (p = .5), 10.67 ± 9.9 ms (p = .19), 14.14 ± 9.68 ms (p = .16) according to Bazett's, Fridericia's and Framingham Heart Study formulas respectively. There were no statistically significant differences between QTc intervals. No ventricular tachycardia, ventricular fibrillation or significant conduction delay was seen during follow-up. There was no death or worsening heart function.
Conclusion
The 5-day course of HCQ- AZM combination did not lead to clinically significant QT prolongation and other conduction delays compared to baseline ECG in non-ICU COVID19(+) patients.
Keywords: Hydroxychloroquine, Azithromycin, Electrocardiography, Coronavirus disease 2019, QT interval
Introduction
Since reporting of the first case on December 9 in Wuhan, China, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread swiftly in a short time throughout China and the outside [1]. In early March World Health Organization (WHO) declared the SARS-CoV-2 outbreak a pandemic [2]. Due to the lack of specific antiviral medication for treatment and vaccine for prevention “repurposing” drugs emerged as a rescuer to deal with this problem. Several known molecules were started to be tested in different countries [[3], [4], [5]]. Hydroxychloroquine is an analogue of chloroquine and has been used as an antimalarial and antirheumatic drug [6]. Antiviral effects of HCQ had been demonstrated [7,8]. In a recent study HCQ reinforced by azithromycin was associated significantly with viral load reduction in COVID19 (+) patients [3].
Chronic HCQ use demonstrated QT prolongation and refractory ventricular arrhythmia [9], and azithromycin has been reported to be related to QT prolongation, sudden cardiac arrest, and increased cardiac mortality [10,11].Since HCQ is metabolized by cytochrome P450 enzymes and azithromycin inhibits this enzyme [12,13], this adverse effect brings about safety issues. Despite HCQ - AZM combination was found effective and well tolerable in the treatment of COVID19 there is no study assessing the impact of this combination on ECG [14]. In this study, we aimed to evaluate the ECG changes in COVID19(+) patients taking HCQ -AZM combination.
Methods
Study population
Our study was designed as a retrospective observational study. We screened the records of 196 COVID19(+) patients presented to our hospital between March 31 and April 16 and who were followed in inpatient wards and received HCQ - AZM combination therapy. Pregnancy, patients under 18 years and patients who did not have control ECG were exclusion criteria. After exclusion, we included 109 patients in the study. The flowchart was described in Fig. 1 . Patients who had COVID19(+) with asymptomatic or mildly symptomatic (such as the upper respiratory infection) and without comorbidity followed in the outpatient clinic and excluded from the study. Patients with the lower respiratory infection (such as pneumonia or bronchitis), 1 and more comorbidity and age more than 64 years were hospitalized to inpatient ward if SpO2 is more than %90 and hemodynamically stable.
Fig. 1.
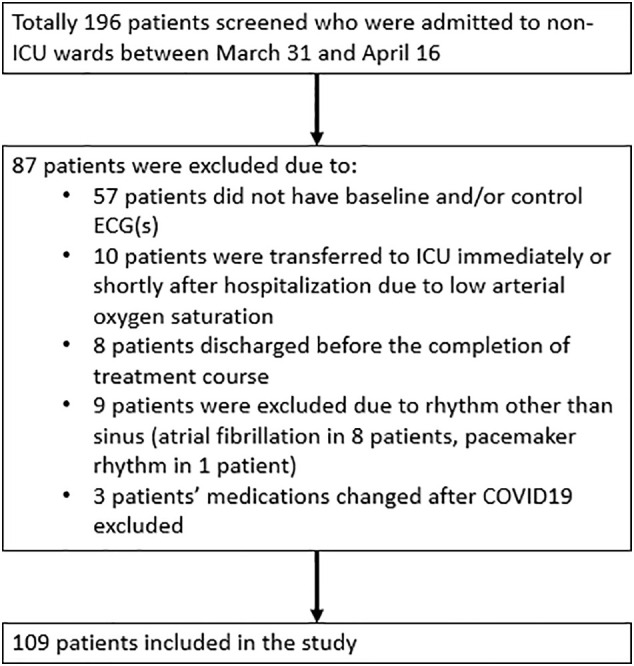
Flow chart of the study population.
Baseline and control ECG were obtained. Control ECGs were repeated 3-6 h after the second HCQ loading dose and 48-72 h after the first dose of the combination. ECGs were assessed in terms of rhythm, PR interval, QRS duration, QT and QTc intervals. Baseline and control ECG findings were compared. Demographic characteristics, laboratory results were recorded. Daily phone call-visit or bed-side visit were performed by attending physician.
Treatment protocol
The treatment protocol was adopted by the national health system and sent to all centers. According to this protocol, most patients after diagnosing COVID19(+) were started Hydroxychloroquine if not any contraindication. Azithromycin was given if there is concomitant pneumonia. Oseltamivir was the part of the protocol until influenza was excluded. The contraindications for HCQ/AZM combination were: 1) QTc > 500 msn (or > 550 msn in bundle branch block) on the baseline ECG; 2) Hypersensitivity.
On the first day HCQ was loaded orally 400 mg b.i.d then 200 mg b.i.d was given for following 4 days. After 500 mg loading dose on the first day, AZM was continued 250 mg od for following 4 days. Enfluvir was received 75 mg bid until influenza was excluded. Hydroxychloroquine and azithromycin combination were given for 5 days if not any contraindications. After that, if the patient remained symptomatic other medications can be given.
Laboratory testing
Blood samples from all patients who required hospitalization were sent to the laboratory to check electrolytes, hemogram, acute phase reactants, kidney and liver functions, troponin I, creatinine kinase – myocardial band (CK- MB), and D-dimer. Demographic characteristics, concomitant diseases, medications were recorded.
ECG recordings
All ECGs were recorded using Mortara ELI 250 device (Welch Allyn, Inc., Skaneateles Falls, NY, USA; standard 12‑lead resting ECG, paper speed of 25 mm/s, the amplitude of 10 mm/V, and a sampling rate of 250 Hz). Patient's 12‑lead ECGs were evaluated before starting HCQ and azithromycin combination. Control ECG was obtained 3 to 6 h after the second HCQ loading dose. QT measurement was performed in leads II, V5 or V6. Measured longest QT interval was used. To exclude interobserver variability all measurements were completed by one cardiologist (NB). In case of problems with measurement, the second cardiologist (AE) measured blindly the QT interval to the first cardiologist. If discrepancy between these two cardiologists was more than %5, the third cardiologist (EK) were invited to resolve the problem. The PR interval was described the interval measured from the onset of the P wave to the beginning of the first point of deflection of the QRS complex. The QRS duration was the interval between the first deflection of the QRS complex and the returning point to the baseline. The QT interval was measured from the onset of the first deflection of QRS complex to the end of T wave. The end of the T wave was determined by the tangent method. QT measurement was performed according to guideline proposed by expert panel [15]. The corrected QT (QTc) interval was calculated by the Bazett's, Fridericia's and Sagie's (Framingham Heart Study) formulas. All measurements were performed manually in EP calipers software (EP Studios, Inc., Version 3.1).
Statistical analysis
Continuous variables were expressed as mean standard deviation, categorical variables were expressed as median with interquartile range. The data was tested by the Kolmogorov – Smirnov test or Shapiro- Wilk test and a visual inspection of histograms for homogeneity. Changes in the baseline, after loading and during maintaining dose were analyzed by Friedman test or repeated measure ANOVA where appropriate. Normally distributed continuous variables were expressed as mean ± standard deviation, non-parametric continuous variables were expressed as median with interquartile range, while percentiles were used for categorical variables. p < .05 was considered as statistically significant. Statistical analyses were performed using SPSS version 22.0 (IBM Inc. USA).
Results
A total of 109 patients eligible for analysis fulfilled the following inclusion criteria: 1) Patients who were in sinus rhythm; 2) Patients >18 years and were followed in in-patient ward; 3) Patients who were started HCQ and azithromycin combination; 4) Patients who had at least 2 control ECGs during the treatment period. Exclusion criteria were: 1) cardiac rhythm other than sinus; 2) Early discharged patients; 3) patients whose combination treatment was changed due to course of the disease (other than cardiac or arrhythmic reasons); 4) Pregnancy. One hundred and nine patients were included in the study. Of them, 48 (44%) were male and the mean age was 57.3 ± 14.4 years (Table 1 ). Laboratory findings were shown in Table 2 . In the baseline ECG mean heart rate (HR) was 86 ± 14 bpm, PR interval was 158.47 ± 25.10 ms, QRS duration was 94.00 ± 20.55 ms, QT interval was 370.09 ± 37.15 ms. Corrected QT interval was 435.28 ± 32.78 ms, 415.67 ± 28.51 ms, 412.07 ± 25.65 ms according to Bazett, Frederica and Framingham Heart Study respectively. In the first on-treatment ECG which was obtained 3–6 h after the second HCQ loading dose HR was 77 ± 12 bpm, PR interval was 156.35 ± 26.00 ms, QRS duration was 97.88 ± 21.73 ms, QT interval was 389.68 ± 42.92 ms. Corrected QT interval was 459.68 ± 38.40 ms, 442.30 ± 40.42 ms, 440.97 ± 39.11 ms according to Bazett, Frederica and Framingham Heart Study respectively. In the second on-treatment ECG which was obtained on day 3 of hospitalization HR was 76 ± 12 bpm, PR interval was 155.53 ± 26.77 ms, QRS duration was 99.18 ± 20.99 ms, QT interval was 397.88 ± 55.66 ms. Corrected QT interval was 441.91 ± 38.71 ms, 426.33 ± 41.19 ms, 426.21 ± 39.68 ms according to Bazett, Frederica and Framingham Heart Study respectively (Table 3, Table 4 ). Compared with baseline QTc interval, QT prolongation ≥50 msn and QTc interval ≥ 500 msn was observed in 2 (1.8%) patients. We analyzed baseline QTc interval and ∆QTc according to serum potassium level (serum K+ < 4.0 mmol/L vs. serum K+ ≥ 4.0 mmol/L). In contrast to higher serum potassium level (K+ ≥ 4.0 mmol/L), lower serum potassium level (serum K+ < 4.0 mmol/L) were associated with statistically significantly longer QT interval. But no difference existed between ∆QTc interval in this subgroup. This may be related to potassium replacement in patients who had lower serum potassium level (serum K+ < 4.0 mmol/L). Detailed results were demonstrated in Table 5 . No ventricular tachycardia, ventricular fibrillation or significant conduction delay was seen during follow-up. There was no death or worsening heart function.
Table 1.
Baseline demographic characteristics of study population.
| Patient characteristics | Value |
|---|---|
| Gender, M, n (%) | 48 (44) |
| Age, year, mean ± SD | 57.3 ± 14.4 |
| Hypertension, n (%) | 49 (45) |
| DM, n (%) | 32 (29.4) |
| CAD, n (%) | 24 (22) |
| HFrEF or HFpEF, n (%) | 10 (9.2) |
| COPD, n (%) | 22 (20.2) |
| Cancer or taking chemoprophylaxis, n (%) |
2 (1.8) |
Tisdale risk score, n (%)
|
93 (85.3) 12 (11) 4 (3.6) |
| Medications which | |
| ACEI or ARB, n (%) | 31 (28.4) |
| CCB, n (%) | 22 (20.2) |
| Diuretics, n (%) | 27 (24.8) |
| Ivabradine, n (%) | 0 (0) |
| Ranolazine, n (%) | 1 (0.9) |
| Amiodarone, n (%) | 1 (0.9) |
| Propafenone, n (%) | 0 (0) |
| Favipiravir, n (%) | 31 (28.4) |
| Oseltamivir. n (%) | 68 (62.4) |
| SSRI, n (%) | 7 (6.4) |
| Tocilizumab, n (%) | 2 (1.8) |
ACEI- angiotensin converting enzyme inhibitory; ARB- angiotensin receptor blocker; CAD- coronary artery disease; CCB- calcium channel blocker; COPD- chronic obstructive pulmonary disease; DM- diabetes mellitus; HFpEF- heart failure with preserved ejection fraction; HFrEF- heart failure with reduced ejection fraction; SSRI- selective serotonin receptor inhibitor;
Table 2.
Baseline laboratory findings of study population.
| Parameters | Variables |
|---|---|
| Hemoglobin, g/dL, mean ± SD | 13.07 ± 1.85 |
| Serum creatinine, mg/dL, mean ± SD | 0.93 ± 0.38 |
| BUN, mg/dL, mean ± SD | 16.82 ± 12.18 |
| eGFR, ml/min, mean ± SD | 79.77 ± 24.34 |
| Serum potassium, mmol/L, mean ± SD | 4.07 ± 0.50 |
| Serum calcium, mg/dL, mean ± SD | 8.95 ± 0.70 |
| Serum magnesium, mg/dL, mean ± SD | 1.99 ± 0.23 |
| Serum natrium, mmol/L, mean ± SD | 137.12 ± 3.03 |
| CRP, mg/dL, median (IQR) | 31.10 (10.31–76.09) |
| Ferritin, mg/dL, median (IQR) | 213.39 (68.43–417.59) |
| ESR, mm/h, median (IQR) | 28 (18–46) |
| Procalcitonin, median (IQR) | 0.21 (0.09–0.35) |
| Serum albumin, median (IQR) | 3.90 (3.53–4.10) |
BUN- blood urine nitrogen; CRP- C reactive protein; ESR- erythrocyte sedimentation rate; IQR- interquartile range; SD- standard deviation;
Table 3.
Changings in electrocardiographic findings during treatment course.
| Baseline ECG | On-treatment first ECG | On-treatment second ECG | |
|---|---|---|---|
| Heart rate, bpm, mean ± SD | 86 ± 14 | 77 ± 12 | 76 ± 12 |
| RR duration, ms, mean ± SD | 739.06 ± 128.84 | 801.59 ± 140.49 | 816.06 ± 161.21 |
| PR interval, ms, mean ± SD | 158.47 ± 25.10 | 156.35 ± 26.00 | 155.53 ± 26.77 |
| QRS duration, ms, mean ± SD | 94.00 ± 20.55 | 97.88 ± 21.73 | 99.18 ± 20.99 |
| QT interval, ms, mean ± SD | 370.09 ± 37.15 | 389.68 ± 42.92 | 397.88 ± 55.66 |
| QTc interval, ms, mean ± SD | |||
|
435.28 ± 32.78 | 459.68 ± 38.40 | 441.91 ± 38.71 |
|
415.67 ± 28.51 | 442.30 ± 40.42 | 426.33 ± 41.19 |
|
412.07 ± 25.65 | 440.97 ± 39.11 | 426.21 ± 39.68 |
| LBBB, n (%) | 4 (3.7) | 4 (3.7) | 4 (3.7) |
| RBBB, n (%) | 3 (2.8) | 3 (2.8) | 3 (2.8) |
| NIVCD, n (%) | 4 (3.7) | 4 (3.7) | 4 (3.7) |
ECG- electrocardiogram; LBBB- left bundle branch block; NIVCD - Nonspecific intraventricular conduction delay; QTc- corrected QT; RBBB- right bundle branch block; SD- standard deviation.
Table 4.
Comparison of electrocardiographic findings during treatment course.
| Parameters | ∆1. on-treatment ECG vs. baseline ECG | P value | ∆2. on-treatment vs. ∆1. on-treatment ECG |
P value | ∆2. on-treatment ECG vs. baseline ECG | P value |
|---|---|---|---|---|---|---|
| Heart rate, bpm, mean ± SEM | 10 ± 1 | < 0.001 | 1 ± 1 | 0.4 | 10 ± 1 | <0.001 |
| RR duration, ms, mean ± SD | 62.53 ± 9.42 | <0.001 | 14.47 ± 14.17 | 0.29 | 77 ± 24.95 | 0.009 |
| PR interval, ms, mean ± SD | −2.12 ± 18.90 | 0.65 | −0.82 ± 9.79 | 0.73 | −2.94 ± 19.93 | 0.55 |
| QRS duration, ms, mean ± SEM | 3.88 ± 8.37 | 0.074 | 1.29 ± 8.51 | 0.54 | 5.18 ± 8.94 | 0.03 |
QTc interval, ms, mean ± SEM
|
24.40 ± 2.99 26.64 ± 3.12 28.90 ± 2.97 |
<0.001 <0.001 < 0.001 |
−17.76 ± 3.94 −15.96 ± 3.94 −14.76 ± 3.65 |
<0.001 0.001 0.001 |
6.64 ± 9.60 10.67 ± 9.9 14.14 ± 9.68 |
0.5 0.19 0.16 |
SD- standard mean; SEM- standard error of mean;
*minus “- “indicates shortened duration. †Bold indicates statistically significant value. SE.
Table 5.
Comparison of mean baseline QTc and ∆QTc interval according to baseline serum potassium level.
| Serum K+ < 4.0 mmol/L N = 42 |
Serum K+ ≥ 4.0 mmol/L N = 67 |
P value | |
|---|---|---|---|
| QTc and ∆QTc, ms, Mean ± SD | |||
| QTc by Bazett | 451.46 ± 33.44 | 435.82 ± 25.50 | 0.007 |
| ∆QTc by Bazett | 8.66 ± 37.03 | 7.26 ± 26.97 | 0.82 |
| QTc by Fridericia | 425.69 ± 30.64 | 410.23 ± 26.26 | 0.006 |
| ∆QTc by Fridericia | 13.43 ± 39.07 | 11.41 ± 27.77 | 0.75 |
| QTc by FHS | 423.46 ± 28.28 | 409.91 ± 2.98 | 0.009 |
| ∆QTc by FHS | 13.53 ± 37.85 | 10.91 ± 25.69 | 0.67 |
FHS- Framingham Heart Study.
Bold indicates significant value.*
Discussion
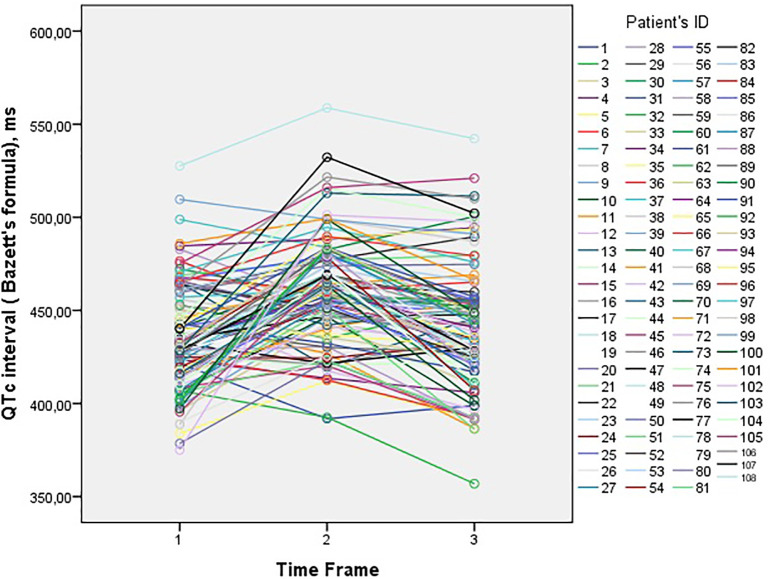
In our study, we showed that the 5-day course of HCQ-AZM combination does not cause significant QT prolongation and other conduction delays and this protocol was safe in terms of malignant cardiac arrhythmias. The changes in QTc interval (according to Bazett's formula) was demonstrated in Fig. 2 . Our results can be summarized as followings:
Fig. 2.
The changes in QTc intervals at the three times points (before starting HCQ/AZM combination, 3–6 h after the second HCQ loading dose and 48-72 h after the first dose of the combination).
1) The risk for QT prolongation with this combination is not frequent. 2) The QT prolongation that was seen after loading doses of HCQ (800 mg) and AZM (500 mg) were shortened during maintenance doses. Given that this trend in the QT interval, it may be suggested that QT prolongation was the result of the acute effect of HCQ and this was dose-related. We could not find a similar outcome in the previous studies. Tett et al. reported similar results with chloroquine [16]. 3) Serum potassium level was lower who had QT prolongation >50 ms in comparison to whom QT prolongation <50 ms. 4) HCQ lowered serum potassium level and this may exacerbate hypokalemia. Hypokalemia per se with other QT-prolonging drugs can worsen myocardial repolarization.
Two potassium ion channels, delayed rectifier K+ current (Ikr (rapid) and Iks (slow)) primarily carry out myocardial repolarization. Virtually Ikr was blocked by QT-prolonging drugs [17]. Ikr blockade produces prolongation of the action potential by delaying in phase 3. This increased duration is reflected by QT prolongation. De Bruin et al. established a clear correlation between the drug's ability to block Ikr and its potential to induce malignant ventricular arrhythmias and sudden cardiac death [18].
Hydroxychloroquine is a chloroquine analogue. Its pharmacokinetics vary widely in different diseases. Bioavailability can range from 25 to 100%. Mean absorption half-life is about 4 h and 40% of drug binds to serum proteins (mostly to albumin). Hydroxychloroquine metabolizes in the liver and excretes from the kidney as metabolites and unchanged from [16]. Hydroxychloroquine impacts on the cell membrane and causes potassium inflow [19]. Hypokalemia following HCQ use can be interpreted by this effect [20]. During our study, we observed a prominent decrease in serum potassium level after loading dose compared to the maintenance dose. Baseline and control (after the loading dose of HCQ dose) serum potassium were 4.13 ± 1.11 mmol/L and 4.0 ± 1.03 mmol/L respectively (p = .02).
QT prolongation, QRS widening was reported as a potential adverse effect of HCQ. Profound bradycardia or advanced AV block and other serious adverse effects were rare [21]. Cardiomyopathy with azithromycin has been reported [21].
Recently conducted chloroquine (CQ) study was stopped prematurely due to increased mortality rate with high dose CQ (the cumulative dose 12 g) in comparison to low dose (the cumulative dose 2.7 g) [22]. Hydroxychloroquine is less toxic than CQ [16]. In our study, there were no significant cardiac adverse effects with HCQ and it was well tolerated. Concomitantly, 75% of patients received oseltamivir and 8% favipiravir.
Azithromycin is a macrolide. Oral bioavailability is low and affected by foods. After taken 500 mg azithromycin orally it takes 2 h to reach serum peak concentration. Binding to plasma protein is low. Similar to other macrolides azithromycin interacts with the cytochrome P-450 and can influence other drugs metabolisms [23]. Hydroxychloroquine metabolizes by the cytochrome enzymes partly and this rises concern about drug interaction when used together. There were no significant interactions before in clinical practice [24]. In our study, there was no significant QT prolongation despite at least 68% of patients received three QT-prolonging medications. Along with HCQ/AZM, 7 (6.4%) patients were received SSRI, 1 (0.9%) patient received amiodarone and 1 (0.9%) patient received ranolazine. No difference was observed on ECGs of these patients compared to other patients.
Azithromycin is known as the safest macrolide in terms of cardiac events [25], this can be derived from unique monophasic action potential configuration compared with clarithromycin and erythromycin. But conflicting studies exist regarding the cardiovascular safety of azithromycin [26]. The QT prolongation and proarrhythmic effects that were reported previously were induced by azithromycin [10,[27], [28], [29]]. Ray et al. reported the increased cardiovascular mortality rate especially in patients who had cardiovascular risk factors with the 5- day course of azithromycin in comparison to amoxicillin [11]. However, Mortensen et al. determined that in comparison to other antibiotics azithromycin was safe and did not increase cardiac arrhythmias and heart failure among older population [30]. In Danish adult cohort study, azithromycin was not associated with increased cardiovascular risk as compared with penicillin V in young and middle-aged adults [31].
There are some limitations to our study. The sample size was small and designed as a single center study. We could not compare our outcomes with other protocols. The QT interval can be affected by several factors including medications, metabolic status, hypoxia, ischemia and underlying pathologies. Patients who were followed in the intensive care unit and who was intubated can be susceptible to QT-prolonging medications. Hence our results should not be generalized to all patients who are a candidate for HCQ and azithromycin combination. We did not perform a power analysis to calculate sample size that we need to predict the prevalence of significant QT prolongation following HCQ and azithromycin combination. However, our study demonstrated that prolonged QT interval after HCQ and azithromycin loading dose generally shortened during the maintenance period. By increasing the number of patients and centers attended the study, our results need to be confirmed.
Conclusion
The 5-day course of HCQ -AZM combination did not lead to significant QT prolongation and other conduction delays compared to baseline ECG in non-ICU COVID19(+) patients.
Acknowledgments
Acknowledgement
I would like to express my special thanks to Sally Sleiman MSc, MBA and Patrick Schnegelsberg MD, PhD for making English proofreading of my article and Hande Sisman for the assistance of data collecting.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019:2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH . 2020. WHO director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. Geneva, Switzerland. [Google Scholar]
- 3.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;104787 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. & Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang G., Sassaroli M., Louie M., Chen H., Stecher V.J., Sperber K. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin. Ther. 1996;18(6):1080–1092. doi: 10.1016/s0149-2918(96)80063-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang L.-F., Lin Y.-S., Huang N.-C. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J. Interf. Cytokine Res. 2015;35(3):143–156. doi: 10.1089/jir.2014.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.-Y., Wang F.-L., Lin C.-C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin. Toxicol. 2006;44(2):173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 10.Kezerashvili A., Khattak H., Barsky A., Nazari R., Fisher J.D. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J. Interv. Card. Electrophysiol. 2007;18(3):243–246. doi: 10.1007/s10840-007-9124-y. [DOI] [PubMed] [Google Scholar]
- 11.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.Y., Vinayagamoorthy N., Han K. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheum. 2016;68(1):184–190. doi: 10.1002/art.39402. [DOI] [PubMed] [Google Scholar]
- 13.Fleet J.L., Shariff S.Z., Bailey D.G. Comparing two types of macrolide antibiotics for the purpose of assessing population-based drug interactions. BMJ Open. 2013;3(7) doi: 10.1136/bmjopen-2013-002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment. Circulation. 2020. [DOI] [PubMed]
- 15.Anderson M.E., Al-Khatib S.M., Roden D.M., Califf R.M., Institute DCR Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am. Heart J. 2002;144(5):769–781. doi: 10.1067/mhj.2002.125804. [DOI] [PubMed] [Google Scholar]
- 16.Tett S., McLachlan A., Day R., Cutler D. Insights from pharmacokinetic and pharmacodynamic studies of hydroxychloroquine. Agents Actions Suppl. 1993;44:145–190. [PubMed] [Google Scholar]
- 17.Gupta A., Lawrence A.T., Krishnan K., Kavinsky C.J., Trohman R.G. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am. Heart J. 2007;153(6):891–899. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 18.De Bruin M., Pettersson M., Meyboom R., Hoes A., Leufkens H. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur. Heart J. 2005;26(6):590–597. doi: 10.1093/eurheartj/ehi092. [DOI] [PubMed] [Google Scholar]
- 19.Pruchnicki S., Good T., Walson P. Severe hydroxychloroquine poisoning reversed with diazepam. J. Toxicol. Clin. Toxicol. 1996;33:582. [Google Scholar]
- 20.Clemessy J.-L., Borron S., Baud F., Favier C., Hantson P., Vicaut E. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;346(8979):877–880. doi: 10.1016/s0140-6736(95)92711-5. [DOI] [PubMed] [Google Scholar]
- 21.Yogasundaram H., Putko B.N., Tien J. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 2014;30(12):1706–1715. doi: 10.1016/j.cjca.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Borba M., de Almeida Val F., Sampaio V.S. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study) medRxiv. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 23.Singlas E. Clinical pharmacokinetics of azithromycin. Pathologie-biologie. 1995;43(6):505–511. [PubMed] [Google Scholar]
- 24.Lode H. The pharmacokinetics of azithromycin and their clinical significance. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10(10):807–812. doi: 10.1007/BF01975832. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein E.J., Owens R.C., Jr., Nolin T.D. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin. Infect. Dis. 2006;43(12):1603–1611. doi: 10.1086/508873. [DOI] [PubMed] [Google Scholar]
- 26.Milberg P., Eckardt L., Bruns H.-J. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J. Pharmacol. Exp. Ther. 2002;303(1):218–225. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- 27.HUANG B.H., WU CH, HSIA CP, YIN CHEN C. Azithromycin-induced torsade de pointes. Pacing Clin. Electrophysiol. 2007;30(12):1579–1582. doi: 10.1111/j.1540-8159.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 28.Russo V., Puzio G., Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. ACTA BIOMEDICA-ATENEO PARMENSE. 2006;77(1):30. [PubMed] [Google Scholar]
- 29.Matsunaga N, Oki Y, Prigollini A. A case of QT-interval prolongation precipitated by azithromycin. The New Zealand Medical Journal (Online). 2003;116(1185). [PubMed]
- 30.Mortensen E.M., Halm E.A., Pugh M.J. Association of Azithromycin with Mortality and Cardiovascular Events among Older Patients Hospitalized with Pneumonia. JAMA. 2014;311(21):2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svanström H., Pasternak B., Hviid A. Use of azithromycin and death from cardiovascular causes. N. Engl. J. Med. 2013;368(18):1704–1712. doi: 10.1056/NEJMoa1300799. [DOI] [PubMed] [Google Scholar]