Abstract
Background
Neuroimaging technology is being developed to enable non-invasive mapping of the latency distribution of cortical projection pathways in white matter, and correlative clinical neurophysiological techniques would be valuable for mutual verification. Interhemispheric interaction through the corpus callosum can be measured with interhemispheric facilitation and inhibition using transcranial magnetic stimulation.
Objective
To develop a method for determining the latency distribution of the transcallosal fibers with transcranial magnetic stimulation.
Methods
We measured the precise time courses of interhemispheric facilitation and inhibition with a conditioning-test paired-pulse magnetic stimulation paradigm. The conditioning stimulus was applied to the right primary motor cortex and the test stimulus was applied to the left primary motor cortex. The interstimulus interval was set at 0.1 ms resolution. The proportions of transcallosal fibers with different conduction velocities were calculated by measuring the changes in magnitudes of interhemispheric facilitation and inhibition with interstimulus interval.
Results
Both interhemispheric facilitation and inhibition increased with increment in interstimulus interval. The magnitude of interhemispheric facilitation was correlated with that of interhemispheric inhibition. The latency distribution of transcallosal fibers measured with interhemispheric facilitation was also correlated with that measured with interhemispheric inhibition.
Conclusions
The data can be interpreted as latency distribution of transcallosal fibers. Interhemispheric interaction measured with transcranial magnetic stimulation is a promising technique to determine the latency distribution of the transcallosal fibers. Similar techniques could be developed for other cortical pathways.
Keywords: Corpus callosum, Interhemispheric facilitation and interhemispheric inhibition, Latency distribution, Motor evoked potential, Primary motor cortex, Transcranial magnetic stimulation
Highlights
-
•
Interhemispheric facilitation and inhibition were tested using magnetic stimulation.
-
•
Time courses of interhemispheric interactions were measured with 0.1 ms resolution.
-
•
Interhemispheric interaction increases with increment in interval between two stimuli.
-
•
Latency distributions measured by facilitation and inhibition were correlated.
-
•
Latency distribution of cortical fibers can be verified by brain stimulation technique.
Introduction
A fundamental issue in understanding connectivity between neurons in different cortical areas is the distribution of latencies in white matter pathways that connect these areas. Neuroimaging technology is currently being developed to enable non-invasive mapping of the cortical projection pathways on a derived millisecond scale [1,2]. In particular, our group developed a diffusion weighted magnetic resonance imaging pipeline with a non-Gaussian model [3] for the latency distribution mapping of the cortical fibers (latency connectome) [[4], [5], [6], [7]]. Recently, we performed a latency connectome measurement in the peripheral nervous system using a collision technique and confirmed that correlative neurophysiological techniques are capable of validating the neuroimaging techniques in living humans [8]. However, the validation of the neuroimaging result has never been attempted in the central nervous system with clinical neurophysiological techniques to assess the latency distribution in white matter pathways.
The corpus callosum is the largest white matter structure with a major bundle of commissural fibers connecting the two hemispheres [[9], [10], [11]]. Interhemispheric facilitation and inhibition refer to the neurophysiological mechanisms in which one hemisphere facilitates and inhibits the opposite hemisphere through transcallosal fibers. Interhemispheric facilitation and inhibition between homologous primary motor cortices can be measured by transcranial magnetic stimulation with a conditioning-test paired-pulse paradigm [12,13](Fig. 1 A) wherein a conditioning pulse facilitates the motor evoked potential generated by a test stimulus at interstimulus intervals of 3–6 ms [14,15] and inhibits it at interstimulus intervals of 7–15 ms [16,17]. In this proof-of-principle study, we attempted to establish the latency distribution of the transcallosal fibers by monitoring the changes in transcallosal interactions with motor cortical stimulation. We measured the precise time course of interhemispheric facilitation and inhibition at 0.1 ms resolution. Because different pyramidal neurons in the primary motor cortex (on the conditioning stimulus side) are connected to the transcallosal fibers with different diameters (Fig. 1A and B), the magnitudes of interhemispheric facilitation or inhibition at various interstimulus intervals should reflect the proportions of different transcallosal fibers arriving at the test side. Changes in motor evoked potential amplitude from stimulation on the test side should follow a linear function within the middle part of motor evoked potential recruitment curve [12,18,19]. Therefore, the variation of the conditioned motor evoked potential should be able to be used to track the magnitudes of interhemispheric facilitation and inhibition with various interstimulus intervals and be further translated into a latency distribution of transcallosal fibers with different diameters and conduction velocities. Our hypothesis is that both interhemispheric facilitation and inhibition will increase with increasement of interstimulus interval (interval between conditioning and test stimuli) in a certain range because more transcallosal inputs mediated by fibers with progressively slower conduction velocities (activated by conditioning stimulus) arrive at the opposite hemisphere after longer interval. Based on our hypothesis, we also predict that the magnitude of interhemispheric facilitation will be correlated with the magnitude of interhemispheric inhibition and that the latency distributions of transcallosal fibers measured by interhemispheric facilitation and inhibition will be same.
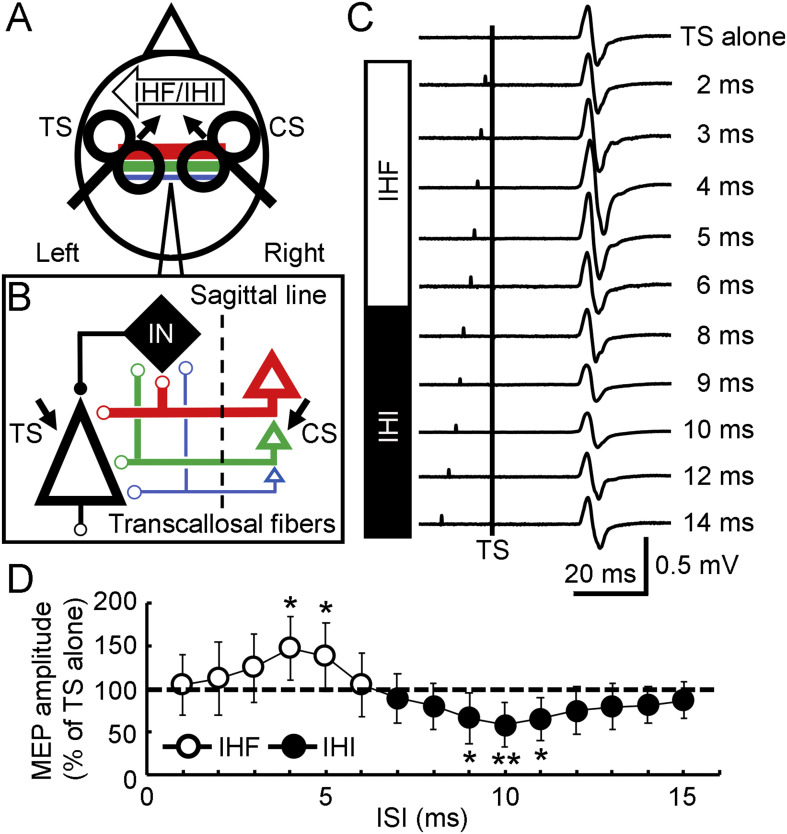
Fig. 1.
Latency distribution of the transcallosal fibers tested with interhemispheric interaction. (A) Experimental setup. IHF and IHI from the right primary motor cortex to the left primary motor cortex tested with a paired-pulse transcranial magnetic stimulation paradigm were used to verify the latency distribution of transcallosal fibers with different diameters (color lines). The first CS was given to the right primary motor cortex. The second TS was given to the left primary motor cortex. The small arrows close to the coil show the induced current direction in the brain. (B) Hypothesis. CS activates multiple pyramidal neurons (triangles with different sizes) in the right primary motor cortex. These pyramidal neurons are connected to the transcallosal fibers with different diameters (color lines with different thickness). Pyramidal neurons (black triangle) in the left primary motor cortex are directly influenced by transcallosal inputs, leading to IHF. Local inhibitory interneurons (black rhombus) in the left cortex are also activated by transcallosal inputs, leading to IHI with longer latency. Dashed line indicates the mid-sagittal line. Facilitatory and inhibitory interactions are marked with small open and filled circles, respectively. (C) Example recordings. Average of 10 trials. All trials included a TS (vertical line). TS alone generated a MEP of about 0.5 mV in amplitude (first row). A preceding CS produced IHF at short ISIs (2–6 ms, second to sixth rows). IHI was induced at longer ISIs (8–14 ms, bottom rows). (D) IHF and IHI (N = 12) tested at ISIs of 1–15 ms with 1 ms resolution. Abscissa indicates the ISI. Ordinate indicates MEP amplitude induced by the paired-pulse stimulation. It is expressed as a percentage value of the mean MEP amplitude evoked by CS-TS to that evoked by TS alone (dashed line). Values above 100% indicate IHF (open circle) and values below 100% indicate IHI (filled circle). ∗P < 0.05, ∗∗P < 0.01, post hoc paired t-test with Bonferroni’s correction comparing MEP with paired-pulse stimulation to that with TS alone. CS = conditioning stimulus; IHF = interhemispheric facilitation; IHI = interhemispheric inhibition; IN = local inhibitory interneuron in the left primary motor cortex; ISI = interstimulus interval; MEP = motor evoked potential; TS = test stimulus. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Methods
Subjects
We studied 12 right-handed (Edinburgh Handedness Inventory, 98.3 ± 5.8) healthy subjects (7 women and 5 men, aged 29.0 ± 12.9 years) [20]. All subjects provided written informed consent, and the clinical protocol (Clinicaltrials.gov Identifier NCT03223636) was approved by the Combined NeuroScience Institutional Review Board at the National Institutes of Health.
Electromyographic recording
Surface electromyograms were recorded from bilateral first dorsal interosseous muscles with 9 mm diameter Ag–AgCl surface electrodes. The active electrode was placed over the muscle belly, and the reference electrode over the metacarpophalangeal joint of the index finger. The signal was amplified (1000 × ), band-pass filtered (20 Hz-2.5 kHz, Neuropack MEB-2300 EMG/NCV/EP Measuring Desktop System, Nihon Kohden, Tokyo, Japan), digitized at 5 kHz by an analog-to-digital interface (Micro1401, Cambridge Electronics Design, Cambridge, UK) and stored in a computer for off-line analysis.
Transcranial magnetic stimulation
Transcranial magnetic stimulation was applied to the primary motor cortex with a custom-made figure-of-eight shaped coil (outside diameter of each loop was 5 cm, handle perpendicular to the coil) connected to a Magstim 200 stimulator (Magstim, Whitland, Dyfed, UK). The current in the joint point of coil pointed backward at 30–45° from the mid-sagittal line. The induced current in the brain was in the posterior-anterior direction, approximately perpendicular to the central sulcus. With this current direction, pyramidal neurons are activated trans-synaptically and produce early indirect waves [21,22]. Interhemispheric facilitation and inhibition from the right primary motor cortex to the left motor cortex were measured. The test stimulus was applied to the left primary motor cortex and conditioning stimulus was applied to the right primary motor cortex. The optimal position for activation of the target muscle was marked with a pen as the motor hot spot. We used “0.5 mV” intensity both for the conditioning and test stimuli. The “0.5 mV” intensity was defined as the lowest stimulus intensity needed to generate MEPs of more than 0.5 mV in at least 5 out of 10 trials in the target muscle when the muscle was completely relaxed.
Experimental design
The time courses of interhemispheric facilitation [14] and interhemispheric inhibition [16,17] were tested in two rounds. The first round tested the time course in 1 ms resolution. Interstimulus intervals of 1–15 ms were selected. Ten trials for each interstimulus interval with paired-pulse stimulation and twenty trials for test alone (total of 170 trials) were delivered in random order. Interstimulus intervals for maximal interhemispheric facilitation and inhibition were identified. A precise time course with 0.1 ms resolution was tested in the second round. Interstimulus intervals were determined individually in each subject. The range was from 2 ms before to 1 ms after the maximal interhemispheric facilitation and inhibition identified in the first round of experiment. Ten trials for each interstimulus interval with paired-pulse stimulation and twenty trials for test alone (total of 330 trials) were delivered in random order. Interhemispheric facilitation and inhibition were studied in separate runs.
Data analysis and statistical analysis
Values are reported as mean ± standard deviation. Motor evoked potential amplitudes were measured peak-to-peak. The amplitude evoked by paired-pulse stimulation was expressed as a percentage of the mean motor evoked potential amplitude of test alone. Values below 100% indicate inhibition and values above 100% indicate facilitation. In addition, the magnitude of interhemispheric facilitation or inhibition was calculated as the percentage difference between conditioned and test motor evoked potentials. Therefore, the magnitude of interhemispheric facilitation was a positive value and that of interhemispheric inhibition was a negative value. A repeated measures analysis of variance was used to examine the effects of interstimulus interval on interhemispheric facilitation and inhibition. Post hoc paired t-test with Bonferroni’s correction was used to examine at which interstimulus intervals a conditioned motor evoked potential (with paired-pulse stimulation) was different from the test motor evoked potential. For the second round of experiments with 0.1 ms time resolution of interstimulus interval, we identified the intervals showing maximal interhemispheric facilitation and inhibition and defined them as time 0 in each subject. We realigned the time course in each subject. The relationship between maximal interhemispheric facilitation and maximal inhibition was examined by Pearson correlation coefficient. The latency distribution of transcallosal fibers was obtained by calculating the proportions of various groups of fibers with different diameters. The proportion was represented by the percentage value of the changes in interhemispheric facilitation (or inhibition) with the minimal increase in interstimulus interval (0.1 ms) divided by the maximal interhemispheric facilitation (or inhibition). Only the data with interstimulus intervals before that for the maximal facilitation and inhibition were used to calculate the latency distribution. The relationship between the proportion of the largest group of transcallosal fibers measured with interhemispheric facilitation and that measured with interhemispheric inhibition was also examined by Pearson correlation coefficient. The significance level was set at P < 0.05. The Statistical Package for the Social Sciences version 22.0 software (International Business Machines Corporation) was used for statistical analysis.
Results
Stimulus intensity and test size of motor evoked potential
The resting motor threshold for the left primary motor cortex (49.4 ± 11.5% of stimulator output) and that for the right primary motor cortex (49.9 ± 12.4% of stimulator output) were similar (t11 = 0.20, P = 0.849). The stimulus intensity for generating 0.5 mV motor evoked potential was also similar for the left (58.8 ± 12.1% of stimulator output) and right primary motor cortices (60.6 ± 14.4% of stimulator output) (t11 = 0.83, P = 0.424). The test motor evoked potential in the right first dorsal interosseous muscle was 0.61 ± 0.13 mV in amplitude. The conditioning motor evoked potential measured in the left hand was 0.58 ± 0.11 mV.
Time courses of interhemispheric facilitation and inhibition
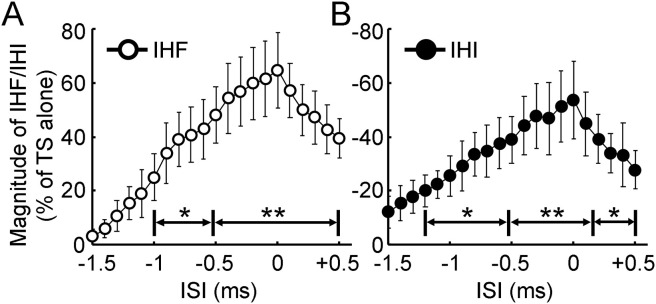
Fig. 1C showed that conditioning stimulation facilitated the test motor evoked potential at interstimulus intervals of about 2–6 ms (interhemispheric facilitation) and inhibited it at intervals of about 8–15 ms (interhemispheric inhibition). In addition, Fig. 1D showed that both interhemispheric facilitation and inhibition increased with initial increment in interstimulus interval. Further increase in interstimulus interval led to the reduction in both interhemispheric facilitation and inhibition. Analysis of variance revealed that the motor evoked potential with paired-pulse stimulation varied with different interstimulus intervals tested in 1 ms resolution (F14,154 = 9.81, P < 0.001). With the precise time course in 0.1 ms resolution, it was identified that the interstimulus interval for maximal interhemispheric facilitation was 4.5 ± 0.4 ms and that for the maximal interhemispheric inhibition was 9.6 ± 0.6 ms. We defined the time point for maximal facilitation or inhibition as time 0 and realigned the time course in each subject. As the precise time courses were tested in two experimental runs, the time courses for interhemispheric facilitation and inhibition were analyzed separately. We only recorded the precise time courses 2 ms before and 1 ms after the presumed maximal interhemispheric facilitation and inhibition (identified from the first round of experiment). Therefore, we were eventually able to obtain a precise time course with interstimulus intervals 1.5 ms before (−1.5 ms) and 0.5 ms after the true maximal interhemispheric facilitation and inhibition in all subjects. We performed an analysis of variance with the realigned time course and found that interhemispheric facilitation increased gradually before reaching the maximal value (F20,220 = 84.31, P < 0.001, Fig. 2 A). Post hoc paired t-test confirmed that conditioned motor evoked potential was larger than the test motor evoked potential at interstimulus intervals of −1.0 to −0.6 ms (P < 0.05) and −0.5 to 0.5 ms (P < 0.01) around the interval for maximal facilitation (defined as time 0). Similarly, with the realigned time course we also found that interhemispheric inhibition increased gradually before reaching the maximal value (F20,220 = 97.28, P < 0.001, Fig. 2B). Post hoc t-test confirmed that significant interhemispheric inhibition occurred at interstimulus intervals of −1.2 to −0.6 ms (P < 0.05), −0.5 to 0.1 ms (P < 0.01) and 0.2 to 0.5 ms (P < 0.05) around the interval for maximal inhibition.
Fig. 2.
Precise time courses of interhemispheric facilitation and inhibition. (A) IHF and (B) IHI (N = 12) tested at ISIs 1.5 ms before and 0.5 ms after the time points for maximal facilitation and inhibition with 0.1 ms resolution. Abscissa indicates the ISI. Time 0 was defined as the ISI with maximal IHF or IHI in each subject. Ordinate indicates the magnitude of IHF (open circle) or IHI (filled circle). It is expressed as a percentage difference between motor evoked potential amplitude induced by CS-TS paired-pulse stimulation and that induced by TS alone (defined as 100%). The value for IHF is positive and that for IHI is negative. ∗P < 0.05, ∗∗P < 0.01, post hoc paired t-test with Bonferroni’s correction comparing motor evoked potential with paired-pulse stimulation to that with TS alone. CS = conditioning stimulus; IHF = interhemispheric facilitation; IHI = interhemispheric inhibition; ISI = interstimulus interval; TS = test stimulus.
Latency distribution of transcallosal fibers
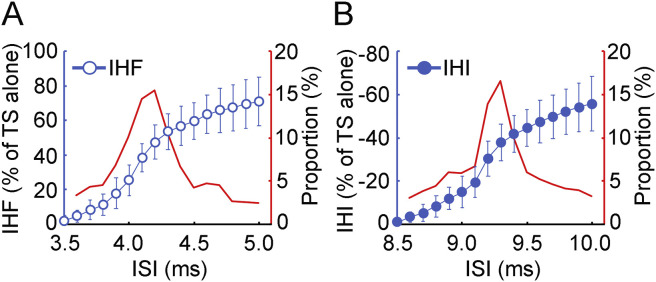
The latency distribution of transcallosal fibers was derived from the continuous changes in the magnitude of interhemispheric facilitation (or inhibition) with increment in the interstimulus interval. Fig. 3 showed the latency distribution of transcallosal fibers measured by precise time courses of interhemispheric facilitation and inhibition in one subject. The proportion of the nerve fibers with certain conduction velocity measured by both facilitation and inhibition increased gradually and reached the maximal value at interstimulus interval of 0.6–0.7 ms before that for maximal facilitation and inhibition. The interstimulus interval for detecting the largest group of transcallosal fibers with interhemispheric facilitation was 3.8 ± 0.4 ms and that with interhemispheric inhibition was 9.0 ± 0.5 ms. Importantly, both the proportion of the largest group of transcallosal fibers and the shape of the latency distribution curve measured by interhemispheric facilitation and those measured by interhemispheric inhibition were similar.
Fig. 3.
Latency distribution measurement. Latency distribution of transcallosal fibers measured with (A) IHF and (B) IHI in one subject. The ISI range from no IHF (or IHI) to the maximal IHF (or IHI) was shown. Abscissa indicates the ISI. Left ordinate (blue) in each panel indicates the magnitude of IHF (blue open circle) or IHI (blue filled circle). It is expressed as a percentage difference between motor evoked potential amplitude induced by CS-TS paired-pulse stimulation and that induced by TS alone (defined as 100%). The value for IHF is positive and that for IHI is negative. Right ordinate (red) in each panel indicates the proportion of the nerve fibers with certain conduction velocity in the whole bundle. The proportion for a group of nerve fibers (red curve) was represented by the percentage of the difference in IHF (or IHI) with the minimal increase in ISI (0.1 ms) divided by the maximal IHF (or IHI). The curve was smoothed with a two-point moving average. Note that the peak proportion of transcallosal fibers measured with IHF and that with IHI were similar. CS = conditioning stimulus, IHF = interhemispheric facilitation, IHI = interhemispheric inhibition, ISI = interstimulus interval, TS = test stimulus. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
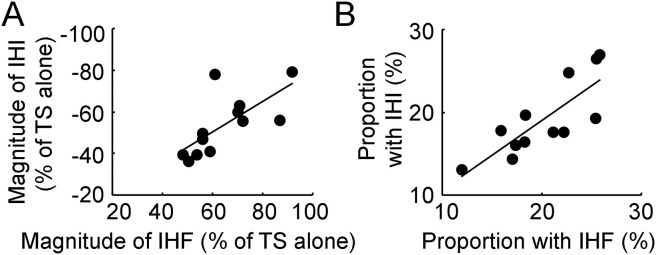
In addition, it was found that the magnitude of maximal interhemispheric facilitation was correlated with that of interhemispheric inhibition (R = −0.699, F1,10 = 9.56, P = 0.011, Fig. 4 A). The proportion of the largest group of transcallosal fibers measured with interhemispheric facilitation was also correlated with that measured with interhemispheric inhibition (R = 0.801, F1,10 = 17.90, P = 0.002, Fig. 4B).
Fig. 4.
Correlation analysis for measurements with interhemispheric interaction. (A) Correlation between IHF and IHI at the peak interstimulus intervals. Abscissa indicates the magnitude of maximal IHF and ordinate indicates the magnitude of maximal IHI. They are expressed as a percentage difference between motor evoked potential amplitude induced by CS-TS paired-pulse stimulation and that induced by TS alone. The value for IHF is positive and that for IHI is negative. (B) Correlation between the proportion of the largest group of transcallosal fibers measured with IHF and that measured with IHI. Abscissa indicates the proportion of transcallosal fibers measured with IHF and ordinate indicate that measured with IHI. They are expressed as a percentage value of the largest change in IHF (or IHI) with the minimal increase in interstimulus interval (0.1 ms) divided by the maximal IHF (or IHI). The solid lines indicate significant correlation between two different variables with P < 0.05. CS = conditioning stimulus, IHF = interhemispheric facilitation, IHI = interhemispheric inhibition, TS = test stimulus.
Discussion
This is the first study to measure the presumed cortical latency distribution of a white matter tract with a neurophysiological technique. Our major finding is that both interhemispheric facilitation and inhibition increase gradually with increment in interstimulus interval before reaching their maximal values. The magnitude of interhemispheric facilitation is correlated with that of interhemispheric inhibition. The latency distributions of transcallosal fibers measured with interhemispheric facilitation and inhibition are same.
Interhemispheric interactions with transcallosal inputs
Using a classical paired-pulse paradigm with transcranial magnetic stimulation we observed a time course of interhemispheric facilitation at short interstimulus intervals and interhemispheric inhibition at longer intervals (Fig. 1C and D). Our results with interstimulus intervals tested in 1 ms resolution supported the opinion of previous studies that both interhemispheric facilitation and inhibition were through corpus callosum [[14], [15], [16], [17]]. In addition, the precise time course with 0.1 ms resolution further explored that interhemispheric facilitation and inhibition started to emerge at interstimulus intervals of more than 1.5 ms before the peak and increased gradually with increments in the interval (Fig. 2). Our findings are compatible with the notion that myelinated fibers in corpus callosum are neuronal elements mediating interhemispheric facilitation and inhibition [11,[23], [24], [25]]. It is likely that interhemispheric facilitation is produced with direct activation of local pyramidal neurons (on the side of test stimulus) by facilitatory drives through transcallosal fibers (activated by the conditioning stimulus) [11,14,15,17,23]. On the other hand, interhemispheric inhibition is modulated by the same intracortical circuits as the pyramidal neurons on the side of test stimulus [[26], [27], [28], [29]], indicating that interhemispheric inhibition is produced via the synaptic transmissions at a group of local inhibitory interneurons activated by the transcallosal facilitatory drive.
Relationship between interhemispheric facilitation and inhibition
We found interhemispheric facilitation and inhibition decreased with further increase in interstimulus intervals after the maximal facilitation and inhibition (Fig. 2). Reductions both in facilitation and inhibition are likely due to the decay of excitatory postsynaptic potential produced by transcallosal inputs. In addition, the recruitment of pyramidal neurons mediating transcallosal interaction is complex and the net result measured with the motor evoked potential in a peripheral muscle represents the complex interplay among different factors. In this regard, it was reported that interhemispheric facilitation was induced when a conditioning stimulus with very low intensity (60% of active motor threshold) was applied 6 ms before the test stimulus with posterior-anterior current direction. Slight increase in conditioning intensity (80% active motor threshold) led to the interhemispheric facilitation at interstimulus intervals of both 6 and 8 ms but with the test stimulus applied in the opposite anterior-posterior current direction [15]. Another study performed during target muscle contraction reported interhemispheric facilitation at 4 and 5 ms interstimulus intervals while the facilitation only occurred when a test stimulus with an anterior-posteriorly directed current was preceded by a conditioning stimulus at 5% or 10% above active motor threshold [14]. It is known that the recruitment of pyramidal neurons in the corticospinal tract follows Henneman’s size principle and the corticospinal axons vary in diameter [30] since more pyramidal neurons in the corticospinal tract could be activated if the primary motor cortex is stimulated with higher stimulus intensity [12,19]. Pyramidal neurons mediating transcallosal interactions and those mediating corticospinal projections likely have similar anatomical and physiological properties because they modulate the local intracortical circuits [29,31] and are modulated by local intracortical circuits in a similar manner [26,32] although the two groups of pyramidal neurons are located in different cortical layers [33]. These results might be explained by the fact that a limited number of transcallosal fibers with very low firing threshold are activated by a low intensity conditioning stimulus. Therefore, the interhemispheric facilitation observed in the previous studies mediated by the low threshold transcallosal fibers were only detectable within a narrow time window and required precise experimental conditions. On the other hand, our conditioning stimulus with suprathreshold intensity activated multiple transcallosal fibers with different diameters (different firing thresholds) and the precise time course determined the interhemispheric facilitation mediated by different transcallosal fibers at corresponding intervals. Furthermore, reduced magnitude of interhemispheric facilitation at long interstimulus intervals may also be related to the arrival of the fastest inhibitory drive (interhemispheric inhibition) on the side of test stimulus. The underlying mechanisms for interhemispheric inhibition is complex as more neuronal elements (the local inhibitory interneuron shown in Fig. 1B or more neurons) are involved [27,28]. Indeed, we found that interhemispheric inhibition started early in the time course when the interstimulus interval with the precise resolution was tested (Fig. 2B, more than 10% inhibition at interstimulus interval of 1.5 ms before the maximal interhemispheric inhibition). Importantly, we found gradually increased interhemispheric facilitation and inhibition with suprathreshold stimulus and observed the reliable time courses of transcallosal interactions. However, future studies testing a detailed recruitment curve of conditioning stimulus (at intensity higher than “0.5 mV” in particular) with a similar precise time course might reveal a latency distribution of transcallosal fibers in a wider range.
Latency distribution of transcallosal fibers
Cortical fibers vary considerably in diameter [[34], [35], [36]]. We performed the measurements of interhemispheric interactions to calculate the latency distribution of transcallosal fibers. One of our major findings was that interhemispheric facilitation and inhibition were highly correlated (Fig. 4A). More importantly, the distribution curves derived from interhemispheric facilitation and inhibition were same (Fig. 3) and the proportions of fibers measured with two methods were also correlated (Fig. 4B). The results strongly supported our hypothesis (Fig. 1A and B) that the magnitude of interhemispheric facilitation or interhemispheric inhibition is determined by the activation of transcallosal fibers with different conduction velocities. However, it should be mentioned that the derivation of precise cortical latencies along the transcallosal fibers depends on further elucidation of the underlying mechanisms of interhemispheric facilitation and inhibitions. It is still not clear whether direct or oligosynaptic connections and which neuronal transmitters are involved in the conduction of transcallosal inputs [11,14,15,17,23]. In addition, our results were obtained from the changes in motor evoked potentials measured in a hand muscle. A basic assumption of our proof-of-principle study is that different transcallosal fibers with same diameter make the same contribution to the measurements of interhemispheric interactions. A similar assumption seems correct when the conduction velocity distribution of a peripheral nerve is measured with compound muscle action potential in a collision test using a neurophysiological approach [8,[37], [38], [39]]. However, different magnitudes of interhemispheric interactions measured in the present study may not follow a linear function with changes in motor evoked potential amplitude. Although we found strong correlation between interhemispheric inhibition and interhemispheric facilitation (Fig. 4), it might still be argued that interhemispheric inhibition with relatively weak test stimulus intensity (“0.5 mV”) pushed the conditioned motor evoked potential to the low end of linear part of stimulus intensity recruitment curve. Future study should test a range of test stimulus intensities to examine whether the determined latency distribution of transcallosal fibers is the same.
Technical limitations1
Several other confounding factors should be taken into consideration. We used relatively low stimulus intensity (“0.5 mV”) in the resting state to reduce the spreading current into a wide area. However, it might be discussed that different latencies of the transcallosal fibers measured by interhemispheric facilitation and inhibition were simply due to fibers with different lengths rather than fibers with different diameters because magnetic stimulation actives pyramidal neurons at different sites. Similarly, the explanation of our results may be complicated by the factor that various pyramidal neurons on the conditioning side are located at cortical layers with different depth and are in preference to different current directions which produce multiple descending volleys [21,22]. The recruitments of early and later indirect waves and the modulations of these waves involving transcallosal inputs activated by the conditioning stimulus might be different [27,40]. The recruitment of pyramidal neurons on the test stimulus side with different descending corticospinal volleys may also be complex. As discussed in the former sections, previous studies reported that interhemispheric facilitation was observed in a narrow time window with specific preference for test stimulus current direction [14,15] while interhemispheric inhibition was detectable in a wide range of conditioning stimulus intensities and test stimulus directions [14]. These studies raised the possibility that early and later components of descending volleys that largely change the latency of motor evoked potential have different sensitivity to the transcallosal inputs [41]. It should be noted that the latency distribution derived from the present study was based on the measurement of changes in motor evoked potential amplitudes and not latencies. Therefore, longer latency measured in the motor evoked potential induced by an anterior-posteriorly directed current should not change the latency distribution measured in the present study. However, if magnetic stimulations with anterior-posterior and posterior-anterior current directions activate different populations of pyramidal neurons [42,43], future studies performed with test stimuli in both current directions may identify different patterns of latency distributions of transcallosal fibers targeting separate groups of pyramidal neurons. Furthermore, the temporal and spatial summation along various transcallosal fibers with same conduction velocity may produce large within-subject and between-subject variation on the excitability of pyramidal neurons on the side of test stimulus. In addition, different magnitudes of interhemispheric facilitation and inhibition in various muscles and contribution to interhemispheric facilitation and inhibition with pathways outside corpus callosum might also be confounding factors [16,17,44].
Verification of neuroimaging techniques
Water diffusion parallel to axon bundles is relatively free and follows Gaussian displacement distribution. This was used in our group to develop the diffusion tensor imaging technique with k-space encoding to measure neuronal fibers and provide a means to visualize white matter pathways [4,45]. Previous studies performed by other groups demonstrated that both transcallosal fibers connecting bilateral primary motor cortices [23] and transcallosal fibers connecting bilateral parietal cortices [46] had high correlation between the measurement with diffusion tensor imaging and that with interhemispheric inhibition. These studies suggested that functional measurements using transcranial magnetic stimulation may provide ground truth data to verify the neuroimaging pipeline. Cortical latency connectome is a comprehensive map of neuronal connections between different nodes in the cortical network at the time scale of impulses being transmitted. To obtain the cortical latency connectome with structural and functional data, our group developed the neuroimaging pipeline with novel type of diffusion weighted magnetic resonance imaging techniques (non-Gaussian q-space model with large q-values) to determine the mean and average nerve fiber diameter distribution in the central nervous systems [[4], [5], [6],45,47]. The present proof-of-principle study was the first step to verify the neuroimaging pipeline using neurophysiological techniques. However, the production of believable cortical latency connectome will depend on consistency between neurophysiological and neuroimaging data [[4], [5], [6], [7]]. Future studies will focus on the determination of cortical latency distributions in various white matter tracts. The discrimination of normal and abnormal brain networks, elucidation of development, degeneration and trauma using brain stimulation techniques are also crucial tasks for the latency connectome project.
Conclusion
Our study is the first to determine the presumed latency distribution of a major bundle of cortical projection fibers. We conclude that interhemispheric interaction measured with a classical transcranial magnetic stimulation paradigm is a promising technique to determine the conduction velocity distribution of the transcallosal fibers.
Research data linking
The research data for each subject is available in Mendeley Data. Citation of dataset: Ni, Zhen; Leodori, Giorgio; Vial, Felipe; Zhang, Yong; Avram, Alexandru; Pajevic, Sinisa; Basser, Peter; Hallett, Mark (2020),“BRS-D-20-00320”, Mendeley Data, V4; https://doi.org/10.17632/9zdh7xt3ks.4; URL: https://data.mendeley.com/datasets/9zdh7xt3ks/4.
CRediT authorship contribution statement
Zhen Ni: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - original draft, Writing - review & editing. Giorgio Leodori: Conceptualization, Investigation, Methodology, Writing - original draft, Writing - review & editing. Felipe Vial: Investigation, Methodology, Writing - review & editing. Yong Zhang: Investigation, Methodology, Writing - review & editing. Alexandru V. Avram: Investigation, Writing - review & editing. Sinisa Pajevic: Investigation, Writing - review & editing. Peter J. Basser: Conceptualization, Investigation, Supervision, Writing - review & editing. Mark Hallett: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing - original draft, Writing - review & editing.
Declaration of competing interest
All authors declare no conflict of interest. We further confirm that all subjects provided written informed consent and any aspect of the work covered in this study (clinical protocol, Clinicaltrials.gov Identifier NCT03223636) that has involved human subjects has been conducted with the ethical approval of the Combined NeuroScience Institutional Review Board at the National Institutes of Health.
Acknowledgement
This work was done at the National Institutes of Health. This work was supported by BRAIN INITIATIVE Grant #1-R24-MH-109068-01, and the National Institute of Neurological Disorders and Stroke and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Intramural Research Programs.
Footnotes
None of the future studies suggested here could be performed now due to the COVID-19 pandemic.
References
- 1.Wedeen V.J., Rosene D.L., Wang R. The geometric structure of the brain fiber pathways. Science. 2012;335:1628–1634. doi: 10.1126/science.1215280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drakesmith M., Harms R., Rudrapatna S.U., Parker G.D., Evans C.J., Jones D.K. Estimating axon conduction velocity in vivo from microstructural MRI. Neuroimage. 2019;203:116186. doi: 10.1016/j.neuroimage.2019.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaghan P.T., Coy A., MacGowan D., Packer K.J., Zelaya F.O. Diffraction-like effects in NMR diffusion studies of fluids in porous solids. Nature. 1991;351:467–469. [Google Scholar]
- 4.Basser P.J., Pajevic S., Pierpaoli C., Duda J., Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Avram A.V., Sarlls J.E., Barnett A.S. Clinical feasibility of using mean apparent propagator (MAP) MRI to characterize brain tissue microstructure. Neuroimage. 2016;127:422–434. doi: 10.1016/j.neuroimage.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assaf Y., Blumenfeld-Katzir T., Yovel Y., Basser P.J. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komlosh M.E., Ozarslan E., Lizak M.J. Mapping average axon diameters in porcine spinal cord white matter and rat corpus callosum using d-PFG MRI. Neuroimage. 2013;78:210–216. doi: 10.1016/j.neuroimage.2013.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Z., Vial F., Avram A.V. Measuring conduction velocity distributions in peripheral nerves using neurophysiological techniques. Clin Neurophysiol. 2020;131:1581–1588. doi: 10.1016/j.clinph.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzaniga M.S. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 10.Luders E., Thompson P.M., Toga A.W. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarei M., Johansen-Berg H., Smith S., Ciccarelli O., Thompson A.J., Matthews P.M. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Ni Z., Müller-Dahlhaus F., Chen R., Ziemann U. Triple-pulse TMS to study interactions between neural circuits in human cortex. Brain Stimul. 2011;4:281–293. doi: 10.1016/j.brs.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Hanajima R., Ugawa Y., Machii K. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumer T., Bock F., Koch G. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572:857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferbert A., Priori A., Rothwell J.C., Day B.L., Colebatch J.G., Marsden C.D. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni Z., Gunraj C., Nelson A.J. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cerebr Cortex. 2009;19:1654–1665. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- 18.Boroojerdi B., Battaglia F., Muellbacher W., Cohen L.G. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–937. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- 19.Rothwell J.C., Thompson P.D., Day B.L., Boyd S., Marsden C.D. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- 20.Oldfield R.C. The assessment and analysis of handedness: the Edingburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko K., Kawai S., Fuchigami Y., Morita H., Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- 22.Di Lazzaro V., Oliviero A., Saturno E. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- 23.Wahl M., Lauterbach-Soon B., Hattingen E. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofer S., Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Aboitiz F., Scheibel A.B., Fisher R.S., Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 26.Avanzino L., Teo J.T., Rothwell J.C. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583:99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R., Yung D., Li J.-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kukaswadia S., Wagle-Shukla A., Morgante F., Gunraj C., Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol. 2005;563:915–924. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daskalakis Z.J., Christensen B.K., Fitzgerald P.B., Roshan L., Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemon R.N. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Dahlhaus F., Liu Y., Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H., Gunraj C., Chen R. The effects of inhibitory and facilitatory intracortical circuits on interhemispheric inhibition in the human motor cortex. J Physiol. 2007;580:1021–1032. doi: 10.1113/jphysiol.2006.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fame R.M., MacDonald J.L., Macklis J.D. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasi S., Caminiti R., Innocenti G.M. Areal differences in diameter and length of corticofugal projections. Cerebr Cortex. 2012;22:1463–1472. doi: 10.1093/cercor/bhs011. [DOI] [PubMed] [Google Scholar]
- 35.Hursh J.B. Conduction velocity and diameter of nerve fibers. Am J Physiol. 1939;127:131–139. [Google Scholar]
- 36.Sherman D.L., Brophy P.J. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 37.Hopf H.C. Electromyographic study on so-called mononeuritis. Arch Neurol. 1963;9:307–312. doi: 10.1001/archneur.1963.00460090113014. [DOI] [PubMed] [Google Scholar]
- 38.Stalberg E., van Dijk H., Falck B. Standards for quantification of EMG and neurography. Clin Neurophysiol. 2019;130:1688–1729. doi: 10.1016/j.clinph.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Ingram D.A., Davis G.R., Swash M. Motor nerve conduction velocity distributions in man: results of a new computer-based collision technique. Electroencephalogr Clin Neurophysiol. 1987;66:235–243. doi: 10.1016/0013-4694(87)90072-1. [DOI] [PubMed] [Google Scholar]
- 40.Di Lazzaro V., Profice P., Ranieri F. I-wave origin and modulation. Brain Stimul. 2012;5:512–525. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Di Lazzaro V., Oliviero A., Profice P. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- 42.Ni Z., Charab S., Gunraj C. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol. 2011;105:749–756. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- 43.Di Lazzaro V., Rothwell J.C. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol. 2014;592:4115–4128. doi: 10.1113/jphysiol.2014.274316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris-Love M.L., Perez M.A., Chen R., Cohen L.G. Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. J Neurophysiol. 2007;97:2511–2515. doi: 10.1152/jn.01331.2006. [DOI] [PubMed] [Google Scholar]
- 45.Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch G., Cercignani M., Bonni S. Asymmetry of parietal interhemispheric connections in humans. J Neurosci. 2011;31:8967–8975. doi: 10.1523/JNEUROSCI.6567-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komlosh M.E., Özarslan E., Lizak M.J. Mapping average axon diameters in porcine spinal cord white matter and rat corpus callosum using d-PFG MRI. Neuroimage. 2013;78:210–216. doi: 10.1016/j.neuroimage.2013.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]